Abstract
Transducin (T), the GTP-binding protein of the retina activates the cGMP phosphodiesterase system, and presents analogies with the proteins GS and Gi which respectively mediate adenylate cyclase activation and inhibition by hormone receptors. These proteins are all comprised of an alpha subunit carrying the GTP-binding site and a beta gamma subunit made of two peptides. The beta peptide (35 kd) appears similar in the three proteins. We demonstrate here that purified T beta gamma inhibits adenylate cyclase from human platelet membranes. This inhibition was observed when adenylate cyclase was stimulated by GTP, prostaglandin E1 (PGE1), NaF and forskolin, but not when stimulated by GTP(gamma)S. In the presence of GTP and forskolin, the T beta gamma-induced maximal inhibition was not additive with the alpha 2-receptor-induced adenylate cyclase inhibition mediated by Gi. Both inhibitions were suppressed at high Mg2+ concentrations, which as also known to dissociate T beta gamma from T alpha-GDP. This suggests that these adenylate cyclase inhibitions are due to the formation of inactive complexes of GS alpha-GDP with T beta gamma or Gi beta gamma. T beta gamma-induced inhibition did not require detergent and could be suppressed by simple washing. T beta gamma effects are dependent on its concentration rather than on its total amount. This suggests that T beta gamma can operate in solution with no integration into the membrane. Similar inhibitory effects of T beta gamma are observed on adenylate cyclase from anterior pituitary and lymphoma S49 cell lines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Jakobs K. H. Epinephrine inhibits adenylate cyclase and stimulates a GTPase in human platelet membranes via alpha-adrenoceptors. FEBS Lett. 1981 Aug 3;130(2):235–238. doi: 10.1016/0014-5793(81)81128-3. [DOI] [PubMed] [Google Scholar]
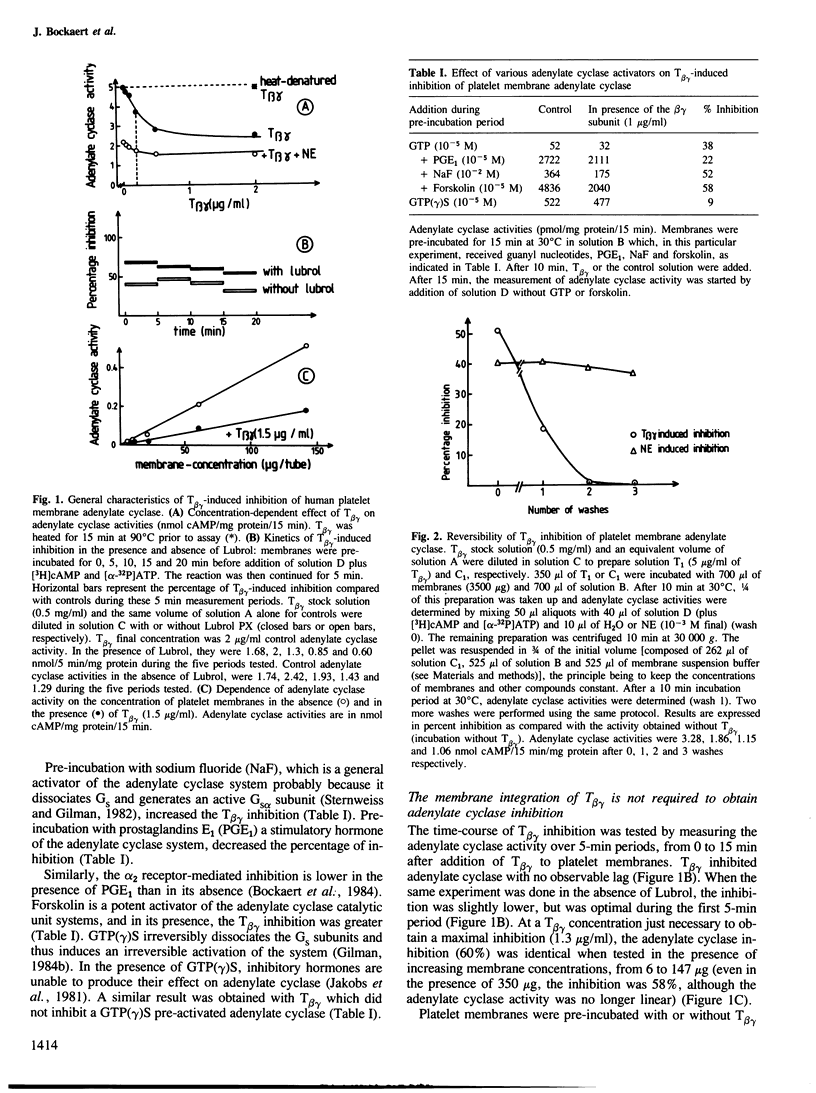
- Bockaert J., Cantau B., Sebben-Perez M. Hormonal inhibition of adenylate cyclase. A crucial role for Mg2+. Mol Pharmacol. 1984 Sep;26(2):180–186. [PubMed] [Google Scholar]
- Bockaert J., Hunzicker-Dunn M., Birnbaumer L. Hormone-stimulated desensitization of hormone-dependent adenylyl cyclase. Dual action of luteninizing hormone on pig graafian follicle membranes. J Biol Chem. 1976 May 10;251(9):2653–2663. [PubMed] [Google Scholar]
- Bockaert J., Sebben-Perez M. Adenylate cyclase inhibition by hormones. The Mg2+ hypothesis. FEBS Lett. 1983 Sep 5;161(1):113–116. doi: 10.1016/0014-5793(83)80741-8. [DOI] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Pfister C., Chabre M. Guanine nucleotides and magnesium dependence of the association states of the subunits of transducin. FEBS Lett. 1984 Dec 10;178(2):228–232. doi: 10.1016/0014-5793(84)80606-7. [DOI] [PubMed] [Google Scholar]
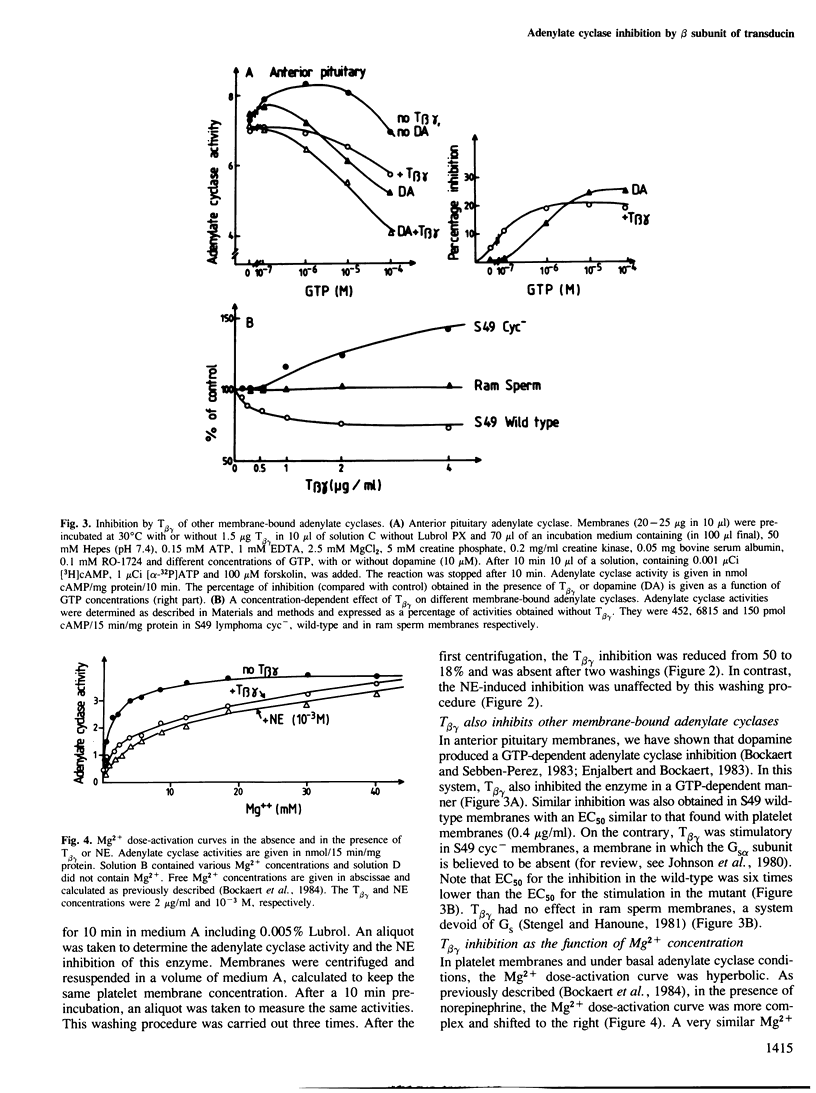
- Enjalbert A., Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Mol Pharmacol. 1983 May;23(3):576–584. [PubMed] [Google Scholar]
- Fung B. K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983 Sep 10;258(17):10495–10502. [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. Guanine nucleotide-binding regulatory proteins and dual control of adenylate cyclase. J Clin Invest. 1984 Jan;73(1):1–4. doi: 10.1172/JCI111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. D., Codina J., Risinger R., Birnbaumer L. Identification of a gamma subunit associated with the adenylyl cyclase regulatory proteins Ns and Ni. J Biol Chem. 1984 Feb 25;259(4):2039–2042. [PubMed] [Google Scholar]
- Insel P. A., Stengel D., Ferry N., Hanoune J. Regulation of adenylate cyclase of human platelet membranes by forskolin. J Biol Chem. 1982 Jul 10;257(13):7485–7490. [PubMed] [Google Scholar]
- Iyengar R., Abramowitz J., Bordelon-Riser M., Blume A. J., Birnbaumer L. Regulation of hormone-receptor coupling to adenylyl cyclase. Effects of GTP and GDP. J Biol Chem. 1980 Nov 10;255(21):10312–10321. [PubMed] [Google Scholar]
- Iyengar R., Birnbaumer L. Hormone receptor modulates the regulatory component of adenylyl cyclase by reducing its requirement for Mg2+ and enhancing its extent of activation by guanine nucleotides. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5179–5183. doi: 10.1073/pnas.79.17.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. Inhibition of adenylate cyclase by hormones and neurotransmitters. Adv Cyclic Nucleotide Res. 1981;14:173–187. [PubMed] [Google Scholar]
- Jakobs K. H., Schultz G. Occurrence of a hormone-sensitive inhibitory coupling component of the adenylate cyclase in S49 lymphoma cyc- variants. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3899–3902. doi: 10.1073/pnas.80.13.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Farfel Z., Bourne H. R. Genetic analysis of hormone-sensitive adenylate cyclase. Adv Cyclic Nucleotide Res. 1980;13:1–37. [PubMed] [Google Scholar]
- Kanaho Y., Tsai S. C., Adamik R., Hewlett E. L., Moss J., Vaughan M. Rhodopsin-enhanced GTPase activity of the inhibitory GTP-binding protein of adenylate cyclase. J Biol Chem. 1984 Jun 25;259(12):7378–7381. [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984 Mar 25;259(6):3568–3577. [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Smigel M. D., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and the inhibition of adenylate cyclase in S49 lymphoma cyc- and wild type membranes. J Biol Chem. 1984 Mar 25;259(6):3586–3595. [PubMed] [Google Scholar]
- Kimura N., Shimada N. GDP does not mediate but rather inhibits hormonal signal to adenylate cyclase. J Biol Chem. 1983 Feb 25;258(4):2278–2283. [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Gilman A. G. The regulatory components of adenylate cyclase and transducin. A family of structurally homologous guanine nucleotide-binding proteins. J Biol Chem. 1983 Jun 10;258(11):7059–7063. [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Gilman A. G. The guanine nucleotide activating site of the regulatory component of adenylate cyclase. Identification by ligand binding. J Biol Chem. 1982 Oct 10;257(19):11416–11423. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution, activity, and properties of the 35,000-dalton (beta) subunit. J Biol Chem. 1983 Sep 25;258(18):11361–11368. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Stengel D., Hanoune J. The catalytic unit of ram sperm adenylate cyclase can be activated through the guanine nucleotide regulatory component and prostaglandin receptors of human erythrocyte. J Biol Chem. 1981 Jun 10;256(11):5394–5398. [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Wheeler G. L., Yamazaki A., Bitensky M. W. A GTP-protein activator of phosphodiesterase which forms in response to bleached rhodopsin. J Cyclic Nucleotide Res. 1981;7(2):95–104. [PubMed] [Google Scholar]
- Van Dop C., Medynski D., Sullivan K., Wu A. M., Fung B. K., Bourne H. R. Partial cDNA sequence of the gamma subunit of transducin. Biochem Biophys Res Commun. 1984 Oct 15;124(1):250–255. doi: 10.1016/0006-291x(84)90944-6. [DOI] [PubMed] [Google Scholar]


