Abstract
Background
Background: Alzheimer’s disease (AD) is the most common form of old age dementia. The formation of amyloid plaques (Aβ), neurofibrillary tangles and loss of basal forebrain cholinergic neurons are the hallmark events in the pathology of AD.
Literature Review
Cholinergic system is one of the most important neurotransmitter system involved in learning and memory which preferentially degenerates in the initial stages of AD. Activation of cholinergic receptors (muscarinic and nicotinic) activates multiple pathways which result in post translational modifications (PTMs) in multiple proteins which bring changes in nervous system. Cholinergic receptors-mediated PTMs “in-part” substantially affect the biosynthesis, proteolysis, degradation and expression of many proteins and in particular, amyloid precursor protein (APP). APP is subjected to several PTMs (proteolytic processing, glycosylation, sulfation, and phosphorylation) during its course of processing, resulting in Aβ deposition, leading to AD. Aβ also alters the PTMs of tau which is a microtubule associated protein. Therefore, post-translationally modified tau and Aβ collectively aggravate the neuronal loss that leads to cholinergic hypofunction.
Conclusion
Despite the accumulating evidences, the interaction between cholinergic neurotransmission and the physiological significance of PTM events remain speculative and still needs further exploration. This review focuses on the role of cholinergic system and discusses the significance of PTMs in pathological progression of AD and highlights some important future directions.
Keywords: Acetylcholine, Alzheimer’s disease, muscarinic receptors, nicotinic receptors, post translational modifications
Alzheimer’s Disease
Neurodegenerative diseases are devastating conditions with progressive degeneration of nerve cells resulting in abnormal mental functioning specially dementia [1]. Degenerative diseases of the brain were long considered among the most ambiguous and troublesome of all diseases [2]. In 1906, Alois Alzheimer for the first time described the neuropathological features in the brain of a patient Auguste D., suffering from dementia. Later on, Emil Kraepelin in 1910 renamed the same pathology as “Alzheimer’s Disease” to differentiate the general memory impairment from the common senile dementia [3].
Pathological Features of Alzheimer’s Disease
Major hallmarks of AD include basal forebrain cholinergic hypofunction [4], extracellular accumulation of beta-amyloid known as amyloid or “senile” plaques [5] and intracellular neurofibrillary tangles (NFTs) accumulation [6, 7]. Senile plaques are the extracellular aggregates of beta-amyloid protein (Aβ) derived from cleavage of amyloid precursor protein (APP) via the action of β- and γ-secretase [8], while neurofibrillary tangles (NFTs) consist of hyperphosphorylated tau protein, present inside the neurons [9]. Beside plaques and NFTs, synaptic dysfunction is one of the most critical aspects of dementia [10, 11]. It has been found that synapses involving acetylcholine (ACh), glutamate and serotonin are primarily impaired in AD [12, 13]. The loss of basal cholinergic neurons is associated with severe neurodegeneration and cell loss in the nucleus basalis complex [14]. Cortex and hippocampus receive their major cholinergic input from nucleus basalis of Meynert and diagonal band of broncha, respectively [15]. The degeneration of basal forebrain cholinergic neurons is considered to be the earliest pathological event along with plaque and tangle formation [16, 17]. Lesions of the cholinergic basal nuclei in rats result in a number of memory deficits [18] and affect memory and cognition in primates [19]. The basal forebrain cholinergic deficits positively correlate with cognitive [20] and non cognitive behavioral deficits [21] observed in AD patients. But the dilemma, that why the basal forebrain cholinergic neurons are among the first targets in AD pathology, still needs to be solved [22].
Cholinergic Receptors
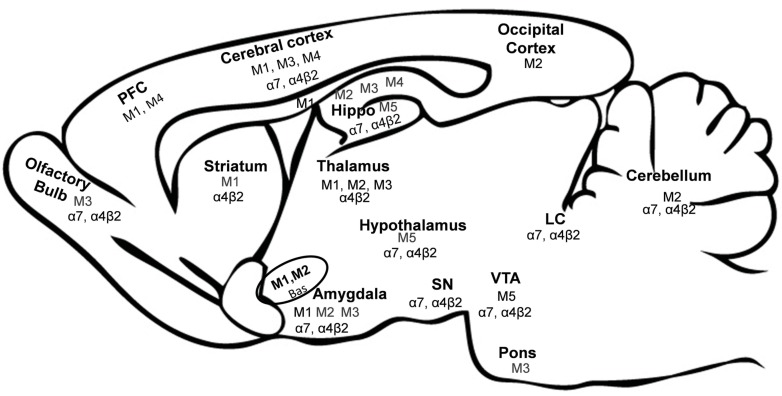
The ACh receptor (AChR) is a vital membrane protein on which ACh acts as a neurotransmitter. The cholinergic receptors are broadly categorized as muscarinic ACh receptors (mAChR) and nicotinic ACh receptors (nAChR) on the basis of their exogenous agonists. The expression of these receptors varies in different brain areas (Fig. 1). Nicotinic receptors are found in the neuromuscular junction, autonomic ganglia and various places in the CNS, though, with different composition. While muscarinic receptors are found to be expressed in the brain both at the pre-synaptic and post-synaptic nerve terminals and parasympathetic effector organs [23].
Fig. (1).
Expression of Muscarinic and Nicotinic Receptors in Brain. Abbreviations: bas: Nucleus basalis, Hippo: hippocampus, LC: locus coeruleus, PFC: Prefrontal cortex, SN: substantia nigra, VTA: ventral tegmental area [39, 40, 44, 51, 52, 76].
Muscarinic Receptors: Classification, Location and Brain Functions
The mAChR belong to the family of seven transmembrane receptors coupled to G-proteins (GPCRs), regulating a variety of physiological processes [24]. These receptors are comprised of single polypeptides which form seven transmembrane domains forming a central pore. ACh binds at a site inside this pore to activate the signaling cascade via G-proteins [25]. In the central nervous system (CNS), the muscarinic system plays important role in the regulation of many sensory, motor and autonomic processes [26]. Moreover, mAChRs have established roles in cholinergic transmission as well as learning and memory [12].
Muscarinic receptors are further sub-divided into five types M1-M5 encoded by five genes, m1–m5 [27]. The five mAChR subtypes are similar with the exception of the third intracellular loop while their signaling features are different, so these subtypes are further categorized into two groups [28-30] which determine the specific coupling preferences of these receptors [31]. The M1-like subfamily (M1, M3 and M5) is coupled to Gαq/11 protein which causes activation of phospholipase C. Stimulation of M1-like subfamily receptors leads to regulation of different proteins and their functions by the process of phosphorylation. Whereas the M2-like subfamily (M2 and M4) is coupled to Gi/o, which inhibits adenylate cyclase [32]. The stimulation of M2 and M4 receptors causes a reduced cytosolic cAMP level [33]. The intracellular muscarinic signaling responses include activation of protein kinases, phospholipases A2 and D (releasing arachidonic acid and choline, respectively) and regulation of calcium and potassium channels [34].
The mAChRs are widely distributed throughout the body peripherally as well as centrally. In the hippocampus and cerebral cortex, mAChRs are involved in cognitive processes such as memory [35-37]. While in the striatum and motor cortex, these receptors are involved in motor function [38].
M1 receptors are abundantly present in the hippocampus, neocortex, striatum, amygdala, thalamus [39] and prefrontal cortex [40]. M1 receptor knock-out mouse showed elevated levels of Aβ peptides in brain [41] and increased aggregation of amyloid plaques which leads to impaired memory consolidation in this model [42].
M2 receptors are auto-receptors for ACh release [43], present on presynaptic cholinergic neurons, and abundantly found in the cerebellum, thalamus [44] and nucleus basalis while lower levels are found in the hippocampus, amygdala and caudate putamen [39, 45].
The expression of M3 is relatively low i-e; 5-10% of total mAChRs in brain [46]. It is found in several brain regions, including cortex, amygdala, hippocampus, olfactory bulb, striatum, thalamus and pons [39]. M3 is involved in the regulation of neurotransmitter release, including dopamine, GABA and glycine as well as endocannabinoids [47, 48], suggesting its critical role in regulating other types of neurotransmission and learning and memory.
Relatively low levels of M4 receptors are expressed in brain as compared to other mAChR subtypes and are localized to hippocampus, including CA1 region and dentate gyrus [39], while the highest levels are in the caudate putamen [45] and prefrontal cortex [40] where they play role in the feedback control of neurotransmitter release [49] and cognitive processes [50].
M5 mAChRs has been found at low levels in the brain, particularly in the ventral tegmental area [51, 52], hippocampus and substantia nigra [13]. These receptors play an important role in facilitating muscarinic agonist-induced dopamine release from the nerve terminal [53]. So as a consequence, presence of M5 receptor is important for dopamine release and might be involved in facilitating dopamine-mediated and reward related physiological functions.
Involvement in Alzheimer’s Disease
Normal processing of the APP is under the control of cholinergic inputs [54, 55]. So altered cholinergic inner-vations could lead to abnormal processing of β-amyloid and possible formation of potentially neurotoxic fragments leading to neuritic plaque formation [56-58]. It has been observed that ACh esterase (AChE) accelerates the aggregation of Aβ into insoluble amyloid fibrils via unknown mechanism [59]. Basal forebrain cholinergic neuronal fibres are lost in AD at a later stage [60, 61]. This neuronal loss may be a result of Aβ neurotoxicity to the cholinergic terminals followed by retrograde degeneration [62]. Long-term exposure to micromolar concentration of Aβ is toxic to cholinergic neurons [63]. There are several reports on loss of cholinergic fibers and nerve terminals in AD and reduced cholinergic receptors [12, 64] but the relationship between Aβ and cholinergic deficit is poorly understood [62]. Due to the deterioration of cholinergic neurons in the brain of AD patients, there is a considerable loss of nicotinic receptors and certain muscarinic receptors particularly in the cortex and hippocampus [65-67], leading to impaired neurotransmitter release. In the cortical pyramidal neurons the activation of muscarinic receptors is known to enhance GABAergic transmission. The GABAergic inhibition is cruical for execution of certain memory forms by controlling the information flow in cortical circuits. Therefore, cholinergic hypofunction leads to cognitive impairment in AD patients [68].
Cholinergic hypofunction is a hallmark of AD [69, 70]. Specially, M1 and, M2 are down-regulated in hippocampus and cortex [45] and M4 appears to be down-regulated in cortex [71]. Down-regulation of M1, M2 and M4 is responsible for cognition deficits as well as impaired ACh release which exacerbates the AD symptoms. In another study, M1 receptors remained unchanged in AD patients, but M1/G-protein coupling was considerably decreased in the frontal cortex which was linked with the progression of cognitive impairment [72]. M1 receptors being involved in modulation of cognition, and are found to be the therapeutic targets for AD treatment. M1 agonists may alter the proteolysis of APP resulting in significantly reduced Aβ levels in cortex and hippocampus [73]. The M1/M3 activation increases non amyloidogenic pathway of APP processing. Therefore, the hypofunction of these receptors might increase Aβ generation leading to severe AD pathology [74]. It is reported that M1 receptor signal transduction-related functions are compromised in AD [72, 75, 76] but in another study cortical M1 receptor was increased in AD [77]. M2 receptor is increased in AD suggesting, that the presynaptic M2 receptors are preserved or up-regulated resulting in reduction in neurotransmitter release [71], but in another study M2 receptors were significantly reduced in hippocampus of AD brains [78]. Learning and memory associated with fear conditioning was declined in M3 knock-out mice [79]. M3 receptor levels were found to be decreased in the entorrhinal cortex and hippocampus [78]. Alteration in mAChR subtypes has important implications in cognitive control as well as ACh regulation. Impaired M1 receptor exacerbates AD-related cognitive decline, while disruption of M2/M4 receptors negatively regulates the ACh release as well the cognitive learning.
Among the cholinergic markers, the activity of choline acetyltransferase (ChAT) is greatly reduced in AD and is related to severity of disease [62, 80, 81]. Reduction in ChAT activity has been correlated with the numbers of neurofibrillary tangles in AD [82], suggesting a strong relationship between cholinergic transmission and AD. Loss of cholinergic neurons causes a significant reduction of ChAT activity (up to 95%) in the neocortex [83, 84] and hippocampus [85], that has been related to a marked decrease of ACh levels in these regions [86].
Agents that block mAChRs disturb cognitive functions and cause temporary loss of short term memory [12, 87-89]. Recently, muscarinic receptor family has shown clinical effectiveness in recovering cognitive impairment associated with AD [90, 91]. The role of Gq-coupled M1 and M3 receptors has been confirmed in modifying non-amyloidogenic pathway of APP processing while Gi-coupled M2 and M4 receptors promote amyloidogenic pathway [54, 92]. It reflects that the muscarinic receptors are among the excellent targets for the treatment of AD.
Muscarinic Agonists for Alzheimer’s disease treatment
mAChR are considered to be of prime importance among key drug discovery targets for the cure of AD (Table 1) [93-95]. Muscarinic agonists delay the progression of AD by decreasing β-amyloid aggregation, reducing tau phosphorylation and improving cognitive behavior [73, 94, 96, 97]. Cholinergic system modulation improves synaptic function by increasing synaptic protein expression at various stages of disease [98], improves synaptic plasticity [99] and also suppresses inflammatory response [100].
Table 1.
M1 Allosteric modulators/agonists under clinical trials.
| M1 Agonist | Therapeutic Effects | Refs. |
|---|---|---|
| AF150(S) | Decreases β-amyloid levels in CSF | [102] |
| AF267B | Restores cognitive and behavioural impairments, decreases Aβ aggregation and tau hyperphosphorylation | [61] |
| AF102B | Decreases CSF β-amyloid level in AD | [84] |
| TBPB | Activation of non-amyloidogenic pathway for APP processing and reduced Aβ synthesis in vitro. | [81] |
| BQCA | Restores discrimination-based learning in a transgenic mouse model of AD, control non-amyloidogenic pathway of APP in vitro | [103] |
| AC-260584 | Improves cognitive performance in animal model | [104] |
| 77-LH-28-1 | An agonist at rat hippocampal M1 receptors, increases cell firing | [105] |
AD: Alzheimer’s disease, APP: Amyloid Precursor Protein, CSF: Cerebrospinal fluid, M1: Muscarinic receptor 1, BQCA: Benzylquinolone carboxylic acid.
M1 receptor is considered to be an important therapeutic target as it is abundant in the hippocampus and cerebral cortex, where the cholinergic hypofunction is well-defined in AD. This receptor subtype is concerned with short-term memory [13]. Moreover, stimulation of M1 muscarinic receptors reduces the production of β-amyloid by activating α-secretase as this leads to non-amyloidogenic pathway [101].
Nicotinic Acetylcholine Receptors
The nicotinic ACh receptors (nAChRs) are ligand gated ion channels. These receptors are formed by assembly of five subunits, around a central pore, in homomeric or heteromeric conformation [106]. The standard subunits include α2-α9 and β2-β4 [107]. The neural subunits capable of forming heteromeric nAChRs with αβ subunit combinations are α2-α6 and β2-β4. Whereas subunits α7-α9 make functional homomeric nAChRs [106]. The β subunits alone are incapable of forming functional receptor while α2-α6 alone can only make receptors with very weak response to ligand. This indicates that only the combination of α and β receptors make a fully functional receptor [108]. It is also reported that α subunits contain agonist recognition and binding site. The β subunits are helpful to increase affinity towards agonist and to stabilize the whole receptor [108]. Individual nAChR subunits can combine in different stichiometries but, (α7)5, (α4)2(β2)3 and (α4)3(β2)2 nAChR are the most common receptor types in central nervous system [109]. Each subunit of nAChRs contains four transmembrane domains (M1-M4), two hydrophilic extracellular segments (N- and C- terminals) and an intracellular loop between M3 and M4 transmembrane domains [110]. This intracellular loop has putative phosphorylation sites [110]. The second transmembrane domain, M2, aligns along the centre to make the central pore [106]. Two molecules of ligand must bind to the receptor to allow opening of the central pore and permeation of cations (Ca+2, Na+, K+) [110].
Location and Function of Nicotinic Acetylcholine Receptors
Among many different possible combinations of the nicotinic receptor subunits the α4β2 and α7 type receptors are most abundant in the mammalian brain [107]. In the rodent brain α4β2 receptor type is reported to be the most abundant of all the nicotinic receptor subtypes and is found to be expressed in all layers of cerebral cortex and hippocampus in rat [111]. The α7 receptor subtype is highly expressed by basal forebrain neurons, and the innervations projecting towards hippocampus from basal forebrain [112, 113].
The function of the nAChRs is regulated by the binding of ligand, nicotine or ACh, to the receptor. ACh binds to extracellular N-terminal domain of receptor at boundary between α and non-α subunits [114]. The binding causes an influx of different cations, especially Ca+2 ion, inside the cell [115]. This nAChR mediated Ca+2 entry causes a marked increase in intracellular Ca+2 concentration, which is adequate to initiate Ca+2 sensitive processes [110].
Involvement in Alzheimer’s disease
There are contradictory reports about the expression of most abundant nAChR subtypes, α4β2 and α7, during AD in different brain areas. Some studies report an increase in the expression of nAChRs in AD [116-118] while others report nAChRs decrease during progression of AD [108, 119-121]. It was reported in a study that at mRNA level, the expression of both receptor types remains the same in control and AD patient brain cortices while at protein level there is a 30% decrease in the expression of α4β2 and α7 receptor subtypes. The difference observed in α4β2 and α7 receptor expression at protein level and mRNA level might be due to a change at translational or post translational level during nAChR biosynthesis [122], but the exact mechanism is not known and needs to be investigated. Similar observation was made when autopsy samples of cerebral cortex from AD patients were studied [123]. But they reported a 40% decrease in α4 receptor expression and 17% decrease in α7 receptor expression. Decreased expression of nAChRs causes a deficiency in the binding sites for nicotine and ACh which leads to cognitive deficit in AD [123]. Contrary to these observations that report a decrease in nicotinic receptor expression, another study [124] reported an increase in α7 mRNA expression in patients of AD, while no difference in the expression of α4 was observed by them. An increased expression of nicotinic receptor protein was also reported in animal models [125, 126]. This difference in expression studies might be due to age dependent biphasic effect on nicotinic receptor expression, during AD, in animal model. As it is reported that nAChR expression increases 3-4 fold at 9 months of age and then a decrease in expression is observed at 12 months of age [127].
It is reported that in early AD the initial Aβ aggregation overlaps with the α7 receptor expression in basal forebrain cholinergic system [128]. This early co-localization of the α7 receptors and Aβ may be due to high affinity binding between these two components [117, 129]. Receptor binding experiments have also shown co-precipitation of Aβ1-42 and α7 nAChRs [113]. Because these co-precipitates are resistant to detergent treatment which shows that a high affinity binding takes place between Aβ1-42 and α7 nAChRs [117]. Aβ1-42 also binds to heteromeric nAChRs but with 5000 times lesser affinity as compared to α7- and is known to block whole cell and single channel currents in CA1 stratum radiatum interneurons in rat hippocampal slices [130].
It is now known that Aβ1-42 at high (nM) concentrations leads to nAChR inactivation and thus can disrupt synaptic plasticity and cognitive functions [131]. However, at low (pM) concentration the Aβ1-42 plays neuromodulatory role and activate nAChRs, thus modulating synaptic plasticity and enhancing cognitive functions [132]. Low levels of Aβ and short exposure time help to activate different neuro-modulatory pathways in nAChR dependant manner but extended exposure at higher doses causes a dysregulation of these signal transduction pathways, possibly through desensitization of receptor, leading to cell death which in turn impairs learning and memory [133].
α7 nAChRs are reported to be involved in induction of long term potentiation (LTP) and long term depression (LTD), two forms of synaptic plasticity. As reported by Gu and Yakel schaffer collateral (SC) CA1 plasticity is dependent on α7 receptor. In this experiment when septal cholinergic input was activated 100ms or 10ms prior to SC stimulation caused induction of LTP or LTD which was blocked by α7 antagonist MLA but not by non-α7 antagonist DHβE. Moreover, this synaptic plasticity was disrupted by 10nM or 100nM Aβ. These results suggest that inactivation of α7 receptors by Aβ has negative effects on synaptic plasticity [134] which results in impaired learning and memory.
The fact that α7 nAChRs are present in glial cells, particularly astrocytes, suggests that these receptors also have an important role in inflammation process. Work by Nagele et al. show that α7 nAChRs and Aβ were found to be intensely co-localized with green fluorescence activated protein (GFAP) positive (activated) astrocytes in AD brains. Since the authors also found ChAT, they proposed a model that α7 and Aβ are phagocytized by activated astrocytes in the vicinity of neural remnants. As a result astrocyte viability is compromised with increased accumulation of neuronal debris in astrocytes. This results in selective lysis of the astrocytes lead to astrocyte derived amyloid plaque formation [135]. The Aβ peptide is also reported to activate caspase 3 and induce astrocyte apoptosis [136] leading to higher rate of astrocyte apoptosis as compared to neuronal cells [137]. Thus, apoptosis of astrocytes may positively contribute to pathogenesis of AD [138].
Α7 Nicotinic Acetylcholine Receptor Targeting Drugs for Treatment of Alzheimer’s Disease
Although there is no preventive treatment available for AD but there is a continuous urge in the scientific community for the search of novel therapeutic strategies that can alleviate pathological symptoms of AD. The α7 nAChRs are pentameric ligand gated ion channels with selective permeability to Na+ and Ca+2 ions [139]. These receptors have exceptionally high Ca+2 permebility [140] as compared to other ligand gated ion channels. The Ca+2 acts as a second messenger and activates many signaling pathways in the cell and also mediates neurotransmitter release [141]. Due to high vulnerability of cholinergic neurons (specifically those having high amount of α7 nAChRs), high affinity binding between α7 nAChRs–Aβ and exceptionally high calcium ion selectivity of α7 nAChRs these receptors have become an attractive target for the treatment of AD [140].
For drug target both α7 nAChR agonists and antagonists are under investigation. The α7 nAChR agonists are of more therapeutic interest for the pharmacologist. Antagonists of α7 nAChRs have lower practical impact as compared to its agonists [109]. Table 2 lists some of the agonists, targeting α7 nAChR, under clinical trials for treatment of AD.
Table 2.
α7 Nicotinic acetylcholine receptor agonists and antagonists under clinical testing for the treatment of AD.
| α7 Nicotinic Acetylcholine Receptor Agonists | ||
|---|---|---|
| Name | Therapeutic effects | Refs. |
| EVP-6124 | Activates α7 nAChRs and is used for the treatment of mild to moderate AD, under phase 3 clinical trials | [142] |
| AZD-0328 | Activates α7 nAChRs and enhances dopamine release. Used for the treatment of AD and is under clinical testing. | [143] |
| ABT-107 | Treatment of AD and cognitive deficits associated with schizophrenia, under testing, not comercially available | [144] |
| GTS-21 | Treatment of AD and cognitive deficits associated with schizophrenia, experimental testing for anti-inflammatory potency, under clinical testing | [145] |
Amyloid Precursor Protein and its Post translational Modifications and Processing in Alzheimer’s Disease
Although the relationship between protein dysfunction and neurodegeneration remains elusive [146-148], protein aggregation has evolved as an emerging theme in diseases such as AD. Several heavily debated hypotheses exist to sequentially interlink all these phenomena under one event; aggregation of toxic Aβ is considered to be the driving force of AD pathology. Aβ, peptides of 40 or 42 amino acids, are derived from the sequential proteolytic cleavage of β-amyloid precursor protein (APP). Two mutually exclusive pathways exist for proteolytic processing of APP; while cleavage at residue Lys 16 by α-secretase results in the generation of soluble APP (sAPP) peptides, altered cleavage by β- and γ-secretases results in the formation of the 40-42 amino acid which coalesces to form insoluble, extracellular Aβ [149].
With recent paradigm shift, post-translational modifications (PTMs) of pathology associated proteins have become a valuable tool in the evaluation of the structural and functional alterations governing the neurodegenerative diseases [150]. PTMs significantly contribute to proteome expansion with each variant displaying a starkly different property such as phosphorylation of Tau protein in neurofibrillary tangles (NFT) or alternative cleavage of post-translationally modified APP into different forms of Aβ [150]. It comes in good agreement with studies implicating aberrant PTMs in AD pathogenesis [151].
APP is post-translationally modified by sulfation, phosphorylation, glycosylation, including both N- and O-linked glycosylation and proteolytic processing. In fact it is the O-glycosylated version of APP that is preferentially secreted. The most interesting correlation of PTMs with AD pathophysiology is “glycosylation” whereby oligosaccharide side chains attach themselves at N&O- linked sites on the nascent APP, in the endoplasmic reticulum. Two putative N-linked oligosaccharide attachment sites (Asn 467 and Asn 496) have been identified. Accumulating evidence proposes that only the former is occupied under normal conditions [152]. It has been suggested that the oligosaccharide side chains have a pivotal role in the protein processing. In AD, the major lesion associated proteins, APP and Tau, and their respective metabolites undergo altered N- and O-glycosylation [153].
Since Aβ can be produced by cultured cells, this has left us with a powerful model system for analyzing the aberrant PTMs, leading to Aβ formation in cells. Mutant Lec 8 strain, CHO cell lines are reported to have a defect in the CMP-NeuNAc transport system, which has shown an increase in asialo-oligosaccharide expression [154]. Tunicamycin and brefeldin A, soluble inhibitors of glycosylation have demonstrated to reduce APP secretion when N-glycosylation and sialylation were inhibited [152]. Altered protein glycosylation, via tunicamycin or mannosidase inhibition, has shown the disruption of axonal sorting in both in vitro and in vivo [155]. A similar result was observed with another model, when the asparagine residues, i.e. the sites of N Glycosylation, were removed [156]. McFarlane et al., [157] used mannosidase I and II inhibitors, 2-deosxymannojirimycin (dMan) and swainsonine respectively, for the investigation of the different effects of mannose and other complex sugars on APP processing. They observed that the treatment of AtT-20 mouse pituitary cells with dMan or swainsonine in vitro, prevented the N-linked sugars to mature which resulted in a significant decrease in APP secretion from the cell to the cell membrane.
Taken together these results confirm the previous lectin studies that suggested the preferential transfer of mature APP from the perinuclear region to cell membrane, where the high-mannose containing forms were retained in the ER/Golgi complex. If the APP is retained in the perinuclear region, it may have implications in its processing and the generation of Aβ [158]. Hence we can say that the impairment of APP maturation of the oligosaccharide chains, causing the retention of APP in the perinuclear region leads to an uprise in Aβ concentration in the cell due to holoprotein buildup in cellular compartments [159].
Decreased secretion of sAPP has been associated with generation of oligomannosyl oligosaccharides mediated altered APP glycosylation state. This was observed to be coupled with a parallel increase in the deposition of the cellular protein within the cell [154, 160]. Conversely, conjugation of terminal sialic acid residues to the glycan was shown to increase sAPP levels [161, 162]. Activation of Protein kinase C (PKC) has been widely reported to alter APP processing [163, 164]. Sialyltransferase enzyme trans-fected cells have demonstrated a direct relationship between the sialylation potential of APP and the fold stimulation of sAPP, following PKC activation [154]. Mutations altering the APP glycosylation state have been linked to an increased Aβ 42/Aβ 40 ratio, such as Swedish and London mutations. Both of these mutations account for altered N-glycosylation of APP, with an increased content of bisecting GlcNAc [165]. In accordance with this, GlcNActransferase III mRNA expression has reportedly been increased in AD brains [161].
Several studies have reported the presence of O-glycosylation sites and their functional role in APP [166-169]. Though elusive, the role of O-Glycosylastion has been proposed in proteolytic processing of APP by α-secretase, β-secretase and γ-secretase. In addition, studies have shown that it is the O-glycosylated APP that is preferentially secreted [168, 170]. Tyrosine O-glycosylation has been reported in Aβ 1–15 to Ab1–20 but not in full-length (Aβ1–38 to Aβ 1–42) Aβ fragments [167]. An increase in the shorter Aβ fragments cerebrospinal fluid (CSF) from AD patients and non-demented controls showed to carry the tyrosine-linked glycan in AD patients. APP is also O-GlcNAcylated [171], which affects proteolytic processing of APP, thereby increasing sAPP and decreasing Aβ secretion [172]. These results suggest that the post-translational modification of APP by glycosylation is a key event in determining the processing of the protein and may have significant implications in understanding the initial deposition and kinetics of amyloid aggregation in a pathological situation like AD.
Role of Cholinergic System in APP Post Translational Modifications
It has been established that APP, γ-secretase and altered glycosylation can lead towards misfolding and AD pathology. Moreover, cholinergic system has a pivotal role in learning and memory, and its deficits are also part of AD pathology. But the relationship between APP and cholinergic neurons has not been elucidated. However, recent evidence obtained from mice and cell lines implies that the cognitive decline occurs due to loss of cholinergic neurons and APP processing [173]. Experimental evidence obtained from the studies on these model organisms suggests that activity of cholinergic neurotransmission might have an impact on APP processing. Moreover, the APP phosphorylation on threonine 668 (P-APP) may also influence the APP metabolism. The Aβ production significantly reduced due to mutation or inhibition of T668 kinase inhibitors. It is suggested that the T668 phosphorylation may facilitate the β-secretase (BACE) 1 cleavage of APP to increase Aβ generation [174]. In addition, p35- and p25-mediated Cdk5 activities lead to discrete APP (Thr668) phosphorylation, where the overexpression of both p35 and p25, increases the secretion of Aβ, as well sAPP (beta), and sAPP (alpha) [175]. The APP T688 phosphorylation also regulates the nuclear translocation of APP intracellular domain that also contributes towards neurodegeneration [176].
Alterations in APP metabolism significantly aids in the long-lasting effects of AChE inhibitors. However, complete inhibition is lethal as the natural physiology of the neuron will also be inhibited [177]. The potentiation of the central cholinergic system can be a potential tool and a promising strategy for therapeutics by modulating AChE that ultimately increases the ACh concentration in the brain [178]. However, despite the accumulating evidences, the interaction between cholinergic neurotransmission and APP processing and the physiological significance of PTM events remain speculative and still needs further exploration.
Post Translational Modifications and Tau
Several studies have suggested and proposed two major hypotheses about the relationship of tau and Aβ through which tau may facilitate Aβ-induced impairments [179]. According to the first hypothesis, physiological forms of tau may cause abnormal neural network activity via diverse pathogenic triggers [180, 181], while the second hypothesis proposed that the Aβ might change the PTM or distribution of tau, making it an active mediator of Aβ-induced neuronal dysfunction [182]. A detailed characterization of tau PTMs may assist to strategize the plausible mechanisms to combat the consequences of the pathological processes associated with tau.
Although the phosphorylation of tau is well understood, with identified phosphorylated sites i.e. Ser-68, Thr-69, and Thr-71 [183], it also involves cross talk among diverse and sometimes competing PTMs which have still not been well studied including O-glycosylation, ubiquitination, acetylation and methylation [179].
Methylation of tau on lysine and arginine residues has been recently reported [184, 185]; however, the functional effects are still unknown. Methylation inhibits tau aggregation by increasing the amount of proteins required to form aggregates by increasing tau’s dissociation rate from fibrils and decreasing the flexible extension rate. Moreover, methylation also delays the aggregation rate by diminishing the filament nucleation, which is the rate limiting step in the formation of neurofibrillary tangles [184]. Interestingly, the increased demethylation of protein phosphatase 2A (PP2A) (L309) results in reduced PP2A activity in AD brain, mediated by Aβ overproduction (or estrogen deficiency in mice), leading to compromised dephosphorylation of abnormally hyperphosphorylated tau [186].
In addition, tau phosphorylation which is directly mediated by phosphotransferases, there is a complex regulatory control on tau aggregation by competing modifications like O-linked β-N-acetylglucosaminylation (OGlcNAcylation). The OGlcNAcylation significantly regulates tau phosphorylation by decreasing the phosphorylation levels, thus depressing the neurofibrillary lesion formation and aggregation [187].
Furthermore, the ubiquitination of tau at Lys-6, Lys-11 and Lys-48 also modulates the intracellular tau levels in AD brains [188]. Studies have also shown significant regulating effects of increased acetylation on tau in AD which also counteracts the ubiquitination and degradation of phosphorylated tau [189].
Muscarinic acetylcholine receptor and tau hyperphosphorylation
Post-translational modifications play an important role in the structure and function of GPCRs. Although N-linked glycosylation is the most common posttranslational modification of GPCRs but limited data is available regarding their role in mAChRs [190]. Among all mAChRs, M3 undergoes few important modifications, such as N-glycosylation and disulfide bond formation [191]. The absence of M3 N-glycosylation promotes receptor trafficking impairment, generates ER stress and thus leads to an increased susceptibility for cell disruption [191]. Impaired neurotransmission which is highly evident in many neurodegenerative disorders is also perturbed due to lack of N-glycosylation of M3 [192]. On the contrary, N-glycosylation of the M2 is not required for cell surface localization or ligand binding [193].
It has been observed that activation of M1 receptor decreases tau phosphorylation. Fisher et al., demonstrated the plausible mechanism of M1-mediated decrease in tau phosphorylation [194]. M1- agonists improved cognition and behavior, decreased the hyperphosphorylated tau and the number of neurons containing aggregated tau and paired helical filaments (PHFs), and decreased the inflammation [194].
Nicotinic acetylcholine receptor and tau hyperphosphorylation
The α7-Aβ binding activates α7 nAChR and increases tau hyperphosphorylation via ERK-MAPK and JNK-1-MAPK activation [195]. The ERK-MAPK activation leads to phosphorylation of two proline directed MAPK-targeted serine and threonine residues (S202, T181) while the activation of JNK-1-MAPK pathway phosphorylates the T231 along with S202 and T181 residues on tau [195]. Activation of α7 nAChR via Aβ mediates the phosphorylation of GSK 3β at tyrosine 216 which also results in phosphorylation of S202 on tau [196]. The phosphorylated S202 and T181 residues can lead to microtubule instability as they are involved in binding kinetics of tau-microtubule, resulting in the formation of NFTs [197]. The Aβ induced tau hyper-phosphorylation can be blocked by α7 nAChR selective antagonist, methyllycaconitine (MLA) [133].
Moreover, increase in tau phosphorylation was observed after nAChR activation via application of nAChR agonists. It is evident that chronic nicotine treatment in transgenic model of AD causes an upregulation of nAChRs which results in activation of p38 MAP kinase which in turn phosphorylates tau and exacerbates tau pathology [198]. Similarly, increase in tau phosphorylation was also observed after activation of nAChRs via AChE inhibitors and nAChR agonists, however, this requires nAChR mediated Ca+2 entry inside the cell as Ca+2 removal by ethylene glycol tetra acetic acid (EGTA) prevents increased tau hyperphosphorylation [199]. Taken together these studies highlight the nAChR-Aβ interaction, mediating tau hyperphosphorylation and neurofibrillary tangle formation (Fig. 2).
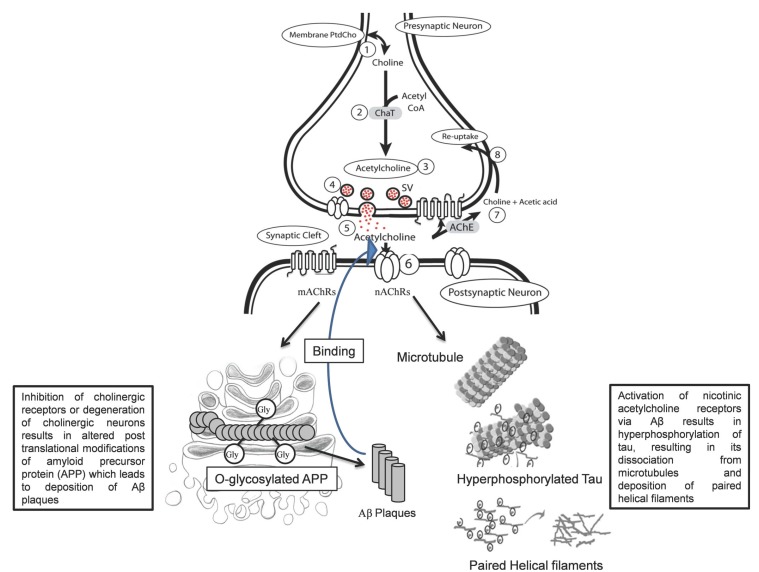
Fig. (2).
Cholinergic Synapse and PTMs of APP and Tau. Abbreviations: ChaT: Choline acetylTransferase, PtdCho: Phosphotidylcholine, SV: Synaptic vesicles, AChE: Acetylcholine esterase, ACh: acetylcholine, mAChR: muscarinic ACh receptor, nAChR: nicotinic ACh receptor, 1) PtdCho provides the choline precursor and acetyl moiety from acetyl CoA, 2) in the presence of ChaT 3) the ACh is synthesized, 4) Storage of ACh in synaptic vesicles, 5) Release of ACh in the synaptic cleft, 6) Action of ACh on postsynaptic cholinergic receptor, 7) Degradation of ACh via AChE into choline and acetate ion, 8) Reuptake of choline into the presynaptic nerve terminal by choline transporter.
CONCLUSION
AD, despite been extensively studied for the last many years, is a constellation of consequences which is still to be explored, to devise effective therapeutic strategies. The dysfunctional cholinergic system in AD has been linked to ACh deficits, which emphasizes therapeutic research to focus on effective approaches for maintaining its level in brain. Muscarinic cholinergic receptors play a significant role in regulating CNS circuits, particularly involving learning and memory. Muscarinic receptor subtypes with different distributions in CNS, provides the opportunity to take each receptor as a drug target. The available cholinergic compounds lack the subtype-specificity and effectiveness that favors the side effects and may influence cognitive effects because of weak or differing actions. However, some selective allosteric modulators of ACh have demonstrated therapeutic potential that can be used as a better therapeutic approach. Additionally, a rather unique aspect of involvement of PTMs and their potential effect on muscarinic and nicotinic cholinergic receptors, gives a new dimension to study the pathological consequences where these cholinergic receptors are involved. The distinct and influential association of several competing PTMs with cholinergic receptors provide a detailed understanding of complex regulation of cholinergic system under several PTMs that may govern the diverse pathological mechanisms and associated consequences in AD. Future studies are required to focus on the specific role of cholinergic neurotransmission and PTMs in AD, to further validate drug targets and effective therapeutics.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Jucker M., Walker L.C. Neurodegeneration: Amyloid-β pathology induced in humans. Nature. 2015;525(7568):193–194. doi: 10.1038/525193a. [http://dx. doi.org/10.1038/525193a]. [PMID: 26354478]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe D.J., Schenk D. Alzheimers disease: molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [http://dx.doi.org/10.1146/annurev.pharmtox.43.100901.140248]. [PMID: 12415125]. [DOI] [PubMed] [Google Scholar]
- 3.Zilka N., Novak M. The tangled story of Alois Alzheimer. Bratisl. Lek Listy (Tlacene Vyd) 2006;107(9-10):343–345. [PMID: 17262985]. [PubMed] [Google Scholar]
- 4.Fu W., Jhamandas J.H. β-amyloid peptide activates non-α7 nicotinic acetylcholine receptors in rat basal forebrain neurons. J. Neurophysiol. 2003;90(5):3130–3136. doi: 10.1152/jn.00616.2003. [http://dx.doi.org/10.1152/ jn.00616.2003]. [PMID: 12890800]. [DOI] [PubMed] [Google Scholar]
- 5.Glenner G., Wong C. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Alzheimer Dis. Assoc. Disord. 1988;2(2):134. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 6.Zheng W-H., Bastianetto S., Mennicken F., Ma W., Kar S. Amyloid β peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 2002;115(1):201–211. doi: 10.1016/s0306-4522(02)00404-9. [http://dx.doi.org/10.1016/S0306-4522(02) 00404-9]. [PMID: 12401334]. [DOI] [PubMed] [Google Scholar]
- 7.Grundke-Iqbal I., Iqbal K., Tung Y-C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [http://dx. doi.org/10.1073/pnas.83.13.4913]. [PMID: 3088567]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh D.M., Selkoe D.J. A beta oligomers - a decade of discovery. J. Neurochem. 2007;101(5):1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [http://dx.doi.org/10.1111/j.1471-4159.2006.04426.x]. [PMID: 17286590]. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., OBanion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimers disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [http://dx.doi.org/10.1016/S0197-4580(00)00124-X]. [PMID: 10858586]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimers disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [http://dx.doi.org/10.1002/ana.410300410]. [PMID: 1789684]. [DOI] [PubMed] [Google Scholar]
- 11.DeKosky S.T., Scheff S.W. Synapse loss in frontal cortex biopsies in Alzheimers disease: correlation with cognitive severity. Ann. Neurol. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [http://dx.doi.org/10.1002/ana. 410270502]. [PMID: 2360787]. [DOI] [PubMed] [Google Scholar]
- 12.Coyle J.T., Price D.L., DeLong M.R. Alzheimers disease: a disorder of cortical cholinergic innervation. Science. 1983;219(4589):1184–1190. doi: 10.1126/science.6338589. [http://dx.doi.org/10.1126/science.6338589]. [PMID: 6338589]. [DOI] [PubMed] [Google Scholar]
- 13.Levey A.I. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1996;93(24):13541–13546. doi: 10.1073/pnas.93.24.13541. [http://dx. doi.org/10.1073/pnas.93.24.13541]. [PMID: 8942969]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geula C., Mesulam M.M. Cortical cholinergic fibers in aging and Alzheimers disease: a morphometric study. Neuroscience. 1989;33(3):469–481. doi: 10.1016/0306-4522(89)90399-0. [http://dx.doi.org/10.1016/0306-4522(89)90399-0]. [PMID: 2636703]. [DOI] [PubMed] [Google Scholar]
- 15.Mufson E.J., Ginsberg S.D., Ikonomovic M.D., DeKosky S.T. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J. Chem. Neuroanat. 2003;26(4):233–242. doi: 10.1016/s0891-0618(03)00068-1. [http://dx. doi.org/10.1016/S0891-0618(03)00068-1]. [PMID: 14729126]. [DOI] [PubMed] [Google Scholar]
- 16.Pearson R.C., Sofroniew M.V., Cuello A.C., Powell T.P., Eckenstein F., Esiri M.M., Wilcock G.K. Persistence of cholinergic neurons in the basal nucleus in a brain with senile dementia of the Alzheimers type demonstrated by immuno- histochemical staining for choline acetyltransferase. Brain Res. 1983;289(1-2):375–379. doi: 10.1016/0006-8993(83)90046-x. [http://dx.doi.org/10.1016/0006-8993(83) 90046-X]. [PMID: 6362777]. [DOI] [PubMed] [Google Scholar]
- 17.Bowen D.M., White P., Spillane J.A., Goodhardt M.J., Curzon G., Iwangoff P., Meier-Ruge W., Davison A.N. Accelerated ageing or selective neuronal loss as an important cause of dementia? Lancet. 1979;1(8106):11–14. doi: 10.1016/s0140-6736(79)90454-9. [PMID: 83462]. [DOI] [PubMed] [Google Scholar]
- 18.Sarter M., Bruno J.P. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol. Aging. 2004;25(9):1127–1139. doi: 10.1016/j.neurobiolaging.2003.11.011. [http://dx.doi.org/10.1016/j.neurobiolaging.2003.11.011]. [PMID: 15312959]. [DOI] [PubMed] [Google Scholar]
- 19.Ridley R.M., Murray T.K., Johnson J.A., Baker H.F. Learning impairment following lesion of the basal nucleus of Meynert in the marmoset: modification by cholinergic drugs. Brain Res. 1986;376(1):108–116. doi: 10.1016/0006-8993(86)90904-2. [http://dx.doi.org/10.1016/0006-8993(86)90904-2]. [PMID: 3087582]. [DOI] [PubMed] [Google Scholar]
- 20.DeKosky S.T., Harbaugh R.E., Schmitt F.A., Bakay R.A., Chui H.C., Knopman D.S., Reeder T.M., Shetter A.G., Senter H.J., Markesbery W.R. Cortical biopsy in Alzheimers disease: diagnostic accuracy and neurochemical, neuropathological, and cognitive correlations. Ann. Neurol. 1992;32(5):625–632. doi: 10.1002/ana.410320505. [http://dx.doi.org/10.1002/ana.410320505]. [PMID: 1360195]. [DOI] [PubMed] [Google Scholar]
- 21.Minger S.L., Esiri M.M., McDonald B., Keene J., Carter J., Hope T., Francis P.T. Cholinergic deficits contribute to behavioral disturbance in patients with dementia. Neurology. 2000;55(10):1460–1467. doi: 10.1212/wnl.55.10.1460. [http://dx.doi.org/10.1212/WNL.55.10.1460]. [PMID: 11094098]. [DOI] [PubMed] [Google Scholar]
- 22.Davies P., Maloney A.J. Selective loss of central cholinergic neurons in Alzheimers disease. Lancet. 1976;2(8000):1403. doi: 10.1016/s0140-6736(76)91936-x. [http://dx.doi.org/10.1016/S0140-6736(76)91936-X]. [PMID: 63862]. [DOI] [PubMed] [Google Scholar]
- 23.Kato G., Agid Y. Acetylcholine receptors. Nouv. Presse Med. 1979;8(29):2407–2411. [PMID: 226931]. [PubMed] [Google Scholar]
- 24.Lefkowitz R.J. Seven transmembrane receptors: a brief personal retrospective. Biochim. Biophys. Acta. 2007;1768(4):748–755. doi: 10.1016/j.bbamem.2006.11.001. [http://dx.doi.org/10.1016/j.bbamem.2006.11.001]. [PMID: 17173855]. [DOI] [PubMed] [Google Scholar]
- 25.Hulme E.C., Curtis C.A., Wheatley M., Aitken A., Harris A.C. Localization and structure of the muscarinic receptor ligand binding site. Trends Pharmacol. Sci. 1989;(Suppl.):22–25. [PMID: 2694518]. [PubMed] [Google Scholar]
- 26.Koshimizu H., Leiter L.M., Miyakawa T. M4 muscarinic receptor knockout mice display abnormal social behavior and decreased prepulse inhibition. Mol. Brain. 2012;5:10. doi: 10.1186/1756-6606-5-10. [http://dx. doi.org/10.1186/1756-6606-5-10]. [PMID: 22463818]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonner T.I., Buckley N.J., Young A.C., Brann M.R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237(4814):527–532. doi: 10.1126/science.3037705. [http://dx.doi.org/10. 1126/science.3037705]. [PMID: 3037705]. [DOI] [PubMed] [Google Scholar]
- 28.Hulme E.C., Birdsall N.J., Buckley N.J. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [http://dx.doi.org/10.1146/annurev.pa.30.040190.003221]. [PMID: 2188581]. [DOI] [PubMed] [Google Scholar]
- 29.Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 1996;10(1):69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [http://dx.doi.org/10. 1615/CritRevNeurobiol.v10.i1.40]. [PMID: 8853955]. [DOI] [PubMed] [Google Scholar]
- 30.Wess J., Liu J., Blin N., Yun J., Lerche C., Kostenis E. Structural basis of receptor/G protein coupling selectivity studied with muscarinic receptors as model systems. Life Sci. 1997;60(13-14):1007–1014. doi: 10.1016/s0024-3205(97)00041-6. [http://dx.doi.org/10.1016/S0024-3205(97)00041-6]. [PMID: 9121341]. [DOI] [PubMed] [Google Scholar]
- 31.Wess J. Molecular basis of muscarinic acetylcholine receptor function. Trends Pharmacol. Sci. 1993;14(8):308–313. doi: 10.1016/0165-6147(93)90049-p. [http://dx. doi.org/10.1016/0165-6147(93)90049-P]. [PMID: 8249149]. [DOI] [PubMed] [Google Scholar]
- 32.Matsui M., Yamada S., Oki T., Manabe T., Taketo M.M., Ehlert F.J. Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci. 2004;75(25):2971–2981. doi: 10.1016/j.lfs.2004.05.034. [http://dx.doi.org/10.1016/j.lfs.2004.05.034]. [PMID: 15474550]. [DOI] [PubMed] [Google Scholar]
- 33.Caulfield M.P., Birdsall N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;50(2):279–290. [PMID: 9647869]. [PubMed] [Google Scholar]
- 34.Lanzafame A.A., Christopoulos A., Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors Channels. 2003;9(4):241–260. [http://dx.doi.org/10.1080/ 10606820308263]. [PMID: 12893537]. [PubMed] [Google Scholar]
- 35.Bartus R.T., Dean R.L., III, Beer B., Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [http://dx.doi.org/10.1126/science.7046051]. [PMID: 7046051]. [DOI] [PubMed] [Google Scholar]
- 36.Bartus R.T., Johnson H.R. Short-term memory in the rhesus monkey: disruption from the anti-cholinergic scopolamine. Pharmacol. Biochem. Behav. 1976;5(1):39–46. doi: 10.1016/0091-3057(76)90286-0. [http://dx.doi.org/10.1016/0091-3057(76)90286-0]. [PMID: 825880]. [DOI] [PubMed] [Google Scholar]
- 37.Everitt B.J., Robbins T.W. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [http://dx.doi.org/10.1146/ annurev.psych.48.1.649]. [PMID: 9046571]. [DOI] [PubMed] [Google Scholar]
- 38.Howe A.R., Surmeier D.J. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J. Neurosci. 1995;15(1 Pt 1):458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [PMID: 7823150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levey A.I., Kitt C.A., Simonds W.F., Price D.L., Brann M.R. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 1991;11(10):3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [PMID: 1941081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crook J.M., Tomaskovic-Crook E., Copolov D.L., Dean B. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmanns areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am. J. Psychiatry. 2001;158(6):918–925. doi: 10.1176/appi.ajp.158.6.918. [http://dx.doi.org/10.1176/appi.ajp.158.6.918]. [PMID: 11384900]. [DOI] [PubMed] [Google Scholar]
- 41.Anagnostaras S.G., Murphy G.G., Hamilton S.E., Mitchell S.L., Rahnama N.P., Nathanson N.M., Silva A.J. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 2003;6(1):51–58. doi: 10.1038/nn992. [http://dx.doi.org/10.1038/nn992]. [PMID: 12483218]. [DOI] [PubMed] [Google Scholar]
- 42.Davis A.A., Fritz J.J., Wess J., Lah J.J., Levey A.I. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo. J. Neurosci. 2010;30(12):4190–4196. doi: 10.1523/JNEUROSCI.6393-09.2010. [http://dx.doi.org/10.1523/JNEUROSCI.6393-09.2010]. [PMID: 20335454]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamberini M.T., Bolognesi M.L., Nasello A.G. The modulatory role of M2 muscarinic receptor on apomorphine-induced yawning and genital grooming. Neurosci. Lett. 2012;531(2):91–95. doi: 10.1016/j.neulet.2012.09.052. [http://dx.doi.org/10.1016/j.neulet.2012.09.052]. [PMID: 23041487]. [DOI] [PubMed] [Google Scholar]
- 44.Piggott M., Owens J., OBrien J., Paling S., Wyper D., Fenwick J., Johnson M., Perry R., Perry E. Comparative distribution of binding of the muscarinic receptor ligands pirenzepine, AF-DX 384, (R,R)-I-QNB and (R,S)-I-QNB to human brain. J. Chem. Neuroanat. 2002;24(3):211–223. doi: 10.1016/s0891-0618(02)00066-2. [http://dx.doi.org/10.1016/ S0891-0618(02)00066-2]. [PMID: 12297267]. [DOI] [PubMed] [Google Scholar]
- 45.Flynn D.D., Ferrari-DiLeo G., Mash D.C., Levey A.I. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimers disease. J. Neurochem. 1995;64(4):1888–1891. doi: 10.1046/j.1471-4159.1995.64041888.x. [http://dx.doi.org/10.1046/j.1471-4159.1995.64041888.x]. [PMID: 7891119]. [DOI] [PubMed] [Google Scholar]
- 46.Levey A.I., Edmunds S.M., Heilman C.J., Desmond T.J., Frey K.A. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience. 1994;63(1):207–221. doi: 10.1016/0306-4522(94)90017-5. [http://dx.doi.org/10.1016/0306-4522(94)90017-5]. [PMID: 7898649]. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W., Yamada M., Gomeza J., Basile A.S., Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J. Neurosci. 2002;22(15):6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [PMID: 12151512]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohno-Shosaku T., Matsui M., Fukudome Y., Shosaku J., Tsubokawa H., Taketo M.M., Manabe T., Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur. J. Neurosci. 2003;18(1):109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [http://dx. doi.org/10.1046/j.1460-9568.2003.02732.x]. [PMID: 12859343]. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W., Basile A.S., Gomeza J., Volpicelli L.A., Levey A.I., Wess J. Characterization of central inhibitory muscarinic auto- receptors by the use of muscarinic acetylcholine receptor knock-out mice. J. Neurosci. 2002;22(5):1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [PMID: 11880500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzavara E.T., Bymaster F.P., Felder C.C., Wade M., Gomeza J., Wess J., McKinzie D.L., Nomikos G.G. Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol. Psychiatry. 2003;8(7):673–679. doi: 10.1038/sj.mp.4001270. [http://dx.doi.org/10.1038/sj.mp.4001270]. [PMID: 12874603]. [DOI] [PubMed] [Google Scholar]
- 51.Vilaró M.T., Palacios J.M., Mengod G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci. Lett. 1990;114(2):154–159. doi: 10.1016/0304-3940(90)90064-g. [http://dx.doi.org/10.1016/0304-3940(90)90064-G]. [PMID: 2395528]. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda R.P., Ciesla W., Flores L.R., Wall S.J., Li M., Satkus S.A., Weisstein J.S., Spagnola B.V., Wolfe B.B. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol. Pharmacol. 1993;43(2):149–157. [PMID: 8429821]. [PubMed] [Google Scholar]
- 53.Yamada M., Lamping K.G., Duttaroy A., Zhang W., Cui Y., Bymaster F.P., McKinzie D.L., Felder C.C., Deng C.X., Faraci F.M., Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2001;98(24):14096–14101. doi: 10.1073/pnas.251542998. [http://dx.doi.org/10.1073/pnas.251542998]. [PMID: 11707605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nitsch R.M., Slack B.E., Wurtman R.J., Growdon J.H. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258(5080):304–307. doi: 10.1126/science.1411529. [http://dx.doi.org/10.1126/science.1411529]. [PMID: 1411529]. [DOI] [PubMed] [Google Scholar]
- 55.Buxbaum J.D., Oishi M., Chen H.I., Pinkas-Kramarski R., Jaffe E.A., Gandy S.E., Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc. Natl. Acad. Sci. USA. 1992;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. [http://dx.doi.org/10.1073/pnas.89.21. 10075]. [PMID: 1359534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kosik K.S. Alzheimers disease: a cell biological perspective. Science. 1992;256(5058):780–783. doi: 10.1126/science.1589757. [http://dx.doi.org/10.1126/ science.1589757]. [PMID: 1589757]. [DOI] [PubMed] [Google Scholar]
- 57.Mullan M., Crawford F. Genetic and molecular advances in Alzheimers disease. Trends Neurosci. 1993;16(10):398–403. doi: 10.1016/0166-2236(93)90007-9. [http://dx.doi.org/10.1016/0166-2236(93)90007-9]. [PMID: 7504354]. [DOI] [PubMed] [Google Scholar]
- 58.Selkoe D.J. Physiological production of the beta-amyloid protein and the mechanism of Alzheimers disease. Trends Neurosci. 1993;16(10):403–409. doi: 10.1016/0166-2236(93)90008-a. [http://dx.doi.org/10.1016/0166-2236(93)90008-A]. [PMID: 7504355]. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez A., Alarcón R., Opazo C., Campos E.O., Muñoz F.J., Calderón F.H., Dajas F., Gentry M.K., Doctor B.P., De Mello F.G., Inestrosa N.C. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimers fibrils. J. Neurosci. 1998;18(9):3213–3223. doi: 10.1523/JNEUROSCI.18-09-03213.1998. [PMID: 9547230]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitehouse P.J., Price D.L., Struble R.G., Clark A.W., Coyle J.T., Delon M.R. Alzheimers disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215(4537):1237–1239. doi: 10.1126/science.7058341. [http://dx.doi.org/10.1126/science.7058341]. [PMID: 7058341]. [DOI] [PubMed] [Google Scholar]
- 61.Mufson E.J., Bothwell M., Kordower J.H. Loss of nerve growth factor receptor-containing neurons in Alzheimers disease: a quantitative analysis across subregions of the basal forebrain. Exp. Neurol. 1989;105(3):221–232. doi: 10.1016/0014-4886(89)90124-6. [http://dx.doi.org/10.1016/0014-4886(89)90124-6]. [PMID: 2548888]. [DOI] [PubMed] [Google Scholar]
- 62.Boncristiano S., Calhoun M.E., Kelly P.H., Pfeifer M., Bondolfi L., Stalder M., Phinney A.L., Abramowski D., Sturchler-Pierrat C., Enz A., Sommer B., Staufenbiel M., Jucker M. Cholinergic changes in the APP23 transgenic mouse model of cerebral amyloidosis. J. Neurosci. 2002;22(8):3234–3243. doi: 10.1523/JNEUROSCI.22-08-03234.2002. [PMID: 11943824]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kar S., Slowikowski S.P., Westaway D., Mount H.T. Interactions between β-amyloid and central cholinergic neurons: implications for Alzheimers disease. J. Psychiatry Neurosci. 2004;29(6):427–441. [PMID: 15644984]. [PMC free article] [PubMed] [Google Scholar]
- 64.Perry E.K., Johnson M., Kerwin J.M., Piggott M.A., Court J.A., Shaw P.J., Ince P.G., Brown A., Perry R.H. Convergent cholinergic activities in aging and Alzheimers disease. Neurobiol. Aging. 1992;13(3):393–400. doi: 10.1016/0197-4580(92)90113-c. [http://dx.doi.org/10.1016/0197-4580 (92)90113-C]. [PMID: 1625768]. [DOI] [PubMed] [Google Scholar]
- 65.Giacobini E. Cholinergic receptors in human brain: effects of aging and Alzheimer disease. J. Neurosci. Res. 1990;27(4):548–560. doi: 10.1002/jnr.490270416. [http://dx.doi.org/10.1002/jnr.490270416]. [PMID: 2079716]. [DOI] [PubMed] [Google Scholar]
- 66.Greenamyre J.T., Maragos W.F. Neurotransmitter receptors in Alzheimer disease. Cerebrovasc. Brain Metab. Rev. 1993;5(2):61–94. [PMID: 8392361]. [PubMed] [Google Scholar]
- 67.Perry E.K., Morris C.M., Court J.A., Cheng A., Fairbairn A.F., McKeith I.G., Irving D., Brown A., Perry R.H. Alteration in nicotine binding sites in Parkinsons disease, Lewy body dementia and Alzheimers disease: possible index of early neuropathology. Neuroscience. 1995;64(2):385–395. doi: 10.1016/0306-4522(94)00410-7. [http://dx.doi.org/10.1016/ 0306-4522(94)00410-7]. [PMID: 7700528]. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed T., Gilani A-H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimers disease. Pharmacol. Biochem. Behav. 2009;91(4):554–559. doi: 10.1016/j.pbb.2008.09.010. [http://dx.doi.org/10.1016/j.pbb.2008.09.010]. [PMID: 18930076]. [DOI] [PubMed] [Google Scholar]
- 69.Barrantes F.J., Borroni V., Vallés S. Neuronal nicotinic acetylcholine receptor-cholesterol crosstalk in Alzheimers disease. FEBS Lett. 2010;584(9):1856–1863. doi: 10.1016/j.febslet.2009.11.036. [http://dx.doi.org/10.1016/j. febslet.2009.11.036]. [PMID: 19914249]. [DOI] [PubMed] [Google Scholar]
- 70.Medeiros R., Kitazawa M., Caccamo A., Baglietto-Vargas D., Estrada-Hernandez T., Cribbs D.H., Fisher A., LaFerla F.M. Loss of muscarinic M1 receptor exacerbates Alzheimers disease-like pathology and cognitive decline. Am. J. Pathol. 2011;179(2):980–991. doi: 10.1016/j.ajpath.2011.04.041. [http://dx.doi.org/10.1016/j.ajpath.2011.04.041]. [PMID: 21704011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiozaki K., Iseki E., Uchiyama H., Watanabe Y., Haga T., Kameyama K., Ikeda T., Yamamoto T., Kosaka K. Alterations of muscarinic acetylcholine receptor subtypes in diffuse lewy body disease: relation to Alzheimers disease. J. Neurol. Neurosurg. Psychiatry. 1999;67(2):209–213. doi: 10.1136/jnnp.67.2.209. [http://dx.doi.org/10.1136/ jnnp.67.2.209]. [PMID: 10406992]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsang S.W., Lai M.K., Kirvell S., Francis P.T., Esiri M.M., Hope T., Chen C.P., Wong P.T. Impaired coupling of muscarinic M1 receptors to G-proteins in the neocortex is associated with severity of dementia in Alzheimers disease. Neurobiol. Aging. 2006;27(9):1216–1223. doi: 10.1016/j.neurobiolaging.2005.07.010. [http://dx.doi.org/10.1016/j.neurobiolaging.2005. 07.010]. [PMID: 16129514]. [DOI] [PubMed] [Google Scholar]
- 73.Caccamo A., Oddo S., Billings L.M., Green K.N., Martinez-Coria H., Fisher A., LaFerla F.M. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49(5):671–682. doi: 10.1016/j.neuron.2006.01.020. [http://dx.doi.org/10.1016/j.neuron.2006.01. 020]. [PMID: 16504943]. [DOI] [PubMed] [Google Scholar]
- 74.Hashmi A.N., Yaqinuddin A., Ahmed T. Pharmacological effects of Ibuprofen on learning and memory, Muscarinic receptors genes expression and APP isoforms levels in Pre-frontal cortex of AlCl3-induced toxicity mouse model. Int. J. Neurosci. 2014;(0):1–37. doi: 10.3109/00207454.2014.922972. [PMID: 24825584]. [DOI] [PubMed] [Google Scholar]
- 75.Tsang S.W., Pomakian J., Marshall G.A., Vinters H.V., Cummings J.L., Chen C.P., Wong P.T., Lai M.K. Disrupted muscarinic M1 receptor signaling correlates with loss of protein kinase C activity and glutamatergic deficit in Alzheimers disease. Neurobiol. Aging. 2007;28(9):1381–1387. doi: 10.1016/j.neurobiolaging.2006.06.001. [http://dx.doi.org/10.1016/j.neurobiolaging.2006.06.001]. [PMID: 16828202]. [DOI] [PubMed] [Google Scholar]
- 76.Ferrari-DiLeo G., Mash D.C., Flynn D.D. Attenuation of muscarinic receptor-G-protein interaction in Alzheimer disease. Mol. Chem. Neuropathol. 1995;24(1):69–91. doi: 10.1007/BF03160113. [http://dx.doi.org/10. 1007/BF03160113]. [PMID: 7755848]. [DOI] [PubMed] [Google Scholar]
- 77.Harrison P.J., Barton A.J., Najlerahim A., McDonald B., Pearson R.C. Increased muscarinic receptor messenger RNA in Alzheimers disease temporal cortex demonstrated by in situ hybridization histochemistry. Brain Res. Mol. Brain Res. 1991;9(1-2):15–21. doi: 10.1016/0169-328x(91)90125-h. [http://dx.doi.org/10.1016/0169-328X(91)90125-H]. [PMID: 1673214]. [DOI] [PubMed] [Google Scholar]
- 78.Rodríguez-Puertas R., Pascual J., Vilaró T., Pazos A. Autoradiographic distribution of M1, M2, M3, and M4 muscarinic receptor subtypes in Alzheimers disease. Synapse. 1997;26(4):341–350. doi: 10.1002/(SICI)1098-2396(199708)26:4<341::AID-SYN2>3.0.CO;2-6. [http://dx.doi.org/10.1002/(SICI)1098-2396(199708)26:4 <341:AID-SYN2>3.0.CO;2-6]. [PMID: 9215593]. [DOI] [PubMed] [Google Scholar]
- 79.Poulin B., Butcher A., McWilliams P., Bourgognon J.M., Pawlak R., Kong K.C., Bottrill A., Mistry S., Wess J., Rosethorne E.M., Charlton S.J., Tobin A.B. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl. Acad. Sci. USA. 2010;107(20):9440–9445. doi: 10.1073/pnas.0914801107. [http://dx.doi.org/10.1073/pnas.0914801107]. [PMID: 20439723]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pedersen W.A., Kloczewiak M.A., Blusztajn J.K. Amyloid beta-protein reduces acetylcholine synthesis in a cell line derived from cholinergic neurons of the basal forebrain. Proc. Natl. Acad. Sci. USA. 1996;93(15):8068–8071. doi: 10.1073/pnas.93.15.8068. [http://dx.doi.org/10.1073/pnas.93. 15.8068]. [PMID: 8755604]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bierer L.M., Haroutunian V., Gabriel S., Knott P.J., Carlin L.S., Purohit D.P., Perl D.P., Schmeidler J., Kanof P., Davis K.L. Neurochemical correlates of dementia severity in Alzheimers disease: relative importance of the cholinergic deficits. J. Neurochem. 1995;64(2):749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [http://dx.doi.org/10.1046/j. 1471-4159.1995.64020749.x]. [PMID: 7830069]. [DOI] [PubMed] [Google Scholar]
- 82.Wilcock G.K., Esiri M.M., Bowen D.M., Smith C.C. Alzheimers disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J. Neurol. Sci. 1982;57(2-3):407–417. doi: 10.1016/0022-510x(82)90045-4. [http://dx.doi.org/10.1016/ 0022-510X(82)90045-4]. [PMID: 7161627]. [DOI] [PubMed] [Google Scholar]
- 83.Davies P., Maloney A.J. Selective loss of central cholinergic neurons in Alzheimers disease. Lancet. 1976;2(8000):1403. doi: 10.1016/s0140-6736(76)91936-x. [http://dx.doi.org/10.1016/S0140-6736(76)91936-X]. [PMID: 63862]. [DOI] [PubMed] [Google Scholar]
- 84.Bowen D.M., Smith C.B., White P., Davison A.N. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99(3):459–496. doi: 10.1093/brain/99.3.459. [http://dx.doi.org/10.1093/brain/99.3.459]. [PMID: 11871]. [DOI] [PubMed] [Google Scholar]
- 85.Araujo D.M., Lapchak P.A., Robitaille Y., Gauthier S., Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimers disease. J. Neurochem. 1988;50(6):1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [http://dx.doi.org/10.1111/ j.1471-4159.1988.tb02497.x]. [PMID: 3373218]. [DOI] [PubMed] [Google Scholar]
- 86.Bartus R.T., Dean R.L., III, Beer B., Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [http://dx.doi.org/10.1126/science.7046051]. [PMID: 7046051]. [DOI] [PubMed] [Google Scholar]
- 87.Drachman D.A. Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology. 1977;27(8):783–790. doi: 10.1212/wnl.27.8.783. [http://dx.doi.org/10.1212/WNL.27.8.783]. [PMID: 560649]. [DOI] [PubMed] [Google Scholar]
- 88.Sutherland R.J., Whishaw I.Q., Regehr J.C. Cholinergic receptor blockade impairs spatial localization by use of distal cues in the rat. J. Comp. Physiol. Psychol. 1982;96(4):563–573. doi: 10.1037/h0077914. [http://dx.doi.org/10.1037/h0077914]. [PMID: 7119176]. [DOI] [PubMed] [Google Scholar]
- 89.Roldán G., Bolaños-Badillo E., González-Sánchez H., Quirarte G.L., Prado-Alcalá R.A. Selective M1 muscarinic receptor antagonists disrupt memory consolidation of inhibitory avoidance in rats. Neurosci. Lett. 1997;230(2):93–96. doi: 10.1016/s0304-3940(97)00489-8. [http://dx.doi.org/10.1016/S0304-3940(97)00489-8]. [PMID: 9259472]. [DOI] [PubMed] [Google Scholar]
- 90.Bodick N.C., Offen W.W., Levey A.I., Cutler N.R., Gauthier S.G., Satlin A., Shannon H.E., Tollefson G.D., Rasmussen K., Bymaster F.P., Hurley D.J., Potter W.Z., Paul S.M. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 1997;54(4):465–473. doi: 10.1001/archneur.1997.00550160091022. [http://dx.doi.org/10.1001/ archneur.1997.00550160091022]. [PMID: 9109749]. [DOI] [PubMed] [Google Scholar]
- 91.Shekhar A., Potter W.Z., Lightfoot J., Lienemann J., Dubé S., Mallinckrodt C., Bymaster F.P., McKinzie D.L., Felder C.C. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008;165(8):1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [http://dx.doi.org/10.1176/appi.ajp.2008. 06091591]. [PMID: 18593778]. [DOI] [PubMed] [Google Scholar]
- 92.Farber S.A., Nitsch R.M., Schulz J.G., Wurtman R.J. Regulated secretion of beta-amyloid precursor protein in rat brain. J. Neurosci. 1995;15(11):7442–7451. doi: 10.1523/JNEUROSCI.15-11-07442.1995. [PMID: 7472496]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fisher A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimers disease. Neurotherapeutics. 2008;5(3):433–442. doi: 10.1016/j.nurt.2008.05.002. [http://dx. doi.org/10.1016/j.nurt.2008.05.002]. [PMID: 18625455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones C.K., Brady A.E., Davis A.A., Xiang Z., Bubser M., Tantawy M.N., Kane A.S., Bridges T.M., Kennedy J.P., Bradley S.R., Peterson T.E., Ansari M.S., Baldwin R.M., Kessler R.M., Deutch A.Y., Lah J.J., Levey A.I., Lindsley C.W., Conn P.J. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J. Neurosci. 2008;28(41):10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI. 1850-08.2008]. [PMID: 18842902]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bridges T.M., Reid P.R., Lewis L.M., Dawson E.S., Weaver C.D., Wood M.R., Lindsley C.W. Discovery and development of a second highly selective M1 Positive Allosteric Modulator; PAM, 2010. PMID: 21433387. [PubMed] [Google Scholar]
- 96.Beach T.G., Walker D.G., Potter P.E., Sue L.I., Fisher A. Reduction of cerebrospinal fluid amyloid beta after systemic administration of M1 muscarinic agonists. Brain Res. 2001;905(1-2):220–223. doi: 10.1016/s0006-8993(01)02484-2. [http://dx.doi.org/10.1016/S0006-8993(01)02484-2]. [PMID: 11423097]. [DOI] [PubMed] [Google Scholar]
- 97.Nitsch R.M., Deng M., Tennis M., Schoenfeld D., Growdon J.H. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimers disease. Ann. Neurol. 2000;48(6):913–918. [http://dx.doi.org/10.1002/1531-8249(200012)48:6<913:AID-ANA12>3.0.CO;2-S]. [PMID: 11117548]. [PubMed] [Google Scholar]
- 98.Ahmed T., Enam S.A., Gilani A.H. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimers disease. Neuroscience. 2010;169(3):1296–1306. doi: 10.1016/j.neuroscience.2010.05.078. [http://dx.doi.org/10. 1016/j.neuroscience.2010.05.078]. [PMID: 20538041]. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed T., Gilani A.H., Hosseinmardi N., Semnanian S., Enam S.A., Fathollahi Y. Curcuminoids rescue long-term potentiation impaired by amyloid peptide in rat hippocampal slices. Synapse. 2011;65(7):572–582. doi: 10.1002/syn.20876. [http://dx.doi.org/10.1002/syn.20876]. [PMID: 20963814]. [DOI] [PubMed] [Google Scholar]
- 100.Ahmed T., Gilani A-H. A comparative study of curcuminoids to measure their effect on inflammatory and apoptotic gene expression in an Aβ plus ibotenic acid-infused rat model of Alzheimers disease. Brain Res. 2011;1400:1–18. doi: 10.1016/j.brainres.2011.05.022. [http://dx.doi.org/10. 1016/j.brainres.2011.05.022]. [PMID: 21640982]. [DOI] [PubMed] [Google Scholar]
- 101.Wolf B.A., Wertkin A.M., Jolly Y.C., Yasuda R.P., Wolfe B.B., Konrad R.J., Manning D., Ravi S., Williamson J.R., Lee V.M. Muscarinic regulation of Alzheimers disease amyloid precursor protein secretion and amyloid beta-protein production in human neuronal NT2N cells. J. Biol. Chem. 1995;270(9):4916–4922. doi: 10.1074/jbc.270.9.4916. [http://dx.doi.org/10.1074/jbc.270.9.4916]. [PMID: 7876266]. [DOI] [PubMed] [Google Scholar]
- 102.Buiter H.J., Windhorst A.D., Huisman M.C., Yaqub M., Knol D.L., Fisher A., Lammertsma A.A., Leysen J.E. [11C]AF150(S), an agonist PET ligand for M1 muscarinic acetylcholine receptors. EJNMMI Res. 2013;3(1):19. doi: 10.1186/2191-219X-3-19. [http://dx.doi.org/10.1186/2191-219X-3-19]. [PMID: 23514539]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shirey J.K., Brady A.E., Jones P.J., Davis A.A., Bridges T.M., Kennedy J.P., Jadhav S.B., Menon U.N., Xiang Z., Watson M.L., Christian E.P., Doherty J.J., Quirk M.C., Snyder D.H., Lah J.J., Levey A.I., Nicolle M.M., Lindsley C.W., Conn P.J. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J. Neurosci. 2009;29(45):14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [http://dx.doi.org/10.1523/JNEUROSCI. 3930-09.2009]. [PMID: 19906975]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bradley S.R., Lameh J., Ohrmund L., Son T., Bajpai A., Nguyen D., Friberg M., Burstein E.S., Spalding T.A., Ott T.R., Schiffer H.H., Tabatabaei A., McFarland K., Davis R.E., Bonhaus D.W. AC-260584, an orally bioavailable M(1) muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology. 2010;58(2):365–373. doi: 10.1016/j.neuropharm.2009.10.003. [http://dx.doi.org/10.1016/j.neuropharm.2009.10.003]. [PMID: 19835892]. [DOI] [PubMed] [Google Scholar]
- 105.Langmead C.J., Austin N.E., Branch C.L., Brown J.T., Buchanan K.A., Davies C.H., Forbes I.T., Fry V.A., Hagan J.J., Herdon H.J., Jones G.A., Jeggo R., Kew J.N., Mazzali A., Melarange R., Patel N., Pardoe J., Randall A.D., Roberts C., Roopun A., Starr K.R., Teriakidis A., Wood M.D., Whittington M., Wu Z., Watson J. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-281. Br. J. Pharmacol. 2008;154(5):1104–1115. doi: 10.1038/bjp.2008.152. [http://dx.doi.org/10.1038/ bjp.2008.152]. [PMID: 18454168]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dani J.A., Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [http://dx.doi.org/10.1146/annurev.pharmtox.47.120505.105214]. [PMID: 17009926]. [DOI] [PubMed] [Google Scholar]
- 107.Dani J.A. Overview of nicotinic receptors and their roles in the central nervous system. Biol. Psychiatry. 2001;49(3):166–174. doi: 10.1016/s0006-3223(00)01011-8. [http://dx.doi.org/10.1016/S0006-3223(00)01011-8]. [PMID: 11230867]. [DOI] [PubMed] [Google Scholar]
- 108.Tohgi H., Utsugisawa K., Yoshimura M., Nagane Y., Mihara M. Age-related changes in nicotinic acetylcholine receptor subunits α4 and β2 messenger RNA expression in postmortem human frontal cortex and hippocampus. Neurosci. Lett. 1998;245(3):139–142. doi: 10.1016/s0304-3940(98)00205-5. [http://dx.doi.org/10.1016/S0304-3940(98)00205-5]. [PMID: 9605475]. [DOI] [PubMed] [Google Scholar]
- 109.Pohanka M. Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int. J. Mol. Sci. 2012;13(2):2219–2238. doi: 10.3390/ijms13022219. [http://dx.doi.org/10.3390/ijms13022219]. [PMID: 22408449]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lucas-Meunier E., Fossier P., Baux G., Amar M. Cholinergic modulation of the cortical neuronal network. Pflugers Arch. 2003;446(1):17–29. doi: 10.1007/s00424-002-0999-2. [http://dx.doi.org/10.1007/s00424-002-0999-2]. [PMID: 12690458]. [DOI] [PubMed] [Google Scholar]
- 111.Tohgi H., Utsugisawa K., Yoshimura M., Nagane Y., Mihara M. Age-related changes in nicotinic acetylcholine receptor subunits alpha4 and beta2 messenger RNA expression in postmortem human frontal cortex and hippocampus. Neurosci. Lett. 1998;245(3):139–142. doi: 10.1016/s0304-3940(98)00205-5. [http://dx.doi.org/10.1016/S0304-3940(98)00205-5]. [PMID: 9605475]. [DOI] [PubMed] [Google Scholar]
- 112.Hernandez C.M., Dineley K.T. α7 nicotinic acetylcholine receptors in Alzheimers disease: neuroprotective, neurotrophic or both? Curr. Drug Targets. 2012;13(5):613–622. doi: 10.2174/138945012800398973. [http://dx. doi.org/10.2174/138945012800398973]. [PMID: 22300028]. [DOI] [PubMed] [Google Scholar]
- 113.Wang H., Lee D.H., DAndrea M.R., Peterson P.A., Shank R.P., Reitz A.B. β-Amyloid1–42 binds to α7 Nicotinic Acetylcholine Receptor with High Affinity. J. Biol. Chem. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. [http://dx.doi.org/10.1074/jbc.275.8.5626]. [PMID: 10681545]. [DOI] [PubMed] [Google Scholar]
- 114.Resende R.R., Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun. Signal. 2009;7:20. doi: 10.1186/1478-811X-7-20. [http://dx.doi.org/10.1186/1478-811X-7-20]. [PMID: 19712465]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Itier V., Bertrand D. Neuronal nicotinic receptors: from protein structure to function. FEBS Lett. 2001;504(3):118–125. doi: 10.1016/s0014-5793(01)02702-8. [http://dx.doi.org/10.1016/S0014-5793(01)02702-8]. [PMID: 11532443]. [DOI] [PubMed] [Google Scholar]
- 116.Liu Q., Xie X., Lukas R.J., St John P.A., Wu J. A novel nicotinic mechanism underlies β-amyloid-induced neuronal hyper- excitation. J. Neurosci. 2013;33(17):7253–7263. doi: 10.1523/JNEUROSCI.3235-12.2013. [http://dx.doi.org/10.1523/JNEUROSCI.3235-12.2013]. [PMID: 23616534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jones I.W., Westmacott A., Chan E., Jones R.W., Dineley K., ONeill M.J., Wonnacott S. α7 nicotinic acetylcholine receptor expression in Alzheimers disease. J. Mol. Neurosci. 2006;30(1):83–84. doi: 10.1385/JMN:30:1:83. [http://dx.doi.org/10.1385/JMN:30:1:83]. [PMID: 17192639]. [DOI] [PubMed] [Google Scholar]
- 118.Counts S.E., He B., Che S., Ikonomovic M.D., DeKosky S.T., Ginsberg S.D., Mufson E.J. α7 nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch. Neurol. 2007;64(12):1771–1776. doi: 10.1001/archneur.64.12.1771. [http://dx.doi.org/10.1001/ archneur.64.12.1771]. [PMID: 18071042]. [DOI] [PubMed] [Google Scholar]
- 119.Pandya A., Yakel J.L. Allosteric modulator Desformylflustrabromine relieves the inhibition of α2β2 and α4β2 nicotinic acetylcholine receptors by β-amyloid(142) peptide. J. Mol. Neurosci. 2011;45(1):42–47. doi: 10.1007/s12031-011-9509-3. [http://dx.doi.org/10.1007/s12031-011-9509-3]. [PMID: 21424792]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee J., Durst R., Wrolstad R. 4 but not 3 and 7 nicotinic acetylcholine receptor subunits are lost from the temporal cortex in alzheimer's disease. J. Neurochem. 2002 doi: 10.1046/j.1471-4159.1999.0731635.x. [http://dx.doi.org/10.1023/A:1014832520809]. [DOI] [PubMed] [Google Scholar]
- 121.Banerjee C., Nyengaard J.R., Wevers A., de Vos R.A., Jansen Steur E.N., Lindstrom J., Pilz K., Nowacki S., Bloch W., Schröder H. Cellular expression of α7 nicotinic acetylcholine receptor protein in the temporal cortex in Alzheimers and Parkinsons diseasea stereological approach. Neurobiol. Dis. 2000;7(6 Pt B):666–672. doi: 10.1006/nbdi.2000.0317. [http://dx.doi.org/10.1006/nbdi.2000.0317]. [PMID: 11114264]. [DOI] [PubMed] [Google Scholar]
- 122.Wevers A., Monteggia L., Nowacki S., Bloch W., Schütz U., Lindstrom J., Pereira E.F., Eisenberg H., Giacobini E., de Vos R.A., Steur E.N., Maelicke A., Albuquerque E.X., Schröder H. Expression of nicotinic acetylcholine receptor subunits in the cerebral cortex in Alzheimers disease: histotopographical correlation with amyloid plaques and hyperphosphorylated-tau protein. Eur. J. Neurosci. 1999;11(7):2551–2565. doi: 10.1046/j.1460-9568.1999.00676.x. [http://dx.doi.org/10.1046/ j.1460-9568.1999.00676.x]. [PMID: 10383644]. [DOI] [PubMed] [Google Scholar]
- 123.Burghaus L., Schütz U., Krempel U., de Vos R.A., Jansen S.E., Wevers A., Lindstrom J., Schröder H. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res. Mol. Brain Res. 2000;76(2):385–388. doi: 10.1016/s0169-328x(00)00031-0. [http://dx.doi.org/10.1016/S0169-328X(00) 00031-0]. [PMID: 10762715]. [DOI] [PubMed] [Google Scholar]
- 124.Hellström-Lindahl E., Mousavi M., Zhang X., Ravid R., Nordberg A. Regional distribution of nicotinic receptor subunit mRNAs in human brain: comparison between Alzheimer and normal brain. Brain Res. Mol. Brain Res. 1999;66(1-2):94–103. doi: 10.1016/s0169-328x(99)00030-3. [http://dx. doi.org/10.1016/S0169-328X(99)00030-3]. [PMID: 10095081]. [DOI] [PubMed] [Google Scholar]
- 125.Bednar I., Paterson D., Marutle A., Pham T.M., Svedberg M., Hellström-Lindahl E., Mousavi M., Court J., Morris C., Perry E., Mohammed A., Zhang X., Nordberg A. Selective nicotinic receptor consequences in APP(SWE) transgenic mice. Mol. Cell. Neurosci. 2002;20(2):354–365. doi: 10.1006/mcne.2002.1112. [http://dx.doi.org/10.1006/mcne. 2002.1112]. [PMID: 12093166]. [DOI] [PubMed] [Google Scholar]
- 126.Dineley K.T., Westerman M., Bui D., Bell K., Ashe K.H., Sweatt J.D. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimers disease. J. Neurosci. 2001;21(12):4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [PMID: 11404397]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jones I.W., Westmacott A., Chan E., Jones R.W., Dineley K., ONeill M.J., Wonnacott S. α7 nicotinic acetylcholine receptor expression in Alzheimers disease: receptor densities in brain regions of the APP(SWE) mouse model and in human peripheral blood lymphocytes. J. Mol. Neurosci. 2006;30(1-2):83–84. doi: 10.1385/JMN:30:1:83. [http://dx.doi.org/10.1385/JMN:30:1:83]. [PMID: 17192639]. [DOI] [PubMed] [Google Scholar]
- 128.Parri H.R., Hernandez C.M., Dineley K.T. Research update: Alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimers disease. Biochem. Pharmacol. 2011;82(8):931–942. doi: 10.1016/j.bcp.2011.06.039. [http://dx. doi.org/10.1016/j.bcp.2011.06.039]. [PMID: 21763291]. [DOI] [PubMed] [Google Scholar]
- 129.Wang H-Y., Lee D.H., DAndrea M.R., Peterson P.A., Shank R.P., Reitz A.B. β-Amyloid(142) binds to α7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimers disease pathology. J. Biol. Chem. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. [http://dx. doi.org/10.1074/jbc.275.8.5626]. [PMID: 10681545]. [DOI] [PubMed] [Google Scholar]
- 130.Pettit D.L., Shao Z., Yakel J.L. Beta-Amyloid(142) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J. Neurosci. 2001;21(1):RC120–RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [PMID: 11150356]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cleary J.P., Walsh D.M., Hofmeister J.J., Shankar G.M., Kuskowski M.A., Selkoe D.J., Ashe K.H. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 2005;8(1):79–84. doi: 10.1038/nn1372. [http://dx.doi.org/10.1038/nn1372]. [PMID: 15608634]. [DOI] [PubMed] [Google Scholar]
- 132.Puzzo D., Privitera L., Leznik E., Fà M., Staniszewski A., Palmeri A., Arancio O. Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008;28(53):14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.2692-08.2008]. [PMID: 19118188]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dineley K.T. Beta-amyloid peptide--nicotinic acetylcholine receptor interaction: the two faces of health and disease. Front. Biosci. 2006;12:5030–5038. doi: 10.2741/2445. [DOI] [PubMed] [Google Scholar]
- 134.Gu Z., Yakel J.L. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71(1):155–165. doi: 10.1016/j.neuron.2011.04.026. [http://dx.doi.org/10.1016/j.neuron.2011.04.026]. [PMID: 21745645]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nagele R.G., DAndrea M.R., Lee H., Venkataraman V., Wang H-Y. Astrocytes accumulate A β 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971(2):197–209. doi: 10.1016/s0006-8993(03)02361-8. [http://dx.doi.org/10.1016/S0006-8993(03)02361-8]. [PMID: 12706236]. [DOI] [PubMed] [Google Scholar]
- 136.Wang G., Dinkins M., He Q., Zhu G., Poirier C., Campbell A., Mayer-Proschel M., Bieberich E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J. Biol. Chem. 2012;287(25):21384–21395. doi: 10.1074/jbc.M112.340513. [http://dx.doi.org/10.1074/jbc.M112.340513]. [PMID: 22532571]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smale G., Nichols N.R., Brady D.R., Finch C.E., Horton W.E., Jr Evidence for apoptotic cell death in Alzheimers disease. Exp. Neurol. 1995;133(2):225–230. doi: 10.1006/exnr.1995.1025. [http://dx.doi.org/10.1006/exnr. 1995.1025]. [PMID: 7544290]. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y., Hu J., Wu J., Zhu C., Hui Y., Han Y., Huang Z., Ellsworth K., Fan W. α7 nicotinic acetylcholine receptor-mediated neuroprotection against dopaminergic neuron loss in an MPTP mouse model via inhibition of astrocyte activation. J. Neuroinflammation. 2012;9(98):2094–2099. doi: 10.1186/1742-2094-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang H.Y., Lee D.H., Davis C.B., Shank R.P. Amyloid peptide Abeta(142) binds selectively and with picomolar affinity to α7 nicotinic acetylcholine receptors. J. Neurochem. 2000;75(3):1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [http://dx.doi.org/10.1046/j.1471-4159.2000.0751155. x]. [PMID: 10936198]. [DOI] [PubMed] [Google Scholar]
- 140.Kem W.R. The brain α7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimers disease: studies with DMXBA (GTS-21). Behav. Brain Res. 2000;113(1-2):169–181. doi: 10.1016/s0166-4328(00)00211-4. [http://dx.doi.org/10.1016/S0166-4328(00)00211-4]. [PMID: 10942043]. [DOI] [PubMed] [Google Scholar]
- 141.Shen J.X., Yakel J.L. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol. Sin. 2009;30(6):673–680. doi: 10.1038/aps.2009.64. [http://dx.doi.org/10.1038/aps.2009.64]. [PMID: 19448647]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Preskorn S.H., Gawryl M., Dgetluck N., Palfreyman M., Bauer L.O., Hilt D.C. Normalizing effects of EVP-6124, an α-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J. Psychiatr. Pract. 2014;20(1):12–24. doi: 10.1097/01.pra.0000442935.15833.c5. [http://dx.doi.org/10.1097/ 01.pra.0000442935.15833.c5]. [PMID: 24419307]. [DOI] [PubMed] [Google Scholar]
- 143.Sydserff S., Sutton E.J., Song D., Quirk M.C., Maciag C., Li C., Jonak G., Gurley D., Gordon J.C., Christian E.P., Doherty J.J., Hudzik T., Johnson E., Mrzljak L., Piser T., Smagin G.N., Wang Y., Widzowski D., Smith J.S. Selective α7 nicotinic receptor activation by AZD0328 enhances cortical dopamine release and improves learning and attentional processes. Biochem. Pharmacol. 2009;78(7):880–888. doi: 10.1016/j.bcp.2009.07.005. [http://dx.doi.org/10.1016/ j.bcp.2009.07.005]. [PMID: 19615981]. [DOI] [PubMed] [Google Scholar]
- 144.Bitner R.S., Bunnelle W.H., Decker M.W., Drescher K.U., Kohlhaas K.L., Markosyan S., Marsh K.C., Nikkel A.L., Browman K., Radek R., Anderson D.J., Buccafusco J., Gopalakrishnan M. In vivo pharmacological characterization of a novel selective α7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimers disease. J. Pharmacol. Exp. Ther. 2010;334(3):875–886. doi: 10.1124/jpet.110.167213. [http://dx.doi.org/10.1124/jpet.110.167213]. [PMID: 20504913]. [DOI] [PubMed] [Google Scholar]
- 145.Stevens K.E., Cornejo B., Adams C.E., Zheng L., Yonchek J., Hoffman K.L., Christians U., Kem W.R. Continuous administration of a selective α7 nicotinic partial agonist, DMXBA, improves sensory inhibition without causing tachyphylaxis or receptor upregulation in DBA/2 mice. Brain Res. 2010;1352:140–146. doi: 10.1016/j.brainres.2010.06.063. [http://dx.doi.org/10.1016/j.brainres.2010.06.063]. [PMID: 20599427]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hardy J. Expression of normal sequence pathogenic proteins for neurodegenerative disease contributes to disease risk: permissive templating as a general mechanism underlying neurodegeneration. Biochem. Soc. Trans. 2005;33(Pt 4):578–581. doi: 10.1042/BST0330578. [http://dx.doi.org/10.1042/BST0330578]. [PMID: 16042548]. [DOI] [PubMed] [Google Scholar]
- 147.Schedin-Weiss S., Winblad B., Tjernberg L.O. The role of protein glycosylation in Alzheimer disease. FEBS J. 2014;281(1):46–62. doi: 10.1111/febs.12590. [http://dx.doi.org/10.1111/febs.12590]. [PMID: 24279329]. [DOI] [PubMed] [Google Scholar]
- 148.Taylor J.P., Hardy J., Fischbeck K.H. Toxic proteins in neurodegenerative disease. Science. 2002;296(5575):1991–1995. doi: 10.1126/science.1067122. [http://dx.doi.org/10.1126/science.1067122]. [PMID: 12065827]. [DOI] [PubMed] [Google Scholar]
- 149.Poulsen S-A., Watson A.A., Fairlie D.P., Craik D.J. Solution structures in aqueous SDS micelles of two amyloid β peptides of A β(128) mutated at the α-secretase cleavage site (K16E, K16F). J. Struct. Biol. 2000;130(2-3):142–152. doi: 10.1006/jsbi.2000.4267. [http://dx.doi.org/10.1006/ jsbi.2000.4267]. [PMID: 10940222]. [DOI] [PubMed] [Google Scholar]
- 150.Ren R.J., Dammer E.B., Wang G., Seyfried N.T., Levey A.I. Proteomics of protein post-translational modifications implicated in neurodegeneration. Transl. Neurodegener. 2014;3(1):23. doi: 10.1186/2047-9158-3-23. [http://dx.doi.org/10.1186/2047-9158-3-23]. [PMID: 25671099]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zahid S., Khan R., Oellerich M., Ahmed N., Asif A.R. Differential S-nitrosylation of proteins in Alzheimers disease. Neuroscience. 2014;256:126–136. doi: 10.1016/j.neuroscience.2013.10.026. [http://dx.doi.org/10.1016/ j.neuroscience.2013.10.026]. [PMID: 24157928]. [DOI] [PubMed] [Google Scholar]
- 152.McFarlane I., Georgopoulou N., Coughlan C.M., Gillian A.M., Breen K.C. The role of the protein glycosylation state in the control of cellular transport of the amyloid β precursor protein. Neuroscience. 1999;90(1):15–25. doi: 10.1016/s0306-4522(98)00361-3. [http://dx.doi.org/10.1016/ S0306-4522(98)00361-3]. [PMID: 10188930]. [DOI] [PubMed] [Google Scholar]
- 153.Mohorko E., Glockshuber R., Aebi M. Oligosaccharyltransferase: the central enzyme of N-linked protein glycosylation. J. Inherit. Metab. Dis. 2011;34(4):869–878. doi: 10.1007/s10545-011-9337-1. [http://dx.doi.org/10.1007/ s10545-011-9337-1]. [PMID: 21614585]. [DOI] [PubMed] [Google Scholar]
- 154.Georgopoulou N., McLaughlin M., McFarlane I., Breen K.C. The role of post-translational modification in beta-amyloid precursor protein processing. Biochem. Soc. Symp. 2001;(67):23–36. doi: 10.1042/bss0670023. [http://dx.doi.org/10.1042/bss0670023]. [PMID: 11447837]. [DOI] [PubMed] [Google Scholar]
- 155.Haass C., Kaether C., Thinakaran G., Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med. 2012;2(5):a006270. doi: 10.1101/cshperspect.a006270. [http://dx.doi.org/10.1101/cshperspect.a006270]. [PMID: 22553493]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yazaki M., Tagawa K., Maruyama K., Sorimachi H., Tsuchiya T., Ishiura S., Suzuki K. Mutation of potential N-linked glycosylation sites in the Alzheimers disease amyloid precursor protein (APP). Neurosci. Lett. 1996;221(1):57–60. doi: 10.1016/s0304-3940(96)13285-7. [http://dx. doi.org/10.1016/S0304-3940(96)13285-7]. [PMID: 9014180]. [DOI] [PubMed] [Google Scholar]
- 157.McFarlane I., Georgopoulou N., Coughlan C.M., Gillian A.M., Breen K.C. The role of the protein glycosylation state in the control of cellular transport of the amyloid beta precursor protein. Neuroscience. 1999;90(1):15–25. doi: 10.1016/s0306-4522(98)00361-3. [http://dx.doi.org/10.1016/ S0306-4522(98)00361-3]. [PMID: 10188930]. [DOI] [PubMed] [Google Scholar]
- 158.Yang D.S., Tandon A., Chen F., Yu G., Yu H., Arawaka S., Hasegawa H., Duthie M., Schmidt S.D., Ramabhadran T.V., Nixon R.A., Mathews P.M., Gandy S.E., Mount H.T., St George-Hyslop P., Fraser P.E. Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins. J. Biol. Chem. 2002;277(31):28135–28142. doi: 10.1074/jbc.M110871200. [http://dx.doi.org/10.1074/ jbc.M110871200]. [PMID: 12032140]. [DOI] [PubMed] [Google Scholar]
- 159.LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-beta in Alzheimers disease. Nat. Rev. Neurosci. 2007;8(7):499–509. doi: 10.1038/nrn2168. [http://dx.doi.org/10.1038/nrn2168]. [PMID: 17551515]. [DOI] [PubMed] [Google Scholar]
- 160.Menéndez-González M., Pérez-Pinera P., Martínez-Rivera M., Calatayud M.T., Blázquez M.B. APP processing and the APP-KPI domain involvement in the amyloid cascade. Neurodegener. Dis. 2005;2(6):277–283. doi: 10.1159/000092315. [http://dx.doi.org/10.1159/000092315]. [PMID: 16909010]. [DOI] [PubMed] [Google Scholar]
- 161.Akasaka-Manya K., Manya H., Sakurai Y., Wojczyk B.S., Kozutsumi Y., Saito Y., Taniguchi N., Murayama S., Spitalnik S.L., Endo T. Protective effect of N-glycan bisecting GlcNAc residues on beta-amyloid production in Alzheimers disease. Glycobiology. 2010;20(1):99–106. doi: 10.1093/glycob/cwp152. [http://dx.doi.org/10.1093/glycob/cwp152]. [PMID: 19776078]. [DOI] [PubMed] [Google Scholar]
- 162.Nakagawa K., Kitazume S., Oka R., Maruyama K., Saido T.C., Sato Y., Endo T., Hashimoto Y. Sialylation enhances the secretion of neurotoxic amyloid-beta peptides. J. Neurochem. 2006;96(4):924–933. doi: 10.1111/j.1471-4159.2005.03595.x. [http://dx.doi.org/10.1111/j.1471-4159.2005. 03595.x]. [PMID: 16412100]. [DOI] [PubMed] [Google Scholar]
- 163.Mills J., Reiner P.B. Regulation of amyloid precursor protein cleavage. J. Neurochem. 1999;72(2):443–460. doi: 10.1046/j.1471-4159.1999.0720443.x. [http://dx.doi.org/10.1046/j.1471-4159.1999.0720443.x]. [PMID: 9930716]. [DOI] [PubMed] [Google Scholar]
- 164.Zhang H.Y., Yan H., Tang X.C. Huperzine A enhances the level of secretory amyloid precursor protein and protein kinase C-alpha in intracerebroventricular beta-amyloid-(140) infused rats and human embryonic kidney 293 Swedish mutant cells. Neurosci. Lett. 2004;360(1-2):21–24. doi: 10.1016/j.neulet.2004.01.055. [http://dx.doi.org/10.1016/j.neulet. 2004.01.055]. [PMID: 15082169]. [DOI] [PubMed] [Google Scholar]
- 165.Akasaka-Manya K., Manya H., Sakurai Y., Wojczyk B.S., Spitalnik S.L., Endo T. Increased bisecting and core-fucosylated N-glycans on mutant human amyloid precursor proteins. Glycoconj. J. 2008;25(8):775–786. doi: 10.1007/s10719-008-9140-x. [http://dx.doi.org/10.1007/ s10719-008-9140-x]. [PMID: 18521746]. [DOI] [PubMed] [Google Scholar]
- 166.Brinkmalm G., Portelius E., Öhrfelt A., Mattsson N., Persson R., Gustavsson M.K., Vite C.H., Gobom J., Månsson J.E., Nilsson J., Halim A., Larson G., Rüetschi U., Zetterberg H., Blennow K., Brinkmalm A. An online nano-LC-ESI-FTICR-MS method for comprehensive characterization of endogenous fragments from amyloid β and amyloid precursor protein in human and cat cerebrospinal fluid. J. Mass Spectrom. 2012;47(5):591–603. doi: 10.1002/jms.2987. [http://dx.doi.org/10.1002/jms.2987]. [PMID: 22576872]. [DOI] [PubMed] [Google Scholar]
- 167.Halim A., Brinkmalm G., Rüetschi U., Westman-Brinkmalm A., Portelius E., Zetterberg H., Blennow K., Larson G., Nilsson J. Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc. Natl. Acad. Sci. USA. 2011;108(29):11848–11853. doi: 10.1073/pnas.1102664108. [http://dx.doi.org/10.1073/pnas.1102664108]. [PMID: 21712440]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kitazume S., Tachida Y., Kato M., Yamaguchi Y., Honda T., Hashimoto Y., Wada Y., Saito T., Iwata N., Saido T., Taniguchi N. Brain endothelial cells produce amyloid beta from amyloid precursor protein 770 and preferentially secrete the O-glycosylated form. J. Biol. Chem. 2010;285(51):40097–40103. doi: 10.1074/jbc.M110.144626. [http://dx.doi.org/10.1074/jbc.M110.144626]. [PMID: 20952385]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Perdivara I., Petrovich R., Allinquant B., Deterding L.J., Tomer K.B., Przybylski M. Elucidation of O-glycosylation structures of the beta-amyloid precursor protein by liquid chromatography-mass spectrometry using electron transfer dissociation and collision induced dissociation. J. Proteome Res. 2009;8(2):631–642. doi: 10.1021/pr800758g. [http://dx.doi.org/10.1021/pr800758g]. [PMID: 19093876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tomita S., Kirino Y., Suzuki T. Cleavage of Alzheimers amyloid precursor protein (APP) by secretases occurs after O-glycosylation of APP in the protein secretory pathway. Identification of intracellular compartments in which APP cleavage occurs without using toxic agents that interfere with protein metabolism. J. Biol. Chem. 1998;273(11):6277–6284. doi: 10.1074/jbc.273.11.6277. [http://dx.doi.org/10.1074/ jbc.273.11.6277]. [PMID: 9497354]. [DOI] [PubMed] [Google Scholar]
- 171.Griffith L.S., Mathes M., Schmitz B. Beta-amyloid precursor protein is modified with O-linked N-acetylglucosamine. J. Neurosci. Res. 1995;41(2):270–278. doi: 10.1002/jnr.490410214. [http://dx.doi.org/10.1002/jnr.490410214]. [PMID: 7650762]. [DOI] [PubMed] [Google Scholar]
- 172.Jacobsen K.T., Iverfeldt K. O-GlcNAcylation increases non-amyloidogenic processing of the amyloid-β precursor protein (APP). Biochem. Biophys. Res. Commun. 2011;404(3):882–886. doi: 10.1016/j.bbrc.2010.12.080. [http://dx.doi.org/10.1016/j.bbrc.2010.12.080]. [PMID: 21182826]. [DOI] [PubMed] [Google Scholar]
- 173.Zhang X. Cholinergic activity and amyloid precursor protein processing in aging and Alzheimers disease. Curr. Drug Targets CNS Neurol. Disord. 2004;3(2):137–152. doi: 10.2174/1568007043482499. [http://dx.doi.org/10.2174/1568007043482499]. [PMID: 15078189]. [DOI] [PubMed] [Google Scholar]
- 174.Lee M-S., Kao S-C., Lemere C.A., Xia W., Tseng H-C., Zhou Y., Neve R., Ahlijanian M.K., Tsai L-H. APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 2003;163(1):83–95. doi: 10.1083/jcb.200301115. [http://dx.doi.org/10.1083/jcb.200301115]. [PMID: 14557249]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu F., Su Y., Li B., Zhou Y., Ryder J., Gonzalez-DeWhitt P., May P.C., Ni B. Regulation of amyloid precursor protein (APP) phosphorylation and processing by p35/Cdk5 and p25/Cdk5. FEBS Lett. 2003;547(1-3):193–196. doi: 10.1016/s0014-5793(03)00714-2. [http://dx.doi.org/10.1016/S0014-5793(03)00714-2]. [PMID: 12860412]. [DOI] [PubMed] [Google Scholar]
- 176.Chang K-A., Kim H-S., Ha T-Y., Ha J-W., Shin K.Y., Jeong Y.H., Lee J-P., Park C-H., Kim S., Baik T-K., Suh Y.H. Phosphorylation of amyloid precursor protein (APP) at Thr668 regulates the nuclear translocation of the APP intracellular domain and induces neurodegeneration. Mol. Cell. Biol. 2006;26(11):4327–4338. doi: 10.1128/MCB.02393-05. [http://dx.doi.org/10.1128/MCB.02393-05]. [PMID: 16705182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Castro A., Martinez A. Targeting beta-amyloid pathogenesis through acetylcholinesterase inhibitors. Curr. Pharm. Des. 2006;12(33):4377–4387. doi: 10.2174/138161206778792985. [http://dx.doi.org/10.2174/138161206778792985]. [PMID: 17105433]. [DOI] [PubMed] [Google Scholar]
- 178.Pakaski M., Kasa P. Role of acetylcholinesterase inhibitors in the metabolism of amyloid precursor protein. Curr. Drug Targets CNS Neurol. Disord. 2003;2(3):163–171. doi: 10.2174/1568007033482869. [http://dx.doi.org/10.2174/ 1568007033482869]. [PMID: 12769797]. [DOI] [PubMed] [Google Scholar]
- 179.Morris M., Knudsen G.M., Maeda S., Trinidad J.C., Ioanoviciu A., Burlingame A.L., Mucke L. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 2015;18(8):1183–1189. doi: 10.1038/nn.4067. [http://dx.doi.org/10.1038/nn.4067]. [PMID: 26192747]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G-Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimers disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [http://dx.doi.org/10.1126/science.1141736]. [PMID: 17478722]. [DOI] [PubMed] [Google Scholar]
- 181.Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B.C., Christie M.J., Napier I.A., Eckert A., Staufenbiel M., Hardeman E., Götz J. Dendritic function of tau mediates amyloid-β toxicity in Alzheimers disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [http://dx.doi.org/10.1016/j.cell.2010.06.036]. [PMID: 20655099]. [DOI] [PubMed] [Google Scholar]
- 182.Zempel H., Thies E., Mandelkow E., Mandelkow E-M. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of micro- tubules and spines. J. Neurosci. 2010;30(36):11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.2357-10.2010]. [PMID: 20826658]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hanger D.P., Byers H.L., Wray S., Leung K-Y., Saxton M.J., Seereeram A., Reynolds C.H., Ward M.A., Anderton B.H. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J. Biol. Chem. 2007;282(32):23645–23654. doi: 10.1074/jbc.M703269200. [http://dx.doi.org/10.1074/jbc.M703269200]. [PMID: 17562708]. [DOI] [PubMed] [Google Scholar]
- 184.Funk K.E., Thomas S.N., Schafer K.N., Cooper G.L., Liao Z., Clark D.J., Yang A.J., Kuret J. Lysine methylation is an endogenous post-translational modification of tau protein in human brain and a modulator of aggregation propensity. Biochem. J. 2014;462(1):77–88. doi: 10.1042/BJ20140372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guo A., Gu H., Zhou J., Mulhern D., Wang Y., Lee K.A., Yang V., Aguiar M., Kornhauser J., Jia X., Ren J., Beausoleil S.A., Silva J.C., Vemulapalli V., Bedford M.T., Comb M.J. Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol. Cell. Proteomics. 2014;13(1):372–387. doi: 10.1074/mcp.O113.027870. [http://dx.doi.org/10.1074/mcp.O113.027870]. [PMID: 24129315]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou X-W., Gustafsson J-Å., Tanila H., Bjorkdahl C., Liu R., Winblad B., Pei J-J. Tau hyperphosphorylation correlates with reduced methylation of protein phosphatase 2A. Neurobiol. Dis. 2008;31(3):386–394. doi: 10.1016/j.nbd.2008.05.013. [http://dx.doi.org/10.1016/j.nbd.2008.05.013]. [PMID: 18586097]. [DOI] [PubMed] [Google Scholar]
- 187.Liu F., Iqbal K., Grundke-Iqbal I., Hart G.W., Gong C-X. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimers disease. Proc. Natl. Acad. Sci. USA. 2004;101(29):10804–10809. doi: 10.1073/pnas.0400348101. [http://dx.doi.org/10.1073/pnas.0400348101]. [PMID: 15249677]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cripps D., Thomas S.N., Jeng Y., Yang F., Davies P., Yang A.J. Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-Tau is polyubiquitinated through Lys-48, Lys-11, and Lys-6 ubiquitin conjugation. J. Biol. Chem. 2006;281(16):10825–10838. doi: 10.1074/jbc.M512786200. [http://dx.doi.org/10.1074/jbc.M512786200]. [PMID: 16443603]. [DOI] [PubMed] [Google Scholar]
- 189.Min S-W., Cho S-H., Zhou Y., Schroeder S., Haroutunian V., Seeley W.W., Huang E.J., Shen Y., Masliah E., Mukherjee C., Meyers D., Cole P.A., Ott M., Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953–966. doi: 10.1016/j.neuron.2010.08.044. [http://dx.doi.org/10.1016/j.neuron.2010.08.044]. [PMID: 20869593]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nathanson N.M. Synthesis, trafficking, and localization of muscarinic acetylcholine receptors. Pharmacol. Ther. 2008;119(1):33–43. doi: 10.1016/j.pharmthera.2008.04.006. [http://dx.doi.org/10.1016/j.pharmthera.2008.04.006]. [PMID: 18558434]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Romero-Fernandez W., Borroto-Escuela D.O., Alea M.P., Garcia-Mesa Y., Garriga P. Altered trafficking and unfolded protein response induction as a result of M3 muscarinic receptor impaired N-glycosylation. Glycobiology. 2011;21(12):1663–1672. doi: 10.1093/glycob/cwr105. [http://dx.doi.org/10.1093/glycob/cwr105]. [PMID: 21798865]. [DOI] [PubMed] [Google Scholar]
- 192.Peretto I., Petrillo P., Imbimbo B.P. Medicinal chemistry and therapeutic potential of muscarinic M3 antagonists. Med. Res. Rev. 2009;29(6):867–902. doi: 10.1002/med.20158. [DOI] [PubMed] [Google Scholar]
- 193.van Koppen C.J., Nathanson N.M. Site-directed mutagenesis of the m2 muscarinic acetylcholine receptor. Analysis of the role of N-glycosylation in receptor expression and function. J. Biol. Chem. 1990;265(34):20887–20892. [PMID: 2249995]. [PubMed] [Google Scholar]
- 194.Fisher A., Pittel Z., Haring R., Bar-Ner N., Kliger-Spatz M., Natan N., Egozi I., Sonego H., Marcovitch I., Brandeis R. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimers disease. J. Mol. Neurosci. 2003;20(3):349–356. doi: 10.1385/JMN:20:3:349. [http://dx.doi.org/10.1385/JMN:20:3:349]. [PMID: 14501019]. [DOI] [PubMed] [Google Scholar]
- 195.Wang H-Y., Li W., Benedetti N.J., Lee D.H. α 7 nicotinic acetylcholine receptors mediate β-amyloid peptide-induced tau protein phosphorylation. J. Biol. Chem. 2003;278(34):31547–31553. doi: 10.1074/jbc.M212532200. [http://dx.doi.org/10.1074/jbc.M212532200]. [PMID: 12801934]. [DOI] [PubMed] [Google Scholar]
- 196.Hu M., Waring J.F., Gopalakrishnan M., Li J. Role of GSK-3β activation and α7 nAChRs in Abeta(142)-induced tau phosphorylation in PC12 cells. J. Neurochem. 2008;106(3):1371–1377. doi: 10.1111/j.1471-4159.2008.05483.x. [http://dx.doi.org/10.1111/j.1471-4159.2008.05483.x]. [PMID: 18485099]. [DOI] [PubMed] [Google Scholar]
- 197.Xie H., Litersky J.M., Hartigan J.A., Jope R.S., Johnson G.V. The interrelationship between selective tau phosphorylation and microtubule association. Brain Res. 1998;798(1-2):173–183. doi: 10.1016/s0006-8993(98)00407-7. [http://dx.doi.org/10.1016/S0006-8993(98)00407-7]. [PMID: 9666118]. [DOI] [PubMed] [Google Scholar]
- 198.Oddo S., Caccamo A., Green K.N., Liang K., Tran L., Chen Y., Leslie F.M., LaFerla F.M. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimers disease. Proc. Natl. Acad. Sci. USA. 2005;102(8):3046–3051. doi: 10.1073/pnas.0408500102. [http://dx.doi.org/10.1073/pnas.0408500102]. [PMID: 15705720]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hellström-Lindahl E. Modulation of β-amyloid precursor protein processing and tau phosphorylation by acetylcholine receptors. Eur. J. Pharmacol. 2000;393(1-3):255–263. doi: 10.1016/s0014-2999(00)00028-5. [http://dx.doi.org/10. 1016/S0014-2999(00)00028-5]. [PMID: 10771022]. [DOI] [PubMed] [Google Scholar]