Abstract
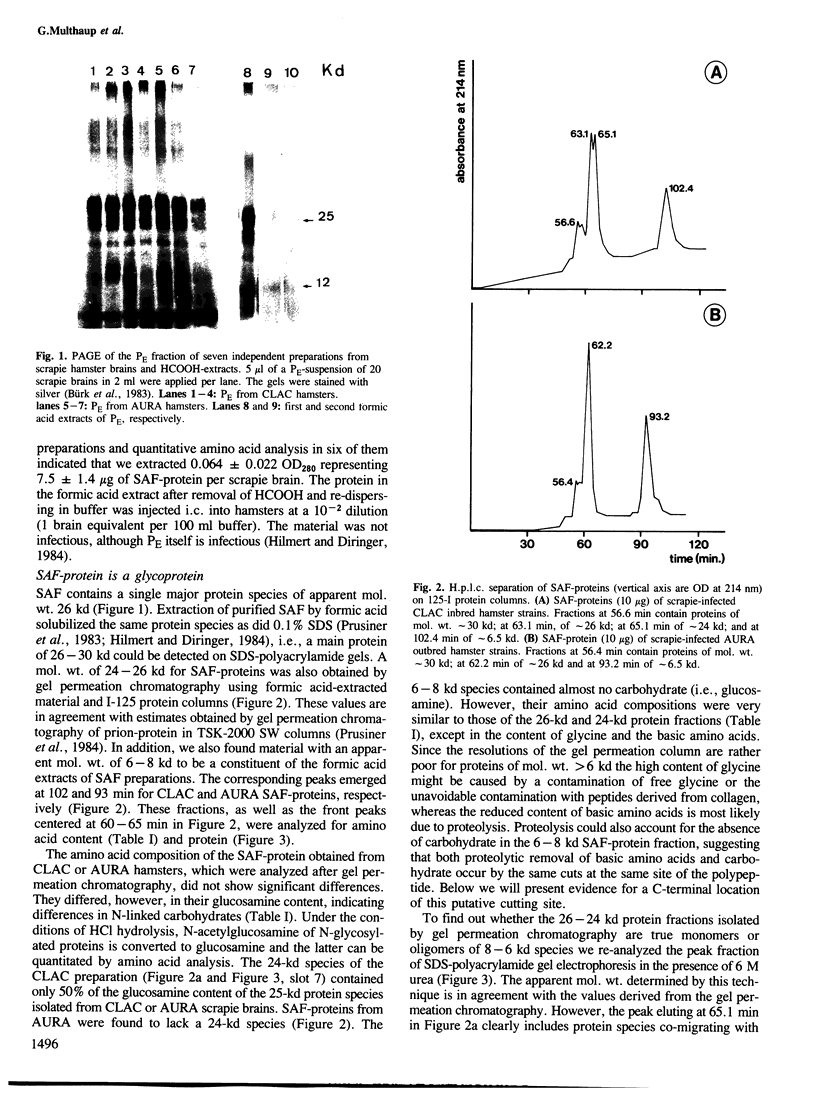
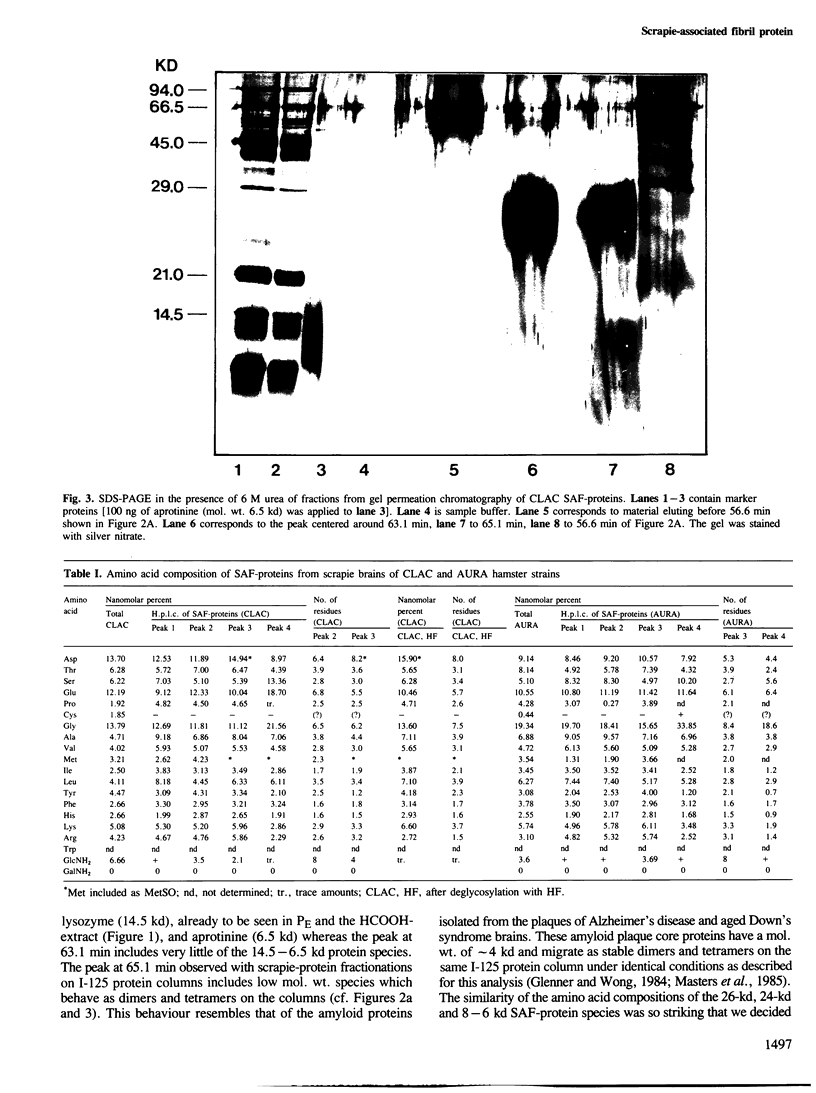
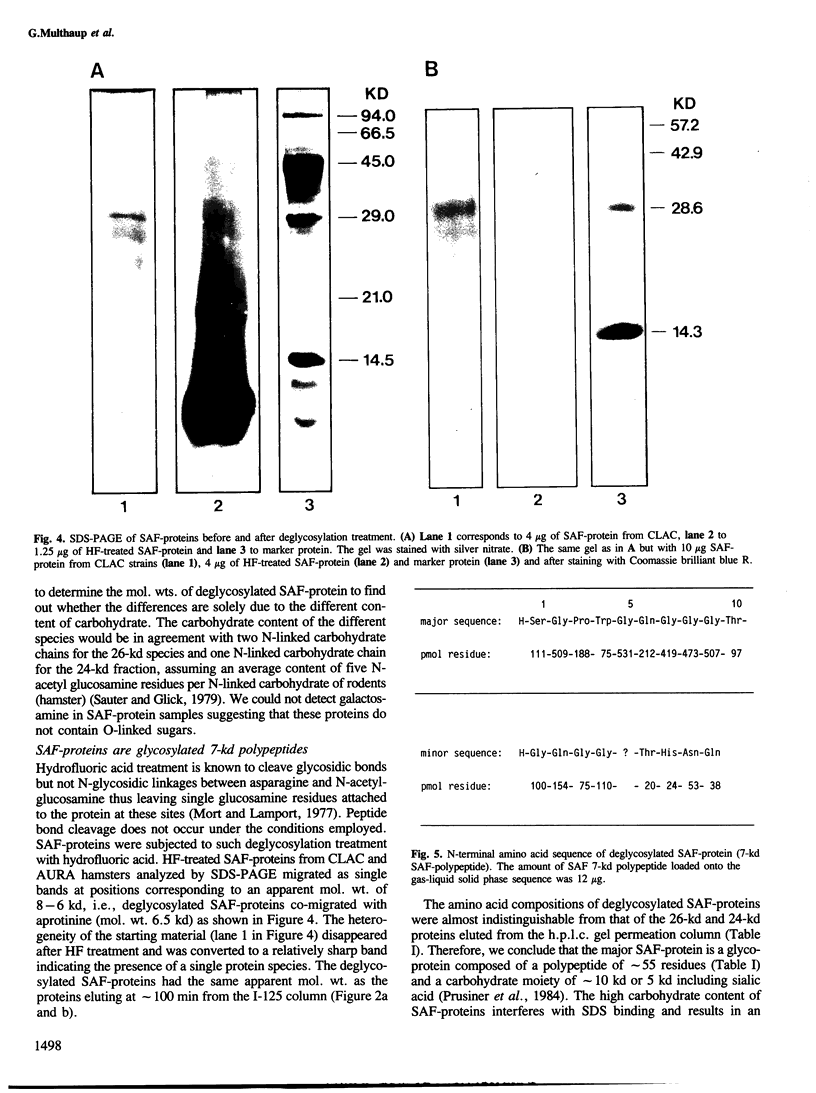
Scrapie-associated fibril protein (SAF-protein) extracted from infectious scrapie-associated fibrils (SAF) isolated from scrapie hamster brains is not infectious. SAF-protein is composed of various mol. wt. species of glycoproteins differing in carbohydrate content rather than amino acid composition. The N-linked carbohydrate chains represent approximately 40-60% of the mol. wt. of SAF-protein. The deglycosylated SAF-protein has a surprisingly low mol. wt. of approximately 7 kd, representing approximately 55 amino acid residues. This size and chemical analyses indicate that SAF-protein is an amyloid-type of protein. The simplest explanation for the available data is that SAF-polypeptide is very likely not to be part of the scrapie agent but that it is, like other amyloid proteins, derived from host-encoded proteins and not infectious. It is suggested that the infectivity of fractions rich in SAF is due to co-purification of scrapie virus and SAF caused by the high carbohydrate content of SAF-protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton D. C., McKinley M. P., Prusiner S. B. Molecular characteristics of the major scrapie prion protein. Biochemistry. 1984 Dec 4;23(25):5898–5906. doi: 10.1021/bi00320a002. [DOI] [PubMed] [Google Scholar]
- Bürk R. R., Eschenbruch M., Leuthard P., Steck G. Sensitive detection of proteins and peptides in polyacrylamide gels after formaldehyde fixation. Methods Enzymol. 1983;91:247–254. doi: 10.1016/s0076-6879(83)91021-2. [DOI] [PubMed] [Google Scholar]
- Cooper J. H. Selective amyloid staining as a function of amyloid composition and structure. Histochemical analysis of the alkaline Congo red, standardized toluidine blue, and iodine methods. Lab Invest. 1974 Sep;31(3):232–238. [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Diringer H., Hilmert H., Simon D., Werner E., Ehlers B. Towards purification of the scrapie agent. Eur J Biochem. 1983 Aug 15;134(3):555–560. doi: 10.1111/j.1432-1033.1983.tb07602.x. [DOI] [PubMed] [Google Scholar]
- Eanes E. D., Glenner G. G. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968 Nov;16(11):673–677. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Ein D., Eanes E. D., Bladen H. A., Terry W., Page D. L. Creation of "amyloid" fibrils from Bence Jones proteins in vitro. Science. 1971 Nov 12;174(4010):712–714. doi: 10.1126/science.174.4010.712. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Rapid analytical and preparative separation of structural polypeptides of poliovirus by reverse-phase high-performance liquid chromatography. J Virol Methods. 1983 May;6(5):283–293. doi: 10.1016/0166-0934(83)90043-5. [DOI] [PubMed] [Google Scholar]
- Hilmert H., Diringer H. A rapid and efficient method to enrich SAF-protein from scrapie brains of hamsters. Biosci Rep. 1984 Feb;4(2):165–170. doi: 10.1007/BF01120313. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977 Feb;34(2):295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Rohwer R. G., Kascsak R., Wisniewski H. M., Somerville R. A., Gibbs C. J., Jr, Gajdusek D. C. Infection-specific particle from the unconventional slow virus diseases. Science. 1984 Jul 27;225(4660):437–440. doi: 10.1126/science.6377496. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Santer U. V., Glick M. C. Partial structure of a membrane glycopeptide from virus-transformed hamster cells. Biochemistry. 1979 Jun 12;18(12):2533–2540. doi: 10.1021/bi00579a016. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]