Abstract
Objective
Cognitive deficits are a common feature of psychiatric disorders. We investigated the nature of disruptions in neural circuitry underlying cognitive control capacities across psychiatric disorders through a transdiagnostic neuroimaging meta-analysis.
Method
We searched PubMed for whole-brain functional neuroimaging articles published through June 2015 that compared activation in patients with Axis I disorders to matched healthy control participants during cognitive control tasks. Tasks that probed performance or conflict monitoring, response inhibition or selection, set shifting, verbal fluency, and recognition or working memory were included. Activation likelihood estimation meta-analyses were conducted on peak voxel coordinates.
Results
The 283 experiments submitted to meta-analysis included 5,728 control participants and 5,493 patients with different disorders (schizophrenia, bipolar or unipolar depression, anxiety, substance use). Transdiagnostically abnormal activation was evident in the left prefrontal cortex, as well as anterior insula, right ventrolateral prefrontal cortex, right intraparietal sulcus, and mid-cingulate/pre-supplementary motor area. Disruption was also observed in a more anterior cluster in the dorsal cingulate cortex, which overlapped with a network of structural perturbation we previously observed in a transdiagnostic meta-analysis of gray matter volume.
Conclusion
These findings demonstrate a common pattern of disruption across major psychiatric disorders that parallels the “multiple demand network” observed in intact cognition. This network interfaces with the anterior-cingulo-insular or “salience network” demonstrated to be transdiagnostically vulnerable to gray matter reduction. Thus, networks intrinsic to adaptive, flexible cognition are vulnerable to broad spectrum psychopathology. Dysfunction in these networks may reflect an intermediate transdiagnostic phenotype, which could be leveraged to advance therapeutics.
Introduction
Cognitive control, or executive functions, refer to those processes integral to the effortful deployment of cognitive resources for flexible, adaptive responding to shifting contingencies—and ultimately accommodating to the demands of daily life. Accordingly, cognitive control capacity predicts socio-occupational sfility and success as well as broader measures of quality of life (1).
Latent variable analysis of neuropsychological performance has shown that intact cognition consists of interrelated executive functions including updating (i.e., monitoring working memory store), inhibition (resisting prepotent responses), and shifting (switching between mental sets). An underlying, largely heritable, common factor reflecting general cognitive control capacity also emerges (2,3). Across various psychiatric disorders, neuropsychological performance is broadly (i.e., domain non-specifically) perturbed, with some variations in severity (4, 5). Evidence from large-scale phenotypic studies has also demonstrated a dimension of general psychopathology that cuts across disorder boundaries (6). This dimension robustly accounts for lifespan functional impairment and prospective psychopathology above and beyond current symptom-based predictions (7,8). Higher loadings on the general psychopathology factor predict worse performance on tasks of working memory and planning as well as limited academic achievement and lower IQ (7). Thus, a general liability for cognitive dyscontrol, which traverses both cognitive domains and diagnostic boundaries, may be a core feature of mental illness.
Evidence for common, largely heritable liabilities to experiencing general psychopathology as well as cognitive dyscontrol prompts the question of whether there are accompanying structural anomalies seated within the neurocircuitry subserving cognitive control. We recently completed a meta-analysis of volumetric differences in Axis I patients and matched control groups (9). Across 193 whole-brain voxel-based morphometry (VBM) studies of nearly 16,000 individuals representing diverse diagnostic classes (schizophrenia, bipolar and unipolar depression, substance use, and anxiety disorders) we found that gray matter loss converged across diagnoses in three regions: the dorsal anterior cingulate and bilateral anterior insula. In an independent sample of healthy participants, we found that lower gray matter volume in these regions predicted worse behavioral performance on measures of higher-level cognitive control, but was unrelated to more rudimentary processing speed. These findings suggest a coordinated structural perturbation of a closely interconnected anterior-cingulo-insular or “salience network” across disorders, likely associated with transdiagnostic deficits in executive function tasks.
The insula and anterior cingulate, as part of the broader “salience network” (10), feature prominently in intact (11) as well as disordered emotional responding (12). However, the insula and anterior cingulate are deployed beyond emotional processing—more generally coordinating dynamic neural network interactions in response to contextual demands (13–15). Critical to cognitive control is their coordination with the fronto-parietal network to function as a superordinate or “multiple demand” cognitive processing network (16–26). That is, in tasks ranging from working memory to inhibiting irrelevant information and selecting competing task-relevant responses (17) the dorsal anterior cingulate and bilateral anterior insula extending to ventrolateral prefrontal cortex are recruited in conjunction with the mid-cingulate cortex extending into pre-supplementary motor area, left dorsolateral prefrontal cortex extending from middle frontal gyrus to inferior frontal junction/gyrus and premotor cortex, and inferior parietal cortex extending into intraparietal sulcus. Findings have been mixed in terms of which multiple demand network nodes show dissociable sensitivity to phasic (i.e., moment-to-moment) versus sustained (i.e., set maintenance) cognitive demands (e.g., 20–22). However, the salience network (often referred to as the cingulo-opercular network in cognitive task literature) and fronto-parietal network reliably coordinate as subnetworks of a broader, coherent multiple demand network. Similar to the latent or common cognitive control factor observed in behavioral measures of cognitive processing, the activity of this network suggests a “common core” recruited across diverse cognitive challenges (18).
Taken together, behavioral and structural evidence implicates transdiagnostic disruptions in the neurocircuitry underlying general cognitive control capacity. In this study, we examined whether there is a parallel transdiagnostic functional impairment in whole brain activation during cognitive control task performance. We hypothesized that deficits would be particularly manifest in the multiple demand network or common core of cognitive processing, including in regions we previously observed as transdiagnostically structurally perturbed (9).
Methods
Experiment Inclusion Criteria and Identification
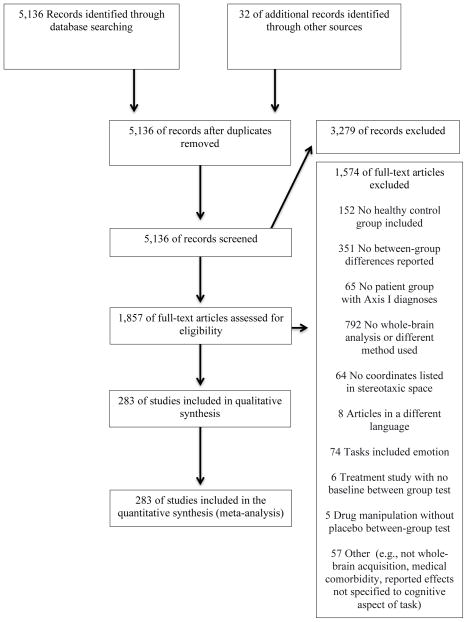
Articles were identified by searching PubMed for functional neuroimaging experiments of cognitive control tasks published through June 2015 that compared patients with Axis I disorders to matched control participants (Figure 1). Experiments were eligible if they (1) examined cognitive control tasks with functional neuroimaging, (2) performed whole-brain analysis, (3) included a comparison between patients with Axis I disorders and matched healthy control participants during cognitive challenges, and (4) reported coordinates in a defined stereotaxic space (e.g., Talairach or Montreal Neurological Institute (MNI) space).
Figure 1.
Flow Diagram of Study Selection in an Analysis of Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders
Experimental procedures must have included diagnostic interview of Axis I patients and control participants, with patient groups exceeding clinical threshold for diagnosis. A psychotic disorders category comprised schizophrenia, schizoaffective, schizophreniform, and delusional disorders. A non-psychotic disorders category comprised bipolar, unipolar (major depression, dysthymia) depressive, anxiety (including obsessive compulsive and posttraumatic stress disorders), and substance use (mixed substance abuse and/or dependence) disorders. Experiments of fully remitted patient samples were excluded.
While individuals with principal depressive or bipolar disorders may also present with psychotic features, these were excluded by criteria in the original experiments. Across disorders, patient participants included those with first episode and chronic disorder manifestations, including inter-episode states of bipolar and psychotic disorders. The substance use disorders included chronic users of a range of substances currently active or abstinent, but not in acute withdrawal. Experiments were selected to capture lifespan patterns and thus included participants ranging from childhood through older adulthood. Axis I diagnoses presenting predominantly in childhood (e.g., attention deficit/hyperactivity disorder) or those associated with altered developmental trajectories of brain structures inherent to expression of disorder phenotypes (e.g., autism spectrum disorders) were excluded.
Articles with experimental tasks probing a wide range of processes related to cognitive control were included, categorized into eight domains: conflict monitoring, performance monitoring, response inhibition, response selection, set shifting, verbal fluency, recognition memory, working memory. A ninth category (“other”) included 18 disparate experiments that did not cohere with one of these domains (Supplementary Table 1). To target substrates of higher order cognitive control, experiments that focused on simple processing speed or orienting in the context of passive perception (e.g., oddball discrimination) were excluded. Cognitive processing experiments with embedded affective manipulations (e.g., affective stimuli, mood induction) were also excluded.
Peak coordinates for whole-brain between group comparisons under cognitive challenge were required. Interactions were included if follow-up tests clarified patterns of patient hyper- versus hypoactivation during cognitive challenge. Experiments reporting results only for small-volume correction or within a region of interest were excluded. Articles with reported contrasts that did not reflect cognitive demand were excluded. If multiple contrasts were reported in a single paper only those pertaining to the most challenging condition were included. All coordinates reported in Talairach space were converted into MNI space (27).
Activation Likelihood Estimation (ALE) Meta-analysis
The revised ALE algorithm, implemented in MATLAB, was used to identify areas of convergence of reported coordinates for patient/control differences in activation during cognitive control tasks higher than expected under a random spatial association (28–30; cf. Supplementary Methods for details). Resulting nonparametric p values were thresholded at a cluster-level familywise-error-corrected threshold of p < .05 (cluster-forming threshold at voxel-level P < .005) and transformed into z scores for display. To avoid results dominated by one or two individual experiments and to have sufficient power to detect moderately sized effects, ALE analyses were limited to those contrasts with at least 20 experiments (31).
We conducted the following analyses:
Pooling across coordinates of hypo- and hyperactivation in patients relative to controls to identify transdiagnostic patterns of “aberrant activation”.
A conjunction between these results and the multiple demand network from three large meta-analyses in healthy participants (25, retrieved through ANIMA (32; http://anima.fz-juelich.de).
A conjunction with the nodes of common gray matter decrease revealed by Goodkind and colleagues (9).
Separate ALE analyses on hyper- or hypoactivation coordinates (i.e. patient>control or control>patient).
Guided by our prior work (9) and phenotypic structural models (33), we distinguished between psychotic and non-psychotic disorders. Given sufficient numbers of experiments (31) we performed ALE by broad diagnostic groupings (i.e., schizophrenia, bipolar and unipolar depression, substance use, and anxiety disorders).
Follow-up analyses on extracted data (probability of voxelwise activation from the modeled activation maps) in significant clusters to examine the contribution of demographic, disorder, medication, and task-related factors.
Nonparametric Wilcoxon Signed Ranks, Kruskal-Wallis, and Mann-Whitney U tests were utilized as warranted.
Results
Final Selected Experiment Set
The final set of experiments consisted of 283 experiments from 251 articles (Figure 1; Supplementary Tables ST1, ST2) covering 11,221 participants (5,493 patient and 5,728 control participants). For a more complete description of included experiments see the supplementary material. The vast majority of experiments (n=260) used functional magnetic resonance imaging (fMRI), followed by 21 positron emission tomography experiments and one each using arterial spin labeling and single-photon emission computerized tomography. Mean ages ranged from 11.2 to 73.3 years. There was near equal representation of experiments by psychotic (n=139) and non-psychotic disorders (n=144). Included experiments also represented an array of cognitive tasks across multiple domains: working memory (n=100), response inhibition (n=42), recognition memory (n=37), conflict monitoring (n=31), verbal fluency (n=17), set shifting (n=15), response selection (n=12), performance monitoring (n=11), and a set of 18 diverse experiments outside of these domains. Most experiments included medicated (n=193) as opposed to unmedicated patients (n=60) while information on medication was lacking for 30 experiments.
Meta-Analysis Results Across Disorders
Activation patterns during cognitive control: Voxelwise Analyses
Transdiagnostic Aberrant Activation
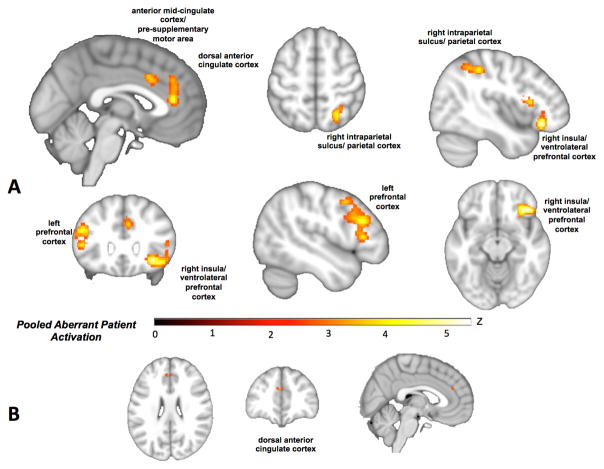
Pooling across patterns of patient hyper- and hypoactivation to assess “aberrant activation” at the whole-brain level revealed patient abnormalities in dorsal anterior cingulate, anterior mid-cingulate cortex/pre-supplementary motor area, right insula (extending to ventrolateral prefrontal cortex) and right intraparietal sulcus, as well as a cluster in the left prefrontal cortex extending from mid-dorsolateral prefrontal to premotor cortex (Figure 2A; Table ST3). This pattern suggests disruption of a network of regions similar to the multiple demand network (25) that may overlap with nodes of transdiagnostic gray matter loss. Furthermore, a broad distribution of disorders and domains contributed to each cluster of convergence (Table ST4).
Figure 2.
A) Regions of transdiagnostic aberrant activation (i.e., pooled across patient hyper- and hypo-activation (red/yellow). B) A conjunction with the regions of gray matter loss observed by Goodkind and colleagues (9) highlights anatomical and functional correspondence in dorsal anterior cingulate (orange).
A conjunction with the multiple demand network identified from meta-analyses of healthy participants (25) highlights overlap in left inferior frontal gyrus/junction, pre- supplementary motor area, right anterior insula/ventrolateral prefrontal cortex, and right intraparietal sulcus (Figure SF1; Table ST5). A conjunction with the regions of transdiagnostic gray matter loss observed by Goodkind et al. (9) shows similar cross-modality disruptions in regions of dorsal anterior cingulate and right insula, with exact correspondence in the dorsal anterior cingulate (Figure 2B; Figure SF2). This suggests two distinct posterior-medial frontal effects, one being disruption within a node of the multiple demand network and one in a more anterior node shown to be especially vulnerable to gray matter loss.
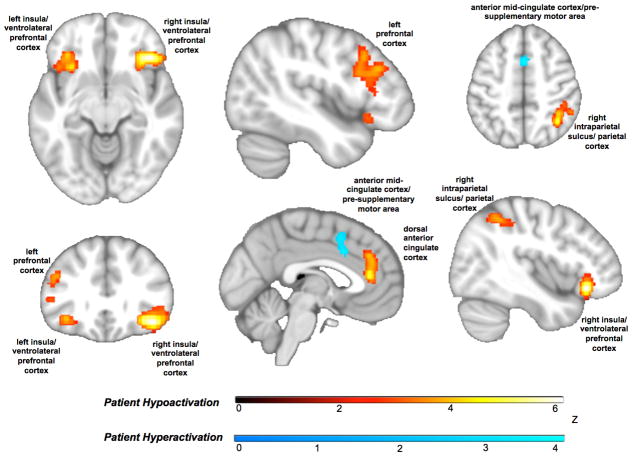
Transdiagnostic Hyper- vs. Hypoactivation
The distinction between anterior and mid-cingulate effects was further underscored when we tested separately for convergent hyper- versus hypoactivation in patients. The more anterior dorsal cingulate overlapping with regions prone to gray matter loss showed patient hypoactivation whereas the anterior mid-cingulate/pre-supplementary motor cortex cluster overlapping with the canonical multiple demand network showed patient hyperactivation (Figure 3; Table ST6). All other regions of the cognitive control circuit showed patient hypoactivation. Bilateral hypoactivation of the insula, extending to ventrolateral prefrontal cortex, was also evident. These patterns persisted when ALE was re-run systematically excluding the experiments of arterial spin labeling and single-photon emission computerized tomography (Figure SF3; Table ST7), the tasks that did not cohere in a domain (Figure SF4; Table ST8), and those of children and older adults (Figure SF5; Table ST9).
Figure 3.
Transdiagnostic patterns of hyper- and hypoactivation in patients. Within the anterior cingulate, hypoactivation (orange) was seen in an anterior dorsal cingulate region that overlaps with a region prone to gray matter loss in our prior work. An anterior mid-cingulate/pre-supplementary motor area cluster that overlaps with the multiple demand network showed patient hyperactivation (blue). All other regions of the cognitive control circuit showed consistent patient hypoactivation (orange).
Next we examined whether factors of age, medication, and behavioral performance might impact these patterns. The majority of experiments represented adulthood (n=248; 18–50 years), followed by childhood/adolescence (n=27; <18 years) and there were few studies of older adulthood (n=8; >50 years). Since excluding children and older adults had little impact on activation patterns but were contributing to convergence (Table ST10), we computed the aberrant activation contrast stratified by age group. No clusters converged in the older adult sample, though this included too few studies for valid for ALE inference (31). By contrast, the childhood/adolescent sample showed strong right anterior insula/ventrolateral prefrontal cortex activation overlapping with the adult sample (Figure SF6; Table ST11)—suggesting a particular role of this node in cognitive dyscontrol from childhood through adulthood.
Next we considered current psychotropic medication status as 68% of the experiments included medicated patients. Medication did not influence patterns of hypoactivation in multiple demand network nodes (Figure SF7; Table ST12). Medicated patients, however, showed hyperactivation specific to the anterior mid-cingulate cortex/pre-supplementary motor cortex, also evident in contribution analyses (Table ST10). Unmedicated patient experiments did not show any (whole-brain significant) hyperactivations. Moreover, accounting for behavioral performance on the scanner task demonstrated that patient hyperactivation in the anterior mid-cingulate/pre-supplementary motor cortex cluster was primarily driven by patient groups that performed on par with, as opposed to worse than, control participants (Figure SF8; Table ST10; ST13). By contrast, patient hypoactivation in multiple demand network nodes were largely similar regardless of whether behavioral performance was impaired.
Accounting for Psychotic & Non-psychotic Disorders
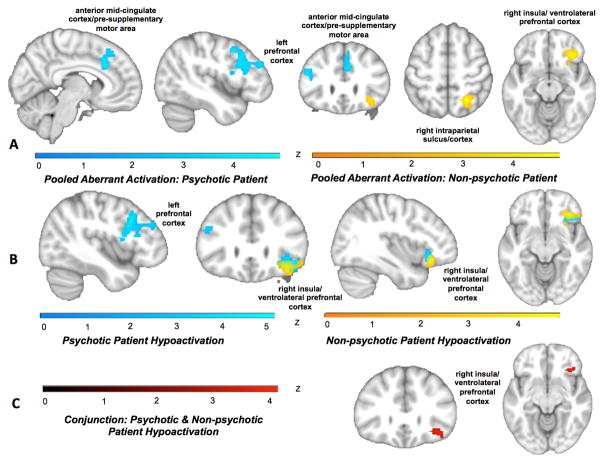
Examining psychotic and non-psychotic disorders separately revealed aberrant activation in psychotic disorders in anterior mid-cingulate/pre-supplementary motor cortex and left prefrontal cortex extending posteriorly from mid-dorsolateral prefrontal to premotor cortex. Non-psychotic disorders showed aberrant activation in right anterior insula/ventrolateral prefrontal cortex and right intraparietal sulcus (Figure 4A; Table ST14; ST15). A contrast revealed that aberrant activation in a posterior portion of the left prefrontal cluster as well as a medial portion of the mid-cingulate/pre-supplementary motor area were more characteristic of psychotic disorders (Figure SF9; Table ST14; ST15), whereas aberrant activation in right intraparietal sulcus and a more anterior portion of the right anterior insula/ventrolateral prefrontal cortex cluster were more specific to non-psychotic disorders.
Figure 4.
Patterns of brain activation in psychotic and non-psychotic disorders. A) Aberrant activation (pooling across hyper- and hypoactivation) emerged for psychotic disorder patients (blue) in anterior mid-cingulate/pre-supplementary motor area and left prefrontal cortex extending posteriorly from mid-dorsolateral prefrontal cortex to inferior frontal gyrus/junction and premotor cortex. Non-psychotic disorders showed aberrant activation (yellow) in right anterior insula/ventrolateral prefrontal cortex and right intraparietal sulcus. B) In separate analyses of psychotic and non-psychotic disorders hyypoactivation in the left mid-dorsolateral prefrontal cortex to inferior frontal gyus/junction and premotor cortex characterized psychotic disorders (blue). Hypoactivation for both disorder classes emerged in right anterior insula/ventrolateral prefrontal cortex (non-psychotic disorders=yellow). Hyperactivation contrasts did not show any significant whole-brain activations. C) A conjunction of hypoactivation across psychotic and non-psychotic disorders revealed shared dysfunction in right anterior insula/ventrolateral prefrontal cortex (red).
No hyperactivation regions survived whole-brain correction for either psychotic or non-psychotic disorders. Hypoactivation specific to psychotic disorders emerged, again, in the left lateral prefrontal cluster (Figure 4B; Table ST16; ST17). Hypoactivation for both disorder classes emerged in right anterior insula/ventrolateral prefrontal cortex, confirmed with a conjunction analysis to correspond to the multiple demand network. Contrasting hypoactivation in psychotic and non-psychotic disorders further highlighted that the right anterior insula/ventrolateral prefrontal cortex extended more anteriorly in non-psychotic disorders, whereas psychotic disorders showed stronger hypoactivation in the posterior portion of the left prefrontal cluster (Figure SF10).
Accounting for Disorders & Task Domains
Schizophrenia spectrum disorders showed a reliable hypoactivation of the left prefrontal cortex as well as right ventrolateral prefrontal cortex clusters consistent with the overall pattern. Substance use patients showed hyperactivation in right posterior parietal cortex (more posterior than the overall pattern) (Figure SF11; Table ST18). Finally, though our focus was on “multiple demand” or “general cognitive” processing, we also assessed the contribution of different domains to the overall convergence. Domain-specific ALE (as well as domain by disorder analyses) was performed for contrasts with at least 20 experiments (31) and these are reported in the supplement (Figures SF12; SF13; Table ST19; ST20). In summary, while transdiagnostic results demonstrate the wide distribution of individual disorders and domains to the ALE maps, refining ALE to specific diagnoses and domains revealed few activations that survived whole-brain correction, likely due to power limitations.
Region of Interest Analyses
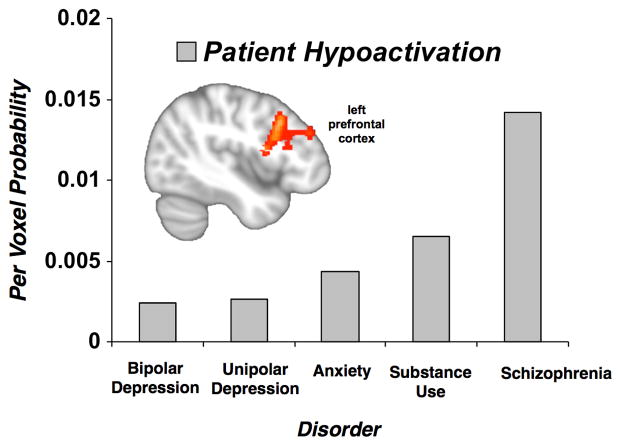
Per voxel probabilities in regions of significant convergence from the multiple demand and salience networks were extracted from the transdiagnostic hyper- and hypoactivation data and examined for effects of age groups, psychotic versus non-psychotic disorders, individual disorder classes, medication status, and behavioral performance. Psychotic relative to non-psychotic disorders showed stronger hypoactivation in left prefrontal cortex (Mann-Whitney U Test, U=10,994.5, p<.05). Specifically, schizophrenia showed substantially more hypoactivation than each of non-psychotic disorders (except substance use disorders), which in turn did not differ from each other (Figure 5). Regarding the transdiagnostic patient hyperactivation in anterior mid-cingulate/pre-supplementary motor cortex, patient samples whose behavioral performance was on par with control participants (mean per voxel probability=1.0) were more likely to show hyperactivation than patient samples that performed worse (mean per voxel probability =0.29) (Mann-Whitney U Test, U=9,270, p<.05). No other group differences were observed in the extracted data.
Figure 5.
Probability of Hypoactivation of Left Prefrontal Cortex by Disorder Class. In voxelwise analysis, patients with psychotic disorders showed a prominent hypoactivation in the left prefrontal cortex (inset). More specifically, extracted per-voxel probability of control > patient activation in this region of interest revealed that schizophrenia, was more likely to show hypoactivation than each of the nonpsychotic disorders (except substance use disorders), which in turn did not differ from each other.
Discussion
In a meta-analysis of cognitive control tasks across Axis I disorders, we observed a transdiagnostic pattern of aberrant brain activation in regions corresponding to the well-established multiple demand network (16–26), including the left prefrontal cortex (from premotor to mid-dorsolateral prefrontal cortex), the right insula extending to ventrolateral prefrontal cortex, right intraparietal sulcus, and anterior mid-cingulate/pre-supplementary motor cortex. Abnormal activation was also observed in a separate more anterior dorsal anterior cingulate cluster (as well as the insula), suggestive of concurrent disruption in regions we previously observed as transdiagnostically prone to reduced gray matter (9).
Unlike patient hypoactivation, patient hyperactivation was isolated to anterior mid-cingulate/pre-supplementary motor cortex activation. Consistent with a role in the implementation and maintenance of task sets (34) as well as the translation to overt action (35), patient hyperactivation in anterior mid-cingulate/pre-supplementary motor cortex was primarily driven by experiments for which predominantly medicated patients performed on par with control participants as opposed to those for which patients performed worse. Increased anterior mid-cingulate/pre-supplementary motor cortex among patients relative to control participants may reflect a compensatory process for maintaining intact performance amidst deficiencies in other network nodes (i.e., proactive/reactive control; 36).
Given that the swath of cortex extending from anterior to mid-cingulate/pre-supplementary motor cortex has been characterized as part of a coherent salience network (10,15), the discordant hypo- and hyperactivation observed here between the more anterior and posterior cingulate respectively might seem unexpected. However, parcellation of the intrinsic functional connectivity of the anterior insula has revealed subnetworks that differentiate these regions. While both ventral and dorsal anterior insula support cognitive processing (36), the dorsal portion is more closely coupled with the anterior mid-cingulate/pre-supplementary motor cortex (marked here by patient hyperactivation) and appears to promote cognitive flexibility (37). The ventral anterior insula (marked here by patient hypoactivation) is more closely coupled with the anterior dorsal cingulate (marked here by corresponding hypoactivation) and relates more to motivational engagement (36). Whole-brain graph theoretic approaches have similarly revealed this distinction leading to speculation that the more anterior cingulate subnetwork is more characteristic of the salience network, whereas the more posterior cingulate subnetwork is more representative of a cingulo-opercular task control network (38).
Differences in the extent of disruption also emerged between psychotic and non-psychotic disorders. Psychotic disorders, particularly schizophrenia, showed pronounced hypoactivation of the left lateral prefrontal cluster, particularly the more posterior portion. Meta-analytic coactivation-based parcellation of this region has suggested that while left prefrontal cortex is broadly recruited for adaptive cognitive control, the predominant processes are typically more top-down moving anteriorly from premotor to mid-dorsolateral prefrontal cortex (39,40). The consistent hypoactivation across this cortical gradient, including the more posterior portion subserving more rudimentary processes may reflect the broad and more severe cross-domain disruption on neuropsychological performance in schizophrenia relative to other disorders (4). In contrast, particularly convergent hypoactivation across disorders emerged in right anterior insula/ventrolateral prefrontal cortex. This network switchboard or hub appears especially vulnerable to both gray matter loss and functional impairment across psychopathology.
Concurrent disruptions in the salience and multiple demand networks highlight a means by which transdiagnostic gray matter reduction in the dorsal anterior cingulate and insula might influence cognitive control capacity and furthermore, how affective and neurocognitive deficits in psychopathology may so often be expressed simultaneously. That is, these highly coordinated regions are sensitive to demands on either cognitive control or emotional processing (17).
Our findings are also consistent with the broader role of anterior cingulate and insular cortices as coordinating network interactions in the service of goal-directed behavior (41–42). For example, recent work on causal interactions among nodes of multiple demand and salience networks (43–44) suggests the anterior insula amplifies salience detection in the anterior and mid-cingulate in a manner proportional to both cognitive demand and individual capacity. In turn this prompts activation of the fronto-parietal subnetwork, particularly lateral prefrontal regions and parietal cortex. Furthermore, a coactivation-based parcellation of the lateral prefrontal cortex across cognitive paradigms (45) revealed two functional subregions, with the anterior region preferentially connected to the anterior cingulate and the posterior region with the intraparietal sulci. In short, accumulating evidence supports strong functional integration among the salience and multiple demand networks and subnetworks during intact cognitive processing and the current findings suggest that their coordination is vulnerable to disruption across disorders.
Limitations
First, the number of included experiments in each of the cognitive domains differed substantially, as did the distribution by disorder. We observed strong evidence of a domain general cognitive control disruption in fronto-parietal-cingular-insular networks, with limited diagnosis-specific effects. The latter may reflect the typically less severe neuropsychological impairments of disorders other than schizophrenia (4) or simply a lack of power due to the limited corpus of published papers for some disorders and/or that ALE probes spatial convergence without accounting for individual effects sizes. Additionally, polythetic diagnostic schemes, comorbidity, and the inherent difficulty with establishing consensus on principal disorder could hamper detection of cognitive control impairment profiles of putatively “pure” disorder manifestations and instead contribute to common patterns. Likely influential factors such as medication types, illness duration, and comorbidity could not be comprehensively assessed due to incomplete reporting across studies. Further, few published study sets in children and older adults render our finding most applicable to (younger) adults. Lastly, while this is the most comprehensive meta-analysis of functional neuroimaging of cognitive processing in Axis I disorders, the included studies do not represent the whole of the extant literature, including the vast number of studies focused on specific ROIs.
Conclusion
Neuropsychological performance, gray matter volume, and now functional brain activation evidence converge to implicate transdiagnostic disruptions in the neurocircuits underlying general cognitive control capacity. Functional disruptions parallel the multiple demand network and its interface with the salience network. Essentially, networks intrinsic to adaptive, flexible cognition are vulnerable to a broad spectrum of psychopathology. These findings highlight a common intermediate phenotype (46–48), which could be leveraged to advance therapeutics. Multimodal interventions that target the foundation of intact, dynamic cognition seated in these frontal-parietal-cingular-insular networks could be powerful for ameliorating not only symptomatic distress but also the often pervasive functional impairments and diminished quality of life prevalent across psychiatric disorders.
Supplementary Material
Acknowledgments
LM was supported by National Institute of Mental Health K23 MH104849. JH was supported by the Max Kade Fellowship provided by the Austrian Academy of Sciences. AE was supported by the Sierra-Pacific Mental Illness Research, Education and Clinical Center (MIRECC) at the Palo Alto VA. SBE was supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/4-1; and LA 3071/3-1), the National Institute of Mental Health (R01-MH074457) the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 604102)
Footnotes
Disclosures: The authors declare no competing financial interests relevant to this research. Dr. Etkin reports consulting funds for unrelated work from Otsuka, Acadia and Takaeda as well as research grant support from Brain Resource Dr. McTeague reports receiving stock options in Joyable.com for unrelated consulting.
References
- 1.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: four general conclusions. Curr Dir Psychol Sci. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008;137(2):201–25. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol. 2015;6:328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008 Jul;69(7):1122–30. doi: 10.4088/jcp.v69n0712. [DOI] [PubMed] [Google Scholar]
- 6.Carragher N, Teesson M, Sunderland M, Newton NC, Krueger R, Conrod PJ, Barrett EL, Champion KE, Nair NK, Slade T. The structure of adolescent psychopathology: a symptom-level analysis. Psychol Med. 2015 doi: 10.1017/S0033291715002470. in press. [DOI] [PubMed] [Google Scholar]
- 7.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, Moffitt TE. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Eaton NR. The hierarchical structure of common mental disorders: Connecting multiple levels of comorbidity, bifactor models, and predictive validity. J Abnorm Psychol. 2015;124(4):1064–78. doi: 10.1037/abn0000113. [DOI] [PubMed] [Google Scholar]
- 9.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes DJ, Duncan NW, Xu J, Northoff G. A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neurosci Biobehav Rev. 2014;45:350–68. doi: 10.1016/j.neubiorev.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J, Beck J, Heller K, Egner T. An insula-frontostriatal network mediates flexible cognitive control by adaptively predicting changing control demands. Nat Commun. 2015;6:8165. doi: 10.1038/ncomms9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct & Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013;80(1):35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013;110(41):16616–21. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neuroscience. 2000;23:475–83. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 19.Duncan J. The multiple-demand MD system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Science. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Science. 2012;16:106–13. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–60. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 23.Crittenden BM, Mitchell DJ, Duncan J. Task encoding across the multiple demand cortex is consistent with a frontoparietal and cingulo-opercular dual networks distinction. Journal of Neuroscience. 2016;36:6147–55. doi: 10.1523/JNEUROSCI.4590-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadaghiani S, D’Esposito M. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cerebral Cortex. 2015;25:2763–73. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller VI, Langner R, Cieslik EC, Rottschy C, Eickhoff SB. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Struct Funct. 2015;220(4):2401–14. doi: 10.1007/s00429-014-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–68. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp. 2012;33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eickhoff SE, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, Bzdok D, Eickhoff CR. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016;137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid AT, Bzdok D, Genon S, Langner R, Müller VI, Eickhoff CR, Hoffstaedter F, Cieslik EC, Fox PT, Laird AR, Amunts K, Caspers S, Eickhoff SB. ANIMA: A data-sharing initiative for neuroimaging meta-analyses. Neuroimage. 2016;124(Pt B):1245–53. doi: 10.1016/j.neuroimage.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 33.Kotov R, Chang SW, Fochtmann LJ, Mojtabai R, Carlson GA, Sedler MJ, Bromet EJ. Schizophrenia in the internalizing-externalizing framework: a third dimension? Schizophr Bull. 2011;37(6):1168–78. doi: 10.1093/schbul/sbq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9(11):856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 36.Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen S. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214(5–6):669–80. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60(4):1947–58. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72(4):665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiers P, Mennes M, Sunaert S. Distributed task coding throughout the multiple demand network of the human frontal-insular cortex. Neuroimage. 2010;52(1):252–62. doi: 10.1016/j.neuroimage.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 40.Muhle-Karbe PS, Derrfuss J, Lynn MT, Neubert FX, Fox PT, Brass M, Eickhoff SB. Co-Activation-Based Parcellation of the Lateral Prefrontal Cortex Delineates the Inferior Frontal Junction Area. Cereb Cortex. 2016;26(5):2225–41. doi: 10.1093/cercor/bhv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen T, Cai W, Ryali S, Supekar K, Menon V. Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biol. 2016;14(6):e1002469. doi: 10.1371/journal.pbio.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai W, Chen T, Ryali S, Kochalka J, Li CS, Menon V. Causal interactions within a frontal-cingulate-parietal network during cognitive control: Convergent evidence from a multisite-multitask Investigation. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen T, Michels L, Supekar K, Kochalka J, Ryali S, Menon V. Role of the anterior insular cortex in integrative causal signaling during multisensory auditory-visual attention. Eur J Neurosci. 2015;41(2):264–74. doi: 10.1111/ejn.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 2013;23(11):2677–89. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74(6):990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspect Psychol Sci. 2011;6(6):589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- 48.Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria Initiative: background, issues, and pragmatics. Psychophysiology. 2016;53(3):286–97. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.