Abstract
In phocid seals, an increase in hematocrit (Hct) accompanies diving and periods of apnea. The variability of phocid Hct suggests that the total red cell mass is not always in circulation, leading researchers to speculate on the means of blood volume partitioning. The histology and disproportionate size of the phocid spleen implicates it as the likely site for RBC storage. We used magnetic resonance imaging on Northern elephant seals to demonstrate a rapid contraction of the spleen and a simultaneous filling of the hepatic sinus during forced dives (P < 0.0001, R2 = 0.97). The resulting images are clear evidence demonstrating a functional relationship between the spleen and hepatic sinus. The transfer of blood from the spleen to the sinus provides an explanation for the disparity between the timing of diving-induced splenic contraction (≈1–3 min) and the occurrence of peak Hct (15–25 min). Facial immersion was accompanied by an immediate and profound splenic contraction, with no further significant decrease in splenic volume after min 2 (Tukey–Kramer HSD, P = 0.05). At the conclusion of the dive, the spleen had contracted to 16% of its predive volume (mean resting splenic volume = 3,141 ml ± 68.01 ml; 3.54% of body mass). In the postdive period, the spleen required 18–22 min to achieve resting volume, indicating that this species may not have sufficient time to refill the spleen when routinely diving at sea, which is virtually continuous with interdive surface intervals between 1 and 3 min.
Phocid seals exhibit a higher hematocrit (Hct) during apnea and diving than during periods of eupneic respiration (1–4). This variation in red cell mass indicates that seals have some method of sequestering red cells during nonapneic events. Bryden and Lim (5) found the Hct values of elephant seal pups to be highly variable and suggested that the spleen acts as a dynamic reservoir for erythrocytes, releasing RBCs into general circulation when required. Qvist et al. (2) recorded a distinct rise in Weddell seal Hct during diving and suggested that the total red cell mass is partitioned between general circulation and the splenic reservoir. The concept of splenic red cell storage is well accepted by many researchers, and a diving-induced sympathetic vasoconstriction is thought to be the stimulus for splenic contraction and subsequent injection of oxygenated RBCs into circulation (2, 4, 6, 7). Strong evidence supports this line of reasoning. Histological and physiological data collected on seal spleens indicate that the organ is capable of considerable RBC storage and that it reacts to catecholamine stimulation (6–9). The ability of the phocid spleen to concentrate RBCs is supported by high Hct values obtained from splenic venous blood during catecholamine-induced contraction (88–93% in hooded seals; 82–88% in harp seals; ref. 7). Ultrasound imaging in Weddell seals demonstrates a correlation of the diving-induced rise in Hct with a postdive reduction in spleen size (6).
Dive and postdive catecholamine levels collected from freely diving Weddell and harbor seals show a significant increase over resting levels (6, 10, 11). In hooded and harp seals, in vitro plethysmographic measurements indicate that α-adrenoreceptor activation with epinephrine results in forceful contraction within 1–3 min of administration. Stimulation of β-receptors and cholinergic receptors did not cause capsular contraction. The contractile effect of epinephrine and nor-epinephrine was largely abolished when the α-adrenergic receptors were first blocked with phentolamine (7). Hurford et al. (6) found that, in vivo, Weddell seal spleens contract on stimulation by exogenous epinephrine infusion. The splenic dimensions obtained during the postepinephrine injection period were similar to those obtained from the postdive period. However, the doses required to obtain an equal degree of contraction are 400 times resting and significantly higher than observed physiological levels. This finding suggests that direct neural stimulation plays a considerable role in diving-induced splenic contraction, but to date this has not been investigated (6). Experiments with feline spleens indicate that the capsule is mainly under neural control, demonstrating significant capsular contraction with neural stimulation alone (12). In a diving seal, the rapidity of contraction indicates that the initial stimulation is likely neural in origin, but contraction may be sustained throughout the dive by circulating catecholamines released from the adrenal gland.
The data clearly support the existence of a diving-induced sympathetic contraction of the spleen and subsequent release of the stored erythrocytes; however, a discrepancy exists in the timing of these two events. Complete splenic contraction occurs within 3 min of catecholamine stimulation, yet peak Hct is not observed until 15–25 min after the spleen has contracted (2, 6). The delay between splenic contraction and changes in peripheral venous Hct may represent an equilibration between the splenic reservoir and the venous circulatory blood pools (6), but no evidence exists to substantiate this belief.
The presence of a large venous reservoir, termed the hepatic sinus, provides another possible source of sequestered RBCs. Formed by the dilation of the hepatic veins, the thin-walled sinus lies caudal to the diaphragm, draining from its midpoint through the diaphragm and into the thoracic portion of the posterior vena cava (13). The inferior vena cava and the hepatic sinus may contain up to one-fifth of the animal's total blood volume and present a significant storage depot of oxygenated blood during dives. During forced diving in northern elephant seals, the oxygen content of sinus blood was observed to decrease more slowly than arterial blood. As the dive progressed, blood oxygen content of sinus blood exceeded arterial values (6.6 and 4.6 vol per 100 ml, respectively).¶ The behavior of the sinus and the elevated blood oxygen content in the venous system has led some to suggest that the hepatic sinus is a controlled oxygen reservoir for use during diving (14, 15).
Filling of the sinus depends on the closure of a muscular vena caval sphincter located on the cranial aspect of the diaphragm. Stimulation of the right phrenic nerve causes forceful contraction of the sphincter (13). Angiographic studies enabled researchers to visualize blood flow and hepatic sinus dynamics during diving. Using harbor seals, Elsner et al. (14) demonstrated a diving-induced accumulation of contrast material within the vena cava below the diaphragm. Small quantities of contrast material were observed to pass through the restricted orifice of the sphincter and enter the heart. In experimental dives using harp seals (Pagophilus groenlandicus), Hol et al. (15) reported a marked constriction of the sphincter occurred 20 sec after commencement of the dive, with dilation of the posterior caval vein and hepatic sinuses occurring before as well as during the 40 sec after constriction. This study also demonstrated a temporary relaxation of the caval sphincter during the dive and subsequent mixing of the blood in the sinus with that returning from the anterior part of the body. These findings provide a potential explanation for the Elsner paradox, where blood oxygen content was observed to be higher in the venous system than in arterial vessels. Although these studies reveal a considerable amount of information regarding the dynamics of the sphincter and sinus during diving, its function in phocid physiology has not been clearly defined.
Under most circumstances, accurate measurements of a flaccid blood-filled structure are difficult to obtain, and this task is further complicated when the structure involves contractile smooth muscle. When invasive surgical procedures or stress-inducing methodologies are used, catecholamine-mediated changes in the smooth muscle are inevitable. Through MRI technology, we were able to acquire sequential images of the spleen and related vasculature during rest, diving, and postdiving periods of a long duration, deep diving seal (16). Volumetric assessment of the spleen and hepatic sinus in vivo during forced diving protocols was achieved through quantitative analysis of these images.
Methodology
Animal Handling.
In April 1997, four male and one female 4-month-old northern elephant seals, Mirounga angustirostris, were collected from Año Nuevo, California and held at the Long Marine Laboratory, University of California Santa Cruz (UCSC) for the duration of their captivity (mean = 94 kg ± 7.65 SD; National Marine Fisheries Service permit #938; UCSC Chancellor's Animal Research Committee protocol #COST97.10–2; Stanford University's Institutional Animal Care and Use Committee protocol #5091). The animals were transported from the holding facility to the Lucas Center for Advanced MR Technology at Stanford University on each day of an MR experiment. At the MR unit, the seal was manually restrained, and four ECG electrodes were glued to the ventral surface with cyanoacrylate adhesive. The animal was placed in a conical nylon jacket and strapped to a restraining board in a prone position. The board then was lifted into a PVC half-pipe, which served as a fluid containment unit to prevent water damage to the magnet. Once in position on the magnet bed, the ECG leads were connected and the strap of the respiratory bellows was threaded under the animal at the level of the diaphragm.
MR Imaging.
All images were collected by using a high-performance 1.5 T system (Signal Horizon Echo Speed, General Electric Medical Systems, Milwaukee, WI). MR imaging uses the magnetic properties of hydrogen and its interaction with both a large external magnetic field and radio waves to produce highly detailed images. The tissue is magnetized through placement of the animal in the magnet, and a radio frequency pulse is then emitted, exciting the region of interest. Two independent processes, termed T1 and T2, govern the relaxation of the signal. Due to the varying molecular structure of the tissues within an organism, each tissue exhibits a characteristic spin density and specific T1 and T2 relaxation times, which provide the basis for MR image contrast. Controlling of the contrast is accomplished through the timing of radio frequency pulses (known as TR, or repetition time), as well as alteration of the time interval between exciting the spins and sampling the MR signal (referred to as T1 or T2 weighting). For this study, a single-shot fast spin echo (SSFSE) pulse sequence was used in the sagittal plane to localize the splenic anatomy, whereas images used for analysis were obtained in the axial plane. SSFSE is an ultra-fast, T2-weighted sequence, with subsecond single-slice data acquisition. As the name suggests, SSFSE is a single-shot technique where all of the data required for a complete image is acquired in a single TR. The total acquisition time for a typical SSFSE image is 300–1,000 ms. The ability to acquire an image in less than 1 sec overcomes most of the motion artifact problems associated with older techniques such as conventional FSE. Scan parameters used were a flip angle of 90, echo time 185, 0.5 NEX, 48 × 48 field of view, 26–32 contiguous 15-m thick slices with no overlap, 256 × 256 matrix.
Diving Protocol.
Before imaging, animals were fitted with a diving helmet manufactured from a 35-cm Plexiglas tube, inner neoprene seal, and a secondary outer latex neck seal. The animal was allowed to acclimate for ≈30 min, during which time a vacuum hose was attached to the helmet to ensure sufficient airflow through the open valves. At the initiation of a diving experiment, the vacuum hose was removed and the helmet was filled with cold water. Timing of the dive commenced when the animal's nostrils were completely submerged and continued through until the helmet was drained and the first inspiration occurred.
Each seal was subjected to four sequential dives (diving time ranged from 5 min 38 sec to 7 min 23 sec; interdive time 20 min 13 sec to 26 min 21 sec). Forced dive durations were slightly less than the mean dive durations reported for freely diving pups of a similar age class (17). Imaging the entire spleen required 28–32 axial images. The acquisition time for each series of splenic images was less than 1 min. Images were acquired sequentially during the dive and continued throughout the dive at 1-min intervals until the dive was terminated. Images also were obtained in the predive and postdive periods.
Blood Sampling.
Collection of blood samples during diving could not be achieved in the MR unit, as the caudal end of the animal was not accessible during MRI data acquisition. Consequently, the restraint procedure and diving protocols were repeated at Long Marine Lab, and blood samples were drawn from the extradural intervertebral vein during the predive, dive, and postdive period (predive samples were obtained during initial needle placement; dive samples were drawn at 1, 3, 5, and 6 min; postdive samples at 2, 5, and 10 min). Two animals were released immediately after MR assessment, therefore blood was only obtained from three animals.
Serial blood samples were collected by using a 14-gauge, 13-cm spinal needle inserted between the lumbar vertebrae (approximately L4-L5, 5 cm cranial to the pelvis). A Vacutainer (Becton Dickinson) blood collection tube holder was attached to the needle via a multisampler hub, and samples were collected into a heparinized Vacutainer tube (Becton Dickinson). The heparinized tubes were placed on a blood tube oscillator before the transfer of two subsamples into heparinized microhaematocrit tubes. The microhaematocrit tubes were sealed (Critoseal, Sherwood Services), and the samples were centrifuged at 4,500 rpm for 15 min. Hct readings were obtained via comparison of the packed red cell level with a standard Hct card. Values expressed are an average of two subsample values for each time interval.
Image Assessment.
Images were analyzed by using National Institutes of Health IMAGE 1.6.1. The border of the spleen or hepatic sinus was drawn on each image, and the area of the selected region was derived (1 pixel = 0.1875 cm2). Volume of the selected region was calculated by multiplying the area of the region by 1.5 (slice thickness = 1.5 cm) (see Fig. 6 and Movies 1 and 2, which are published as supplemental data on the PNAS web site, www.pnas.org). Organ or sinus volume was obtained by summing the volume of all slices; images were analyzed out of sequence to avoid bias. Statistical analysis was conducted by using JMP 3.2.1. Changes in splenic volume were assessed by using ANOVA and all pairs comparison [Tukey–Kramer honestly significant difference (HSD)]; comparison of spleen and sinus volume to baseline was made by using the Dunnett's method. Splenic mass was calculated by summing the mass of the contracted spleen and the blood mass [(resting splenic volume − end dive volume) × specific gravity of northern elephant seal pup blood (1.07 g/ml; ref. 18)]. Sinus and spleen data were bootstrapped by using 10,000 repeats.
Results
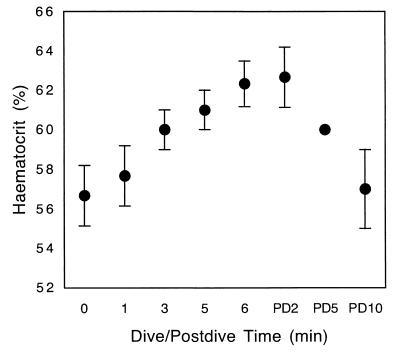
Hct values obtained during forced diving showed a significant increase over resting values for dive min 5 and 6. Hct had returned to resting by postdive min 10 (ANOVA, F(3,8)= 11.01, P = 0.003; Tukey–Kramer HSD, P = 0.05). Values show a trend toward a continued increase for the duration of the dive (Fig. 1). The highest values were observed at postdive min 2 (62.7 ± 1.58%).
Figure 1.
Hct values obtained from northern elephant seal pups during forced diving (n = 3). Diving Hct is significantly different from predive Hct in min 5 and 6. A trend toward a continued increase in Hct during the dive is observed and values returned to predive levels by postdive (PD) min 10 (ANOVA, F(3,8) = 11.01, P = 0.003; Tukey–Kramer HSD, P = 0.05).
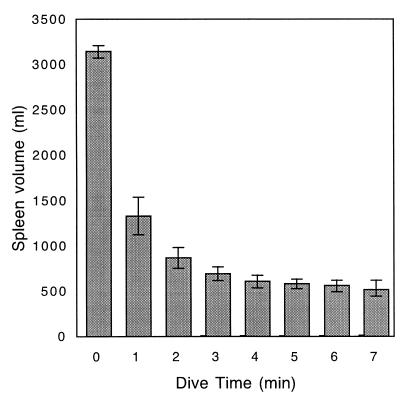
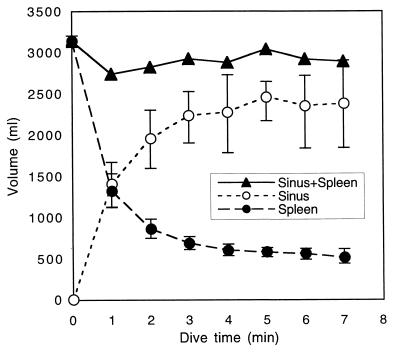
Mean resting splenic volume was 3,141.34 ml ± 68.01 ml SD, whereas end dive splenic volume (min 7) was 513.10 ml ± 87.12 ml SD. For the purpose of calculating splenic mass, the total volume of the splenic reservoir is assumed to be 100% RBCs (2,628 ml × 1.07 g/ml = 2812.22 g). Resting splenic mass was 3,325.31 g, or 3.54% of body mass (splenic blood reservoir mass + end dive spleen mass). In all animals, splenic contraction was initiated immediately upon facial immersion (Fig. 2) and no further significant reduction was observed after dive min 2 (Fig. 3; ANOVA F(6,28)= 34.10, P < 0.0001; Tukey–Kramer HSD, no significant decrease in volume after min 2, P = 0.05). Concomitantly, an increase in hepatic sinus volume also was observed, suggesting a direct shift of blood from the spleen to the sinus (P < 0.0001, R2 = 0.97). The combined volume of the spleen and hepatic sinus did not differ significantly from resting splenic volume at any time during the dive (Fig. 4; sinus volume + spleen volume is not significantly different from resting splenic volume; ANOVA, F(7,32) = 0.24, P = 0.97; comparison to baseline, Dunnett's method, P = 0.05). Once dilated, the hepatic sinus volume remained constant throughout the dive (no significant change in volume after min 1; ANOVA, F(7,32)= 12.94, P < 0.0001, Tukey–Kramer HSD, P = 0.05). As the dive progressed, a loss of signal intensity from the region of the hepatic sinus was observed (Fig. 5).
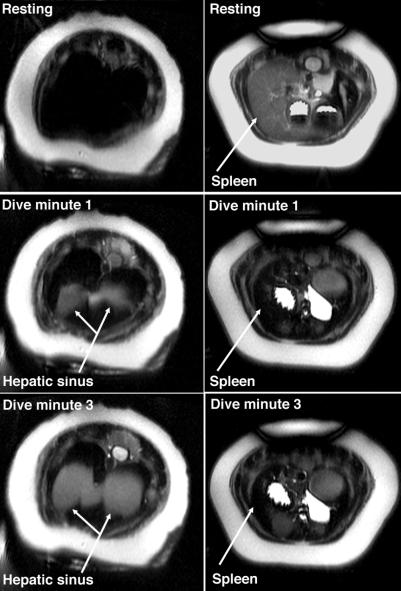
Figure 2.
Thoracic images of a northern elephant seal during rest and simulated diving. (Left) Images are from the region immediately caudal to the diaphragm. (Right) Images are 12 cm caudal to the diaphragm. Rapid contraction of the spleen and simultaneous filling of the hepatic sinus are observed.
Figure 3.
Northern elephant seal spleen volume during rest (min 0) and diving (min 1–7) was obtained by using MR imaging techniques (n = 5). Each animal had four dives. Splenic volume does not decrease significantly after min 2 (ANOVA, F(6,28) = 33.94, P < 0.0001). Error bars indicate 95% confidence interval (bootstrap analysis, 10,000 repeats).
Figure 4.
Graph of northern elephant seal splenic volume and hepatic sinus volume at each min of a 7-min dive (n = 5). Combined spleen and hepatic sinus volume does not differ significantly from spleen volume at rest (ANOVA, F(7,32) = 0.24, P = 0.97; comparison to baseline, Dunnett's method, P = 0.05).
Figure 5.
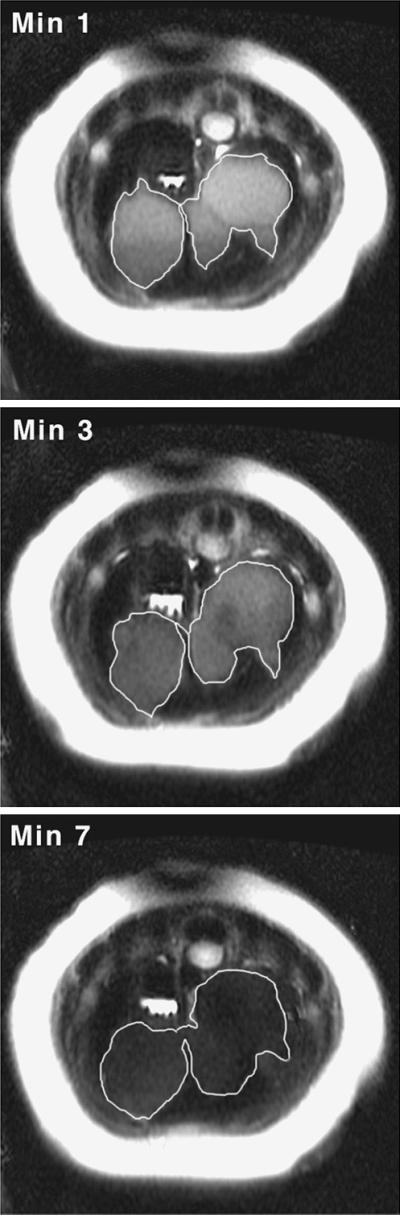
Axial images of a northern elephant seal pup during forced diving. The hepatic sinus dilates rapidly upon submergence and remains dilated throughout the dive. Loss of hepatic sinus signal intensity is observed as the dive progresses. Axial images were obtained from the thoracic region immediately caudal to the diaphragm. Dark crescent shape in the upper left quadrant of the image is the spleen; the margins of the hepatic sinus are outlined in white.
In the postdive period, the spleen gradually dilated, achieving its maximum volume at 18–22 min after the dive. Immediately after the dive, increased respiratory motion and diaphragm movement hampered quantification of the hepatic sinus volume. However, tachypnea had subsided by postdive min 4 and no evidence of hepatic sinus dilation or vena caval distension was observed.
Discussion
This study provides in vivo measurements of splenic contraction in a phocid and confirms that forced diving in northern elephant seal pups results in rapid contraction of the spleen and emptying of the concentrated RBCs into the venous system. Moreover, facial immersion-induced splenic contraction is accompanied by an acute increase in hepatic sinus volume, signifying a transfer of splenic RBCs into the sphincter-controlled sinus. The strong correlation of these events (R2 = 0.97) suggests that these two reservoirs act in concert to provide the diving seal with a means of RBC storage as well as a mechanism for controlled distribution of RBCs into general circulation.
The interplay between the spleen and hepatic sinus serves to explain a number of observed physiological events. Although the spleen has long been suspected as the source of the RBCs released during diving, the rate of splenic contraction has presented an apparent contradiction to the gradual diving-induced rise in Hct (2, 6). In this study, maximal Hct occurred after the 7-min dive had concluded, whereas the spleen had released the majority of its RBCs by dive min 2. The involvement of the sphincter-controlled sinus serves to delay the release of RBCs into general circulation and may abrogate the potentially deleterious effects of an acute rise in red cell mass. In northern elephant seal pups, contraction of the spleen in the first minute of the dive would result in an increase in vena caval blood volume at a rate of 23.6 ml/sec (min 0 to min 1 decrease in splenic volume = 1,417 ml/60 sec). Relocating the RBCs from the spleen into the sphincter-controlled venous sinus results in a gradual metering of oxygenated RBCs into the heart (14, 19), protecting it from a drastic increase in right ventricular pressure at a time when diving bradycardia is most profound (20).
The protective function of the caval sphincter is supported by Harrison's research (21), where placement of a clamp on the vena cava near the sphincter did not alter heart rate appreciably in an anaesthetized harbor seal, suggesting that sphincter-induced venous occlusion does not affect nondiving cardiac frequency. However, prevention of sphincter function via phrenic nerve transsection caused a delay in the onset of bradycardia (22), possibly because of the increase in right ventricular end-diastolic pressure that would result from the combination of peripheral vasoconstriction, splenic contraction, and unrestricted inferior vena caval flow. It was stated that animals with denervated sphincters “disliked diving for more than 2 min,” indicating that the perturbation of flow dynamics caused by sphincter dysfunction was significant enough to disrupt the mammalian diving response (22).
In addition to revealing the correlation between splenic contraction and hepatic sinus volume, the MR images also provide information on aspects of blood flow dynamics during diving. The observed maintenance of hepatic sinus volume over the course of the dive indicates that flow into the sinus is approximately equal to the flow leaving the sinus through the caval sphincter. At the beginning of a dive, hepatic sinus Hct is higher than that observed in general circulation because of the release of concentrated splenic RBCs (7, 8). Based on our present understanding of caval sphincter function, the pulsatile release of oxygenated venous blood into circulation and subsequent replacement by less concentrated (and less oxygenated) blood would result in an overall dilution of the hepatic sinus contents. This situation supports Elsner et al.'s observations¶ of higher oxygen content in the sinus than that observed in general arterial circulation.
Although the volume of the hepatic sinus does not change appreciably in the latter part of the dive, the signal intensity is seen to diminish (Fig. 5). A possible explanation for the loss of signal intensity is a reduction in oxyhaemoglobin signal. When water protons in blood are subject to a MR pulse sequence, the protons respond by precessing rapidly at a rate determined in part by the precise value of its local magnetic field. Slight differences in local fields cause the protons to precess at different rates, leading to a rapid loss of signal as they cancel one another. The time until cancellation, called T2*, is shortened by the presence of deoxyhemoglobin due to the wide variations in magnetic field caused by the iron atoms. The oxygen-carrying form of hemoglobin (oxyhaemoglobin) is not paramagnetic, so oxygen-rich blood exhibits longer T2* times, and thus an increased signal intensity (23). Over the course of a dive, reduction in the absolute oxyhaemoglobin content within the sinus occurs through a decrease in sinus Hct, and/or a decrease in PO2 due to deoxygenation. The release of oxygenated RBCs from the sinus and subsequent influx of deoxygenated blood of lower Hct would provide a combination of effects that may lead to the observed signal loss. Without further experimental analysis the specific cause of signal loss in this instance cannot be ascertained, but is likely caused by an overall reduction in the blood oxygen content within the sinus.
In the postdive period, image acquisition was hampered in the first 3 min by respiratory motion. By postdive min 4, tachypnea had subsided and no evidence of hepatic sinus dilation or vena caval distension was observed, suggesting that the caval sphincter was no longer occluding venous flow and the entire blood volume had entered active circulation. The spleen, however, did not return to its predive volume until 18–22 min after the dive had terminated. The significant delay in the resequestration of RBCs into the phocid spleen results in an extended period of elevated Hct and suggests that these animals would maintain a high circulating red cell mass throughout a diving bout, where surface intervals are normally 1–3 min in duration. The maintenance of an elevated circulating blood volume between dives has been observed in phocids both in the field (Weddell seals; refs. 1–3) and the laboratory (northern elephant seal pups; ref. 24). This observation has a number of ramifications. In the tachycardic postdive period, the rise in cardiac output combined with the high circulating blood volume realized by the delayed resequestration may enable the animal to increase the degree of vasodilation. The increase in cardiac output would facilitate a reduction in peripheral resistance and lead to a more rapid delivery of reoxygenated red cells to the tissues (cardiac output = mean arterial blood pressure/total peripheral resistance). Hyperperfusion of tissues benefits the animal during short interdive surface intervals by ensuring rapid replenishment of hemoglobin and myoglobin oxygen stores (25), as well as the removal of metabolic byproducts from previously ischaemic areas. Immediate dilation of the spleen and removal of RBCs from active circulation at this time might in fact be detrimental to the animal, delaying reoxygenation of the total red cell mass and increasing the surface interval.
For a diving phocid, there is an obvious benefit to increasing oxygen storage through elevation of Hct; however, there is also a potential cost associated with the subsequent increase in viscosity (blood flow is inversely related to blood viscosity; blood viscosity is exponentially related to Hct). It has been suggested that phocids may use the spleen to store RBCs when the benefit of increased oxygen is offset by the cost of transporting blood of higher viscosity (18, 26). In an environment where oxygen is not limiting, removal of a substantial portion of the red cell mass from active circulation would serve to diminish viscosity, resulting in a reduced cost of blood transport.
However, the ability to increase circulating red cell mass while on land may also have provided a selective advantage to the northern elephant seal. During the haul-out, the rise in available on-board oxygen stores resulting from splenic contraction would effectively extend the period of apnea and allow the animal to reduce the overall respiratory rate (25). These periods of nonventilation are important during the fast, as a significant quantity of water is conserved through apneustic breathing patterns (27). The reduction in respiratory water loss may provide a positive selective force for the continued availability of these sequestered RBCs. Significant metabolic savings also are realized through apneustic breathing, as they result in an overall reduction in heart rate and oxygen consumption (28). When an increase in oxygen delivery to the muscles is required, brief periods of high viscosity may be tolerable due to an increase in blood velocity. In a manner similar to that observed in racehorses, the seal may increase blood volume and oxygen availability to the tissues through splenic contraction, reducing viscosity by increasing shear rate through vasodilation and tachycardia (29). In the northern elephant seal, it appears that the spleen confers advantages in both the terrestrial and aquatic environs by facilitating a change in circulating red cell mass.
The demonstrated relationship between splenic contraction and hepatic sinus volume in northern elephant seal pups reveals the functional significance of the sinus and the dynamics of the spleen during forced diving. The elegant system of storage, transfer, and metering of RBCs provided by the spleen/sinus interaction allows the seal to maintain a higher circulating Hct during periods of hypoxia, yet effectively reduce Hct and circulating blood volume when oxygen is not limiting, thus avoiding any deleterious effects of increased blood viscosity.
Supplementary Material
Acknowledgments
We thank Drs. Dorian Houser and Suzi Kohin for their advice and assistance with animal handling and transport and Dr. Lorie Pelc for on-site technical assistance and support. Valuable field assistance was provided by Javier Janz and a legion of volunteers from the University of California, Santa Cruz and Stanford University. This study was funded through the Natural Sciences and Engineering Research Council Canada (to P.W.H.), National Institutes of Health Grant P41 RR09784, and the Center for Advanced MR Technology at Stanford.
Abbreviations
- Hct
hematocrit
- FSE
fast spin echo
- HSD
honestly significant difference
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Eisner, R. W., Scholander, P. F., Craig, A. B., Dimond, E. G., Irving, L., Pilson, M., Johansen, K. & Bradstreet, E. (1964) Physiologist 7, 124 (abstr.).
References
- 1.Kooyman G L, Wahrenbrock E A, Castellini M A, Davis R W, Sinnett E E. J Comp Physiol B. 1980;138:335–346. [Google Scholar]
- 2.Qvist J, Hill R D, Schneider R C, Falke K J, Liggins G C, Guppy M, Elliot R L, Hochachka P W, Zapol W M. J Appl Physiol. 1986;61:1560–1569. doi: 10.1152/jappl.1986.61.4.1560. [DOI] [PubMed] [Google Scholar]
- 3.Castellini M A, Davis R W, Kooyman G L. Physiol Zool. 1988;61:379–386. [Google Scholar]
- 4.Zapol W M, Hill R D, Qvist J, Falke K, Schneider R C, Liggins G C, Hochachka P W. Undersea Biomed Res. 1989;16:363–373. [PubMed] [Google Scholar]
- 5.Bryden M M, Lim G H K. Comp Biochem Physiol. 1969;28:139–148. doi: 10.1016/0010-406x(69)91328-0. [DOI] [PubMed] [Google Scholar]
- 6.Hurford W E, Hochachka P W, Schneider R C, Guyton G P, Stanek K, Zapol D G, Liggins G C, Zapol W M. J Appl Physiol. 1996;80:298–306. doi: 10.1152/jappl.1996.80.1.298. [DOI] [PubMed] [Google Scholar]
- 7.Cabanac A, Folkow L P, Blix A S. J Appl Physiol. 1997;82:1989–1994. doi: 10.1152/jappl.1997.82.6.1989. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher U, Welsch U. Am J Anat. 1987;179:356–368. doi: 10.1002/aja.1001790406. [DOI] [PubMed] [Google Scholar]
- 9.Cabanac A J, Messelt E B, Folkow L P, Blix A S. J Zool. 1999;248:75–81. [Google Scholar]
- 10.Hance A J, Robin E D, Halter J B, Lewiston N, Robin D A, Cornell L, Caligiuri M, Thoedore J. Am J Physiol. 1982;242:R528–R532. doi: 10.1152/ajpregu.1982.242.5.R528. [DOI] [PubMed] [Google Scholar]
- 11.Hochachka P W, Liggins G C, Guyton G P, Schneider R C, Stanek K S, Hurford W E, Creasy R K, Zapol D G, Zapol W M. Comp Biochem Physiol. 1995;112:361–375. doi: 10.1016/0305-0491(96)85239-4. [DOI] [PubMed] [Google Scholar]
- 12.Greenway C V. Am J Physiol. 1979;5:H238–H243. doi: 10.1152/ajpheart.1979.236.2.H238. [DOI] [PubMed] [Google Scholar]
- 13.Harrison R J, Tomlinson J D W. Proc Zool Soc London. 1956;126:205–233. [Google Scholar]
- 14.Elsner R, Hanafee W N, Hammond D D. Am J Physiol. 1971;220:1155–1157. doi: 10.1152/ajplegacy.1971.220.5.1155. [DOI] [PubMed] [Google Scholar]
- 15.Hol R, Blix A S, Myhre H O. Rapp P-V Reun Cons Int Explor Mer. 1975;169:423–432. [Google Scholar]
- 16.Le Boeuf B J, Costa D P, Huntley A C, Feldkamp S D. Can J Zool. 1988;66:446–458. [Google Scholar]
- 17.Le Boeuf B J, Morris P A, Blackwell S B, Crocker D E, Costa D P. Can J Zool. 1996;74:1632–1644. [Google Scholar]
- 18.Castellini J M, Castellini M A. Physiol Zool. 1993;66:619–627. [Google Scholar]
- 19.Zapol W M. Sci Am. 1987;256:100–105. doi: 10.1038/scientificamerican0687-100. [DOI] [PubMed] [Google Scholar]
- 20.Ronald K, McCarter R, Selley L J. In: Functional Anatomy of Marine Mammals. Harrison R J, editor. Vol. 3. New York: Academic; 1977. pp. 235–270. [Google Scholar]
- 21.Harrison R J. Nature (London) 1960;188:1068–1070. doi: 10.1038/1881068a0. [DOI] [PubMed] [Google Scholar]
- 22.Harrison R J, Tomlinson J D W. Mammalia. 1960;24:386–399. [Google Scholar]
- 23.Parrish T. In: Physics of MR Imaging. Finn J P, editor. Vol. 7. Philadelphia: Saunders; 1999. pp. 765–782. [Google Scholar]
- 24.Thorson P H. Ph.D thesis. Santa Cruz: Univ. of California; 1993. [Google Scholar]
- 25.Castellini M A. In: Elephant Seals: Population Ecology, Behavior, and Physiology. Boeuf B J L, Laws R M, editors. Berkeley: Univ. of California Press; 1994. pp. 343–353. [Google Scholar]
- 26.Elsner R, Meiselman H J. Mar Mamm Sci. 1995;11:93–96. [Google Scholar]
- 27.Blackwell S B. Ph.D thesis. Santa Cruz: Univ. of California; 1996. [Google Scholar]
- 28.Kohin S, Williams T M, Ortiz C L. Respir Physiol. 1999;117:59–72. doi: 10.1016/s0034-5687(99)00050-x. [DOI] [PubMed] [Google Scholar]
- 29.Persson S G B. In: Equine Exercise Physiology. Snow D H, Persson S G B, Rose R J, editors. Vol. 1. Oxford: Burlington; 1983. pp. 324–327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.