Abstract
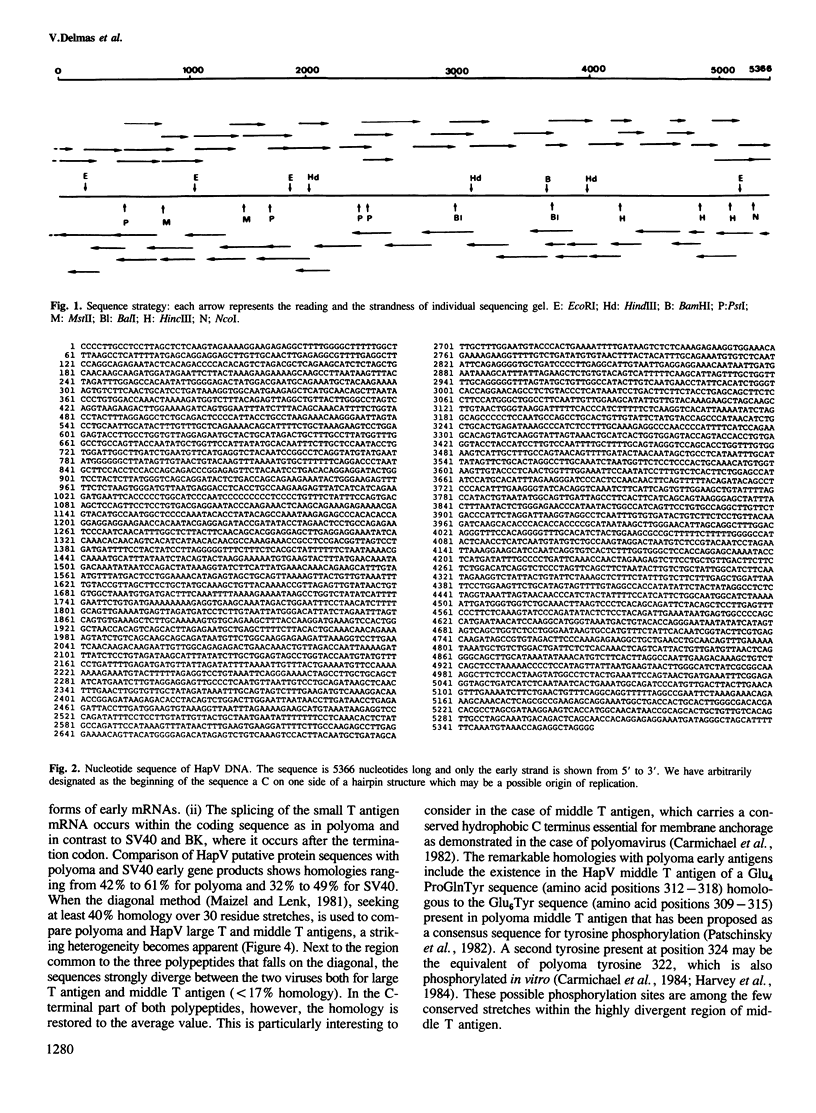
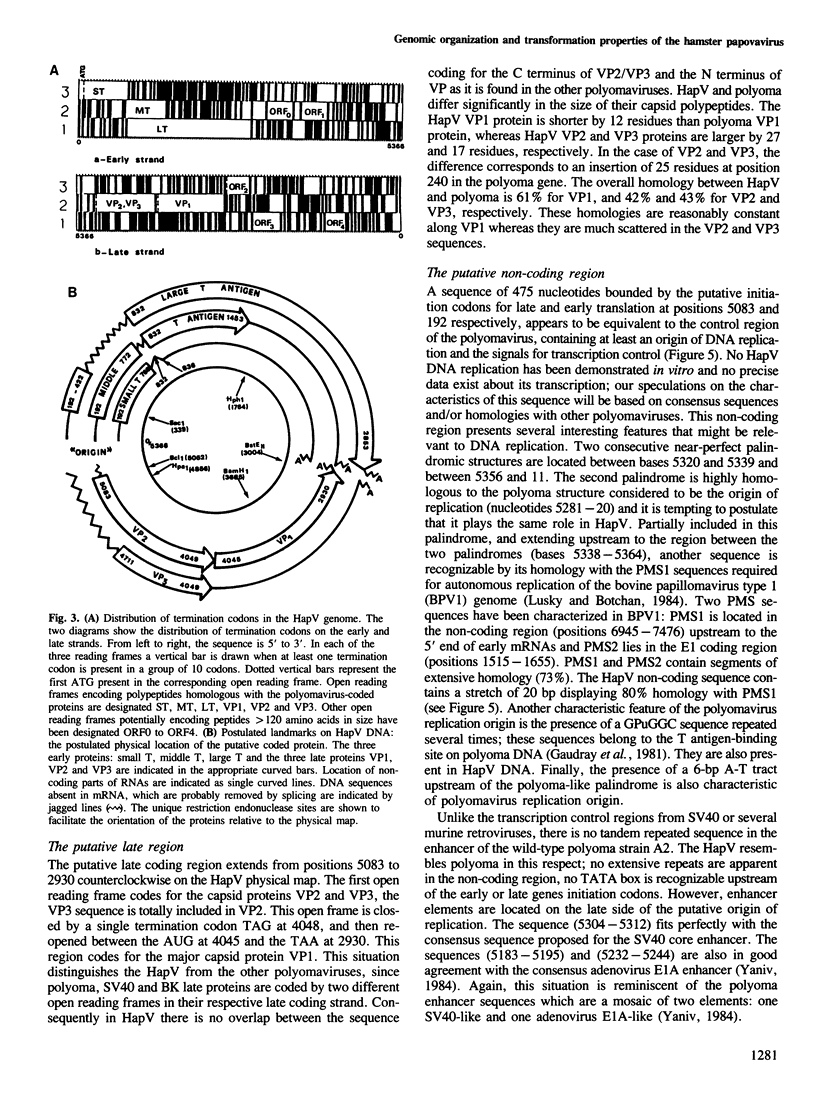
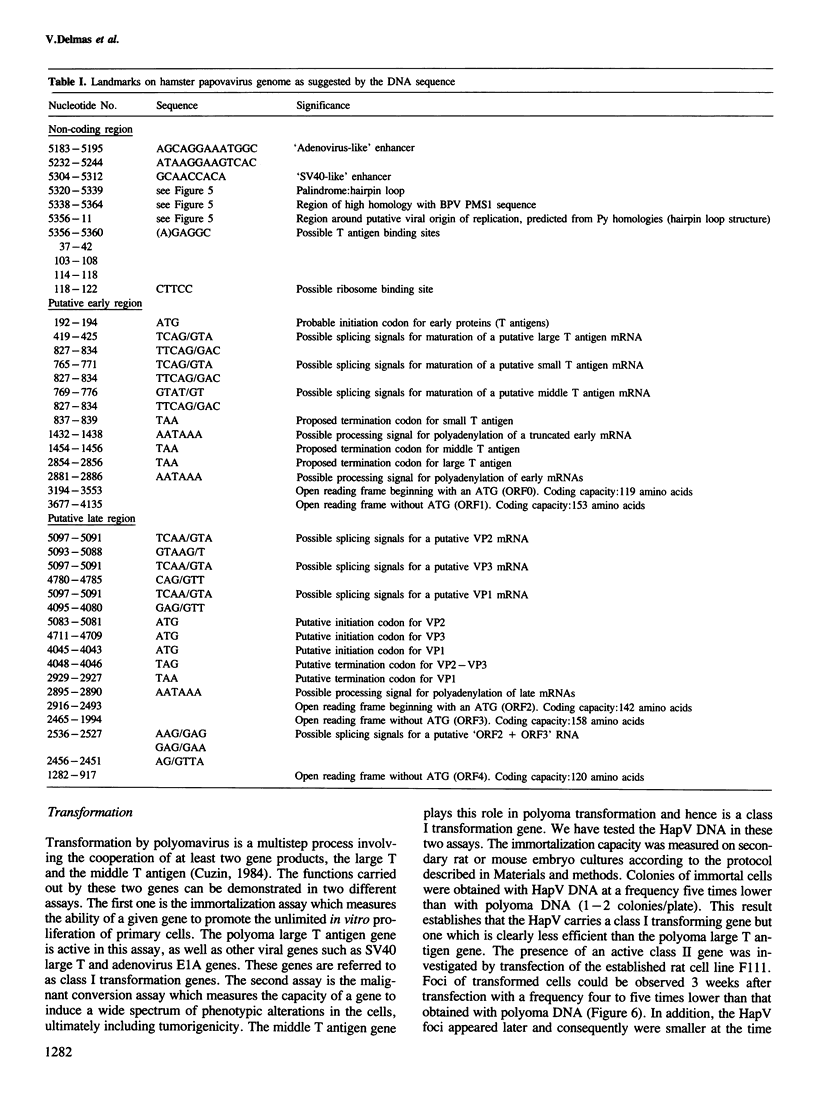
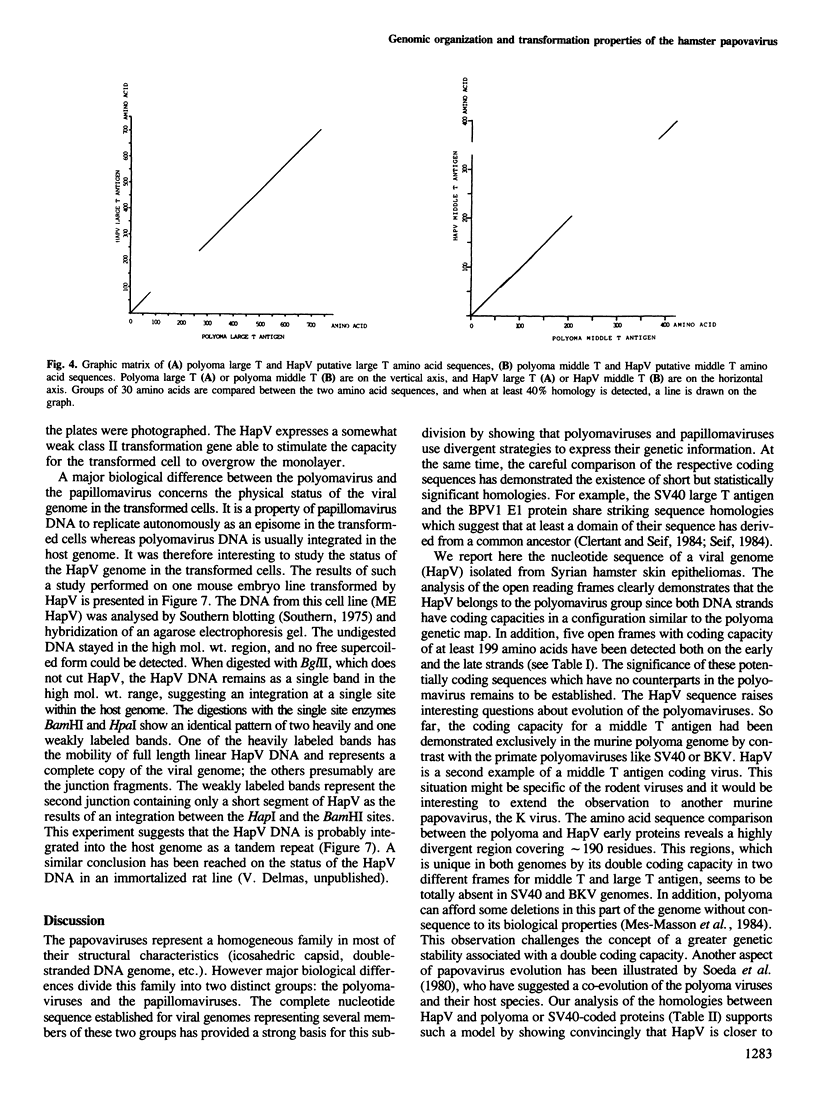
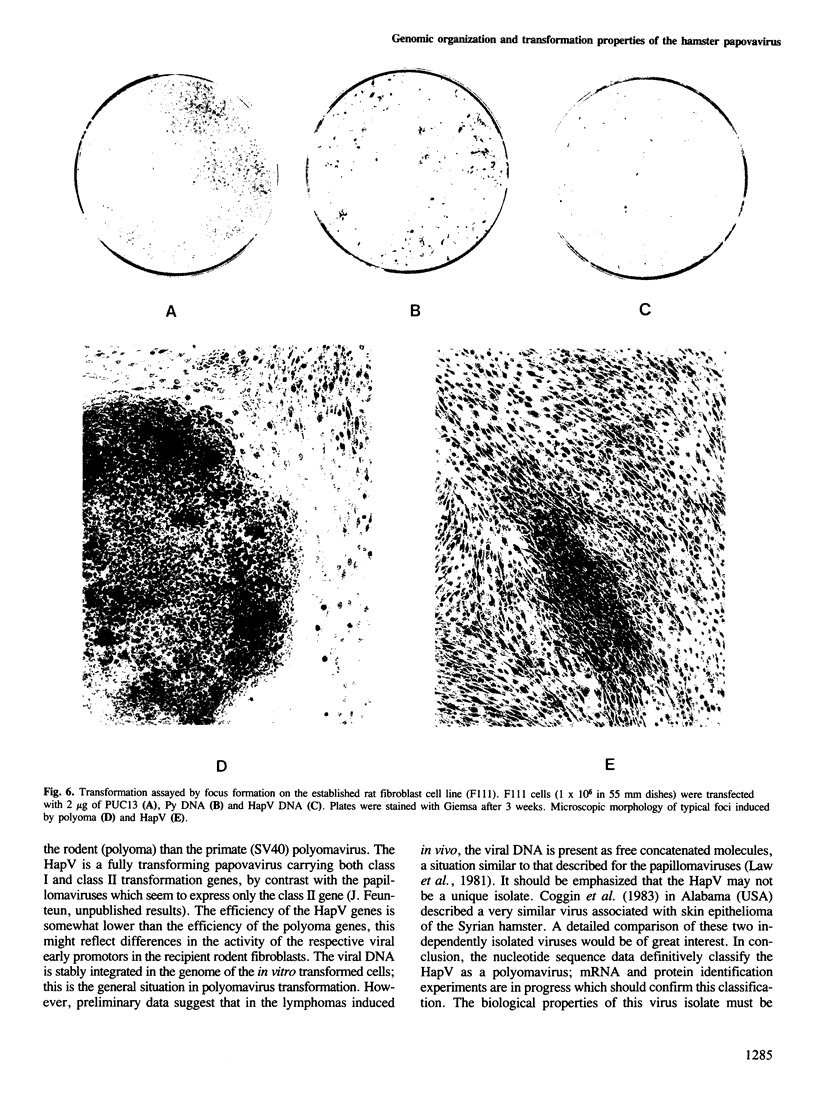
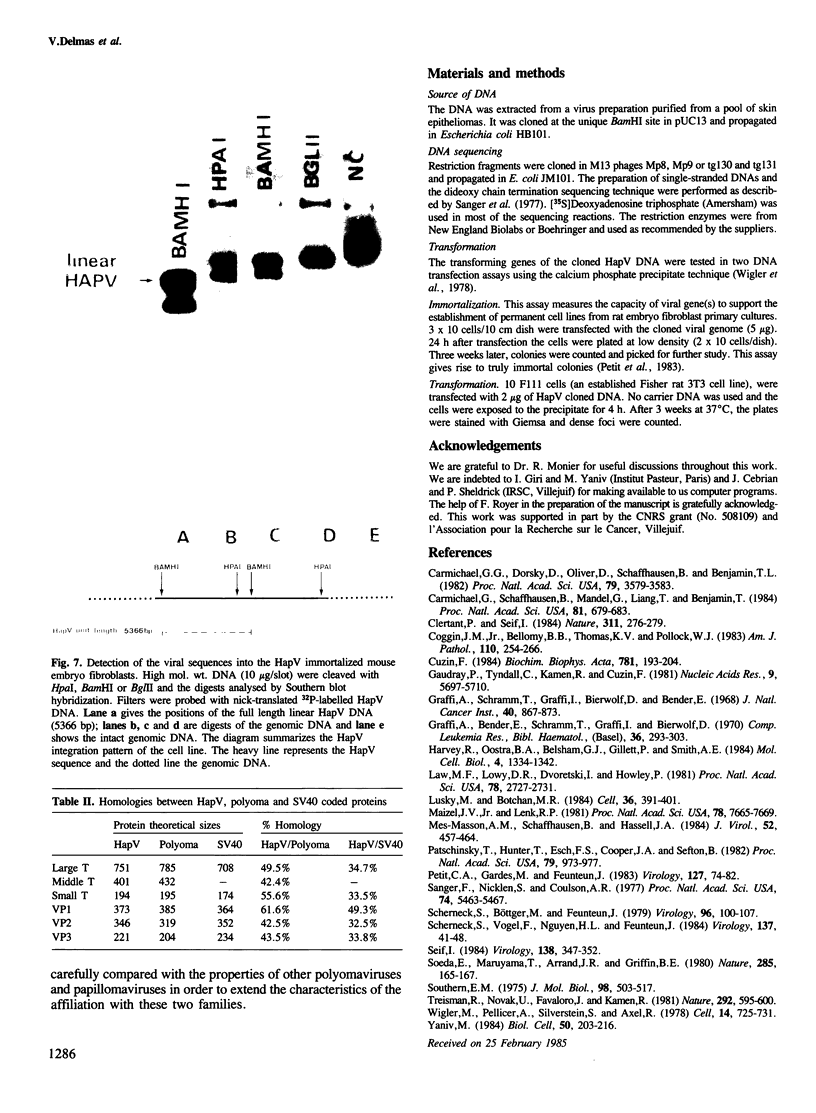
The hamster papovavirus (HapV) is associated with multiple skin epitheliomas of the Syrian hamster. We have sequenced its genome. It is a double-stranded circular DNA of 5366 bp. The hypothetical genomic organization deduced from this nucleotide sequence is clearly of the polyoma type with the two strands coding in the opposite directions from a noncoding region that shows some of the features of a replication origin and a transcription control region. The amino acid sequences predicted from the open reading frames show an average of 50% homology with polyoma-coded polypeptides. The HapV is, after polyoma, the second example of a papovavirus coding for a middle T antigen. The cloned DNA can immortalize primary rat embryo cells and transform an established rat cell line. The viral DNA is stably integrated into the host genome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G., Schaffhausen B. S., Mandel G., Liang T. J., Benjamin T. L. Transformation by polyoma virus is drastically reduced by substitution of phenylalanine for tyrosine at residue 315 of middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1984 Feb;81(3):679–683. doi: 10.1073/pnas.81.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clertant P., Seif I. A common function for polyoma virus large-T and papillomavirus E1 proteins? Nature. 1984 Sep 20;311(5983):276–279. doi: 10.1038/311276a0. [DOI] [PubMed] [Google Scholar]
- Coggin J. H., Jr, Bellomy B. B., Thomas K. V., Pollock W. J. B-cell and T-cell lymphomas and other associated diseases induced by an infectious DNA viroid-like agent in hamsters (Mesocricetus auratus). Am J Pathol. 1983 Mar;110(3):254–266. [PMC free article] [PubMed] [Google Scholar]
- Cuzin F. The polyoma virus oncogenes. Coordinated functions of three distinct proteins in the transformation of rodent cells in culture. Biochim Biophys Acta. 1984 Apr 5;781(3):193–204. doi: 10.1016/0167-4781(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Tyndall C., Kamen R., Cuzin F. The high affinity binding site on polyoma virus DNA for the viral large-T protein. Nucleic Acids Res. 1981 Nov 11;9(21):5697–5710. doi: 10.1093/nar/9.21.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffi A., Bender E., Schramm T., Graffi I., Bierwolf D. Studies on the hamster papilloma and the hamster virus lymphoma. Bibl Haematol. 1970;(36):293–303. doi: 10.1159/000391720. [DOI] [PubMed] [Google Scholar]
- Graffi A., Schramm T., Graffi I., Bierwolf D., Bender E. Virus-associated skin tumors of the Syrian hamster: preliminary note. J Natl Cancer Inst. 1968 Apr;40(4):867–873. [PubMed] [Google Scholar]
- Harvey R., Oostra B. A., Belsham G. J., Gillett P., Smith A. E. An antibody to a synthetic peptide recognizes polyomavirus middle-T antigen and reveals multiple in vitro tyrosine phosphorylation sites. Mol Cell Biol. 1984 Jul;4(7):1334–1342. doi: 10.1128/mcb.4.7.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes-Masson A. M., Schaffhausen B., Hassell J. A. The major site of tyrosine phosphorylation in polyomavirus middle T antigen is not required for transformation. J Virol. 1984 Nov;52(2):457–464. doi: 10.1128/jvi.52.2.457-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Esch F. S., Cooper J. A., Sefton B. M. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C. A., Gardes M., Feunteun J. Immortalization of rodent embryo fibroblasts by SV40 is maintained by the A gene. Virology. 1983 May;127(1):74–82. doi: 10.1016/0042-6822(83)90372-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherneck S., Böttger M., Feunteun J. Studies on the DNA of an oncogenic papovavirus of the Syrian hamster. Virology. 1979 Jul 15;96(1):100–107. doi: 10.1016/0042-6822(79)90176-4. [DOI] [PubMed] [Google Scholar]
- Seif I. Sequence homology between the large tumor antigen of polyoma viruses and the putative E1 protein of papilloma viruses. Virology. 1984 Oct 30;138(2):347–352. doi: 10.1016/0042-6822(84)90359-3. [DOI] [PubMed] [Google Scholar]
- Soeda E., Maruyama T., Arrand J. R., Griffin B. E. Host-dependent evolution of three papova viruses. Nature. 1980 May 15;285(5761):165–167. doi: 10.1038/285165a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Yaniv M. Regulation of eukaryotic gene expression by transactivating proteins and cis acting DNA elements. Biol Cell. 1984;50(3):203–216. doi: 10.1111/j.1768-322x.1984.tb00268.x. [DOI] [PubMed] [Google Scholar]