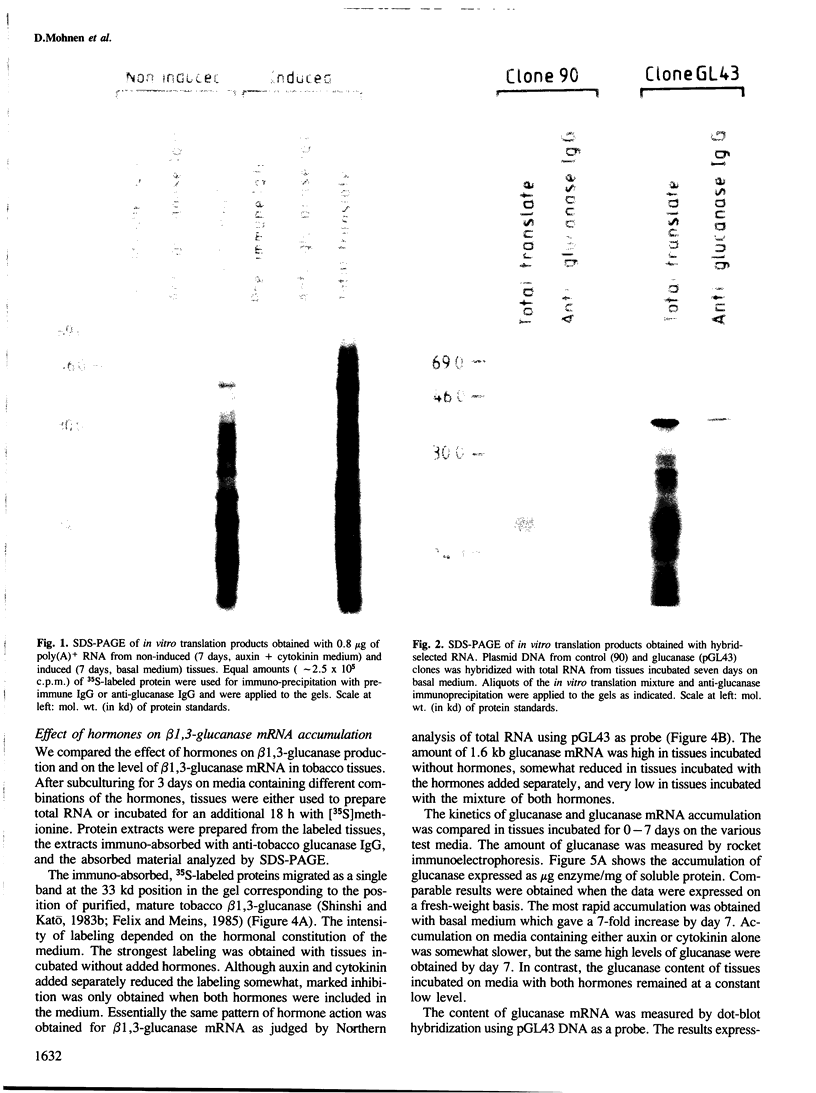
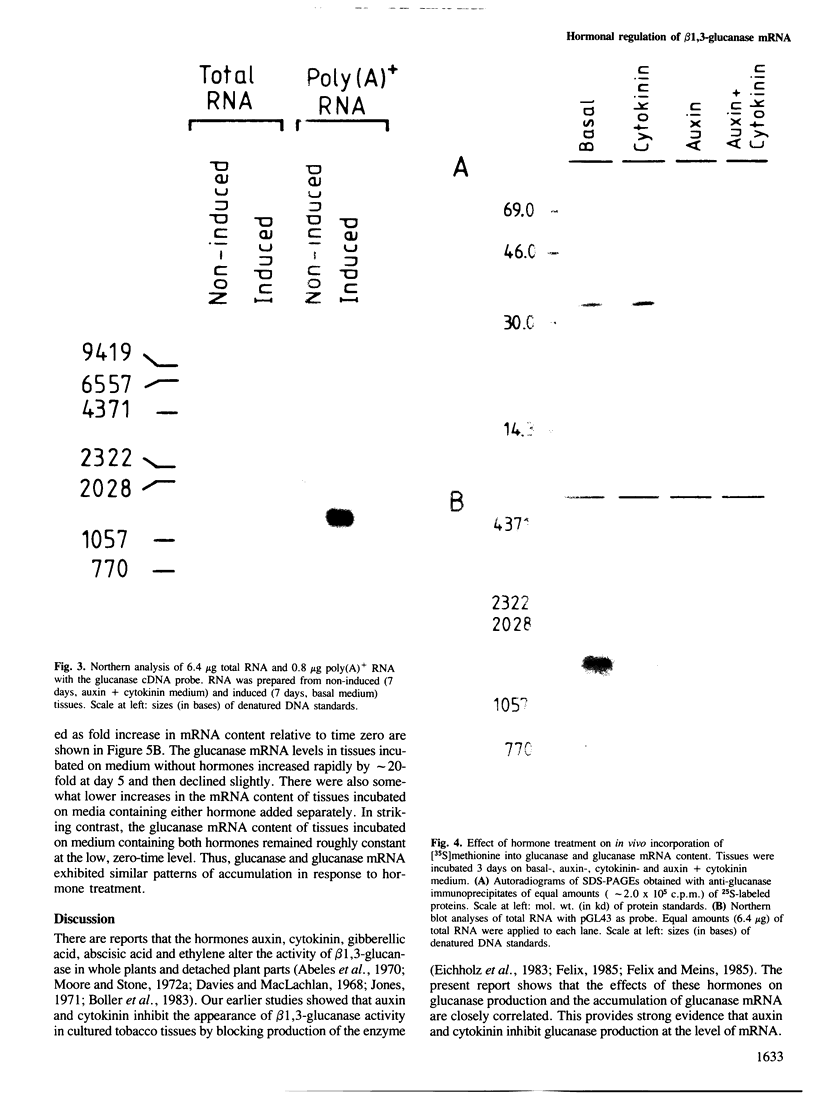
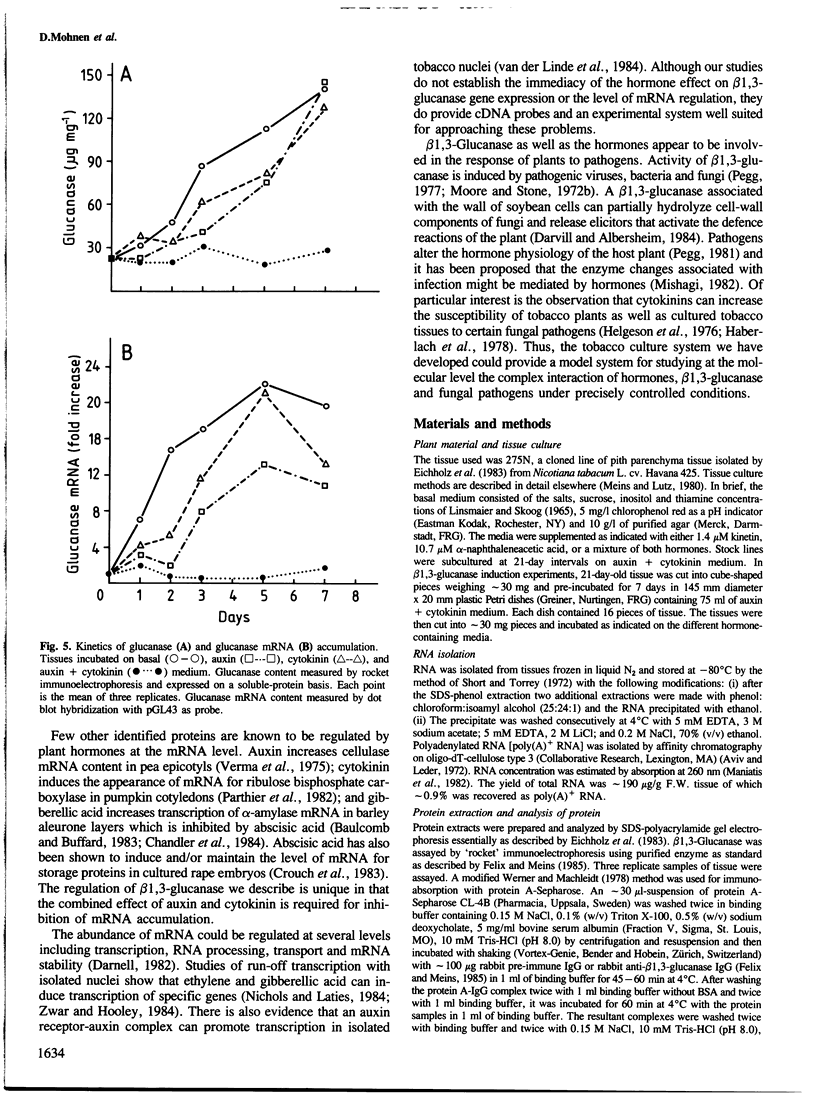
Abstract
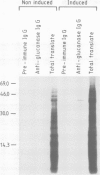
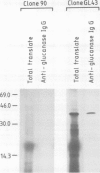
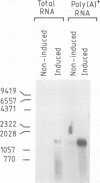
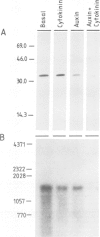
We describe the isolation of a cDNA clone of β1,3-glucanase mRNA from Nicotiana tabacum L. cv. `Havana 425' and its use to measure the kinetics of mRNA accumulation in cultured tobacco tissues treated with the plant hormones auxin and cytokinin. Northern blot analysis showed that the tissues contain a single ˜1.6 kb-sized β1,3-glucanase mRNA. The levels of β1,3-glucanase and β1,3-glucanase mRNA increase by up to seven- and 20-fold, respectively, over a 7-day period in tissues subcultured on hormone-free medium and medium containing auxin or cytokinin added separately. Over the same interval of time, the content of both the enzyme and its mRNA remains at a constant low level in tissues subcultured on medium containing both auxin and cytokinin. The results show that auxin and cytokinin block β1,3-glucanase production at the level of the mRNA.
Keywords: auxin; cytokinin; β1,3-glucanase; cDNA clone; Nicotiana tabacum (tissue culture)
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Forrence L. E. Temporal and hormonal control of beta-1,3-glucanase in Phaseolus vulgaris L. Plant Physiol. 1970 Apr;45(4):395–400. doi: 10.1104/pp.45.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Cochet M., Perrin F., Gannon F., Krust A., Chambon P., McKnight G. S., Lee D. C., Mayo K. E., Palmiter R. Cloning of an almost full-length chicken conalbumin double-stranded cDNA. Nucleic Acids Res. 1979 Jun 11;6(7):2435–2452. doi: 10.1093/nar/6.7.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch M. L., Tenbarge K. M., Simon A. E., Ferl R. cDNA clones for Brassica napus seed storage proteins: evidence from nucleotide sequence analysis that both subunits of napin are cleaved from a precursor polypeptide. J Mol Appl Genet. 1983;2(3):273–283. [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Davies E., Maclachlan G. A. Effects of indoleacetic acid on intracellular distribution of beta-glucanase activities in the pea epicotyl. Arch Biochem Biophys. 1968 Dec;128(3):595–600. doi: 10.1016/0003-9861(68)90068-4. [DOI] [PubMed] [Google Scholar]
- Haberlach G. T., Budde A. D., Sequeira L., Helgeson J. P. Modification of disease resistance of tobacco callus tissues by cytokinins. Plant Physiol. 1978 Oct;62(4):522–525. doi: 10.1104/pp.62.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L. Gibberellic Acid-enhanced Release of beta-1,3-Glucanase from Barley Aleurone Cells. Plant Physiol. 1971 Mar;47(3):412–416. doi: 10.1104/pp.47.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Rapid Metabolic Changes in the Wounding Response of Leaf Discs following Excision. Plant Physiol. 1976 Jan;57(1):80–84. doi: 10.1104/pp.57.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Aspart L., Chartier Y. Auxin-Induced Regulation of Protein Synthesis in Tobacco Mesophyll Protoplasts Cultivated In Vitro: II. Time Course and Level of Auxin Control. Plant Physiol. 1984 Aug;75(4):1034–1039. doi: 10.1104/pp.75.4.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. E., Stone B. A. Effect of infection with TMV and other viruses on the level of a -1,3-glucan hydrolase in leaves of Nicotiana glutinosa. Virology. 1972 Dec;50(3):791–798. doi: 10.1016/0042-6822(72)90433-3. [DOI] [PubMed] [Google Scholar]
- Riezman H., Hase T., van Loon A. P., Grivell L. A., Suda K., Schatz G. Import of proteins into mitochondria: a 70 kilodalton outer membrane protein with a large carboxy-terminal deletion is still transported to the outer membrane. EMBO J. 1983;2(12):2161–2168. doi: 10.1002/j.1460-2075.1983.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Hay R., Witte C., Nelson N., Schatz G. Yeast mitochondrial outer membrane specifically binds cytoplasmically-synthesized precursors of mitochondrial proteins. EMBO J. 1983;2(7):1113–1118. doi: 10.1002/j.1460-2075.1983.tb01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- Short K. C., Torrey J. G. Cytokinins in seedling roots of pea. Plant Physiol. 1972 Feb;49(2):155–160. doi: 10.1104/pp.49.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]
- Werner S., Machleidt W. Isolation of precursors of cytochrome oxidase from Neurospora crassa: application of subunit-specific antibodies and protein A from Staphylococcus aureus. Eur J Biochem. 1978 Sep 15;90(1):99–105. doi: 10.1111/j.1432-1033.1978.tb12579.x. [DOI] [PubMed] [Google Scholar]