Abstract
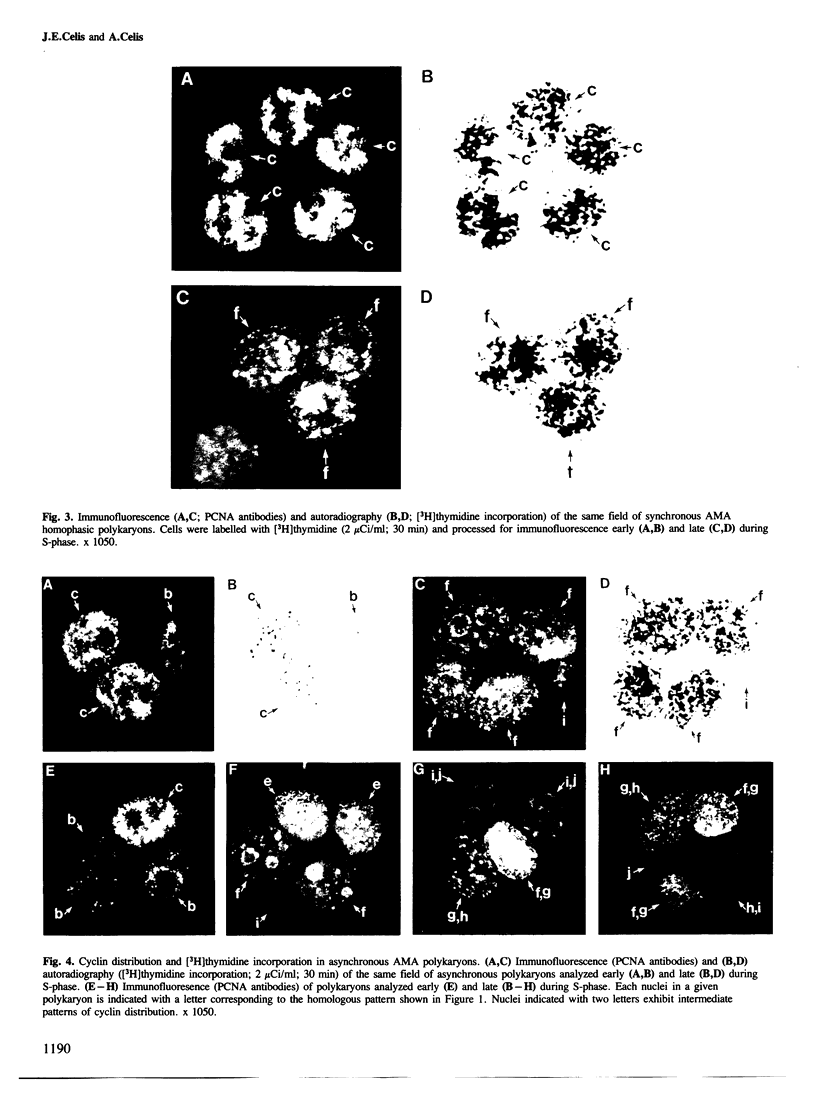
Nuclear patterns of cyclin (PCNA) distribution that subdivide S-phase (determined using PCNA autoantibodies specific for this protein) as well as [3H]thymidine incorporation followed by autoradiography have been used to determine the S-phase synchrony of homophasic polykaryons produced by polyethylene glycol (PEG)-induced fusion of populations of mitotic transformed human amnion cells (AMA) exhibiting the following average distribution of phases: prophase, 9%, metaphase, 60% (including early and late prometaphase), anaphase, 3.8%, telophase, 26.2% and interphase, 1%. Both synchronous and asynchronous polykaryons were generated from these fusions; the latter being frequently observed only amongst populations of multinucleated cells having three or more nuclei. These results are taken to imply that individual nuclei in these polykaryons can control cyclin distribution and DNA synthesis in spite of the fact that they share a common cytoplasm.
Full text
PDF
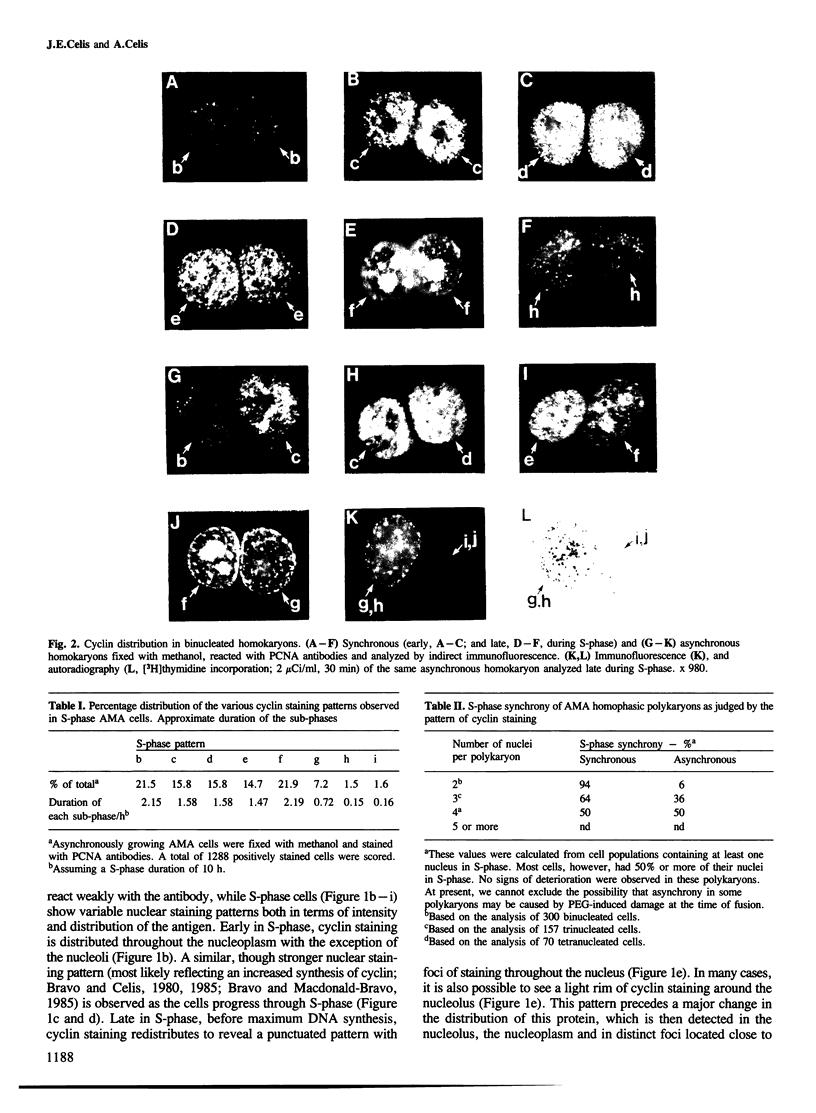



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellatin J., Bravo R., Celis J. E. Changes in the relative proportion of transformation-sensitive polypeptides in giant HeLa cells produced by irradiation with lethal doses of x-rays. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4367–4370. doi: 10.1073/pnas.79.14.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolund L., Ringertz N. R., Harris H. Changes in the cytochemical properties of erythrocyte nuclei reactivated by cell fusion. J Cell Sci. 1969 Jan;4(1):71–87. doi: 10.1242/jcs.4.1.71. [DOI] [PubMed] [Google Scholar]
- Bravo R., Bellatin J., Celis J. E. [35S]-methionine labelled polypeptides from HELA cells. Coordinates and percentage of some major polypeptides. Cell Biol Int Rep. 1981 Jan;5(1):93–96. doi: 10.1016/0309-1651(81)90162-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis A., Mosses D., Celis J. E. Distribution of HeLa cells polypeptides in cytoplasts and karyoplasts. Cell Biol Int Rep. 1981 May;5(5):479–489. doi: 10.1016/0309-1651(81)90175-2. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Changes in the nuclear distribution of cyclin (PCNA) during S-phase are not triggered by post-translational modifications that are expected to moderately affect its charge. FEBS Lett. 1985 Mar 25;182(2):435–440. doi: 10.1016/0014-5793(85)80349-5. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Human proteins sensitive to neoplastic transformation in cultured epithelial and fibroblast cells. Clin Chem. 1982 Apr;28(4 Pt 2):949–954. [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Up-dated catalogue of HeLa cell proteins: percentages and characteristics of the major cell polypeptides labeled with a mixture of 16 14C-labeled amino acids. Clin Chem. 1982 Apr;28(4 Pt 2):766–781. [PubMed] [Google Scholar]
- Bravo R. Coordinated synthesis of the nuclear protein cyclin and DNA in serum-stimulated quiescent 3T3 cells. FEBS Lett. 1984 Apr 24;169(2):185–188. doi: 10.1016/0014-5793(84)80315-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Bellatin J., Larsen P. M., Arevalo J., Celis J. E. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res. 1981 Dec;136(2):311–319. doi: 10.1016/0014-4827(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985 Mar;4(3):655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Induction of the nuclear protein 'cyclin' in quiescent mouse 3T3 cells stimulated by serum and growth factors. Correlation with DNA synthesis. EMBO J. 1984 Dec 20;3(13):3177–3181. doi: 10.1002/j.1460-2075.1984.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. B., Hanks S. K., Murphy E. C., Jr, Rao P. N. Early initiation of DNA synthesis in G1 phase HeLa cells following fusion with red cell ghosts loaded with S-phase cell extracts. Exp Cell Res. 1985 Jan;156(1):251–259. doi: 10.1016/0014-4827(85)90279-4. [DOI] [PubMed] [Google Scholar]
- Burns E. R. Synchronous and asynchronous DNA synthesis in multinucleated Ehrlich ascites tumor cells compared with multinucleated cells cultured from frog lung. Exp Cell Res. 1971 May;66(1):152–156. doi: 10.1016/s0014-4827(71)80023-x. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Bravo R., Larsen P. M., Fey S. J. Cyclin: a nuclear protein whose level correlates directly with the proliferative state of normal as well as transformed cells. Leuk Res. 1984;8(2):143–157. doi: 10.1016/0145-2126(84)90135-8. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Bravo R. Synthesis of the nuclear protein cyclin in growing, senescent and morphologically transformed human skin fibroblasts. FEBS Lett. 1984 Jan 2;165(1):21–25. doi: 10.1016/0014-5793(84)80006-x. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Fey S. J., Larsen P. M., Celis A. Expression of the transformation-sensitive protein "cyclin" in normal human epidermal basal cells and simian virus 40-transformed keratinocytes. Proc Natl Acad Sci U S A. 1984 May;81(10):3128–3132. doi: 10.1073/pnas.81.10.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo H., De Lustig E. S., Schajowicz F. Growth and maintenance of human giant-cell bone tumors (osteoclastomas) in continuous culture. Oncology. 1970;24(2):146–159. doi: 10.1159/000224514. [DOI] [PubMed] [Google Scholar]
- Graves J. A. DNA synthesis in heterokaryons formed by fusion of mammalian cells from different species. Exp Cell Res. 1972 Jun;72(2):393–403. doi: 10.1016/0014-4827(72)90007-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Harris H. DNA synthesis and mitosis in fused cells. 3. HeLa-Ehrlich heterokaryons. J Cell Sci. 1969 Nov;5(3):645–697. doi: 10.1242/jcs.5.3.645. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Harris H. DNA synthesis and mitosis in fused cells. I. HeLa homokaryons. J Cell Sci. 1969 Nov;5(3):603–624. doi: 10.1242/jcs.5.3.603. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Harris H. DNA synthesis and mitosis in fused cells. II. HeLa-chick erythrocyte heterokaryons. J Cell Sci. 1969 Nov;5(3):625–643. doi: 10.1242/jcs.5.3.625. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Mullinger A. M. The induction of DNA synthesis in the chick red cell nucleus in heterokaryons during the first cell cycle after fusion with HeLa cells. J Cell Sci. 1975 Aug;18(3):455–490. doi: 10.1242/jcs.18.3.455. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- Mose-Larsen P., Bravo R., Fey S. J., Small J. V., Celis J. E. Putative association of mitochondria with a subpopulation of intermediate-sized filaments in cultured human skin fibroblasts. Cell. 1982 Dec;31(3 Pt 2):681–692. doi: 10.1016/0092-8674(82)90323-3. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970 Jan 10;225(5228):159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Sandberg A. A., Sofuni T., Takagi N., Moore G. E. Chronology and pattern of human chromosome replication, IV. Autoradiographic studies of binucleate cells. Proc Natl Acad Sci U S A. 1966 Jul;56(1):105–110. doi: 10.1073/pnas.56.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy P. F., Wakonig-Vaartaja T., Winn R., Clarkson B. D. Asynchronous DNA synthesis and asynchronous mitosis in multinuclear ovarian cancer cells. Cancer Res. 1974 May;34(5):991–996. [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M., Gordon L., Beeson M. Carcinogen-transformed human cells are inhibited from entry into S phase by fusion to senescent cells but cells transformed by DNA tumor viruses overcome the inhibition. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5287–5291. doi: 10.1073/pnas.79.17.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki Y., Deng J. S., Tan E. M. A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med. 1981 Dec 1;154(6):1899–1909. doi: 10.1084/jem.154.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerveld A., Freeke M. A. Cell cycle of multinucleate cells after cell fusion. Exp Cell Res. 1971 Mar;65(1):140–144. doi: 10.1016/s0014-4827(71)80059-9. [DOI] [PubMed] [Google Scholar]
- Yanishevsky R. M., Prescott D. M. Late S phase cells (Chinese hamster ovary) induce early S phase DNA labeling patterns in G1 phase nuclei. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3307–3311. doi: 10.1073/pnas.75.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]