Abstract
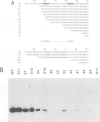
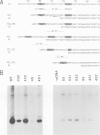
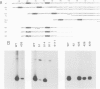
A transcription extract from purified spinach chloroplast was used to test chloroplast DNA sequences for their function as promoter elements. Chloroplast tRNA genes are correctly transcribed in the extract by a soluble RNA polymerase, and precursor molecules are processed into mature tRNAs. Transcription of the spinach chloroplast tRNA2Met gene (trnM2) in vitro requires 5' upstream DNA sequences. Deletion of 5' DNA sequences with exonuclease Bal31 was used to establish the 5' boundary of the promoter region. This boundary is part of a DNA sequence with partial homology to the prokaryotic -35 region. Seventeen base pairs downstream from this sequence a DNA sequence occurs which is homologous to the prokaryotic -10 region. We used synthetic oligonucleotides fused to trnM2 5' deletion mutants to create insertions, deletions and base substitutions in these regions. Internal deletion mutants demonstrated that the -10 promoter element is also required for transcription in vitro. The arrangement of DNA sequences recognised by the chloroplast RNA polymerase resembles the prokaryotic promoter organization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley W., Smith H. J., Bogorad L. RNA polymerases of maize: partial purification and properties of the chloroplast enzyme. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2412–2416. doi: 10.1073/pnas.68.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat J. F., Laulhere J. P., Mache R. Transcription activity of a DNA-protein complex isolated from spinach plastids. Eur J Biochem. 1979 Jul;98(1):285–292. doi: 10.1111/j.1432-1033.1979.tb13187.x. [DOI] [PubMed] [Google Scholar]
- Briat J. F., Mache R. Properties and characterization of a spinach chloroplast RNA polymerase isolated from a tanscriptionally active DNA-protein complex. Eur J Biochem. 1980 Oct;111(2):503–509. doi: 10.1111/j.1432-1033.1980.tb04966.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deno H., Shinozaki K., Sugiura M. Nucleotide sequence of tobacco chloroplast gene for the alpha subunit of proton-translocating ATPase. Nucleic Acids Res. 1983 Apr 11;11(7):2185–2191. doi: 10.1093/nar/11.7.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesel A. J., Crouse E. J., Gordon K., Bohnert H. J., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Fractionation and identification of spinach chloroplast transfer RNAs and mapping of their genes on the restriction map of chloroplast DNA. Gene. 1979 Aug;6(4):285–306. doi: 10.1016/0378-1119(79)90070-2. [DOI] [PubMed] [Google Scholar]
- Dron M., Robreau G., Le Gal Y. Isolation of a chromoid from the chloroplast of Chlamydomonas reinhardi. Exp Cell Res. 1979 Mar 15;119(2):301–305. doi: 10.1016/0014-4827(79)90357-4. [DOI] [PubMed] [Google Scholar]
- Greenberg B. M., Narita J. O., DeLuca-Flaherty C., Gruissem W., Rushlow K. A., Hallick R. B. Evidence for two RNA polymerase activities in Euglena gracilis chloroplasts. J Biol Chem. 1984 Dec 10;259(23):14880–14887. [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Prescott D. M., Greenberg B. M., Hallick R. B. Transcription of E. coli and Euglena chloroplast tRNA gene clusters and processing of polycistronic transcripts in a HeLa cell-free system. Cell. 1982 Aug;30(1):81–92. doi: 10.1016/0092-8674(82)90014-9. [DOI] [PubMed] [Google Scholar]
- Hallick R. B., Lipper C., Richards O. C., Rutter W. J. Isolation of a transcriptionally active chromosome from chloroplasts of Euglena gracilis. Biochemistry. 1976 Jul 13;15(14):3039–3045. doi: 10.1021/bi00659a016. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan M. A., Dudock B. S. The gene for a spinach chloroplast isoleucine tRNA has a methionine anticodon. J Biol Chem. 1982 Oct 10;257(19):11191–11194. [PubMed] [Google Scholar]
- Krebbers E. T., Larrinua I. M., McIntosh L., Bogorad L. The maize chloroplast genes for the beta and epsilon subunits of the photosynthetic coupling factor CF1 are fused. Nucleic Acids Res. 1982 Aug 25;10(16):4985–5002. doi: 10.1093/nar/10.16.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G. DNA sequence requirements for the accurate transcription of a protein-coding plastid gene in a plastid in vitro system from mustard (Sinapis alba L.). EMBO J. 1984 Aug;3(8):1697–1704. doi: 10.1002/j.1460-2075.1984.tb02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982 Jun;29(2):537–550. doi: 10.1016/0092-8674(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Pirtle R., Calagan J., Pirtle I., Kashdan M., Vreman H., Dudock B. The nucleotide sequence of spinach chloroplast methionine elongator tRNA. Nucleic Acids Res. 1981 Jan 10;9(1):183–188. doi: 10.1093/nar/9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M., Jagendorf A. T. Wheat leaf RNA polymerases. I. Partial purification and characterization of nuclear, chloroplast and soluble DNA-dependent enzymes. Arch Biochem Biophys. 1971 Oct;146(2):635–648. doi: 10.1016/0003-9861(71)90172-x. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. Sequence of the intercistronic region between the ribulose-1, 5-bisphosphate carboxylase/oxygenase large subunit and coupling factor beta subunit gene. Nucleic Acids Res. 1982 Aug 25;10(16):4923–4934. doi: 10.1093/nar/10.16.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Smith H. J., Bogorad L. The polypeptide subunit structure of the DNA-dependent RNA polymerase of Zea mays chloroplasts. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4839–4842. doi: 10.1073/pnas.71.12.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Sugita M., Sugiura M. A putative gene of tobacco chloroplast coding for ribosomal protein similar to E. coli ribosomal protein S19. Nucleic Acids Res. 1983 Mar 25;11(6):1913–1918. doi: 10.1093/nar/11.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Westhoff P., Alt J., Herrmann R. G. Localization of the genes for the two chlorophyll a-conjugated polypeptides (mol. wt. 51 and 44 kd) of the photosystem II reaction center on the spinach plastid chromosome. EMBO J. 1983;2(12):2229–2237. doi: 10.1002/j.1460-2075.1983.tb01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6260–6264. doi: 10.1073/pnas.79.20.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Clegg M. T. The barley chloroplast DNA atpBE, trnM2, and trnV1 loci. Nucleic Acids Res. 1984 Mar 12;12(5):2549–2559. doi: 10.1093/nar/12.5.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]