Abstract
Cardiovascular disease is a recognized contributor to the pathogenesis of Alzheimer’s disease (AD). Heart failure (HF) is a cardiovascular subtype that can be used to model the contribution of cardiovascular disease to AD. Neuroimaging research indicates that HF patients exhibit a diverse range of structural brain alterations and epidemiological studies suggest HF may be an important risk factor for AD. The neural alterations observed in HF may overlap with those observed in AD and contribute to increased risk of AD in HF patients. To examine this possibility, we reviewed structural MRI studies in persons with HF. We examined subcortical brain regions affected in the early stages of AD (medial temporal lobes), as well as cortical alterations that typically occur in the later stages of AD. Our review indicates that patients with HF exhibit greater neural atrophy and white matter microstructural alterations of nearly every region of the Papez circuit (e.g., hippocampus, cingulate gyrus, thalamus, mammillary bodies, and fornix), as well-significant alterations in cortical and cerebellar regions. Based on animal research and past work in AD patients, the mechanisms for structural brain changes in HF may stem from reductions in cerebral blood flow subsequent to cardiac deficiency. This review supports the hypothesis that HF may contribute to AD risk via widespread structural brain changes, including many of the same regions affected by AD. Case-controlled prospective neuroimaging studies with long-term follow-ups are needed to clarify the risk of AD in HF and elucidate the neural underpinnings of AD risk in HF.
Keywords: Heart failure, Alzheimer’s disease, Cerebrovascular disease, MRI, DTI, Medial temporal lobe
Introduction
Heart failure (HF) affects more than 5 million American adults with 825,000 new cases diagnosed each year [1]. HF prevalence rates are estimated to increase by 46 % over approximately the next 15 years due to the rising rates of cardiovascular disease risk factors that precede HF (e.g., hypertension, diabetes, obesity) [1]. HF is also most common in older adults, and thus, HF occurrence in this cohort will most certainly rise in the upcoming years in light of the forecasted estimates that 20 % of the US populations will be >65 years by 2030. This pattern is troubling, as HF is associated with elevated mortality risk, recurrent hospital readmissions, and decreased functional independence [1–3]. Although the prevalence and prognostic outcomes of HF vary across HF subtypes (e.g., left vs. right; congestive HF), extant studies do not consistently classify study participants by HF subtypes. Therefore, in the current review, the term HF is used broadly, encompassing multiple distinct forms of cardiac failure.
HF in older adults exacerbates the risk of neurological conditions that accompany aging, including vascular dementia [4] and Alzheimer’s disease (AD) [5]. Vascular dementia is primarily characterized by cognitive decline in a stepwise manner, a temporal relationship between cerebrovascular disease and cognitive impairment, and neuroimaging and clinical evidence of cerebrovascular disease. The neurocognitive profile of vascular dementia is typically characterized by impairments in processing speed and executive function, as well as memory, although the specific domains associated with CVD ultimately depend on the brain regions impacted by disease-related neuropathology (i.e., single or multiple large cortical infarcts, small vessel ischemic damage, or lacunar infarcts). In contrast, AD has a progressive, gradual onset. Impairment in episodic memory (rapid forgetting of novel information) is the hallmark symptom of AD, along with difficulties in expressive language, with subsequent decline in executive functions and visuospatial abilities as AD progresses. Pure AD is believed to involve accumulation and poor clearance of amyloid-beta and neurofibrillary tangles in the absence of cerebrovascular insult. Nevertheless, 20–40 % of dementia cases in older adults are considered mixed AD and vascular dementia (e.g., combination of ischemic lesions and AD pathology) [6].
Given the cardiovascular nature of HF, it is not surprising that HF is indeed a well-established risk factor for neurological insult that underpins vascular dementia such as stroke and micro- and macrovascular diseases. Yet, substantial evidence shows that sporadic AD is often preceded by cardiovascular disease [7] and there is evidence that suggests cardiovascular disease may trigger AD-specific pathology. For example, cardiovascular disease and related risk factors predict plasma Abeta40 levels [8], as well as increased amyloid-beta burden, in the brain [9, 10]. HF, in particular, is one cardiovascular subtype that has been used to model the contribution of cardiovascular disease to AD risk, and emerging evidence suggests that HF is an independent risk factor for AD [5]. For instance, in a large population-based study (>6000 participants), relative to older adults without baseline HF, those with baseline HF incidence exhibited nearly a twofold increased risk of developing probable AD, as defined by gradual onset, progressive worsening, and lack of any other causes of dementia [5]. Prior to the onset of dementia, nearly 80 % of patients with symptomatic HF also demonstrate a pattern of mild forms of cognitive impairment [11] that is similar to what is often observed in patients with AD. Patients with chronic HF exhibit performance reductions on tasks tapping episodic memory and executive functions relative to healthy and medical controls [12]. Furthermore, progressive worsening is also observed within the domains of episodic memory, as well as executive functions in HF patients [13–16]. Taken together, there is reason to believe that HF increases risk of AD because: (1) HF has been shown to increase risk of AD in the absence of any other causes of dementia, and (2) HF patients exhibit progressive cognitive decline, including within the domain of episodic memory, which is considered a hallmark of AD (as opposed to a stepwise pattern of cognitive decline seen in vascular dementia) [17].
Neural underpinnings of Alzheimer’s disease: role of cerebral hypoperfusion
AD risk and related cognitive impairments in HF may in part stem from the negative and independent physiological effects of cardiac dysfunction on the brain, particularly of those regions implicated in AD. The neural underpinnings of AD involve adverse structural brain changes to the medial temporal lobe (MTL), in addition to other subcortical and cortical structural changes [18–20]. Functional brain alterations are also associated with AD [21, 22], which may contribute to the observed structural changes in AD. There is some evidence in the animal literature to support this notion. For instance, induction of chronic cerebral hypoperfusion (via bilateral placements on the carotid arteries in mice) leads to white matter changes in the short term with longer-term changes including hippocampal atrophy and neuronal death [23]. The histological changes also resulted in behavioral impairments, such as deficits in working and reference memory (memory for constant, stable information).
While ischemic-producing reductions in cerebral perfusion (e.g., stroke) often underpin vascular dementia, cerebral blood flow also declines with increasing age and such gradual disruptions in cerebral hemodynamics appear to play a critical role in the pathogenesis of AD [7]. For example, AD patients exhibit global cerebral blood flow reductions that worsens with disease progression relative to controls [24, 25]. Regional cerebral blood flow has also been identified as a clinical predictor of the development of AD, as decreases in MTL perfusion (hippocampus and amygdala) predict conversion from questionable AD to AD [26]. Although some controversy remains regarding whether cerebral hypoperfusion is a cause or consequence of AD [27], growing research suggests that cerebral hypoperfusion is at least one critical contributor to AD-related structural brain changes [25, 27, 28]. Disturbances in hemodynamics can lead to glucose and oxygen deprivation, which are critical for normal brain cell function. Alterations of glucose and oxygen can in turn lead to a cascade of biochemical disturbances that ultimately lead to metabolic and tissue damage, including alterations to hallmark brain regions of AD such as the hippocampus—a region highly sensitive to hypoxic episodes [25, 29]. These biochemical changes can trigger neurodegeneration and cognitive decline in AD.
Cerebral hypoperfusion in heart failure
Based on these findings, it is plausible that factors that negatively impact cerebral perfusion in older adults may increase AD risk. The critically attained threshold of cerebral hypoperfusion (‘CATCH’) theory states that aging in conjunction with vascular risk factors leads to chronic cerebral hypoperfusion that eventually falls below a critical threshold and triggers glucose and oxygen deprivation [30]. HF appears to be one putative factor that may cause brain perfusion to drop below this critical threshold to increase risk of AD. Reduced cardiac pumping efficiency in patients with HF leads to decreased forward outflow of blood from the heart resulting in a decline in perfusion of blood to the rest of the body, including the brain. Cerebral blood flow reductions from cardiac inadequacy are further compounded by the negative effects of comorbid medical and clinical conditions (e.g., hypertension, diabetes, sleep apnea, and depression) on the integrity and plasticity of the arterial structure.
In patients with HF, there is evidence for reduced cerebral blood flow to bilateral hippocampus, parahippocampal gyrus, and right posterior cingulate cortex [31, 32], regions often associated with AD. There is also evidence of reduced global brain perfusion in HF, which has been observed in AD. For instance, patients with chronic HF had up to 31 % reduction in resting cerebral blood flow relative to age-matched healthy controls [33]. Another study showed that patients with mild-to-moderate HF had reduced blood flow velocity of the middle cerebral artery relative to healthy controls, 47.3 versus 56.1 cm/s, respectively [34].
Objective of the current review
The negative impact of HF on cerebral hemodynamics may yield structural brain changes that contribute to the increased risk of AD in older adults with HF. Here, we reviewed structural neuroimaging studies of HF patients to assess whether brain changes that are evident in HF overlap with brain alterations observed in AD. First, we review the neuroimaging literature in HF with a focus on subcortical brain regions affected in the early stages of AD [35–37] such as the MTL (hippocampus, parahippocampal gyrus, and amygdala), as well as other structures within the Papez circuit that are closely connected to the medial temporal lobes (fornix, thalamus, mammillary bodies, and the cingulate gyrus). We then review cortical alterations, which are typically affected in the later stages of AD, in patients with HF. Finally, we discuss the possible mechanisms of brain alterations in HF, particularly as it relates to AD, and propose directions for future research.
Literature review criteria
To identify relevant articles, a literature search was performed on PubMed using key words such as “heart failure,” “neuroimaging,” “MRI,” “DTI,” “brain,” “white mater hyperintensities,” or “Alzheimer’s disease.” Reference sections of articles were also examined to identify additional neuroimaging studies in HF. Study inclusion criteria included a sample mean left ventricular ejection fraction of <40 % and human HF participants with a mean age ≥50 years. In the review of structural neuroimaging studies in HF, only those that employed healthy and/or noncardiac controls were included in order to determine whether abnormal brain changes in HF extend beyond those associated with the normative aging process. For most studies, HF participants were excluded for a past history of stroke or infarct observed on participants’ brain image, with two exceptions. One study [38] had one HF patient with a history of stroke, but it did not result in residual motor symptoms. Another study examined global hippocampal volume, but also examined hippocampal infarcts [39]. Overall, there was minimal evidence for stroke in the neuroimaging studies reviewed here, indicating that noted brain alterations are unlikely attributable to processes most commonly associated with vascular dementia (e.g., large cortical or subcortical infarcts). All studies included participants with systolic dysfunction, and most samples were participants with stable HF. Some studies also involved participants with congestive HF, and while clinical outcomes (including cognitive) for this subgroup may be distinct from clinically stable HF patients, the mechanisms and effects of reduced cardiac output on the brain likely remain similar. We provide the specific characteristics of the HF samples when available.
Neuroimaging in heart failure
A variety of structural neuroimaging methods have been implemented to examine brain alterations associated with HF. Studies have most commonly used MRI T1-weighted imaging to examine volume and gray matter density, with additional studies using fluid attention inversion recovery (FLAIR), proton density, MRI T2 relaxometry, and diffusion tensor imaging (Table 1). A combination of image processing approaches has been employed to detect brain changes on MRI scans in HF, including voxel-based morphometry, manual tracing of region of interests, and semiquantitative visual assessment methods (e.g., Scheltens rating scale). MR sequence type and analysis approach are described for each study.
Table 1.
Structural neuroimaging studies of older adults with HF
| Author | Year | Imaging modality | HF participants (healthy/noncardiac control group) | Mean age of HF patients (SD) | % Female | Mean LVEF, % |
|---|---|---|---|---|---|---|
| Almeida et al. | 2012 | T1 | 35 (64) | 69.2 (±9.0) | 22.9 | 30.4 |
| Almeida et al.a | 2013 | T1 | 19 (65) | 67.7 (±8.9) | 26.3 | 33.1 |
| Alves et al.b | 2005 | T2, FLAIR | 17 (18) | 73.7 (±5.4) | 47.1 | 39.8 |
| Kumar et al. | 2009 | T1 | 17 (50) | 54.4 (±8.1) | 29.4 | 28.0 |
| Kumar et al. | 2011 | DTI | 16 (26) | 55.1 (±7.8) | 25.0 | 27.7 |
| Pan et al. | 2013 | T1, PD | 17 (50) | 54.4 (±8.1) | 29.4 | 28.0 |
| Vogels et al. | 2007 | T1, T2, FLAIR | 58 (42) | 68.7 (±9.1) | 26.0 | 27.0 |
| Woo et al. | 2003 | T1 | 9 (27) | 51.0 (±10.0) | 33.3 | 27.0 |
| Woo et al. | 2009 | T2 relaxometry | 13 (49) | 54.6 (±8.3) | 30.8 | 28.0 |
HF heart failure, PD proton density, FLAIR fluid-attenuated inversion recovery, DTI diffusion tensor imaging, fMRI functional magnetic resonance imaging
This study reports cross-sectional and longitudinal findings
a subsample of HF (n = 10) and healthy control (n = 14) participants completed MRI protocols
Papez circuit alterations in HF
Medial temporal lobes
Neuroimaging studies in patients with HF demonstrate structural alterations within the MTL that are similar to what is observed in AD [40] (Fig. 1a–d). In a relatively large study, Vogels et al. [41] used a visual rating scale of 0 to 4 (higher scores representative of more atrophy) to quantify global MTL atrophy in 58 patients with stable HF and 42 age-matched healthy controls. After adjustment for demographic (e.g., age, gender) and medical variables (e.g., hypertension, diabetes), greater MTL atrophy was observed in HF participants [mean (SD) = 1.1 (0.9)] relative to healthy controls [mean (SD) = 0.4 (0.6)]. This study provided some of the first evidence that HF was associated with decline in a region commonly associated with memory function and implicated in AD.
Fig. 1.
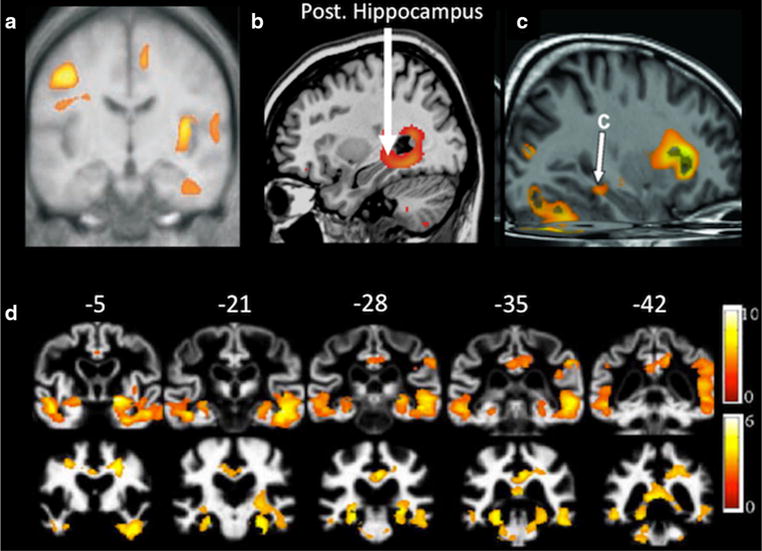
Comparison of MTL atrophy in patients with HF and AD. a Rostral coronal view of greater gray matter loss in the inferior temporal lobe of the parahippocampal and fusiform gyri in HF patients relative to controls (adapted from Figure 2 published in Journal of Applied Physiology, 95, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM, Regional brain gray matter loss in heart failure, 677–684, 2003). b Higher T2 relaxation values observed in the posterior hippocampus of HF patients relative to controls (adapted from Figure 4 published in Journal of Cardiac Failure, 15, Woo, MA, Kumar R, Macey PM, Fonarow GC, Harper RM, Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure, 214–223, 2009). c Increase axial diffusivity of the ventral cingulum bundle and hippocampus in HF patients as compared to controls (adapted from Figure 2 published in Journal of the Neurological Sciences, 307, Kumar R, Woo MA, Macey RM, Fonarow GC, Hamilton MA, Harper RM, Brain axonal and myelin evaluation in heart failure, 106–113, 2011). d Gray matter (a) and white matter (b) loss in patients with AD relative to controls (adapted from Figure 2 published in Journal of Alzheimer’s Disease, 19, Canu E, McLaren DG, Fitzgerald ME et al., Microstructural diffusion changes are independent of macrostructural volume loss in moderate to severe Alzheimer’s disease, 963–976, 2010, with permission from IOS press)
To further clarify these findings, subsequent studies of HF patients have examined the structural integrity of specific MTL structures, which are differentially affected by aging [42] and AD [43]. Pan et al. [39] implemented visual assessment rating procedures to examine hippocampal atrophy in stable HF patients. The visual assessment grading scale consisted of 0 = no atrophy to 3 = severe atrophy. HF patients exhibited greater right, but not left-sided, hippocampal atrophy relative to healthy controls. Specifically, patients with HF exhibited an average right hippocampal grade of 1.53 (SD = 0.94), while healthy controls had an average grade of 0.80 (SD = 0.86). Another study using voxel-based T2 relaxometry also demonstrated higher T2 values (representative of cell body/membrane damage) in the hippocampus in persons with stable HF relative to age-matched controls [44]. In addition to the above cross-sectional findings, a recent longitudinal study used voxel-based morphometry and revealed greater gray matter loss of the left and right parahippocampal gyri over a two-year period in HF patients compared to their healthy counterparts using T1-weighted MRI [45]. Regional volumetric differences in the hippocampus and surrounding regions in patients with HF relative to controls are presented in Fig. 1a–c.
Structural alterations in HF patients have also been observed in critical MTL white matter pathways. For instance, using diffusion tensor imaging, Kumar et al. [46] observed increased axial diffusivity, considered to represent axonal injury, in white matter pathways such as the fornix and cingulum bundle in hemodynamically stable patients with HF (Fig. 1c) relative to healthy controls.
Cingulate gyrus
Patients with HF also exhibit abnormalities of limbic lobe structures such as the cingulate gyrus. For instance, greater atrophy of the anterior, subgenu, and posterior cingulate in stable HF patients compared to healthy control participants has been observed in voxel-based T2-relaxometry studies [38, 44]. Consistent with these findings, the anterior cingulate gyrus was one of two subcortical structures in which volume loss was observed in a sample of 35 patients with stable HF [47]. There is also evidence for greater atrophy of the left and right posterior cingulate over a two-year period when compared to healthy controls [45]. Diffusion tensor imaging studies further demonstrate reduced white matter integrity to the cingulate cortices in persons with HF relative to age-matched controls [46].
Mammillary bodies and fornix
Kumar et al. [48] conducted region of interest analyses on limbic system structures such as the mammillary bodies and the fornix. Mammillary body volumes were quantified using manual tracing procedures, and voxel counts were used to calculate fornix cross-sectional areas. Relative to healthy controls, medically treated HF patients exhibited smaller volume of both the left and right mammillary bodies and fornix cross-sectional areas; these effects remained significant after age, gender, and total intracranial volume were taken into account. Using a 0 (normal) to 3 (mostly decreased) visual grading scale, Pan et al. [39] also revealed smaller right mammillary body volume in HF [mean (SD) grade = 1.18 (1.13)] versus healthy controls [mean (SD) grade = 0.52 (0.74)]. In addition, higher T2 relaxation values have been documented in the output fibers of the fornix to the septum [44].
Thalamus
There is recent evidence of structural decline in thalamic nuclei in persons with HF. Woo et al. [44] employed voxel-based relaxometry in stable HF patients and healthy controls and found HF persons exhibited higher T2 relaxation values of an area extending from the septum to the anterior thalamus (as well as to the hypothalamus and cortical areas that influence hypothalamic activity). Patients with HF have also been shown to exhibit both higher axial and radial diffusivity of the bilateral anterior thalamus [46]. In addition to the anterior thalamus, smaller volume of the right and left thalamus and the right dorsal midbrain and into the posterior and right medial thalamus has also been observed in HF patients versus healthy controls [38, 45].
Summary
Relative to control participants, persons with HF exhibit structural alterations of nearly every region of the Papez circuit. MTL and related limbic system structures have integral roles in episodic memory abilities [49–51] and have been proposed to serve as diagnostic markers of early AD [52, 53]. These findings raise the possibility that HF may increase AD risk via insult to subcortical brain structures that are typically affected in the early stages of AD.
Cortical and cerebellar alterations in HF
As shown in Fig. 2, patients with HF exhibit patterns of cortical alterations that overlap with cortical alterations observed in AD [54], including lateral temporal and parietal regions. Next, we review cortical and cerebellar changes in HF.
Fig. 2.
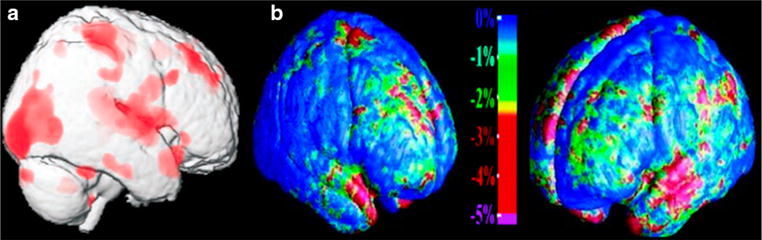
Comparison of cortical atrophy in patients with HF and AD. a HF patients exhibit greater cortical atrophy relative to controls (adapted from Figure 6 published in Journal of Applied Physiology, 95, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM, Regional brain gray matter loss in heart failure, 677–684, 2003). b Cortical atrophy in AD (adapted from Figure 4 published in Journal of Neuroscience, 23, Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW, Dynamics of Gray Matter Loss in Alzheimer’s Disease, 994–1005, 2003)
Frontal lobe
Almeida et al. [47] observed that when compared to healthy controls, atrophy was particularly prevalent in the right inferior frontal gyrus, left and middle superior frontal gyri, the right middle frontal gyrus, and the precentral frontal gyri of HF patients. Voxel-based morphometry analytic approaches have also demonstrated smaller volumes in HF patients compared to healthy individuals of the right caudal orbitofrontal cortex and the ventral and superior lateral frontal cortex [38]. Likewise, left frontal cortical atrophy, as examined by visual assessment, has also been found in HF patients relative to controls [39]. MRI T2-relaxometry studies have provided evidence for left and right atrophy of the ventral medial prefrontal cortices in this population [44], and myelin damage (i.e., increased radial diffusivity) has also been observed in the bilateral frontal regions in persons with HF when compared to controls [46].
Temporal lobe
There is widespread volume loss of the temporal lobe cortices in HF. Neuroimaging studies that utilized voxel-based morphometry have revealed specific gray matter loss in the following temporal lobe regions among patients with HF: the right superior temporal lobe gyrus, right middle temporal lobe gyrus, and the inferior temporal lobe cortical areas that surround the hippocampus and extend into the parahippocampus and fusiform gyrus [38, 47]. A longitudinal study also suggests the possibility of accelerated atrophy of the left superior and middle temporal gyri [45]. Myelin damage has also been observed in the right superior temporal white matter of HF patients relative to control subjects [46].
Parietal lobe
There is bilateral volume loss of the parietal and lateral parietal-occipital cortex and regions involving the left precuneus in patients with HF compared to their healthy peers [38, 47]. A study examining brain changes over a two-year time period in HF and healthy individuals also found greater gray matter loss in the right inferior parietal lobule in HF patients [45]. Reductions in white matter microstructure are also evident in the bilateral parietal lobes among HF patients relative to healthy controls [46].
Occipital lobe
To date, there is limited evidence for structural alterations of the occipital lobe in HF patients. One study has reported reduced white matter microstructure in bilateral occipital regions in HF patients relative to control participants [46].
Cerebellum
Cerebellum volume is decreased in HF [38], and T2 relaxation values are higher in the cerebellum among HF patients relative to controls [44]. Regional volume reductions of the cerebellum have been found among the bilateral quadrangular lobules, right fastigial, and deep cerebellar nuclei [38]. Reductions in white matter integrity have been reported in right cerebellar culmen and quadrangular lobule, left pyramid of the vermis and inferior cerebellar peduncle, and caudal cerebellar cortex [46].
Summary
HF patients exhibit reductions in cortical volume and white matter microstructure throughout the cortex, although few studies have reported volumetric reductions in occipital regions. The specific overlap of neural alterations observed in AD and those observed in HF is difficult to assess quantitatively due to the fact that most extant HF studies do not report findings using a specific neuroanatomical template space (e.g., MNI atlas) [55]. Nevertheless, qualitatively, it appears that many of the same neural regions associated with AD are also impacted by HF. It is important to note that reduced cortical volume and white matter alterations are not necessarily specific to AD. In fact, because cortical changes occur in the later stages of AD and the neuroimaging studies reviewed here included nondemented persons, the cortical alterations may reflect non-AD pathology. As such, the additive cortical insult compounded with subcortical alterations to regions sensitive to AD pathology among patients with HF indeed raises the possibility of a mixed dementia etiology.
Discussion
Persons with HF have long been known to be at increased risk of vascular dementia. Cardiovascular disease is also a recognized contributor to AD pathogenesis, and emerging research links HF in particular with increased AD risk. Recent studies have employed a range of structural neuroimaging modalities to clarify the mechanisms for poor neurological outcomes in HF. Findings from these studies show that HF patients exhibit brain alterations in many of the same brain regions impacted by AD, including volume reductions and reduced white matter integrity of Papez circuit structures, as well as other subcortical and cortical regions. Several aspects of these findings warrant discussion.
HF increases risk of vascular dementia via ischemic injury (i.e., single or multiple large cortical infarcts, lacunar infarcts, small vessel disease). It is possible that cerebrovascular disease in HF superimposed on preexisting AD risk factors (e.g., older age, genetics) leads to earlier onset of AD pathology relative to those without HF. This cascade of events would be consistent with a mixed dementia etiology. Alternatively, it is also plausible that the pathophysiological effects of age-related declines in cerebral perfusion on HF independently increase AD risk through mechanisms not yet known, but may involve disruptions in the blood–brain barrier. For example, reduced cerebral blood flow has been shown to alter cerebral beta amyloid metabolism, a hallmark AD pathology, in HF-induced mice [56]. Therefore, alterations in amyloid metabolism in HF may indeed stem from the negative impact of cerebral hypoperfusion on the blood–brain barrier and subsequent dysfunction in amyloid-beta clearance that ultimately triggers a cascade of molecular events (i.e., amyloid cascade hypothesis of AD) and leads to AD-related neurodegeneration (see Erickson and Banks [57] for a review of blood–brain barrier disruption and AD).
Studies in humans and rodents support the notion that cerebral hypoperfusion is one important contributor to structural brain changes in HF. Moreover, cross-sectional and longitudinal work shows that cerebral hypoperfusion in HF predicts poorer cognitive performance, including within the domain of episodic memory [58–60]. Cerebral hypoperfusion in HF is likely a manifestation of cardiac deficiency and reduced forward blood outflow. However, HF is also typically accompanied by vascular comorbidities such as hypertension, type 2 diabetes mellitus, obesity, and sleep apnea. These conditions can also negatively impact cerebral blood flow in HF not only via suppressed cardiac function, but also through micro- and macrovascular insults. Taken together, the extant evidence for cerebral hemodynamic disturbances in HF supports the notion that AD risk and accelerated cognitive declines in HF may involve gradual deterioration in cerebral perfusion levels.
The impact of cerebral blood flow on the brain in HF remains poorly understood due to the reliance on indirect assessment of blood perfusion (i.e., transcranial Doppler), rather than the direct assessment of cerebral blood flow using techniques such as arterial spin labeling MRI, a cost-effective and noninvasive assessment of brain perfusion that allows for regional examination of cerebral blood flow [25, 61]. Regional examination of cerebral blood flow in HF patients may clarify whether perfusion is reduced among brain regions affected in patients with AD (e.g., MTL) [25, 61]. The lack of functional MRI studies in HF is also noteworthy, as functional MRI is a useful biomarker for AD [62]. Functional MRI detects alterations in blood oxygenation, which is associated with neural activity [63], and is often used to detect activation in response to a stimulus [64] or network connectivity [62]. To better understand the neural correlates of memory impairment in AD, functional MRI has been used to examine alterations in neural activation in regions typically associated with AD, such as the hippocampus and parahippocampal gyrus, as well as brain regions not historically associated with AD (e.g., the precuneus) [22]. Although functional MRI has been used to examine the neural substrates of autonomic nervous system impairments among small samples of HF patients [65, 66], no studies of HF patients have examined functional MRI activity associated with task performance (e.g., during episodic memory encoding or retrieval [67, 68] or large-scale patterns of fMRI connectivity) [50]. Alterations of functional MRI activity in HF seem likely given that a recent functional MRI study demonstrated that cardiovascular disease risk factors (e.g., hypertension, diabetes) are associated with task-related hyperactivation of the inferior parietal lobe. The functional MRI activity was correlated with worse performance on a task of executive functions [69] and interpreted as a reduction in neural efficiency.
Lastly, it is possible that cerebral hypoperfusion may lead to structural brain alterations, which in turn lead to cognitive impairment and increased risk of AD in HF patients [70, 71]. However, no single study in humans has confirmed each of these links. If confirmed, such findings may have significant therapeutic implications. In particular, structured exercise is a highly recommended noninvasive behavioral treatment recommendation for the management of HF that improves vascular health. Such vascular benefits may subsequently lead to enhanced cerebral perfusion, thereby minimizing reductions in neural integrity, and positively impact cognition and attenuate dementia risk. Indeed, past work demonstrates changes in cerebral blood flow velocity and increased cognitive function following cardiac rehabilitation in older adults with cardiovascular disease [72]. More broadly, exercise and aerobic fitness are positively associated with cognition, including episodic memory [73, 74] and brain structure and function in healthy older adults [75, 76]. These effects have been observed in brain regions affected by AD that are also negatively impacted in HF, including the MTLs [77–80]. These findings may in part be attributable to cerebral blood flow, as exercise improves cerebral perfusion [72] and increased cerebral blood flow to the hippocampus has been linked with better memory performance [81]. Exercise is also associated with an array of other metabolic, micro- and macrovascular, neurohormonal, and brain-based (e.g., neurogenesis, synaptogenesis) benefits [82, 83] that likely promote neurocognitive function. These findings suggest that exercise may attenuate the negative neural and cognitive decline observed in HF.
Conclusions and future directions
The neuropathological staging of AD initially begins with MTL pathology (e.g., hippocampus) that gradually progresses to isocortical regions [36]. Cardiovascular disease is a well-known contributor to AD pathogenesis, and evidence in HF supports this claim. Neuroimaging studies show that patients with HF exhibit regional reductions in brain volume and white matter microstructure in those same regions typically affected in the early stages of AD, such as the Papez circuit (e.g., hippocampus, cingulate gyrus, thalamus, mammillary bodies, and fornix). Persons with HF also exhibit structural alterations to cortical regions affected in the later stages of AD. The overlap in structural brain alterations between HF and AD suggests that HF may contribute to AD risk via additive pathology to the same regions affected in AD and/or alterations in brain regions not typically associated with AD.
Currently, the specific etiology of adverse neurological outcomes in HF is unclear. While we provide empirical evidence that supports the association between HF and increased AD risk, it remains unclear whether dementia in HF is a manifestation of AD or a cerebrovascular etiology, or the more likely possibility of a mixed dementia. To better differentiate between these etiological considerations, future studies that implement amyloid imaging are needed to delineate the etiological underpinnings between HF and AD risk and determine whether the heightened AD risk occurs through mechanisms other than cerebrovascular disease, such as increased amyloid burden. Prospective multimodal studies employing arterial spin labeling MRI, structural imaging (T1-weighted or diffusion tensor imaging), and amyloid imaging may further elucidate the associations among HF, cerebral perfusion, structural brain indices, and AD-related neuropathology [84]. For instance, such a study could potentially identify the temporal cascade of neural alterations associated with HF, that is, whether reductions in cerebral blood flow precede increases in amyloid burden or alterations in neural structure (reductions in volume or white matter integrity), or whether the onset of structural alterations and amyloid burden occurs at different time points. A better understanding of the impact of HF on the brain may clarify whether cognitive impairments and dementia risk in this population can be attenuated via interventions (e.g., exercise) that positively impact brain structure and function.
Limitations
Some of the extant studies are limited by small sample sizes and use of visual assessment semiquantitative rating scales. The extent and impact of white matter signal abnormalities on cognitive outcomes in persons with HF are unclear due to the lack of FLAIR imaging. Increased white matter hyperintensities increase risk of AD [85], and there is a strong inverse association between total and subcortical white matter hyperintensities with HF severity [40]. HF patients are at elevated risk of white matter hyperintensities in light of cerebral perfusion alterations and increased prevalence of medical conditions (e.g., obesity, diabetes) that affect white matter integrity [40, 86]. White matter hyperintensities may thus represent an important contributor to AD pathogenesis in HF possibly via impairments in neuronal function and contributions to cortical thinning [70, 85], which is a sensitive marker of cognitive decline and AD progression [87, 88]. Lastly, the above review is not completely exhaustive of brain alterations in HF, and extant research demonstrates cortical and subcortical alterations to brain regions that regulate autonomic functions (e.g., thalamus, hypothalamus, insular cortex), motor abilities, and higher cognitive functions (e.g., basal ganglia). Alterations to these brain regions may also contribute to AD risk in HF via nonconventional and/or poorly understood mechanisms.
Acknowledgments
This work was supported by the Department of Veterans Affairs, Rehabilitation Research & Development Service [Career Development Award e7822w awarded to SMH]. The contents of this article do not represent the views of the US Department of Veterans Affairs or the US Government.
Footnotes
Conflict of interest Michael L. Alosco and Scott M. Hayes have no conflicts of interest or financial ties to disclose. The manuscript does not contain clinical studies or patient data.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alosco ML, Spitznagel MB, Cohen R, Sweet LH, Colbert LH, Josephson R, Waechter D, Hughes J, Rosneck J, Gunstad J. Cognitive impairment is independently associated with reduced instrumental ADLs in persons with heart failure. J Cardiovasc Nurs. 2012;27:44–50. doi: 10.1097/JCN.0b013e318216a6cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers JK, Pocock SJ, McMurray JJ, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Swedberg K, Yusuf S. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2014;16:33–40. doi: 10.1002/ejhf.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjelm C, Brostrom A, Dahl A, Johansson B, Fredrikson M, Stromberg A. Factors associated with increased risk for dementia in individuals age 80 years or older with congestive heart failure. J Cardiovasc Nurs. 2014;29:82–90. doi: 10.1097/JCN.0b013e318275543d. [DOI] [PubMed] [Google Scholar]
- 5.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 6.Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc. 2002;50:1431–1438. doi: 10.1046/j.1532-5415.2002.50367.x. [DOI] [PubMed] [Google Scholar]
- 7.De la Toree JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 8.Bates KA, Sohrabi HR, Rodrigues M, Beilby J, Dhaliwal SS, Taddei K, et al. Association of cardiovascular factors and Alzheimer’s disease plasma amyloid-beta protein in subjective memory complainers. J Alzheimers Dis. 2009;17:305–318. doi: 10.3233/JAD-2009-1050. [DOI] [PubMed] [Google Scholar]
- 9.Toledo JB, Toledo E, Weiner MW, Jack CR, Jr, Jagust W, Lee VM, Shaw LM, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative Cardiovascular risk factors, cortisol, and amyloid-B deposition in Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2012;8:483–489. doi: 10.1016/j.jalz.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noh Y, Seo SW, Jeon S, Lee JM, Kim JH, Kim GH. White matter hyperintensities are associated with amyloid burder in APOE4 non-carriers. J Alzheimers Dis. 2014;40:877–886. doi: 10.3233/JAD-130461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajduk AM, Lemon SC, McManus DD, Lessard DM, Gurwitz JH, Spencer FA, Goldberg RJ, Saczynski JS. Cognitive impairment and self-care in heart failure. Clin Epidemiol. 2013;5:407–416. doi: 10.2147/CLEP.S44560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, Ding Y, Kim J, Sloan R, Jaynes H, Shaw RM. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alwerdt J, Edwards JD, Athilingam P, O’Connor ML, Valdes EG. Longitudinal differences in cognitive function among older adults with and without heart failure. J Aging Health. 2013;25:1358–1377. doi: 10.1177/0898264313505111. [DOI] [PubMed] [Google Scholar]
- 14.Almeida OP, Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Two-year course of cognitive function and mood in adults with congestive heart failure and coronary artery disease: the Heart-Mind Study. Int Psychogeriatr. 2012;24:38–47. doi: 10.1017/S1041610211001657. [DOI] [PubMed] [Google Scholar]
- 15.Hjelm C, Dahl A, Brostrom A, Martensson J, Johansson B, Stromberg A. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. J Clin Nurs. 2012;7–8:994–1003. doi: 10.1111/j.1365-2702.2011.03817.x. [DOI] [PubMed] [Google Scholar]
- 16.Van den Hurk K, Reijmer YD, van den Berg E, Alssema M, Mijpels G, Kostense PJ, Stehouwer CD, Paulus WJ, Kamp O, Dekker JM, Biessels GJ. Heart failure and cognitive function in the general population: the Hoorn Study. Eur J Heart Fail. 2011;13:1362–1369. doi: 10.1093/eurjhf/hfr138. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimers disease. Brain. 2009;132:2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiol Aging. 2010;31:244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling RA, Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishio K, Ihara M, Yamasaki N, Kalaria RN, Maki T, Fujita Y, Ito H, Oishi N, Fukuyama H, Miyakawa T, Takahashi R, Tomimoto H. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke. 2010;41:1278–1284. doi: 10.1161/STROKEAHA.110.581686. [DOI] [PubMed] [Google Scholar]
- 24.Mattson N, Tosun D, Insel PS, Simonson A, Jack CR, Jr, Beckett LA, Donohue M, Jagust W, Schuff N, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative Association of brain amyloid-b with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain. 2014;137:1550–1561. doi: 10.1093/brain/awu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin BP, Nair VA, Meier TB, Xu G, Rowley HA, Carlsson CM, Johnson SC, Prabhakaran V. Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis. 2011;26:123–133. doi: 10.3233/JAD-2011-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 27.Mazza M, Marano G, Traversi G, Bria P, Mazza S. Primary cerebral blood flow deficiency and Alzheimer’s disease: shadows and lights. J Alzheimers Dis. 2011;23:375–389. doi: 10.3233/JAD-2010-090700. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Song X, Beyea S, D’Arcy R, Zhang Y, Rockwood K. Advances in perfusion magnetic resonance imaging in Alzheimer’s disease. Alzheimers Dement. 2010;7:185–196. doi: 10.1016/j.jalz.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Kreisman NR, Soliman S, Gozal D. Regional differences in hypoxic depolarization and swelling in hippocampal slices. J Neurophysiol. 2000;83:1031–1038. doi: 10.1152/jn.2000.83.2.1031. [DOI] [PubMed] [Google Scholar]
- 30.de la Torre JC. Critically attained threshold of cerebral hypoperfusion: Can it cause Alzheimer’s disease? AnnN Y Acad Sci. 2000;903:424–436. doi: 10.1111/j.1749-6632.2000.tb06394.x. [DOI] [PubMed] [Google Scholar]
- 31.Alves TC, Rays J, Fraguas R, Jr, Wajngarten M, Telles RM, Duran FL, Meneghetti JC, Robilotta CC, Prando S, De Castro CC, Buchpiguel CA, Busatto GF. Association between major depressive symptoms in heart failure and impaired regional cerebral blood flow in the medial temporal region: a study using 99 m Tc-HMPAO single photon emission computerized tomography (SPECT) Psychol Med. 2006;36:597–608. doi: 10.1017/S0033291706007148. [DOI] [PubMed] [Google Scholar]
- 32.Alves TC, Rays J, Fraguas R, Jr, Wajngarten M, Meneghetti JC, Prando S, Busatto GF. Localized cerebral blood flow reductions in patients with heart failure: a study using 99mTc-HMPAO SPECT. J Neuroimaging. 2005;15:150–156. doi: 10.1177/1051228404272880. [DOI] [PubMed] [Google Scholar]
- 33.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 34.Vogels RL, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P, van der Flier WM, Weinstein HC. Transcranial Doppler blood flow assessment in patients with mild heart failure: correlates with neuroimaging and cognitive performance. Congest Heart Fail. 2008;14:61–65. doi: 10.1111/j.1751-7133.2008.07365.x. [DOI] [PubMed] [Google Scholar]
- 35.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 36.Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand. 1996;93:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 37.Jack CR, Dickson DW, Parisi JE, Xu YC, Cha RH, O’Brien PC. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 39.Pan A, Kumar R, Macey PM, Fonarow GC, Harper RM, Woo MA. Visual assessment of brain magnetic resonance imaging detects injury to cognitive regulatory sites in patients with heart failure. J Card Fail. 2013;19:94–100. doi: 10.1016/j.cardfail.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canu E, McLaren DG, Fitzgerald ME, Bendlin BB, Zoccatelli G, Alessandrini F, Pizzini FB, Ricciardi GK, Beltramello A, Johnson SC, Frisoni GB. Microstructural diffusion changes are independent of macrostructural volume loss in moderate to severe Alzheimer’s disease. J Alzheimers Dis. 2010;19:963–976. doi: 10.3233/JAD-2010-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogels RLC, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail. 2007;9:1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe—a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- 43.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 44.Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15:214–223. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almeida OP, Garrido GH, Etherton-Beer C, Lautenschlager NT, Arnoldda L, Alfonso H, Flicker L. Brain and mood changes over 2 years in healthy controls and adults with heart failure and ischaemic heart disease. Eur J Heart Fail. 2013;15:850–858. doi: 10.1093/eurjhf/hft029. [DOI] [PubMed] [Google Scholar]
- 46.Kumar R, Woo MA, Macey RM, Fonarow GC, Hamilton MA, Harper RM. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011;307:106–113. doi: 10.1016/j.jns.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida OP, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Flicker L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J. 2012;33:1769–1776. doi: 10.1093/eurheartj/ehr467. [DOI] [PubMed] [Google Scholar]
- 48.Kumar R, Woo MA, Birrer BV, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Mammillary bodies and fornix fibers are injured in heart failure. Neurobiol Dis. 2009;33:236–242. doi: 10.1016/j.nbd.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- 50.Hayes SM, Salat DH, Verfaellie M. Default network connectivity in medial temporal lobe amnesia. J Neurosci. 2012;32:14622–14629. doi: 10.1523/JNEUROSCI.0700-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neuropsychiatry Clin Neurosci. 1957;20:11–21. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- 52.Jones BF, Barnes J, Uylings HB, Fox NC, Frost C, Witter MP, Scheltens P. Differential regional atrophy of the cingulate gyrus in Alzheimer disease: a volumetric MRI study. Cereb Cortex. 2006;16:1701–1708. doi: 10.1093/cercor/bhj105. [DOI] [PubMed] [Google Scholar]
- 53.Pedro T, Weiler M, Yasuda CL, D’Abreu A, Damasceno BP, Cendes F, Balthazar ML. Volumetric brain changes in thalamus, corpus callosum and medial temporal structures: mild Alzheimer’s disease compared with amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2012;34:149–155. doi: 10.1159/000342118. [DOI] [PubMed] [Google Scholar]
- 54.Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashburnder J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 56.Hong X, Bu L, Wang Y, et al. Increases in the in risk of cognitive impairment and alterations of B-amyloid metabolism in mouse model of heart failure. PLoS One. 2013;8:e63829. doi: 10.1371/journal.pone.0063829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alosco ML, Spitznagel MB, Cohen R, Raz N, Sweet LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Reduced cerebral perfusion predicts greater depressive symptoms and cognitive dysfunction at a 1-year follow-up in patients with heart failure. Int J Geriatr Psychiatry. 2014;29:428–436. doi: 10.1002/gps.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, van Dulmen M, Hughes J, Rosneck J, Gunstad J. Obesity interacts with cerebral hypoperfusion to exacerbate cognitive impairment in older adults with heart failure. Cerebrovasc Dis Extra. 2012;2:88–98. doi: 10.1159/000343222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jesus PA, Vieira-de-Melo RM, Reis FJ, Viana LC, Lacerda A, Dias JS, Oliveira-Filho J. Cognitive dysfunction in congestive heart failure: transcranial Doppler evidence of microembolic etiology. Arq Neuropsiquiatr. 2006;64:207–210. doi: 10.1590/s0004-282x2006000200007. [DOI] [PubMed] [Google Scholar]
- 61.Aslop DC, Dai W, Grossman M, Detre JA. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer’s disease. J Alzheimers Dis. 2010;20:871–880. doi: 10.3233/JAD-2010-091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sperling R. Potential of functional MRI as a biomarker in early Alzheimer’s disease. Neurobiol Aging. 2011;32:S37–S43. doi: 10.1016/j.neurobiolaging.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 64.Daselaar SM, Iyengar V, Davis SW, Eklund K, Hayes SM, Cabeza RE. Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb Cortex. 2013;25:983–990. doi: 10.1093/cercor/bht289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, Harper RM. Aberrant central nervous system responses to the Valsalva maneuver in heart failure. Congest Heart Fail. 2007;13:29–35. doi: 10.1111/j.1527-5299.2007.05856.x. [DOI] [PubMed] [Google Scholar]
- 66.Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, Harper RM. Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail. 2005;11:437–446. doi: 10.1016/j.cardfail.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayes SM, Baena E, Truong TK, Cabeza R. Neural mechanisms of context effects on face recognition: automatic binding and context shift decrements. J Cogn Neurosci. 2010;22:2241–2554. doi: 10.1162/jocn.2009.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chuang Y, Eldreth D, Erickson KR, Varma V, Harris G, Fried LP, Rebok GW, Tanner EK, Carlson MC. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol Aging. 2014;35:1396–1403. doi: 10.1016/j.neurobiolaging.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sultzer DL, Chen ST, Brown CV, Mahler ME, Cummings JL, Hinkin CH, Mandelkern MA. Subcortical hyperintensities in Alzheimer’s disease: associated clinical and metabolic findings. J Neuropsychiatry Clin Neurosci. 2002;14:262–269. doi: 10.1176/jnp.14.3.262. [DOI] [PubMed] [Google Scholar]
- 71.Appel J, Potter E, Bhatia N, Shen Q, Zhao W, Greig MT, Raj A, Barker WW, Potter H, Schofield E, Wu Y, Loewenstein DA, Duara R. Association of white matter hyperintensity measurements on brain MR imaging with cognitive status, medial temporal atrophy, and cardiovascular risk factors. AJNR Am J Neuroradiol. 2009;30:1870–1876. doi: 10.3174/ajnr.A1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanek KM, Guntad J, Spitznagel MB, Waechter D, Hughes JW, Luyster F, Josephson R, Rosneck J. Improvements in cognitive function following cardiac rehabilitation for older adults with cardiovascular disease. Int J Neurosci. 2011;121:86–93. doi: 10.3109/00207454.2010.531893. [DOI] [PubMed] [Google Scholar]
- 73.Hayes SM, Forman DE, Verfaellie M. Cardiorespiratory fitness is associated with cognitive performance in older but not younger adults. J Gerontol B Psychol Sci Soc Sci. 2014 doi: 10.1093/geronb/gbu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayes SM, Hayes JP, Cadden M, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front Aging Neurosci. 2013;5:31. doi: 10.3389/fnagi.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hayes SM, Salat D, Forman DE, Verfaellie M. Cardiorespiratory fitness is associated with white matter integrity in aging. Ann Clin Transl Neurol. doi: 10.1002/acn3.204. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;15:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erickson KI, Weinstein AM, Lopez AL. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res. 2012;43:615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heo S, Prakash RS, Voss MW, Erickson KI, Ouyang C, Sutton BP, Kramer AF. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 2010;1315:119–1127. doi: 10.1016/j.brainres.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 83.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci. 2011;34:430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Capizzano AA, Acion L, Bekinschtein T, Furman M, Gomila H, Martinez A, Mizrahi R, Starkstein SE. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:822–827. doi: 10.1136/jnnp.2003.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med. 2012;74:682–690. doi: 10.1097/PSY.0b013e318261909c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V, Puel M, Berry I, Fort JC, Celsis P, Alzheimer’s Disease Neuroimaging Initiative Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donix M, Scharf M, Marschner K, Werner A, Sauer C, Gerner A, Nees JA, Meyer S, Donix KL, Von Kummer R, Holthoff VA. Cardiovascular risk and hippocampal thickness in Alzheimer’s disease. Int J Alzheimers Dis. 2013;2013:108021. doi: 10.1155/2013/108021. [DOI] [PMC free article] [PubMed] [Google Scholar]


