Abstract
Mesenchymal stem cells (MSCs) are thought to occupy a perivascular niche where they are exposed to signals originating from vascular cells. This study focused on the effects of endothelial cell (EC)-derived signals on MSC differentiation toward vascular cell lineages. Upon co-culture with two types of ECs, macrovascular (macro) ECs and microvascular (micro) ECs, the former caused MSCs to increase expression of both EC and smooth muscle cell (SMC) markers, while the latter induced expression of EC markers only. These marker changes in MSCs were linked to the extracellular matrixes secreted by the ECs (EC-matrix) rather than soluble EC-secreted factors. Beyond enhanced marker expression, EC-matrix also induced functional changes in MSCs indicative of development of a genuine vascular cell phenotype. These included enhanced incorporation into vessels and cytoskeletal localization of vascular SMC-specific contractile elements. The bioactivity of EC-matrix was sensitive to EDTA washes and required sulfated glycosaminoglycans. However, neither soluble VEGF nor substrate surfaces coated with fibronectin, collagen type IV, or laminin recreated the effects of EC-matrix on MSC vascular differentiation. In conclusion, these results identified EC-matrix as a critical regulator of vascular cell differentiation of MSCs. Elucidating these MSC-EC-matrix interactions and identifying the specific EC-matrix components involved will shed light on the perivascular signals seen by MSCs in vivo.
Keywords: perivascular niche, mesenchymal stem cells, endothelial cells, extracellular matrix
There is strong evidence to suggest that MSCs reside in perivascular niches and associate with blood vessels throughout the tissues of the body [Crisan et al., 2008; da Silva Meirelles et al., 2006; Shi et al., 2003]. In fact, MSCs have been isolated directly from large and small blood vessels [da Silva Meirelles et al., 2006], and vessel walls are the main sites of expression of Stro-1, an MSC-associated marker [Bianco et al., 2001; Shi et al., 2003]. Furthermore, MSCs share key similarities with pericytes, a microvascular cell type analogous to the smooth muscle cells (SMCs) of macrovessels [Crisan et al., 2008; Tintut et al., 2003]. For example, pericytes posses MSC-like differentiation capabilities [Crisan et al., 2008; Doherty et al., 1998; Farrington-Rock et al., 2004; Tintut et al., 2003] and retain multipotency through high passages [Tintut et al., 2003], indicating a capacity for self renewal, a key feature of MSCs. Both cell types express a mixture of MSC- and vascular cell-markers, including Stro-1, CD106, CD146, CD29, CD44, smooth muscle α-actin, 3G5, Sab-1, and Sab-2 [Crisan et al., 2008; Jones et al., 2008; Shi et al., 2003; Tintut et al., 2003; Traktuev et al., 2008]. Pericytes and MSCs exhibit similar fibroblastic morphologies in culture and are unique among adult mesenchymal cells in their ability to support culture of hematopoietic cells [Jones et al., 2008; Shi et al., 2003; Tintut et al., 2003]. MSCs and pericytes also appear to fill similar vessel-support roles, migrating towards vessels in response to endothelial cell (EC)-secreted factors and enhancing vessel formation and stabilization through paracrine interactions [Armulik et al., 2005; Gruber et al., 2005; Traktuev et al., 2008; von Tell et al., 2006]. These similarities between pericytes and MSCs strengthen the case that MSCs are a kind of pericyte.
Thus MSCs, residing in a perivascular niche, should interact extensively with vascular signals in vivo. These signals include physical interactions with the vascular cell types, as well as interactions with the soluble molecules and extracellular matrix (ECM) these cells produce. ECs, the main vascular cell type, form the lumenal lining of blood vessels [Xu et al., 1994]. There are different ECs, depending on their tissue location. For example, macrovascular ECs (macroECs) are found in the larger blood vessels, while microvascular ECs (microECs) are found in the microvasculature, e.g., capillaries [Xu et al., 1994]. The differences among the types of ECs also include their cell-surface markers, their cellular interactions (only macroECs interact with SMCs), and in their matrix interactions.
There is also abundant evidence of signals from the local environment greatly affecting MSC differentiation in what has been called the “milieu-specific nature of MSC differentiation” [Chiu et al., 1995]. MSCs are also capable of differentiating into a wide range of cell types, including the vascular cell types, ECs and SMCs [Rodriguez et al., 2006]. A review of these studies reveals the tendency of MSCs to acquire tissue-specific characteristics when co-cultured with mature cells types or exposed to tissue extracts in vitro [Choi et al., 2005; da Silva Meirelles et al., 2006; Houghton et al., 2004; Lange et al., 2005]. There is evidence to suggest that this tissue-instructive differentiation is actually supported by the tissue-specific composition of the ECM [Philp et al., 2005]. Indeed, interactions with various matrix molecules have been shown to modulate MSC behavior [Bradham et al., 1995; Hashimoto et al., 2006; Salasznyk et al., 2004]. For example, it has been shown that de-cellularized matrix of a tissue is sufficient for differentiation of stem cells into the cells and structures indicative of that tissue [Philp et al., 2005; Salasznyk et al., 2004].
Based on these findings, we hypothesized that signals originating from the main cell type of the vascular environment, ECs, promote MSCs differentiation toward the vascular cell lineages, specifically ECs and SMCs. Furthermore, given the role of the ECM in guiding the tissue-specific nature of MSC differentiation, this study focuses on the effects of the ECM produced by ECs (EC-matrix) on MSC differentiation.
Materials and Methods
Cell culture
The macroEC line HUV-EC-C (American Type Culture Collection, Manassas, VA) and the microEC line HMEC-1 (U.S. Center for Disease Control) were cultured in EC medium (EGM-2-MV medium (Cambrex, East Rutherford, NJ) containing 5% fetal bovine serum (FBS)). Human bone marrow MSCs were isolated from femoral heads obtained during elective joint replacement (IRB approval, University of Washington) as tissue culture plastic (TCP) adherent cell populations, expanded in MSC medium (high-glucose DMEM (Invitrogen, Carlsbad, CA) containing 10% FBS) and passaged at 70–80% confluency. All experiments were performed with passage 3–5 MSCs. Serum-free (SF) culture conditions involved replacement of serum with insulin-transferrin-selenium-x (Invitrogen, Carlsbad, CA) containing 10% FBS).
Real-time RT PCR
MSC RNA samples were isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA), treated with DNase using the Turbo DNA-free kit (Ambion, Austin, TX), and converted to cDNA with Superscript III First-Strand Synthesis Kits (dT primer) (Invitrogen, Carlsbad, CA). Real-time PCR was performed on a BioRad iCycler using SYBR green detection (BioRad, Hercules, CA). Primers for the EC markers PECAM, KDR, and VECAD and the SMC markers smooth muscle α-actin (SMActin), smooth muscle 22α, and smoothelin were designed using MacVector (Cary, NC). mRNA levels of EC and SMC markers analyzed by real-time RT-PCR were normalized to GAPDH values for each time point and/or experimental condition. Results from studies involving time-courses are presented as fold changes to day 0 values.
Direct MSC:EC co-cultures
MSCs were labeled with Vybrant CM-DiI cell-labeling solution (Invitrogen, Carlsbad, CA) according to the manufacture’s instructions. For heterogeneous direct co-cultures, the DiI-labeled MSCs were mixed with unlabeled ECs (either macroECs or microECs) in 1:2 ratios before plating on TCP at 3×104 total cells/cm2. Homogeneous co-cultures of MSCs consisting of labeled and unlabeled cells (1:2) were used as controls (Figure 1A). The co-cultures were maintained in EC medium containing 1% FBS for 10 days. Every two days, cells from both heterogeneous and homogeneous co-cultures were pelleted, resuspended in full MSC medium containing 5% DMSO, and frozen. Day 2 samples were also fixed (PBS-buffered 10% formalin, 15 min) and stained for PECAM using the Blood Vessel Staining Kit (Millipore, Billerica, MA) per the manufacturer’s instructions. At the completion of the experiment, the frozen cells were thawed and washed and resuspended in FACS wash (1% BSA in PBS) at 5×106 cells/ml. The DiI-labeled MSCs from each sample were then FACS-sorted using a three-laser Dako MoFlo high-speed sorter. RNA isolated from the DiI-labeled MSCs from both the heterogeneous and homogeneous co-cultures were analyzed via real-time RT-PCR for expression of EC and SMC markers.
Figure 1.
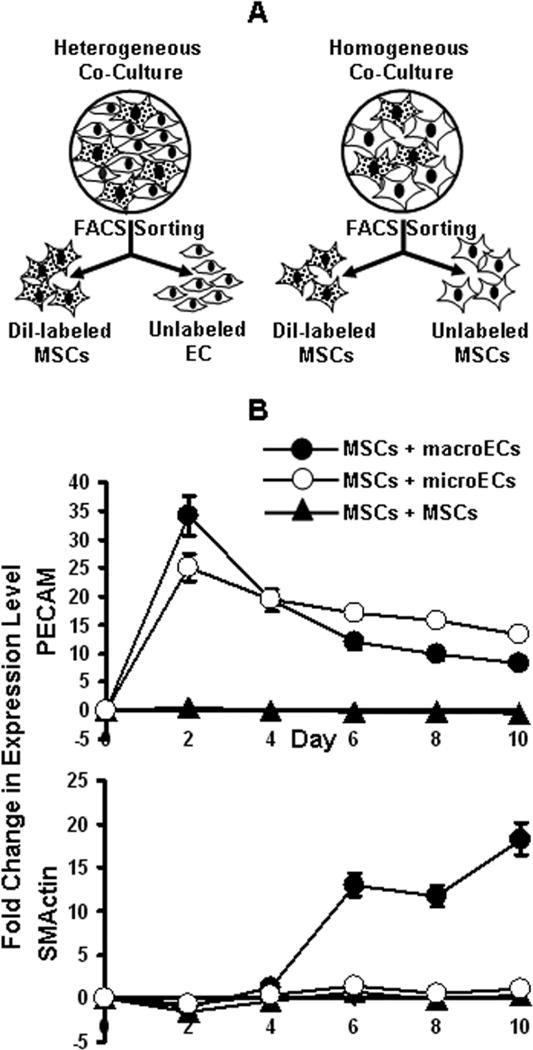
Direct co-culture of MSCs and ECs. (A) DiI-labeled MSCs co-cultured with unlabeled ECs (heterogeneous) or MSCs (homogeneous) were isolated via FACS sorting every 2 days. (B) Real-time RT-PCR analysis of PECAM and SMActin expression by MSCs isolated from co-cultures with (●) macroECs, (○) microECs, or (▲) MSCs. Error bars represent 95% confidence-intervals.
Indirect MSC:EC co-cultures
MSCs were seeded in the wells of tissue-culture plastic (TCP) 6-well dishes. In heterogeneous indirect co-cultures, ECs were seeded in overhanging Transwell inserts (0.4 μm pore membrane) (Corning, Corning, NY) in 2:1 ratios to the MSCs in the wells beneath. As controls, homogenous indirect co-cultures of MSCs were seeded in the Transwell inserts in 2:1 ratios to the MSCs in the bottom wells (Figure 3A). The indirect co-cultures were maintained in EC medium (1% serum) for 10 days. Every two days MSC RNA samples were taken from both types of indirect co-cultures and analyzed via real-time RT-PCR for expression of EC and SMC markers.
Figure 3.
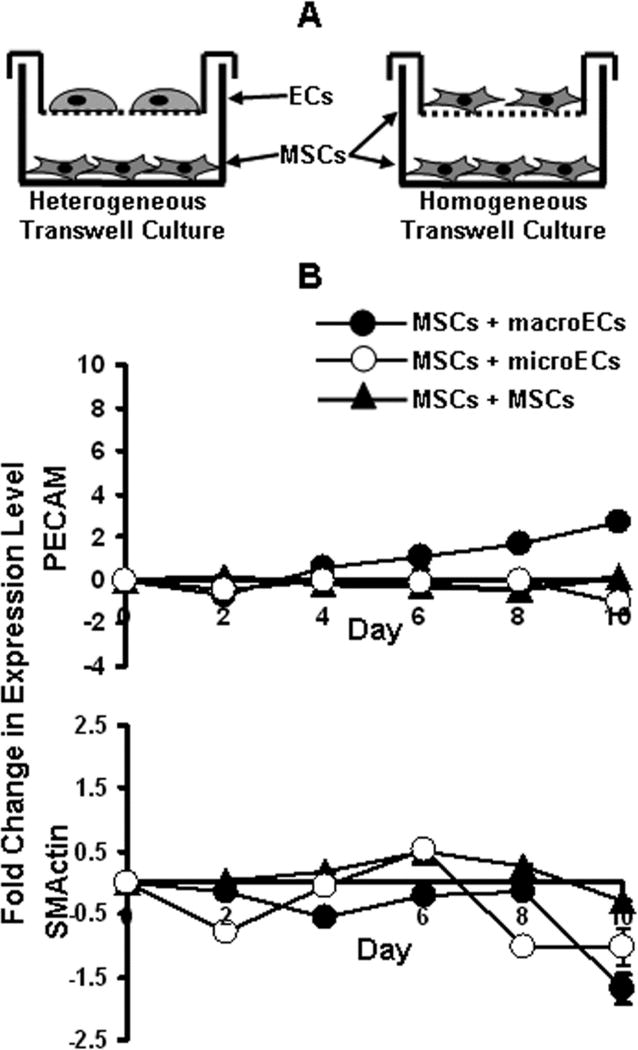
Indirect co-culture of MSCs and ECs. (A) MSCs were indirectly co-cultured with ECs (heterogeneous) or MSCs (homogeneous) using the Transwell culture system. (B) Real-time RT-PCR analysis of PECAM and SMActin expression for MSCs indirectly co-cultured with (●) macroECs, (○) microECs, or (▲) MSCs over 10 days. Error bars represent 95% confidence intervals.
Matrix cultures
MacroEC-matrix and microEC-matrix were derived from macroECs and microECs, respectively, using the following method adapted from Gospodarowicz et al (1983) (Figure 4A) [Gospodarowicz et al., 1983]. EC monolayer cultures were seeded on TCP at a density of 2×104 cells/cm2, cultured for 3 days in full EC medium, washed with HBSS, and cultured for additional 2, 4, 7, or 10 days in SF EC medium. After culture, the cells were lysed for 15 min in H2O, washed with 0.02 M NH4OH in H2O to remove remaining cell debris, and washed 3–6 times with PBS. The resulting surface material, referred to here as EC-matrix, was seeded with MSCs at 1×104 cells/cm2, followed by culture in SF MSC medium for 10 days. MSCs seeded on TCP were used as controls. MSC RNA samples were collected every 2 days and analyzed via real-time RT-PCR for expression of EC and SMC markers. In addition, every 2 days samples were formalin fixed and stained for PECAM expression.
Figure 4.
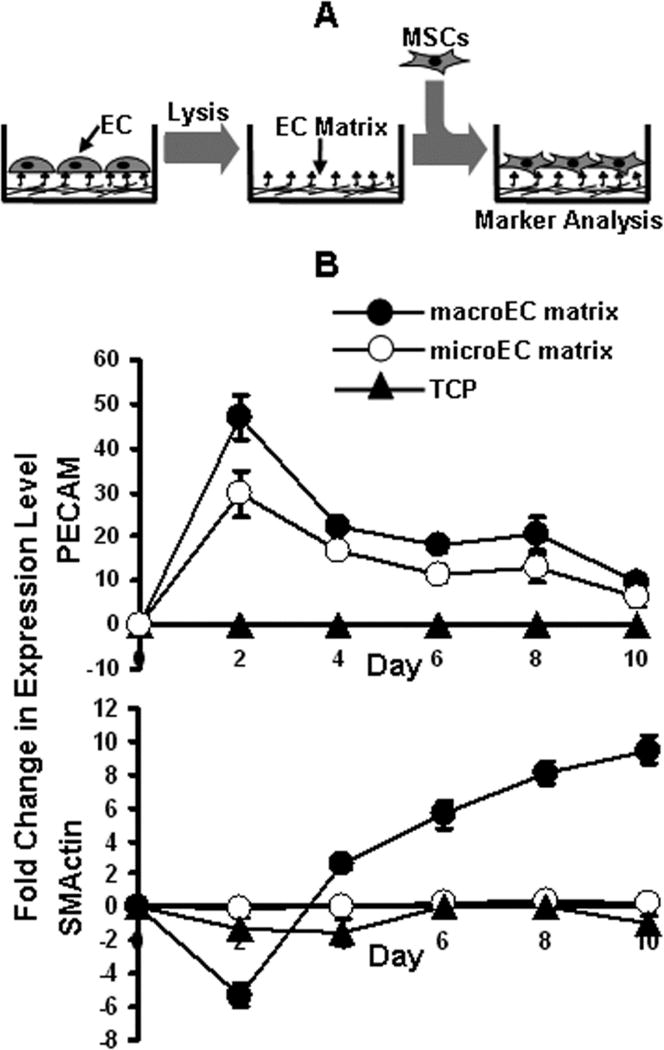
Direct culture of MSCs and EC-matrix. (A) MSCs were directly cultured on de-cellularized EC-matrixes after ECs had been removed via hypotonic lysis in water. (B) Real-time RT-PCR analysis of PECAM and SMActin expression by MSCs cultured on (●) macroEC-matrix, (○) microEC-matrix, or (▲) TCP over 10 days. Error bars represent 95% confidence intervals.
MSC incorporation into EC tubes on Matrigel
MSCs were cultured on either macroEC-matrix or TCP for 10 days, removed, and labeled with CM-DiI (See Direct MSC:EC Co-Cultures section). Meanwhile, two groups of TCP dishes were coated with Matrigel (BD Biosciences, San Jose, CA) according to the manufacturer’s ‘thin gel’ protocol. For one group, macroECs were seeded on Matrigel and cultured for 24 hours in full EC media during which ECs formed a tubular network. DiI-labeled MSCs from either macroEC-matrix or TCP were then seeded on top of the EC tubes and cultured for 24 hours. For the other group of Matrigel-coated plates, DiI-labeled MSCs from either macroEC-matrix or TCP were mixed with macroECs in 1:2 MSC:EC ratios, seeded on the Matrigel, and cultured for 24 hours. Thus, at the completion of the experiment, 4 experimental groups existed: (1) MSCs cultured on TCP, removed, and seeded on Matrigel with pre-formed macroEC tubes; (2) MSCs cultured on macroEC-matrix, removed, and seeded on Matrigel with pre-formed macroEC tubes, (3) MSCs cultured on TCP, removed, mixed with macroECs, and seeded on Matrigel; (4) MSCs cultured on macroEC-matrix, removed, mixed with macroECs, and seeded on Matrigel. Samples of all four groups were formalin fixed, stained with DAPI (Invitrogen, Carlsbad, CA) (1 μg/ml in PBS, 1 min), and imaged at 20× with a Leica fluorescence microscope under brightfield, blue fluorescence, and red fluorescence. The number of orange DiI-labeled MSCs that associated with the blue EC tubes was counted and averaged over 10 regions for each of the four groups.
Subcellular cytosolic and cytoskeletal protein isolation and western blotting
Two groups of MSCs were cultured on either macroEC-matrix or TCP for 10 days. At days 0, 2, 5, and 10, cytosolic and cytoskeletal proteins were isolated from both groups using the Compartment Protein Extraction Kit (Millipore, Billerica, MA) and analyzed for SMActin and smoothelin via western blotting for SMActin and smoothelin expression. Briefly, 5 μg samples of cytosolic and cytoskeletal proteins were resolved using 10% (SMActin) or 8% (smoothelin) SDS-PAGE, transferred to Immobilon-FL PVDF membranes (Millipore, Billerica, MA), and probed with either anti-SMActin (Millipore, Billerica, MA) or anti-smoothelin (Millipore, Billerica, MA) primary antibodies followed by AlexaFluor 488-conjugated secondary antibodies (Invitrogen, Carlsbad, CA). The blots were imaged based on green fluorescence with a Molecular Dynamics Typhoon 9410 Variable Mode Imager.
Growth factor treatments
MSC cultures were cultured in SF MSC medium supplemented with either 0, 25 or 50 ng/ml human VEGF121 (Sigma, St. Louis, MO) or 0, 5, 25, 50 ng/ml human EGF (Sigma, St. Louis, MO) for 10 days. RNA samples were isolated every 2 days for analysis of EC and MSC marker expression.
ECM molecule-coated surfaces
TCP was coated with either laminin (Millipore, Billerica, MA) (2 μg/cm2), collagen type IV (Millipore, Billerica, MA) (10 μg/cm2), a 5:1 mix of laminin and collagen type IV (12 μg/cm2) or fibronectin (Millipore, Billerica, MA) (5 μg/cm2) overnight at 4°C. The surfaces were then washed once with HBSS. MSCs were seeded on the surfaces at 1×104 cells/cm2 and cultured in SF MSC medium for 10 days. Every 10 days, samples were taken for analysis of MSC mRNA expression of EC and SMC markers.
Results
Direct co-culture
DiI-labeled MSCs and unlabeled ECs were directly co-cultured in 1:2 ratios of cell number for 10 days. Homogeneous co-cultures of DiI-labeled MSCs and unlabelled MSCs served as controls (Figure 1A). During the time course, samples of the co-cultures were collected and FACS-sorted, and the DiI-labeled MSCs were separated from the un-labeled cells. Real-time RT-PCR analysis of MSCs directly co-cultured with macroECs revealed increased mRNA expression of the EC markers and of the SMC markers tested compared to controls (Figure 1B). PECAM immunohistochemistry at day 2 also revealed elevated PECAM levels in MSCs directly co-cultured with macroECs (Figure 2). However, distinct differences between the temporal mRNA expression profiles of the EC and SMC markers were observed. Expression of EC markers sharply increased early during co-culture, peaking by day two, but then sharply decreased and remained only slightly elevated for the remainder of the co-culture. Conversely, expression of SMC markers was delayed, beginning at day 4 and then steadily increasing over the remainder of the 10-day co-culture. MSCs directly co-cultured with microECs exhibited increased mRNA expression of EC markers, but did not show increased mRNA expression of the SMC markers tested (Figure 1B).
Figure 2.
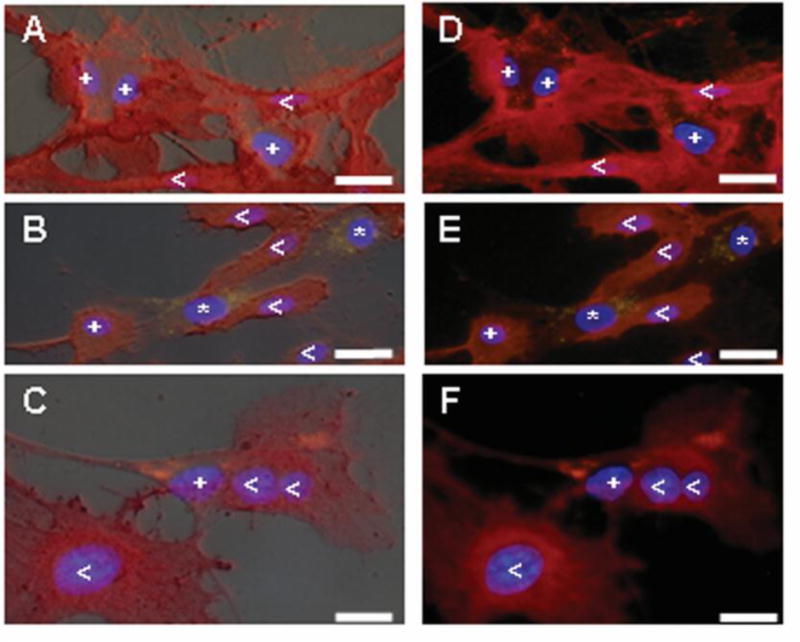
DiI-labeled MSCs co-cultured with macroECs for 2 days were stained with for PECAM expression (TRITC/red). Thus, (<) PECAM-positive ECs appear dark red, (*) PECAM-negative MSCs exhibit yellow punctations, and (+) PECAM-positive MSCs appear red with orange punctations. Blue = DAPI. Images A–C are merged with the corresponding bright-field image. (Bar = 10 μm)
Indirect co-culture
MSCs and either macroECs or microECs were indirectly co-cultured for 10 days using the Transwell system. MSCs indirectly co-cultured with MSCs served as controls (Figure 3A). MSCs indirectly co-cultured with macroECs exhibited only slight enhancement of the EC markers and no increases in the SMC markers tested (Figure 3B). The temporal profiles of these increases occurred gradually over the duration of the experiment, reaching its peak at day 10. This peak value was 10-fold lower than the peak value observed with the direct co-culture experiment. Similarly, MSCs exposed to macroEC-conditioned medium (data not shown) or indirectly co-cultured with micoECs (Figure 3B) did not exhibit increases in either EC or SMC marker expression.
EC-matrix culture
Effects on vascular markers expression
MSCs cultured on de-cellularized macroEC-matrix exhibited enhanced mRNA expression of the EC markers tested compared to control groups cultured on TCP (Figure 4B). The temporal expression profiles of these EC markers closely matched those observed in response to direct co-culture with macroECs; MSCs cultured on macroEC-matrix exhibited sharp increases in mRNA expression of the EC markers. However, these heightened expression levels were not maintained and were followed immediately by sharp decreases. These trends were confirmed with PECAM-staining; more PECAM-positive cells were observed on native matrix for early time points than later during the experiment (data not shown). Culture on EC-matrix also increased mRNA expression of the SMC markers tested (Figure 4B). Again, the temporal expression profiles of SMC markers supported by culture on EC-matrix was similar to those observed in response to direct co-culture with ECs, i.e., the increases in SMC marker expression began later than those observed for the EC markers. Furthermore, the SMC marker expression profiles were more gradual and consistent, absent of the peaks and valleys characteristic of the EC marker curves. Finally, when compared to SMC and EC expression levels in control groups of MSCs cultured on TCP, the magnitude of the increases in SMC marker expression were less substantial than those observed for EC markers.
MSCs cultured on de-cellularized microEC-matrix also exhibited similar trends to those observed in MSCs directly co-cultured with macroECs; microEC-matrix caused increases in EC, but not SMC, marker expression in MSCs (Figure 4B).
Effects on incorporation into macroEC tubes on Matrigel
We next examined whether exposure to macroEC-matrix induced MSCs to incorporate into EC tubes that formed on Matrigel. MSCs cultured on either macroEC-matrix or TCP for 10 days were removed, mixed with macroECs, and seeded on Matrigel. EC formed thin tubular networks on Matrigel, while MSCs previously plated on TCP tended to form bulkier aggregations. MSCs removed from macroEC-matrix exhibited improved incorporation into the EC tubes when mixed with ECs and cultured on Matrigel (Figure 5).
Figure 5.
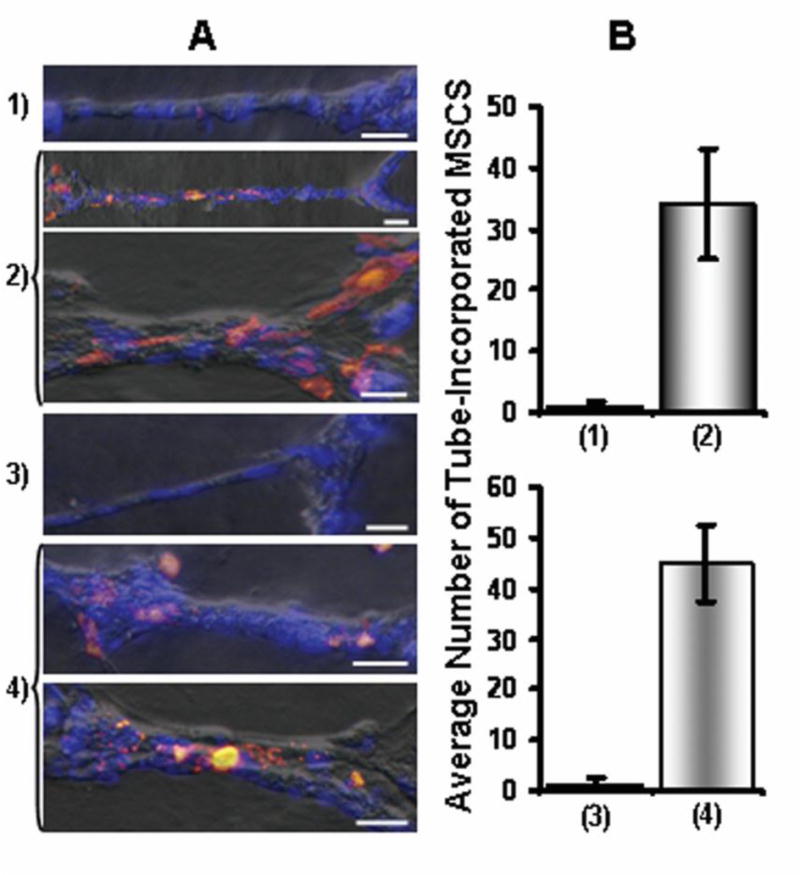
(A) DiI-MSCs (orange) pre-cultured on TCP (1,3) or macroEC-matrix (2,4) were mixed with macroECs and seeded on Matrigel (1,2) or seeded directly on pre-formed EC-tubes (3,4). Blue = DAPI (Bar = 5μm) (B) Averages of tube-incorporated MSCs for the four groups.
This experiment was repeated but, instead of mixing MSCs with ECs before seeding on Matrigel, MSCs were seeded on pre-formed EC tubes. Here, too, MSCs that had been cultured on EC-matrix for 10 days showed greater tube-incorporation than MSCs cultured on TCP (Figure 5).
Effects on subcellular localization of SMC-associated cytoskeletal contractile elements
Cytosolic and cytoskeletal fractions of MSCs cultured on either EC-matrix or TCP for 0, 2, 5, or 10 days were analyzed via western blots for SMActin and smoothelin, cytoskeletal proteins expressed by SMCs (Figure 6). Both SMActin and smoothelin levels increased over time in MSCs cultured on EC-matrix. MSCs cultured on TCP, on the other hand, did not show any changes over the 10-day culture (data not shown). Following 10 days of culture on EC-matrix, both SMActin and smoothelin were largely associated with the cytoskeleton, suggesting the development of a functional SMC phenotype. Furthermore, by day 5, MSC smoothelin expression was predominantly of the vascular SMC isoform that, again, localized to the cytoskeleton (smoothelin exists in two isoforms, a 100 kDa protein found in vascular SMCs and a 59 kDa form specific for visceral SMCs [van der Loop et al., 1997]). Since vascular smoothelin has been shown to be essential for vascular SMC contractility [van der Loop et al., 1997], these findings suggest that MSCs cultured on EC-matrix were specifically differentiating towards a functional vascular SMC phonotype.
Figure 6.
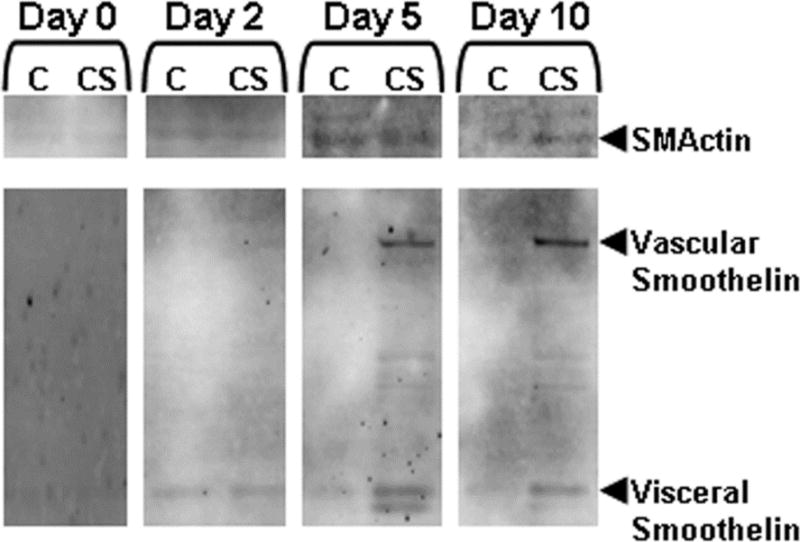
Western blot analysis of SMActin and smoothelin expression and localization to cytosolic (Cy) and cytoskeletal (Cs) compartments in MSCs cultured on EC-matrix over 10 days.
Effects of altering EC matrix production parameters
Culture time of macroECs
Comparison of macroEC-matrix samples collected over 2, 4, 7 or 10 days of macroEC culture showed that EC-matrix-induced enhancement of EC markers in MSCs was culture time-dependent; the longer the time over which the matrix was produced, the more the matrix increased EC marker expression in MSCs (Figure 7A). On the other hand, the ability of EC-matrix to support enhancement of SMC marker expression was not dependent on culture time (Figure 7A).
Figure 7.
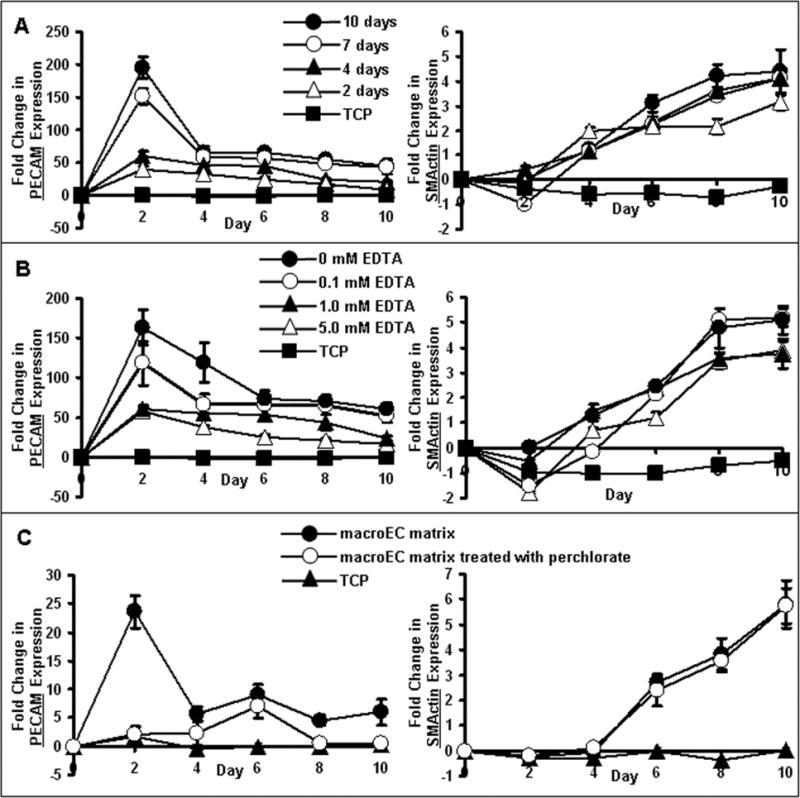
Real-time RT-PCR analysis of MSC expression of PECAM and SMActin in response to culture on (A) EC-matrix produced from macroECs over 2–10 days, (B) EC-matrix washed with/without EDTA, or (C) EC-matrix produced from macroECs cultured with/without 40 mM perchlorate. MSCs cultured on TCP served as controls. Error bars represent 95% confidence intervals.
EDTA extraction
EC-matrixes produced from macroECs cultured on TCP for 7 days and then washed with varying EDTA concentrations (0, 0.1, 1, and 5 mM) were compared for their effects on MSC expression of EC and SMC markers (Figure 7B). Washing with EDTA reduced EC-matrix-enhancement of EC marker expression in MSCs in a concentration-dependent manner (Figure 7B); higher EDTA concentrations resulted in larger drops in EC marker-increases. In contrast, EDTA wash did not affect the ability of the EC-matrix to support increased SMC marker expression in MSCs (Figure 7B).
Perchlorate sensitivity
To investigate whether sulfated glycosaminoglycan components of the EC-matrix were required for its activity, EC-matrixes were produced from EC cultures treated with 40 mM sodium perchlorate, a competitive inhibitor of glycosaminoglycan sulfation [Lin et al., 2000]. MSCs cultured on perchlorate-treated EC-matrixes did not exhibit increased expression of EC markers (Figure 7C), while perchlorate did not affect the ability of EC-matrix to support increases in SMC marker expression in MSCs (Figure 7C).
Lack of effects of VEGF treatment and culture on laminin, collagen type IV or fibronectin
To assess the nature of the active components of EC-matrix, we tested the effects of various growth factors and matrix molecules on MSC expression of vascular markers. Control groups were cultured on uncoated TCP in the absence of VEGF. Experimental groups were cultured either in the presence of VEGF (5, 25 or 50 ng/ml) or on TCP coated with laminin, collagen type IV, a mixture of laminin and collagen IV, or fibronectin. None of the experimental groups enhanced MSC expression of EC or SMC markers compared to controls (data not shown).
Effect of EGF
We next tested the effects of EGF on MSC expression of vascular cell markers. EGF has previously been shown to mediate EC-pericyte interactions, and EGF receptor is one of the most abundant growth factor receptors found on MSCs [Bianco et al., 2001]. Upon culture for 7 days with 0, 5, 25, or 50 ng/ml EGF in SF medium, MSCs exhibited increased expression of the SMC markers SMActin and smoothelin and decreased expression of EC markers PECAM and VECAD (Figure 8).
Figure 8.

Real-time RT-PCR analysis of the effects of 0, 5, 25 or 50 ng/ml EGF on MSC expression of the EC markers PECAM and VECAD and the SMC markers SMActin and smoothelin. Results for each condition are presented as fold changes compared to the 0 ng/ml EGF controls. Error bars represent 95% confidence intervals.
Discussion
Signals from the local environment, either through cell-cell contact, soluble factors, or cell-matrix interactions, profoundly influence MSC differentiation. In fact, this influence often tends to drive MSC differentiation in a tissue-specific manner [Chiu et al., 1995; Philp et al., 2005]. Residing in a perivascular niche, MSCs interact with the local vascular environment, including ECs and EC-derived differentiation signals. In this study, we used ECs to experimentally recreate and analyze the differentiation signals that MSCs would be exposed to in their perivascular niche. We hypothesized that vascular signals would promote MSC differentiation toward the vascular lineages, specifically ECs and SMCs.
In our first set of experiments, MSC differentiation was examined in direct EC-MSC co-cultures. In this set-up, the three sources of EC-derived signals – EC-matrix, EC-secreted soluble factors, and EC-MSC contact – were combined. The results support our hypothesis in that interacting with macroECs caused increased MSC expression of both EC and SMC markers. To study whether the type of EC affected vascular differentiation in MSCs, direct co-culture with microECs was also tested. Interacting with microECs caused increases in EC marker expression only. These results support the tissue-specific trend of MSC differentiation, i.e., macroECs, which interact with SMCs as part of macrovessels in vivo, support MSC differentiation into both ECs and SMCs. MicroECs, which originate from capillaries and do not interact with SMCs, support EC-differentiation only.
To better characterize the effects of ECs on MSC vascular cell differentiation, we deconstructed the local environment created by direct co-culture with ECs into its components, i.e., soluble EC factors and EC-matrix, and tested their effects on MSC expression of vascular cell markers. While soluble EC factors did not affect marker expression, EC-matrix did. In fact, macroEC-matrix alone recreated the temporal characteristics (un-sustained early peak in EC markers, sustained later increases in SMC markers) encountered in macroEC:MSC co-cultures. Repeating these matrix experiments with microEC-matrix, we observed the same differences between macro- and microEC-matrix that we reported for macro- and microECs in the direct co-culture studies; MSCs cultured on macroEC-matrix underwent both EC and SMC-differentiation, while MSCs cultured on microEC-matrix exhibited EC-differentiation only. That the trends observed with the different co-cultures were translated to the different matrices indicated fundamental differences between macro- and micro-EC-matrix compositions.
Our study also linked increased MSC expression of vascular cell markers with the development of functional vascular cell phenotypes. MSCs cultured on EC-matrix exhibited subcellular cytoskeletal protein distributions similar to those of vascular SMCs and improved incorporation into EC tubes on Matrigel. While several studies have used similar functional phenotypes to characterize MSCs and MSC-like cells [Ball et al., 2004; Traktuev et al., 2008], this is the first study to assess the effects of matrix.
We have also characterized the components of EC-matrix responsible for its effects on MSC vascular differentiation. The divergent effects of culture time, EDTA wash, and perchlorate treatment on EC and SMC-differentiation suggest that at least two types of factors are involved in the bioactivity of EC-matrix. EC-differentiation factors are secreted by ECs and accumulate in EC-matrix. Their association with EC-matrix is Ca2+-dependent and is likely mediated via sulfated proteoglycans such as HSPGs. Previous studies have reported that HSPGs contained in EC-matrix binds many bioactive factors, including VEGF, PDGF, and FGF [Kalluri, 2003]. Thus, we propose that the EC-differentiation factor(s) is either an HSPG or a growth factor that binds HSPGs contained within EC-matrix. Other studies linked the differentiation capabilities of vascular matrixes with matrix-bound factors rather than structural components [Philp et al., 2005; Vukicevic et al., 1992]. Similarly, our study showed that, when applied alone, EC-matrix components (laminin, collagen IV, and fibronectin) did not re-create the vascular differentiation effects on MSCs, indicating that a growth factor was responsible. One of the growth factors tested here, VEGF, has been reported to differentiate MSCs into ECs [Oswald et al., 2004]. However, we were unable to replicate these results with the MSC populations and VEGF concentrations tested, suggesting either that VEGF is not the EC-differentiation factor, or that its EC-differentiation activity requires EC-matrix. Many soluble growth factors that bind EC-matrix require HSPGs to interact with their cell-surface receptors for bioactivity [Cohen et al., 1995; Ruoslahti et al., 1991]. Our observation that EC-matrix, but not EC-secreted soluble factors without EC-matrix, induces MSC vascular differentiation suggests that some of these factors require association with EC-matrix to function. We will test this by assaying the activities of the EC-secreted factors in the presence of EC-matrix (or tethered to a surface).
In contrast to the EC-differentiation factors, the matrix-associated SMC-differentiation factors are not affected by EC culture time/EDTA wash/perchlorate treatment, suggesting that their incorporation into EC-matrix does not involve Ca2+ or sulfated proteoglycans. We reasoned, therefore, that the SMC-differentiation factors are structural matrix proteins. However, neither laminin, collagen IV, nor fibronectin induced SMC-differentiation in MSCs. Because a number of structural matrix proteins contain EGF-repeats and interact with cell-surface EGF receptors (EGFRs) [Schenk et al., 2003], and EGFR-mediated signaling is reported to influence MSC differentiation [Tamama et al., 2006], we directly tested the effects of EGF on MSC expression of EC and SMC markers. EGF slightly suppresses expression of EC markers and slightly enhances expression of SMC markers. While these expressional changes were not significant compared to those induced by EC-matrix, they suggest a possible link between SMC-differentiation factors and EGFR-binding/activation. In addition, tethering of the factor(s) to a surface may also enhance biological activity.
In summary, we have shown here that EC-matrix supports MSC differentiation toward EC and SMC lineages. The EC-differentiation factors are likely EC-secreted factors that bind EC-matrix, whereas the SMC-differentiation factors may signal via EGFR. Micro and macroECs differ in the matrices they produce, which in turn affects their influences on MSC differentiation. Further understanding of the roles of EC-matrix and the perivascular niche in regulating MSC differentiation toward the vascular cell lineages has implications in cell-based vascular tissue engineering and regeneration.
Acknowledgments
We gratefully acknowledge James M. Simone of the NIAMS Flow Cytometry Section for his assistance in FACS sorting, and Dr. Paul A. Manner (University of Washington) for providing human clinical specimens.
Contract grant number: NIAMS IRP Z01AR41131.
Footnotes
This manuscript is Part 1 of 2. Part 2 is continued in a manuscript entitled ‘Mesenchymal Stem Cell Modification of Endothelial Matrix Regulates Their Vascular Differentiation’, which has also been submitted to the Journal of Cellular Biochemistry.
References
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Ball SG, Shuttleworth AC, Kielty CM. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol. 2004;36:714–727. doi: 10.1016/j.biocel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Bradham DM, Passaniti A, Horton WE., Jr Mesenchymal cell chondrogenesis is stimulated by basement membrane matrix and inhibited by age-associated factors. Matrix Biol. 1995;14:561–571. doi: 10.1016/s0945-053x(05)80005-8. [DOI] [PubMed] [Google Scholar]
- Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg. 1995;60:12–18. [PubMed] [Google Scholar]
- Choi KS, Shin JS, Lee JJ, Kim YS, Kim SB, Kim CW. In vitro trans-differentiation of rat mesenchymal cells into insulin-producing cells by rat pancreatic extract. Biochem Biophys Res Commun. 2005;330:1299–1305. doi: 10.1016/j.bbrc.2005.03.111. [DOI] [PubMed] [Google Scholar]
- Cohen T, Gitay-Goren H, Sharon R, Shibuya M, Halaban R, Levi BZ, Neufeld G. VEGF121, a vascular endothelial growth factor (VEGF) isoform lacking heparin binding ability, requires cell-surface heparan sulfates for efficient binding to the VEGF receptors of human melanoma cells. J Biol Chem. 1995;270:11322–11326. doi: 10.1074/jbc.270.19.11322. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D, Gonzalez R, Fujii DK. Are factors originating from serum, plasma, or cultured cells involved in the growth-promoting effect of the extracellular matrix produced by cultured bovine corneal endothelial cells? J Cell Physiol. 1983;114:191–202. doi: 10.1002/jcp.1041140208. [DOI] [PubMed] [Google Scholar]
- Gruber R, Kandler B, Holzmann P, Vogele-Kadletz M, Losert U, Fischer MB, Watzek G. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 2005;11:896–903. doi: 10.1089/ten.2005.11.896. [DOI] [PubMed] [Google Scholar]
- Hashimoto J, Kariya Y, Miyazaki K. Regulation of proliferation and chondrogenic differentiation of human mesenchymal stem cells by laminin-5 (laminin-332) Stem Cells. 2006;24:2346–2354. doi: 10.1634/stemcells.2005-0605. [DOI] [PubMed] [Google Scholar]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J Gastroenterol. 2005;11:4497–4504. doi: 10.3748/wjg.v11.i29.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Role of heparan sulfate proteoglycans in cell-cell signaling in Drosophila. Matrix Biol. 2000;19:303–307. doi: 10.1016/s0945-053x(00)00073-1. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288–296. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to Vitronectin and Collagen I Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- van der Loop FT, Gabbiani G, Kohnen G, Ramaekers FC, van Eys GJ. Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vasc Biol. 1997;17:665–671. doi: 10.1161/01.atv.17.4.665. [DOI] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- Xu Y, Swerlick RA, Sepp N, Bosse D, Ades EW, Lawley TJ. Characterization of expression and modulation of cell adhesion molecules on an immortalized human dermal microvascular endothelial cell line (HMEC-1) J Invest Dermatol. 1994;102:833–837. doi: 10.1111/1523-1747.ep12382086. [DOI] [PubMed] [Google Scholar]


