Abstract
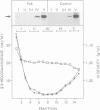
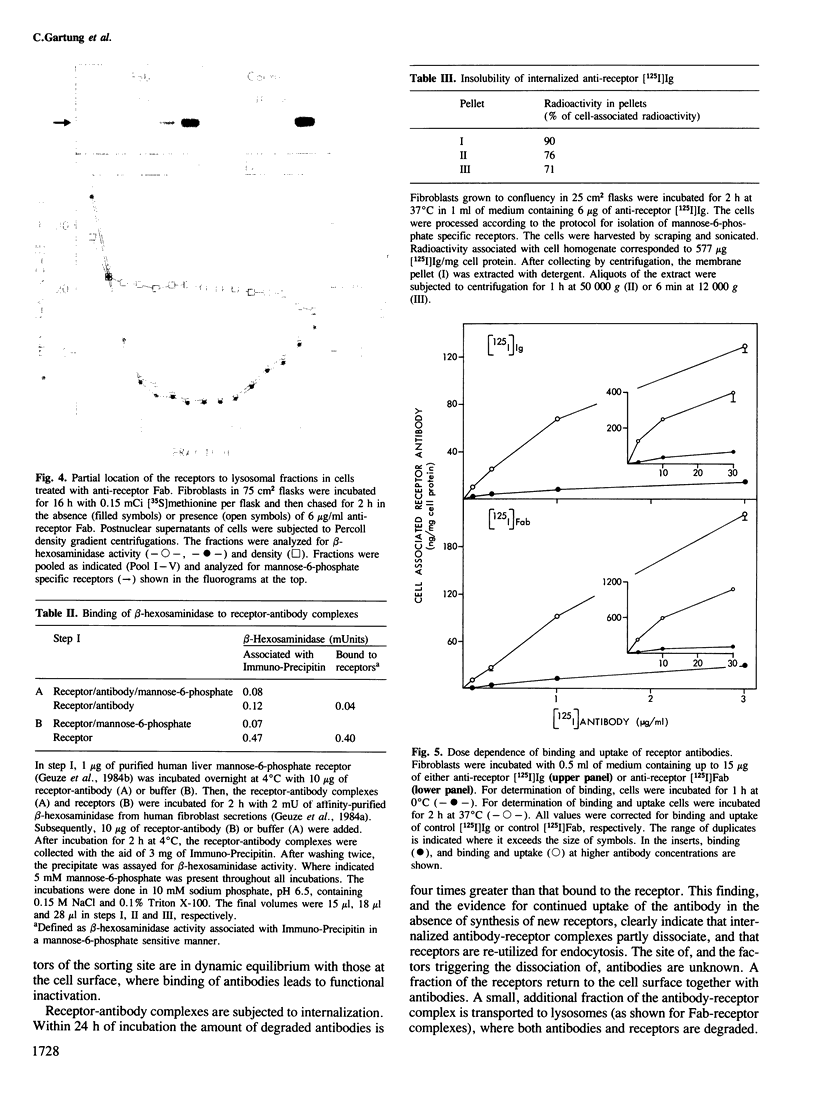
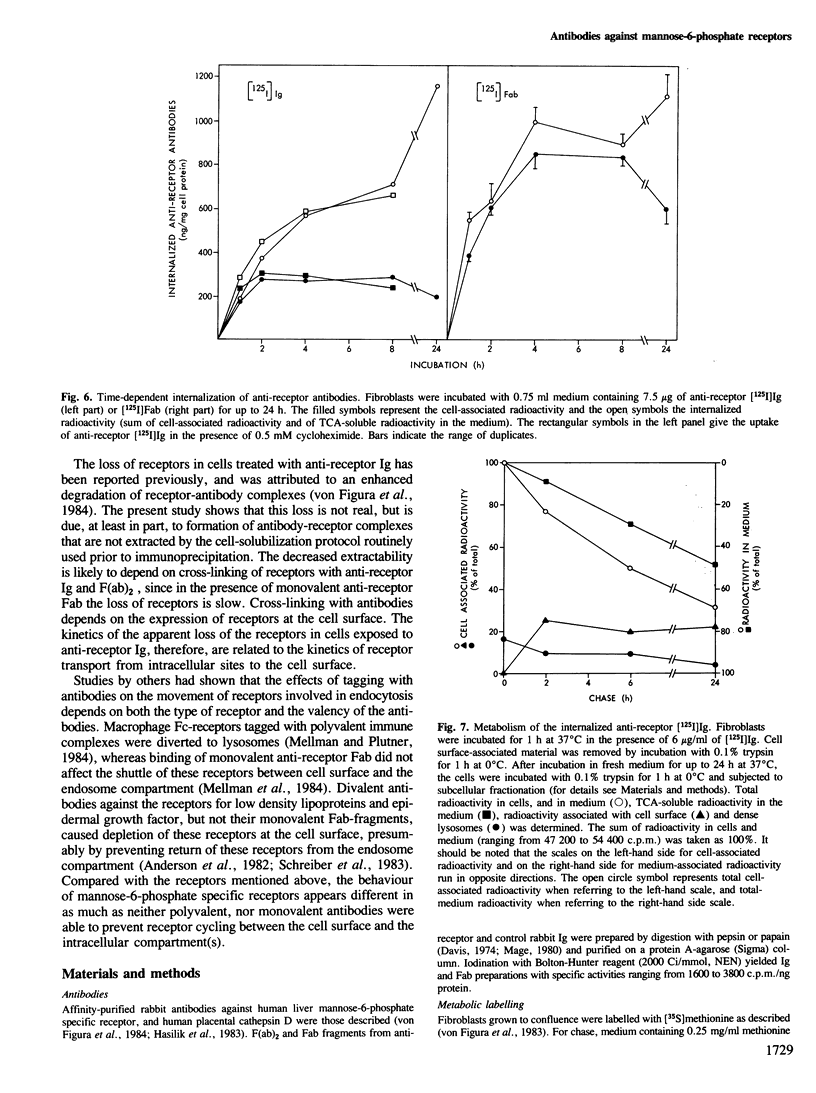
Antibodies against mannose-6-phosphate specific receptors inhibit the receptor-dependent endocytosis of exogenous lysosomal enzymes as well as the sorting of endogenous lysosomal enzymes. This inhibition was correlated with an apparent loss of the receptors. We report here that treatment of cells with the antibody results in the formation of receptor-antibody complexes that are not extracted by the procedure used for the solubilization of receptors prior to immunoprecipitation and detection of the receptor. The apparent loss of receptors is observed with both native antibody and the F(ab)2 fragments, but not with Fab fragments. In contrast the transport of lysosomal enzymes is inhibited by all three forms of the antibody. The inhibition is ascribed to masking by the antibody of the enzyme-binding site in the receptor. The inhibition of the sorting of endogenous lysosomal enzymes by antibodies added to the medium indicates that the mannose-6-phosphate specific receptors at the sorting site are in dynamic equilibrium with those at the cell surface. The receptor-antibody complexes formed at the cell surface appear to cycle between the cell surface and intracellular membranes. A fraction of the internalized antibodies dissociates from the receptors and is degraded after transfer into lysosomes. Complexing with Fab increases the concentration of the receptor in the lysosomes and decreases 2- to 3-fold the half-life of the receptor.
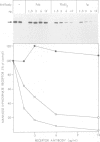
Full text
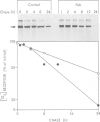
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Brown M. S., Beisiegel U., Goldstein J. L. Surface distribution and recycling of the low density lipoprotein receptor as visualized with antireceptor antibodies. J Cell Biol. 1982 Jun;93(3):523–531. doi: 10.1083/jcb.93.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. J., Farquhar M. G. The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell. 1984 Feb;36(2):295–307. doi: 10.1016/0092-8674(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Davis W. C. Use of antibodies of localization of components on membranes. Methods Enzymol. 1974;32:60–70. doi: 10.1016/0076-6879(74)32009-5. [DOI] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S. Beta-glucuronidase binding to human fibroblast membrane receptors. J Biol Chem. 1980 Jun 10;255(11):5069–5074. [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Hasilik A., Von Figura K. Ultrastructural localization of the mannose 6-phosphate receptor in rat liver. J Cell Biol. 1984 Jun;98(6):2047–2054. doi: 10.1083/jcb.98.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Gieselmann V., Pohlmann R., Hasilik A., Von Figura K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J Cell Biol. 1983 Jul;97(1):1–5. doi: 10.1083/jcb.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Pohlmann R., von Figura K. Inhibition by cyanate of the processing of lysosomal enzymes. Biochem J. 1983 Mar 15;210(3):795–802. doi: 10.1042/bj2100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Cathepsin D and beta-hexosaminidase synthesized in the presence of 1-deoxynojirimycin accumulate in the endoplasmic reticulum. J Biol Chem. 1984 Aug 25;259(16):10129–10135. [PubMed] [Google Scholar]
- Mage M. G. Preparation of Fab fragments from IgGs of different animal species. Methods Enzymol. 1980;70(A):142–150. doi: 10.1016/s0076-6879(80)70045-9. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984 Apr;98(4):1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Plutner H., Ukkonen P. Internalization and rapid recycling of macrophage Fc receptors tagged with monovalent antireceptor antibody: possible role of a prelysosomal compartment. J Cell Biol. 1984 Apr;98(4):1163–1169. doi: 10.1083/jcb.98.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G., Neufeld E. F. Biosynthesis and turnover of the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. J Biol Chem. 1983 Jun 10;258(11):7121–7128. [PubMed] [Google Scholar]
- Schreiber A. B., Libermann T. A., Lax I., Yarden Y., Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983 Jan 25;258(2):846–853. [PubMed] [Google Scholar]
- Ullrich K., Gieselmann V., Mersmann G., Von Figura K. Endocytosis of lysosomal enzymes by non-parenchymal rat liver cells. Comparative study of lysosomal-enzyme uptake by hepatocytes and non-parenchymal liver cells. Biochem J. 1979 Aug 15;182(2):329–335. doi: 10.1042/bj1820329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K., Mersmann G., Weber E., Von Figura K. Evidence for lysosomal enzyme recognition by human fibroblasts via a phosphorylated carbohydrate moiety. Biochem J. 1978 Mar 15;170(3):643–650. doi: 10.1042/bj1700643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. Enhanced breakdown of arylsulfatase A in multiple sulfatase deficiency. Eur J Biochem. 1982 Apr 1;123(2):317–321. doi: 10.1111/j.1432-1033.1982.tb19770.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Gieselmann V., Hasilik A. Antibody to mannose 6-phosphate specific receptor induces receptor deficiency in human fibroblasts. EMBO J. 1984 Jun;3(6):1281–1286. doi: 10.1002/j.1460-2075.1984.tb01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Gieselmann V., Hasilik A. Mannose 6-phosphate-specific receptor is a transmembrane protein with a C-terminal extension oriented towards the cytosol. Biochem J. 1985 Jan 15;225(2):543–547. doi: 10.1042/bj2250543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Hasilik A. Juvenile and adult metachromatic leukodystrophy: partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6066–6070. doi: 10.1073/pnas.80.19.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]