Abstract
Transforming growth factor β (TGF-β) contributes to wound healing and, when dysregulated, to pathological fibrosis. TGF-β and the anti-fibrotic nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ) repress each other’s expression, and such PPARγ downregulation is prominent in fibrosis and mediated, via previously unknown SMAD-signaling mechanisms. Here we show that TGF-β induces association of SMAD3 with both SMAD4, needed for translocation of the complex into the nucleus, and the essential context-sensitive corepressors E2F4 and p107. The complex mediates TGF-β-induced repression by binding to regulatory elements in the target promoter. In the PPARG promoter, we found that the SMAD3-SMAD4 complex binds both to a previously unknown consensus TGF-β inhibitory element (TIE) and also to canonical SMAD-binding elements (SBEs). Furthermore, the TIE and SBEs independently mediated partial repression of PPARG transcription, the first demonstration of a TIE and SBEs functioning within the same promoter. Also, TGF-β-treated fibroblasts contained SMAD complexes that activated a SMAD target gene in addition to those repressing PPARG transcription, the first finding of such dual activity within the same cell. These findings describe in detail novel mechanisms by which TGF-β represses PPARG transcription, thereby facilitating its own pro-fibrotic activity.
Keywords: peroxisome proliferator-activated receptor (PPAR), SMAD transcription factor, TGF-β inhibitory element (TIE), SMAD binding element (SBE), DNA-protein affinity assay, plasmid DNA-protein affinity purification
INTRODUCTION
TGF-β-SMAD signaling leads to versatile biological effects that vary with the cellular context, many of which rest on its ability to alter the cell’s gene expression pattern. TGF-β ligands signal through members of the TGF-β receptor family, a family of transmembrane protein serine/threonine kinases, which are further classified as type I or type II receptors. Binding of TGF-β to the type II receptor induces recruitment of the type I receptor to the complex, where it is subsequently phosphorylated and activated; the type I receptor subunits then phosphorylate R-SMADs.
This activating phosphorylation triggers release of these R-SMADs from cytoplasmic anchors, allowing their nuclear translocation and association with SMAD4, a partner in the assembly of transcriptional complexes. This complex then associates with diverse DNA-binding factors, co-activators and/or co-repressors to regulate induction or suppression of target genes (1–4). Of several hundred direct transcriptional responses elicited by cellular TGF-β signaling, over half represent activation and the rest repression (5). SMAD recruitment to DNA is therefore a key step in determining which genes will be activated or suppressed in response to a TGF-β stimulus. SMADs bind to short (4 bp) DNA sequences known as SMAD-binding elements (SBEs; AGAC and GTCT) via the MH1 domain (6, 7). The effect of TGF-β-SMAD signaling on gene expression relies on the presence of one or more SBEs in the gene’s promoter region (8, 9) or, alternatively, the presence of a sequence known as a TGF-β inhibitory element (TIE) to which SMADs also bind (10).
TGF-β plays a major role in wound healing and the pathological fibrosis that can result when this process is dysregulated (11). These effects can be countered by activation of PPARγ, a ligand-activated nuclear hormone receptor that binds to specific DNA response elements to regulate gene transcription and control a wide range of cellular functions (12). PPARγ regulates fibrosis-related processes including cellular inflammation (13), differentiation (14), and wound-healing (15), and PPARγ agonists exert anti-fibrotic activity in vitro and in a bleomycin-induced murine model of pulmonary fibrosis (14, 16, 17). We recently demonstrated that PPARγ activation dedifferentiates myofibroblasts, increases collagen uptake by alveolar macrophages, and reverses established fibrosis in a murine model (17). TGF-β downregulates PPARγ expression in diverse systems via SMAD signaling (17–19). Co-transfection of SMAD3/4 has been shown to decrease PPARγ promoter activity by ~60% by unidentified mechanisms (18). Using ChIP, we discovered two functional SBEs within the PPARG promoter (17), as likewise were found in hepatic stellate cells (20). The presence of such SBEs thus suggests that TGF-β-activated SMADs may repress PPARγ directly at the transcriptional level.
The present study provides the first comprehensive analysis of the mechanisms by which TGF-β-SMAD signaling represses PPARγ, demonstrating context-specific recruitment of transcription factors to promoter regions in response to TGF-β-SMAD signaling. We also developed a new protein-DNA interaction assay to quantify the affinity of SMADs for regulatory elements in the PPARG promoter and a new plasmid DNA-protein affinity purification method to assess the affinity and functionality of transcriptional co-regulators bound to promoter elements. We identified and mapped the previously unreported functional TIE and the SBEs in the PPARG promoter and their functional roles in mediating the repressive response to TGF-β. Surprisingly, we found that SMAD binding to either the SBEs or the TIE independently represses PPARG transcription, previously unknown in any system. We further discovered that the context-specific corepressor complex, which has the ability to deacetylate histones, associates with the PPARG promoter and induces PPARG repression by chromatin modification. TGF-β-treated human fibroblasts contain not only these transcriptionally repressive SMAD complexes but other SMAD complexes capable of transcriptionally activating appropriate promoters. TGF-β is known to act via SMAD signaling complexes to activate or repress specific genes, but this is the first report demonstrating that such dual activity can occur within the same cell.
EXPERIMENTAL
Cells
Human fetal lung fibroblast (IMR-90) cells were obtained from the Coriell Institute for Medical Research (Camden, NJ), and maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (HyClone, Logan, UT) at 37°C in a humidified atmosphere of 5% CO2 – 95% air. Monolayer cultures at 90% confluence were deprived of serum for 24 h prior to treatment with or without TGF-β (R&D Systems, Minneapolis, MN) as indicated.
siRNA transfection
Cells were incubated for 8 h with a liposome complex containing siRNA and Lipofectamine 3000 (Invitrogen, Carlsbad, CA) under serum- and antibiotic-free conditions. The siRNA, either targeted to the indicated gene (SMAD3: 38376, or SMAD4: 29484) or a scrambled control, was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). After 8 h, fresh DMEM medium with 10% FBS was added and the cells were incubated for a further 16 h. After this 24 h incubation, cells were utilized as described.
RNA isolation and real-time PCR
RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA) and cDNA was generated from 100 ng of total RNA or mRNA using MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA) with random and oligo-dT primers. Real-time PCR was performed using 100 ng cDNA with 2X SYBR Green Master mix (Applied Biosystems) and specific primers for the genes of interest (Supplemental Table 1). These experiments were performed on an AB 7500 fast thermal cycler using a three-step protocol with the melting curve method. The average of each gene cycle threshold (Ct) was determined for each experiment. Relative cDNA levels (2−ΔΔCt) for the genes of interest or the indicated control for each sample were determined using the comparative Ct method, which generates ΔΔCt as the difference between the gene of interest and the housekeeping genes for β-actin and 9S rRNA. Each averaged experimental gene expression sample was compared to the averaged control sample, which was set to 1.
Western blotting
Nuclear proteins were extracted using a nuclear extraction kit (Active Motif, Carlsbad, CA) and their concentrations were determined using the BCA Protein Assay kit (Pierce, Rockford, IL). Western blotting was performed as described previously (21). Primary antibodies against PPARγ (7196), SMAD3 (8332), SMAD4 (7966), E2F4 (511), p107 (250), lamin B1 (20682) and α-tubulin (58666) were from Santa Cruz Biotechnology. The secondary antibodies donkey anti-mouse IR-680RD (926-68072), donkey anti-rabbit IR-680RD (925-68073), donkey anti-goat IR-680RD (926-68074), goat anti-rabbit IR-800CW (925-32211), and goat anti-mouse IR-800CW (926-32210), were from LI-COR (Lincoln, NE). The infrared signal was detected using an Odyssey Infrared Imager (LI-COR).
Nascent RNA capturing
Nascent RNA transcripts were captured by the Click-iT Nascent RNA Capture Kit (Invitrogen) according to the manufacturer’s instructions. IMR-90 lung fibroblasts were cultured with or without TGF-β for the indicated time periods and subsequently pulsed with 0.5 mM 5-ethynyl uridine (5-EU) at 37°C for 1 h. The cells were washed twice with cold PBS and collected. Total RNA was extracted using the RNeasy Mini Kit and mRNA was purified using the Dynabeads mRNA Purification Kit (Invitrogen). A click reaction was performed using 5 μg of 5-EU-labelled mRNA and 0.5 mM biotin azide; the mixture was incubated at room temperature for 30 min. Following overnight precipitation at −70°C, the mRNA was dissolved in 50 μl of RNase-free water. A biotin-labelled EU–mRNA-binding pull-down assay was performed using 50 μl of Dynabeads MyOne Streptavidin beads and the bead-bound mRNA was washed and used as a template for reverse transcription. The captured nascent mRNA was analyzed by RT-PCR as described in RNA isolation and real-time PCR with specific primers for PPARG and ACTA2 (αSMA) (Supplemental Table 1).
We also determined PPARG mRNA decay by using the Click-iT Nascent RNA Capture kit. IMR-90 lung fibroblasts were treated with 5-EU with or without TGF-β at 37°C for 24 h, after which the medium was replaced with growth medium without 5-EU but with TGF-β either present or absent. Total RNAs were isolated at the indicated time points after exchanging the medium and subjected to the click reaction and subsequent purification and analysis as indicated above.
Nuclear run-on (NRO) assay
The nuclear run-on assay was performed as reported previously with minor modifications (22). Cells were treated as indicated; after treatment cells were washed twice with cold PBS and intact nuclei were collected. The NRO transcription reaction was performed with a reaction mixture containing biotin-16-UTP (Sigma). Total RNA was isolated using the RNeasy Mini Kit and the biotin-labelled RNA-binding pull-down assay was performed using Dynabeads M-280 Streptavidin (Invitrogen). The bead-bound RNA was subjected to RT-PCR as described in RNA isolation and real-time PCR with specific primers for PPARG and ACTA2 (αSMA) (Supplemental Table 1).
Bioinformatics analysis
A genomic DNA sequence of ~2.0 kb located immediately upstream from the transcription start site of the human PPARG gene (ID: 5468) was obtained from GenBank. The sequence was analyzed to predict the putative promoter region and to identify putative TIEs, SBEs and PPREs by manual inspection or using MatInspector. Multiple sequence alignment was performed on DNA sequences for the PPARG gene from human (ID: 5468), mouse (ID: 19016) and rat (ID: 25664) using Clustal Omega (23); DNA logos were generated using WebLogo (24).
PPARG promoter, deletion constructs, biotin-labelling and transient transfection assay
The ~2.0 kb DNA sequence located immediately upstream from the transcription start site of the human PPARG gene, the sequence harboring the promoter region, was amplified by PCR from human genomic DNA and cloned into the pGL4-Basic vector (Promega, Madison, WI). In some plasmids the TIE or the SBE region or both elements were deleted. All constructs were sequence verified and utilized as indicated. Plasmids were labelled with biotin using the FastTag Nucleic Acid Labeling kit (Vector Lab Inc., Burlingame, CA) according to the manufacturer’s instructions. Briefly, an equal amount of FastTag reagent was added to plasmid DNA and photocoupled for 30 min with a UV lamp. After coupling, the FastTag reagent was reduced and coupled with biotin maleimide. Biotin-labelled plasmid DNA was further purified using Dynabeads M-280 Streptavidin (Invitrogen), eluted and utilized as described elsewhere.
Transient transfection and luciferase activity assays were performed as previously described (17). Briefly, cells were transiently transfected with the indicated plasmids using Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer’s instructions. Following treatment with TGF-β, luciferase activity was measured using the Dual Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s instructions.
DNA affinity precipitation
Dynabeads M-280 Streptavidin beads (Invitrogen) were washed with cold binding and washing buffer (B&W buffer; 10 mM Tris-HCl, pH 7.5; 1 mM EDTA; 2 M NaCl; 1% protease inhibitor cocktail) and were mixed with 0.5 μM of biotinylated oligonucleotides containing TIE or SBE (Supplemental Table 1), then incubated at room temperature for 30 min. Next, 200 μg of the biotinylated oligonucleotide-coupled beads were added to 30 μg of nuclear proteins and incubated for 30 min at room temperature with rotation. Samples were washed three times with cold B&W buffer. Bead-bound biotinylated oligonucleotide-protein complexes were eluted and subjected to Western blotting as described above with specific antibodies as indicated. An aliquot of the nuclear sample that was not incubated with streptavidin beads was used as the input control sample.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using the SimpleChIP Enzymatic Chromatin Immunoprecipitation kit with magnetic beads (Cell Signaling Technology). Briefly, the DNA-chromatin of the cells was crosslinked with 1% formaldehyde for 10 min at room temperature, the crosslinking was stopped with 0.125 M glycine, and cells were then washed twice with ice cold PBS. Nuclei were pelleted and digested by micrococcal nuclease. Following sonication and centrifugation, an equal amount of sheared chromatin was incubated overnight at 4°C with antibodies against SMAD3 or SMAD4, or against IgG as negative control (Santa Cruz Biotechnology). Then protein G magnetic beads were added and the chromatin was incubated for 2 h at 4°C with rotation. An aliquot of chromatin that was not incubated with an antibody was used as the input control sample. Antibody-bound protein-DNA complexes were eluted and subjected to real-time PCR as described in RNA isolation and real-time PCR with specific primers that amplify the TUBA1A (αTubulin) promoter or SBE and TIE regions in the PPARG promoter region (Supplemental Table 1).
Plasmid DNA-protein affinity purification
IMR-90 lung fibroblasts were transfected with biotinylated-promoter plasmids and treated as indicated. After the treatment period, cellular biotinylated plasmid DNA-bound proteins were crosslinked with 1% formaldehyde for 10 min at room temperature, the crosslinking was stopped with 0.125 M glycine, and cells were washed twice with ice-cold PBS. Nuclei were pelleted and digested using a nuclear extraction kit (Active Motif). One hundred μg of Dynabeads M-280 Streptavidin beads (Invitrogen) were added to equal amounts of nuclear samples and were incubated overnight at 4°C with rotation. Samples were washed three times with cold binding and washing buffer (B&W buffer; 10 mM Tris-HCl, pH 7.5; 1 mM EDTA; 2 M NaCl; 1% protease inhibitor cocktail).
In other experiments PPARG promoter and SMAD reporter plasmids were labelled with biotin maleimide as described earlier. Biotin-labelled plasmid DNA was further purified and coupled to Dynabeads M-280 Streptavidin (Invitrogen) in B&W buffer. To 1 μg/1 mg of bead-bound biotinylated plasmid DNA equal amounts of nuclear proteins were added and incubated for 30 min at room temperature with rotation. Samples were washed three times with cold B&W buffer. Bead-bound biotinylated plasmid DNA-protein complexes from each experiment were eluted and subjected to Western blotting as described above with specific antibodies and also used in the Deacetylation Activity Assay as indicated. An aliquot of the nuclear sample that was not incubated with streptavidin beads was used as the input control sample (Supplemental Fig. S1, flow chart of the assay).
EMSA
SMAD3 and SMAD4 recombinant proteins (1 μg) were incubated with 50 nM of double stranded oligonucleotides end-labeled with infrared dye (IRDye) 700 or 800 as indicated (Supplemental Table 1) in binding buffer (100 mM Tris, 500 mM KCL, 10 mM DTT; pH 7.5), poly dI-dC (1 μg/μl in 10 mM Tris, 1 mM EDTA), 25 mM DTT, and 2.5% Tween 20. Samples were then separated on 5% non-denaturing polyacrylamide gels in 1× Tris-Borate EDTA buffer (130 mM Tris, pH 8.3, 45 mM boric acid, 2.5 mM EDTA). The infrared signal was detected using an Odyssey Infrared Imager (LI-COR).
DNA-protein affinity assay
To quantitate the interactions of SMAD proteins with SBE and TIE regulatory sequences in the PPARG promoter and to analyze the effects of mutations in the sequences, we developed a new assay (Supplemental Fig. S2, flow chart of the assay). One μg of his-tagged SMAD3 or SMAD4 proteins per well in 200 μl PBS were added to nickel coated 96 well plates (Pierce) as needed and incubated at room temperature for 1 h. After incubation, the wells were emptied and washed with 200 μl PBST (PBS supplemented with 0.05% Tween-20) three times to remove the unbound protein. To each well, oligonucleotides end-labeled with infrared dye (IRDye) 700 or 800 (50 pM per well in 200 μl PBST) were added as indicated and incubated at room temperature for 1 h. After incubation, unbound oligonucleotides were removed by washing with 200 μl PBST three times. The infrared signal was detected using an Odyssey Infrared Imager (LI-COR) at the plate template setting. Later Western blot was performed for SMAD3 and SMAD4 proteins in the wells, as described earlier, and quantitative data used to normalize the binding affinity. Data was quantified and analyzed after subtracting the background using Image Studio 2.1 Software (LI-COR) and oligonucleotide affinity was represented as normalized RFUs.
DNA-protein docking studies
Docking studies were performed to predict the interactions between the SMAD3 MH1 domain and PPARG SBE1 (AGTCTAG) or TIE (GATTTGGTGA) elements. Discovery Studio 2.5 (Accelrys Inc., San Diego, CA) was used to prepare and apply CHARMm force field to DNA sequences and SMAD3 MH1 (PDB ID: 1MHD (6)) domain for modelling. Docking was performed in NP Dock server as described previously (25) with default docking parameters and RMSD threshold in the clustering procedure set to 5 Å. After refinement, best scored decoys from the three largest clusters were analyzed and the best pose (highest probability) was used to identify DNA-protein interactions using Discovery Studio software.
Deacetylation activity assay
Deacetylation activity assay was performed to determine the histone deacetylation ability of protein complexes precipitated using the Plasmid DNA-protein affinity purification technique. Eluted protein samples, HDAC1 as a positive control, and HDAC1 + TSA as negative control were incubated with 2 μg of acetylated (K16) histone H4 (Active Motif) for 2 h at room temperature in assay buffer (10 mM Tris-HCl, pH 8; 150 mM NaCl in 50% glycerol) as indicated. Samples were then separated in 10% SDS-PAGE gel and subjected to Western blotting as described above with anti-acetylated lysine (AcK) and anti-histone H4 antibodies to determine the amount of histone acetylation.
Statistical analysis
Data are presented as mean ± SD. Differences between groups were analyzed using an unpaired t-test or analysis of variance, followed by a Bonferroni multiple comparison correction. Analyses were performed using GraphPad Prism 5.03 (GraphPad Software, La Jolla, CA). P < 0.05 was considered significant.
RESULTS
TGF-β-SMAD signaling downregulates PPARγ at the transcriptional level
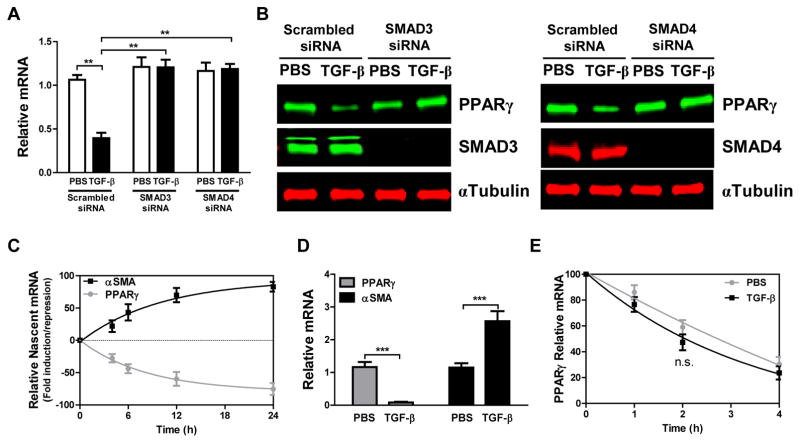
We have previously demonstrated that TGF-β upregulates expression of TβR1 and phosphorylation of SMAD2/3, accompanied by time- and concentration-dependent decreases in PPARG transcription (17). We also demonstrated decreased expression of PPARγ in lung tissue and lung fibroblasts from human idiopathic pulmonary fibrosis (IPF) samples and that knockdown or knockout of PPARγ expression in isolated human and mouse lung fibroblasts induced a profibrotic phenotype (17), whereas treating human fibroblasts with PPARγ agonists blocked TGF-β signaling and actions. Here we investigate the role of SMAD4 in this process, finding that knockdown of either SMAD3 or SMAD4 abrogates the effects of TGF-β on PPARG mRNA (Fig. 1A) and protein (Fig. 1B) expression. We hypothesized that this TGF-β-induced suppression of PPARG mRNA expression occurs at the transcriptional level and reflects recruitment of a SMAD-dependent inhibitory transcription complex to the PPARG promoter. This hypothesis was confirmed by measuring nascent mRNA levels in IMR-90 lung fibroblasts and nuclei obtained from these cells following treatment with or without TGF-β. Nascent PPARG mRNA synthesis was decreased, while nascent ACTA2 (αSMA) mRNA was increased following treatment with TGF-β at all observed time points (Fig. 1C). αSMA expression reflects the fibrosis-related fibroblast-myofibroblast transition. We also assessed amounts of newly synthesized mRNA with a nuclear run-on assay. TGF-β treatment significantly diminished PPARG mRNA synthesis to almost zero whereas it augmented ACTA2 mRNA ~2.5 fold (Fig. 1D). We used EU pulse-chase to assess the complementary hypothesis that TGF-β affected PPARG mRNA decay, and found no significant changes in PPARG mRNA stability in the presence of TGF-β (Fig. 1E). Together these results suggest that TGF-β-SMAD signaling represses PPARγ expression at the transcriptional level by forming a SMAD-dependent inhibitory transcription complex acting at the PPARG promoter.
Figure 1. TGF-β-SMAD signaling downregulates PPARγ at the transcriptional level.
(A, B) IMR-90 human lung fibroblasts were transfected with SMAD3 or SMAD4-targeted siRNA, or with scrambled siRNA as control, and then treated with or without TGF-β (2 ng/ml). Following treatment: (A) PPARG mRNA was measured by real-time PCR, and (B) PPARγ, SMAD3 and SMAD4 protein expression was assessed by Western blotting. (C) IMR-90 fibroblasts were treated with or without TGF-β (2 ng/ml) and nascent mRNA was captured by Click-iT Nascent RNA Capture kit, with relative mRNA levels of PPARG and ACTA2 being measured by real-time PCR. (D) Nuclei isolated from IMR-90 lung fibroblasts were incubated with biotin-16-UTP and a nuclear run-on assay was performed. Labelled, newly synthesized transcripts were collected using streptavidin-conjugated magnet beads and relative levels of PPARG and ACTA2 mRNA were determined by real-time PCR. (E) mRNA stability was determined by incubation with growth medium containing 5-EU for 24 h followed by incubation with unlabeled growth medium for the indicated periods. Total mRNA was then isolated and labelled mRNA captured and analyzed with Click-iT Nascent RNA Capture Kit as described in Experimental Procedures. The data are expressed as the mean ± SD with n = 3–4 and the results were reproduced in two to three independent experiments; **P < 0.01, ***P < 0.001, n.s. = non-significant.
Highly conserved and functional TIE and SBE sequences in the PPARG promoter mediate TGF-β induced transcriptional suppression
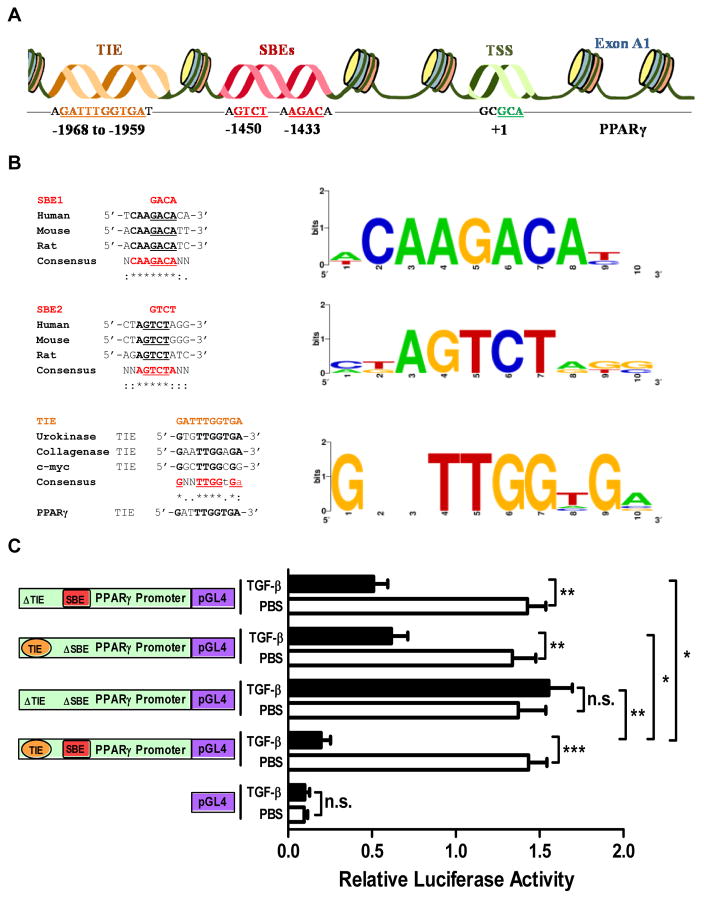
To investigate the basis for PPARγ repression by TGF-β, we analyzed the PPARG promoter for putative transcription factor binding elements that may mediate this response. Our analysis identified a highly conserved TIE between −1969 and −1958 bp and SBE sequences (SBE1: AGAC and SBE2: GTCT) between −1451 and −1429 bp in the PPARG promoter (Fig. 2A). The SBE sequences are identical to most SBEs previously described and are highly conserved across human, mouse, and rat PPARG promoter sequences (Fig. 2B, top and middle). The TIE sequence, which had not previously been reported in the PPARG promoter, is a 100% match to the consensus TIE sequence generated using multiple sequence alignment of TIEs from genes for human urokinase (10), collagenase (26), and c-myc (27) (Fig. 2B, bottom). To investigate the functionality of these binding elements, we performed luciferase assays in IMR-90 lung fibroblasts transfected with reporter constructs incorporating wild-type or mutant (ΔSBE, ΔTIE, or ΔTIE-ΔSBE) PPARG promoters and treated with or without TGF-β. TGF-β treatment significantly decreased luciferase activity of constructs with wild-type promoters, while deletion of both TIE and SBE sites completely abolished the TGF-β response. Notably, deletion of either site but not the other reduced the TGF-β response by about 50%, suggesting that each site functions independently to mediate TGF-β-induced suppression of PPARγ expression (Fig. 2C).
Figure 2. The PPARG promoter contains highly conserved and functional TIE and SBEs.
(A) Bioinformatics analysis identified the existence of a previously unreported putative TGF-β inhibitory element (TIE, orange) and SMAD-binding elements (SBE, red). Numbers refer to the nucleotide position within the promoter region. (B) Sequence alignment shows the high conservation of SBE1 (top, AGACA) and SBE2 (middle, GTCT) in the PPARG promoters of human, mouse and rat. The novel TIE in the PPARG promoter is highly similar to previously identified TIEs in the indicated gene promoters (bottom, GATTTGGTGA). Representative matrix data for each element is shown by Weblogo and were obtained from the CEAS web tool. (C) IMR-90 lung fibroblasts were transfected with luciferase constructs under control of a PPARG promoter encompassing either the wild-type sequence or a mutant (ΔTIE-ΔSBE) with the putative binding sites deleted, or with pGL4, as indicated; the luciferase activities were determined after 24-h in the absence or presence of TGF-β (2 ng/ml). The data are expressed as the mean ± SD with n = 3 and the results were reproduced in two to three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001, n.s. = non-significant.
TGF-β-dependent SMAD complex binds to the TIE and SBEs in the PPARG promoter
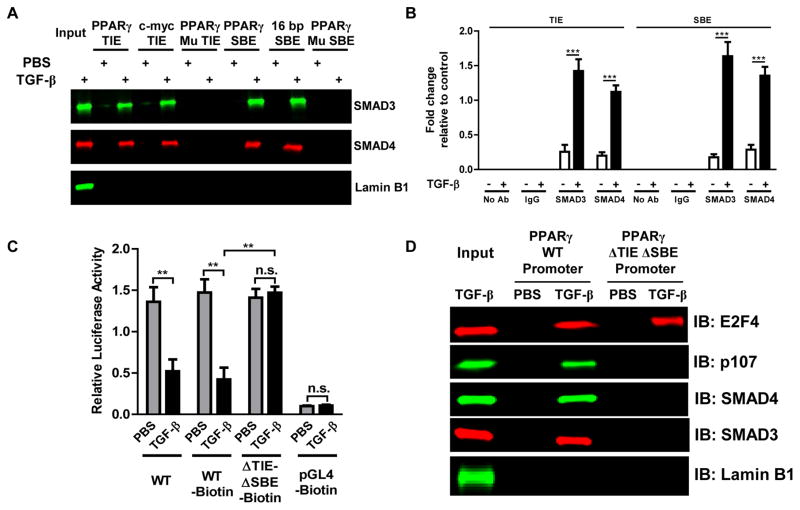
Our results show that TGF-β regulates PPARγ expression via TβR1-SMAD signaling (17) (Fig. 1A, B) and that highly conserved and functional TIE and SBE sequences are present (Fig. 2). To determine whether SMAD proteins physically interact with these SBE and TIE sequences upon TGF-β induction, we used biotinylated double-stranded oligonucleotides corresponding to the TIE or the SBE sequences to precipitate binding proteins from nuclear extracts of IMR-90 lung fibroblasts treated with or without TGF-β. Nuclear extracts from TGF-β-treated cells yielded TIE- and SBE-binding complexes containing SMAD3 and SMAD4 proteins, as determined by Western blotting (Fig. 3A). Mutations in the main sequences of the SBEs or the TIE in the respective oligonucleotides (Supplemental Table 1) inhibited their interaction with these proteins. The TIE from the gene for c-myc and a 16 bp SBE were used as positive controls and nuclear extracts without added nucleotides were loaded as input. Western blot for lamin B1 was performed as a control for non-specific interaction and was not detected in any of the precipitation assays (Fig. 3A). We further investigated the interaction of SMAD proteins with TIE and SBE sequences in the PPARG promoter by ChIP assay using antibodies to SMAD3 and SMAD4, confirming that SMAD3 and SMAD4 proteins exhibit relatively similar affinity for the TIE and SBE sequences in response to TGF-β (Fig. 3B and Supplemental Fig. S3). No direct interaction of E2F4 with the TIE and SBEs was observed (Supplemental Fig. S5). These data show that the TGF-β-induced SMAD3-SMAD4 complex binds independently to the TIE and SBEs in the PPARG promoter.
Figure 3. A TGF-β-activated SMAD3-SMAD4 complex binds to the TIE and the SBEs in the PPARG promoter and mediates PPARγ downregulation through a SMAD-E2F4-p107 transcriptional complex.
(A, B) IMR-90 human lung fibroblasts were treated with or without TGF-β (2 ng/ml). (A) Following treatment, nuclear extracts were obtained and were incubated with the indicated wild-type (WT) or mutant (Mu SBE or Mu TIE) biotinylated double stranded oligonucleotide-coupled beads. Bead-bound biotinylated oligonucleotide-protein complexes were eluted and subjected to Western blot to identify the presence of SMAD3 and SMAD4. (B) Following treatment, chromatin was crosslinked and immunoprecipitated (ChIP) with antibodies to IgG, SMAD3, or SMAD4; the antibody-bound DNA-protein complexes were then subjected to real-time PCR with primers that specifically amplify TIE and SBEs in the PPARG promoter region and the TUBA1A (αTubulin) promoter as a TGF-β-unresponsive control (Supplemental Fig. S3). (C, D) IMR-90 lung fibroblasts were transfected, as indicated, with biotinylated constructs encompassing a luciferase gene under control of a PPARG promoter with wild-type or mutant (ΔTIE-ΔSBE) sequences at the putative binding sites, or with pGL4, then treated with or without TGF-β (2 ng/ml) for 24 h. (C) Luciferase activities were determined. (D) Cellular biotinylated plasmid DNA-bound proteins were crosslinked and precipitated with streptavidin-conjugated magnetic beads; DNA-protein complexes were eluted and subjected to Western blot for E2F4, p107, SMAD3, SMAD4 and lamin B1. The data are expressed as the mean ± SD with n = 3–4 and the results were reproduced in two to three independent experiments; **P < 0.01, ***P < 0.001, n.s.= non-significant.
TGF-β mediates suppression of PPARG gene transcription through a SMAD-E2F4-p107 complex
TGF-β-induced negative gene regulation via SMAD recruitment of E2F and pRB proteins is well known (27–29). Chen et al. previously reported that the TGF-β-activated SMAD-E2F4-p107 complex recognizes a composite SMAD-E2F sequence in the c-myc promoter and represses its expression (29). We performed a series of experiments to investigate the physical association of the E2F4 and p107 transcription regulators to the SMAD complex and their interactions with regulatory binding elements in the PPARG promoter. First, we transfected IMR-90 lung fibroblasts with biotinylated constructs containing wild-type or mutant (ΔTIE-ΔSBE) putative binding sites. Following treatment with TGF-β we checked these constructs’ promoter activity by luciferase assay (Fig. 3C), observing significant reductions in promoter activity with WT but not mutant constructs. In another experiment we precipitated plasmid DNA-protein complexes and analyzed them by Western blot. We found that stimulation with TGF-β induced binding of SMAD-E2F4-p107 to the WT PPARG promoter and that, except for E2F4, this association was completely absent for the mutant (ΔTIE-ΔSBE) promoter. Western blot for lamin B1 was performed as a control for non-specific interaction and was not detected in any of the precipitation assays (Fig. 3D). Further bioinformatics analysis of the PPARG promoter identified a binding site for E2F4 (GCGGGAAA) between −147 to −138 bp that accounts the interaction of E2F4 with the mutant promoter in response to TGF-β. As we did not observe any significant change in luciferase activity in response to TGF-β with the ΔTIE-ΔSBE promoter plasmids (Fig. 3C), we believe that binding of E2F4 solely to its response element does not affect PPARG gene expression. In other experiments we immunoprecipitated endogenous SMAD3 and SMAD4 to determine whether E2F4 and p107 were associated with them. Western blot analysis revealed the association of E2F4 and p107 with SMAD3 and SMAD4 that was increased ~2 fold in response to TGF-β (Supplemental Fig. S4). We also observed that TGF-β increased the association between SMAD3 and SMAD4. These results indicate that TGF-β induces the formation of a SMAD-E2F4-p107 complex in lung fibroblasts and that this complex interacts with putative SBE and TIE binding sequences in the PPARG promoter to suppress its expression at the transcriptional level, while interaction with the E2F binding site has no effect.
Binding of the SMAD complex to TIE and SBEs requires critical nucleotide bases
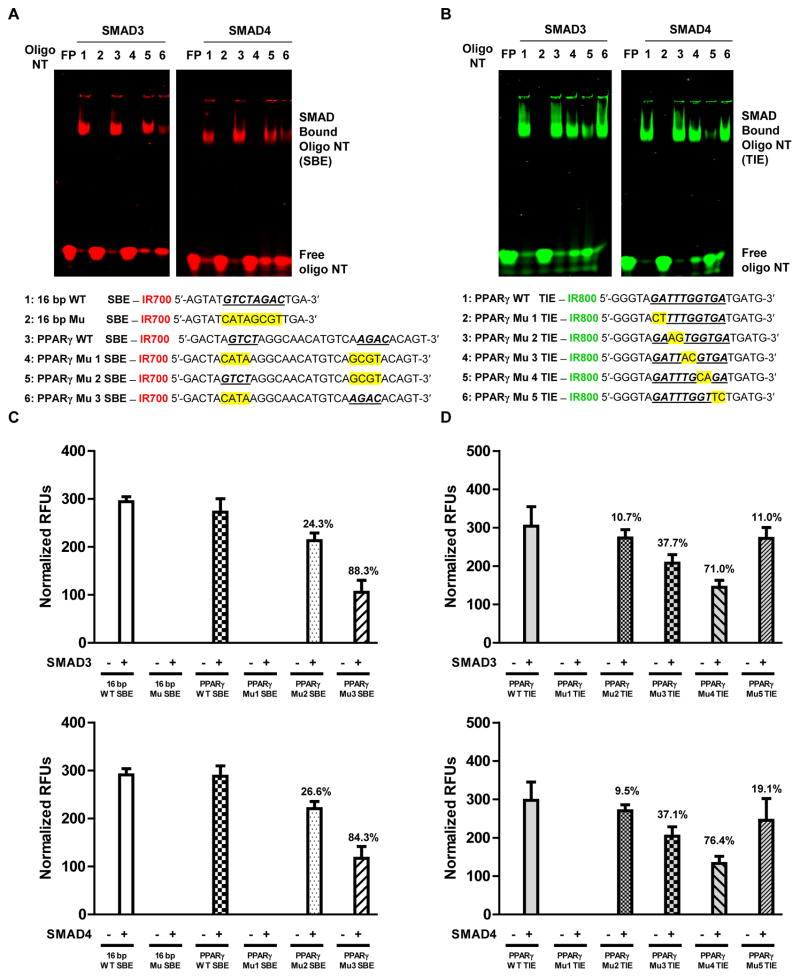
Since SMAD complexes bound to both consensus SBE and TIE sequences with similar affinities, we next explored the biological significance and mapped the specific nucleotide bases with which the SMAD complexes interact. To do this we carried out EMSA and DNA-protein affinity assay experiments with wild-type and the indicated mutated oligonucleotides, each end-labelled with an appropriate infrared dye, in conjunction with DNA-protein docking predictions. In one experiment we performed EMSA and DNA-protein affinity assays with recombinant SMAD3 and SMAD4 using wild-type SBEs and sequences in which one or both of the SBEs were mutated, along with 16 bp wild-type and mutated SBEs as controls. SMAD3 and SMAD4 both shifted wild-type SBE oligonucleotides, with SMAD3 being more efficient. Mutation of both SBEs (Mu 1) completely prevented these effects, whereas mutation of AGAC (Mu 2) had less effect (SMAD3 24.3 %, SMAD4 26.6%) than mutation of GTCT (Mu 3) (SMAD3 88.3 %, SMAD4 84.3%), making GTCT the PPARG promoter’s crucial element for SMAD complex binding (Fig. 4A and C). In another experiment we performed EMSA and DNA-protein affinity assay with recombinant SMAD3 and SMAD4 using WT TIE and TIE with every set of two base pairs mutated as indicated. Both SMADs shifted wild-type TIE oligonucleotides with relatively similar affinity. Mutation of GA→CT at positions 1 and 2 (Mu 1) in TIE completely blocked the shift whereas mutation of TT→AG at positions 3 and 4 (Mu 2) and GA→TC at positions 9 and 10 (Mu 5) had no inhibitory affect. Mutation of TG→AC at positions 5 and 6 had little affect but mutation of GT→CA at position 7 and 8 almost completely blocked the shift. This result indicates that the presence of GA at position 1 and 2, T at position 5, and G at position 7 of TIE are required for a functional SMAD complex binding element (Fig. 4B and D). Together, these results provide a detailed description of the functionality of TIE and SBE sequences in the PPARG promoter.
Figure 4. SMAD3 and SMAD4 binding to the SBEs and TIE requires critical nucleotide bases in the PPARG promoter.
(A, B) Recombinant SMAD3 and SMAD4 were individually incubated with infrared dye IR700 (red) or IR800 (green) end-labeled double stranded oligonucleotides as indicated for: (A) wild-type (WT) or mutated (Mu) SBE; and (B) wild-type (WT) or mutated (Mu) TIE. In each case EMSA was performed as described in Experimental Procedures. (C, D) DNA-protein affinity assay was performed as indicated in the methods (see flow chart in Supplemental Fig. S2) with end-labeled double stranded oligonucleotides for: (C) wild-type (WT) or mutated (Mu) SBE; and (D) wild-type (WT) or mutated (Mu) TIE. The infrared signal was detected using an Odyssey Infrared Imager. SBE and TIE sequences are indicated with bold and underlined text and mutants are indicated with a yellow highlight. Numbers on bar graphs represent percentage difference between groups. The data are expressed as the mean ± SD with n = 3 and the results were reproduced in two to three independent experiments. NT = nucleotides.
DNA-protein docking predictions with a 5 Å RMSD cutoff and analysis of best pose cluster with higher probability further illuminated the hydrogen bond interactions of the SMAD MH1 domain with nucleotides in the SBE and TIE of the PPARG promoter. A highly conserved 11-residue β-hairpin was shown to be embedded in the DNA major groove of these elements in a sequence-specific manner (Fig. 5A and C). Critical contacts of SMAD MH1 domain amino acids with SBE1 and TIE DNA are summarized in Fig. 5B and D. The contact maps shown explain visually all the conserved SMAD MH1 domain amino acids and DNA nucleotides in the binding site. The β-hairpin of the SMAD MH1 domain interacts with the SBE and TIE via 8 and 13 direct hydrogen bond interactions, respectively, and these hydrogen bonds are similar to those previously reported in a SMAD3-SBE crystal structure (6). These results are consistent with our EMSA and DNA-protein affinity assay data and strongly support the hypothesis that R-SMADs bind to specific nucleotide bases in SBE and TIE in the PPARG promoter, and that each of these sequences contains sites where crucial mutations interfere with binding.
Figure 5. SMAD3 MH1 domain interacts with critical nucleotide bases in SBEs and TIE present in the PPARG promoter.
DNA-Protein docking was performed as described in methods. (A, C) Cartoon representation and stereo views of the predicted SMAD3 MH1 domain in complex with (A) SBE and (C) TIE in the PPARG promoter. The SMAD3 MH1 domain is colored by secondary structure type with a sky blue surface, and the amino acid residues involved in DNA contact are depicted as colored stick models. The DNA backbone is depicted as a red (SBE) or orange (TIE) arrow model with same-color semi-transparent surfaces, and bases are drawn as base-colored stick models. Hydrogen bonds are indicated as green dashed lines. (B, D) Contact maps illustrating interacting amino acid residues within 4.0 Å distance from nucleotide bases in (B) SBEs and (D) TIE of the PPARG promoter. Donors are depicted in red and acceptors in blue; and arrow indicates the hydrogen bond interaction.
TGF-β-dependent SMAD transcriptional complexes induce and repress target gene expression in the same cell by recruiting different transcriptional factors
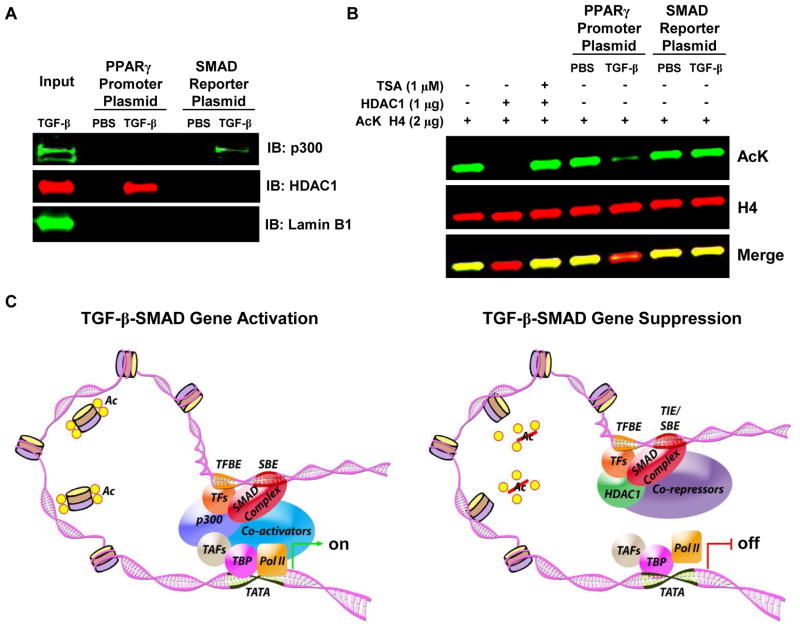
Since activated R-SMAD complexes have been shown to both induce and suppress gene expression when activated by TGF-β, we performed plasmid DNA-protein affinity purification and histone deacetylation activity assays to determine whether the context-specific transcriptional factors p300 and HDACs are recruited to a SMAD reporter promoter responsive to SMAD activation and the PPARG promoter, respectively, and whether they modify histones. Western blot analysis on samples indicated the association of p300 with the SMAD reporter promoter and association of HDAC1 with the PPARG promoter. Western blot for lamin B1 was performed as a control for non-specific interaction and was not detected in any of the precipitation assays (Fig. 6A). To determine whether protein complexes formed on the SMAD reporter promoter and the PPARG promoter have HDAC enzymatic activity, eluted samples were tested for histone deacetylation activity. As a comparison, enzymatic activity was also measured in the presence of recombinant HDAC1 and the HDAC inhibitor Trichostatin A (TSA). As shown in figure 6B, histone 4 (H4) was specifically deacetylated (~90%) by proteins bound to the PPARG promoter but not to the SMAD reporter promoter. Recombinant HDAC1 completely deacetylated H4 and deacetylation was completely abolished by treatment with TSA. These results confirm that TGF-β-dependent SMAD transcriptional complexes recruit p300 to target genes such as ACTA2 (αSMA) to increase their expression and recruit HDACs to target genes that are suppressed, as we show in this study (Fig. 1C, D). In total, our results support the hypothesis that TGF-β-activated R-SMAD transcriptional complexes recruit corepressors and downregulate PPARγ at the transcriptional level while simultaneously recruiting coactivators to upregulate other genes in the same cell (Fig. 6C).
Figure 6. A TGF-β-activated transcriptional complex induces PPARG transcriptional repression by recruiting HDAC1.
IMR-90 human lung fibroblasts were treated with or without TGF-β (2 ng/ml). Following treatment, nuclear extracts were obtained and were incubated with the indicated PPARG promoter or SMAD reporter promoter biotinylated plasmid-coupled beads. Bead-bound biotinylated plasmid-protein complexes were eluted and (A) subjected to Western blot to identify the presence of p300 and HDAC1. (B) A histone deacetylation activity assay was performed with eluted samples and recombinant HDAC1 in the presence and absence of TSA using 2 μg of acetylated (K16) histone H4 in HDAC assay buffer and were then subjected to Western blot to identify the amount of deacetylation under indicated conditions. The results were reproduced in two to three independent experiments. (C) Schematic showing how TGF-β-activated R-SMADs can either activate target gene expression by recruitment of coactivators and other transcription factors that have histone acetylation activity or repress the target gene by recruitment of transcriptional corepressors and transcription factors that have histone deacetylation activity.
DISCUSSION
Tissue fibrosis and fibrosis-related diseases are characterized by abnormal tissue repair and persistent myofibroblast-rich lesions. TGF-β plays a central role in wound healing, fibrosis, and pathogenesis of fibrotic diseases (11, 30). The ability of PPARγ activation to block TGF-β-induced fibrosis has also been studied in a number of systems. Indeed, we have shown that such activation can actually reverse established pulmonary fibrosis in mice (17). Less widely appreciated is that fibrosis and related pathologies also involve TGF-β-induced downregulation of PPARγ expression and activity (17–19, 31), thus reinforcing the cytokine’s pro-fibrotic effect. Loss of PPARγ has been shown to encourage such a pro-fibrotic phenotype in fibroblasts whereas activation ameliorates and reverses these features. We have previously demonstrated negative cross-talk between TGF-β and PPARγ, with each downregulating expression and activity of the other (17). This crosstalk is in accord with observations in other systems, where we (21) and others (32–34) have found PPARγ is downregulated in conditions that it would alleviate if fully expressed.
Such crosstalk suggests that TGF-β-induced downregulation of PPARγ expression is a significant event in the pro-fibrotic TGF-β-signaling pathway; thus understanding the molecular mechanisms that mediate this downregulation may provide insights into ways to modulate uncontrolled tissue repair and myofibroblast formation. Our current studies show that the TGF-β-induced downregulation of PPARγ is transcriptionally controlled, with no effect on PPARG mRNA stability, and is mediated by a SMAD-dependent pathway. SMAD3/4 co-transfection decreases PPARG promoter activity, as others have shown in different contexts (18, 19, 31), but the mechanisms by which R-SMADs regulate PPARG promoter activity remain poorly understood (18).
Our investigations identified a highly conserved TIE (GATTTGGTGA; −1969 to −1958 bp) that had not previously been reported in the PPARG promoter. This sequence matches 100% with the consensus TIE sequence generated using multiple sequence alignment of TIEs from genes for human urokinase, collagenase and c-myc. We also identified SBE (SBE1: AGAC and SBE2: GTCT; −1451 to −1429 bp) sequences that are identical to most SBEs previously described and are highly conserved across human, mouse, and rat PPARG promoter sequences. Luciferase reporter assays confirmed that a TGF-β-activated SMAD complex can downregulate transcription of PPARG and that such downregulation is blocked by appropriate mutations of either the identified TIE or the SBE sequences, with both mutations being required for full effect.
The nucleotide sequences within this novel TIE in the PPARG promoter are completely distinct from those of the classic SBE (27). We also found repression mediated by binding to a classic SBE, which has been less commonly observed. TIE and SBE binding independently orchestrate transcriptional repression of the PPARG promoter, with disruption of either site resulting in only partial abrogation of TGF-β-induced transcriptional inhibition. This contrasts with findings by Wu et al. that the promoter in the gene for lysophosphatidic acid receptor 1 contained 2 TIEs and a classic SBE, but that disruption of one TIE completely abolished TGF-β-induced translational repression, while disruption of the other TIE or the SBE had no effect (35). Involvement of the SMAD-E2F4-p107 complex in TGF-β-induced suppression of other promoters is well known (36), as is binding of this complex to TIEs in promoters of the proto-oncogene c-myc (27) and genes for other proteins (26, 37). Consistent with our hypothesis, both immunoprecipitation and Western blot performed on samples obtained using a plasmid-DNA precipitation assay indicated association of the SMAD-E2F4-p107 complex with the PPARG promoter following TGF-β treatment.
The DNA binding activity of SMAD3 MH1 is known to be required for SMAD-dependent signaling (38). An 8 bp palindromic DNA sequence has been recognized as the SBE (7) and the crystal structure of SMAD3 MH1 bound to this SBE revealed direct hydrogen-bond interactions between sequence-specific DNA bases and a highly conserved amino acid sequence in the SMAD MH1 domain’s β-hairpin loop (6). Our studies support and confirm these findings by showing that mutation of the SBE both abolishes SMAD binding to SBE-containing plasmids and reduces the ability of TGF-β to downregulate PPARG transcription by ~50%. The inhibitory effect of TGF-β is completely abolished by concurrent mutation of the SBEs and the novel TIE that we identified in the PPARG promoter. This is the first instance in any system where TGF-β has been found to induce transcriptional repression via both a TIE and SBEs in the same promoter. We also used site-directed mutagenesis to identify the crucial nucleotides in this previously unreported TIE.
TGF-β-SMAD signaling regulates target gene transcription either positively or negatively depending on the type of transcriptional co-factors recruited by SMADs. These versatile biological effects vary with the cellular context, reflecting the ability of SMADs to, on the one hand, promote cell-specific target gene transcription by interacting with various tissue-related transcription factors and recruiting coactivators such as p300/CBP (39, 40) or, on the other hand, interact with transcriptional corepressors such as TGIF or members of the Ski family that recruit HDACs to SMAD proteins (29, 41, 42) and thus limit gene expression. Specifically, recruitment of HDAC1 to the transcriptional corepressor complex by SMAD (43) and E2F4/5 (44), which we also observed, has previously been reported. Our plasmid DNA-protein affinity purification experiments with SMAD reporter promoter and PPARG promoter plasmids demonstrated that cells stimulated with TGF-β contained both a coactivator complex including p300 that bound to and activated the SMAD reporter promoter plasmid and a corepressor complex containing HDAC1 that bound to the PPARG promoter plasmid. A histone deacetylation assay further demonstrated the ability of the corepressor complex bound to the PPARG promoter to modify histones, an activity that was absent from the coactivator complex bound to the SMAD reporter promoter. These results support the hypothesis that TGF-β-SMAD signaling regulates PPARG promoter by chromatin modification and that this mechanism is totally distinct from that by which TGF-β activates transcription of other genes. To our knowledge, this study is the first to report formation of these two distinct context-specific SMAD transcription complexes in the same cell in response to TGF-β.
In summary, our data show that a TGF-β-activated SMAD repressor complex downregulates PPARγ at the transcriptional level via histone deacetylation after binding to a novel TIE and to SBE sequences in the PPARG promoter. This binding requires specific key nucleotide bases in those regulatory elements. Our findings thus provide direct support for the idea that PPARγ plays a key role in inhibiting the transition from fibroblast to myofibroblast and that TGF-β signaling selectively suppresses PPARγ as a key aspect of this transition. Increasing PPARγ expression and activity in such a context may open new prospects for controlling abnormal tissue repair and fibrosis.
Supplementary Material
Acknowledgments
This work was supported by a Merit Review award from the U.S. Department of Veterans Affairs and National Institutes of Health grants HL093196 and AI125338 (to R.C.R.).
Footnotes
Disclaimer: The contents in this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflict of Interest: The authors declare that they have no conflicts of interest.
Author contributions: SPL ATR and RCR conceived and designed the experiments; SPL and ATR performed the experiments and analyzed the data; ATR SPL and RCR wrote the paper.
References
- 1.Derynck R, Zhang Y, Feng X-H. Transcriptional activators of TGF-β responses: Smads. Cell. 1998;95(6):737–40. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 2.Heldin C-H, Miyazono K, ten Dijke P. TGF-[beta] signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 3.Massagué J. TGF-β signal transduction. Annual review of biochemistry. 1998;67(1):753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 4.Massagué J. How cells read TGF-β signals. Nature reviews Molecular cell biology. 2000;1(3):169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 5.Zavadil J, Bitzer M, Liang D, Yang Y-C, Massimi A, Kneitz S, et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-β. Proceedings of the National Academy of Sciences. 2001;98(12):6686–91. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Wang Y-F, Jayaraman L, Yang H, Massagué J, Pavletich NP. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-β signaling. Cell. 1998;94(5):585–94. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- 7.Zawel L, Le Dai J, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Molecular cell. 1998;1(4):611–7. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 8.Lacerte A, Korah J, Roy M, Yang XJ, Lemay S, Lebrun JJ. Transforming growth factor-beta inhibits telomerase through SMAD3 and E2F transcription factors. Cellular signalling. 2008;20(1):50–9. doi: 10.1016/j.cellsig.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Nagarajan RP, Zhang J, Li W, Chen Y. Regulation of Smad7 promoter by direct association with Smad3 and Smad4. The Journal of biological chemistry. 1999;274(47):33412–8. doi: 10.1074/jbc.274.47.33412. [DOI] [PubMed] [Google Scholar]
- 10.Kerr LD, Miller DB, Matrisian LM. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990;61(2):267–78. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- 11.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18(7):816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 12.Berger J, Moller DE. The mechanisms of action of PPARs. Annual review of medicine. 2002;53:409–35. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 13.Becker J, Delayre-Orthez C, Frossard N, Pons F. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundamental & clinical pharmacology. 2006;20(5):429–47. doi: 10.1111/j.1472-8206.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 14.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, et al. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288(6):L1146–53. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 15.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. The Journal of clinical investigation. 2006;116(3):598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, et al. PPAR-γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L891–901. doi: 10.1152/ajplung.00333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy AT, Lakshmi SP, Zhang Y, Reddy RC. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28(12):5299–310. doi: 10.1096/fj.14-256263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu M, Zhang J, Lin Y, Zhu X, Zhao L, Ahmad M, et al. Early stimulation and late inhibition of peroxisome proliferator-activated receptor gamma (PPAR gamma) gene expression by transforming growth factor beta in human aortic smooth muscle cells: role of early growth-response factor-1 (Egr-1), activator protein 1 (AP1) and Smads. The Biochemical journal. 2003;370(Pt 3):1019–25. doi: 10.1042/BJ20021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J, Ghosh AK, Sargent JL, Komura K, Wu M, Huang QQ, et al. PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PloS one. 2010;5(11):e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng S, Chen A. Disruption of transforming growth factor-beta signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-gamma in rat hepatic stellate cells. American journal of physiology Gastrointestinal and liver physiology. 2007;292(1):G113–23. doi: 10.1152/ajpgi.00200.2006. [DOI] [PubMed] [Google Scholar]
- 21.Lakshmi SP, Reddy AT, Zhang Y, Sciurba FC, Mallampalli RK, Duncan SR, et al. Down-regulated peroxisome proliferator-activated receptor gamma (PPARgamma) in lung epithelial cells promotes a PPARgamma agonist-reversible proinflammatory phenotype in Chronic Obstructive Pulmonary Disease (COPD) The Journal of biological chemistry. 2014;289(10):6383–93. doi: 10.1074/jbc.M113.536805. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Roberts TC, Hart JR, Kaikkonen MU, Weinberg MS, Vogt PK, Morris KV. Quantification of nascent transcription by bromouridine immunocapture nuclear run-on RT-qPCR. Nat Protocols. 2015;10(8):1198–211. doi: 10.1038/nprot.2015.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sievers F, Higgins DG. Clustal omega. Current Protocols in Bioinformatics. 2014:3.13.1–3. 6. doi: 10.1002/0471250953.bi0313s48. [DOI] [PubMed] [Google Scholar]
- 24.Crooks GE, Hon G, Chandonia J-M, Brenner SE. WebLogo: a sequence logo generator. Genome research. 2004;14(6):1188–90. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuszynska I, Magnus M, Jonak K, Dawson W, Bujnicki JM. NPDock: a web server for protein-nucleic acid docking. Nucleic acids research. 2015;43(W1):W425–30. doi: 10.1093/nar/gkv493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White LA, Mitchell TI, Brinckerhoff CE. Transforming growth factor beta inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. Biochimica et biophysica acta. 2000;1490(3):259–68. doi: 10.1016/s0167-4781(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 27.Frederick JP, Liberati NT, Waddell DS, Shi Y, Wang XF. Transforming growth factor beta-mediated transcriptional repression of c-myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Molecular and cellular biology. 2004;24(6):2546–59. doi: 10.1128/MCB.24.6.2546-2559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yagi K, Furuhashi M, Aoki H, Goto D, Kuwano H, Sugamura K, et al. c-myc is a downstream target of the Smad pathway. The Journal of biological chemistry. 2002;277(1):854–61. doi: 10.1074/jbc.M104170200. [DOI] [PubMed] [Google Scholar]
- 29.Chen CR, Kang Y, Siegel PM, Massague J. E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell. 2002;110(1):19–32. doi: 10.1016/s0092-8674(02)00801-2. [DOI] [PubMed] [Google Scholar]
- 30.Verrecchia F, Mauviel A. Transforming growth factor-β and fibrosis. World J Gastroenterol. 2007;13(22):3056–62. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, et al. Hypoxia induces downregulation of PPAR-gamma in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-beta signaling. Am J Physiol Lung Cell Mol Physiol. 2011;301(6):L899–907. doi: 10.1152/ajplung.00062.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culver DA, Barna BP, Raychaudhuri B, Bonfield TL, Abraham S, Malur A, et al. Peroxisome proliferator–activated receptor γ activity is deficient in alveolar macrophages in pulmonary sarcoidosis. American journal of respiratory cell and molecular biology. 2004;30(1):1–5. doi: 10.1165/rcmb.2003-0304RC. [DOI] [PubMed] [Google Scholar]
- 33.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, et al. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L1031–41. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M, Wu R, Dong W, Jacob A, Wang P. Endotoxin Downregulates Peroxisome Proliferator-activated Rreceptor-{gamma} via the Increase in TNF-{alpha} Release. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00340.2007. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Mukherjee A, Lebman DA, Fang X. Gene expression of the lysophosphatidic acid receptor 1 is a target of transforming growth factor beta. Oncogene. 2013;32(26):3198–206. doi: 10.1038/onc.2012.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowalik TF. Smad about E2F. TGFbeta repressionof c-Myc via a Smad3/E2F/p107 complex. Mol Cell. 2002;10(1):7–8. doi: 10.1016/s1097-2765(02)00584-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Yamada O, Kida S, Matsushita Y, Hattori T. Down-regulation of osteopontin mediates a novel mechanism underlying the cytostatic activity of TGF-beta. Cellular oncology (Dordrecht) 2016;39(2):119–28. doi: 10.1007/s13402-015-0257-1. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Johnson K, Chen HJ, Carroll S, Laughon A. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature. 1997;388(6639):304–8. doi: 10.1038/40906. [DOI] [PubMed] [Google Scholar]
- 39.Pouponnot C, Jayaraman L, Massague J. Physical and functional interaction of SMADs and p300/CBP. The Journal of biological chemistry. 1998;273(36):22865–8. doi: 10.1074/jbc.273.36.22865. [DOI] [PubMed] [Google Scholar]
- 40.Feng XH, Zhang Y, Wu RY, Derynck R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-beta-induced transcriptional activation. Genes & development. 1998;12(14):2153–63. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, et al. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. The Journal of biological chemistry. 1999;274(49):35269–77. doi: 10.1074/jbc.274.49.35269. [DOI] [PubMed] [Google Scholar]
- 42.Wotton D, Lo RS, Swaby LA, Massague J. Multiple modes of repression by the Smad transcriptional corepressor TGIF. The Journal of biological chemistry. 1999;274(52):37105–10. doi: 10.1074/jbc.274.52.37105. [DOI] [PubMed] [Google Scholar]
- 43.Kim DW, Lassar AB. Smad-dependent recruitment of a histone deacetylase/Sin3A complex modulates the bone morphogenetic protein-dependent transcriptional repressor activity of Nkx3.2. Molecular and cellular biology. 2003;23(23):8704–17. doi: 10.1128/MCB.23.23.8704-8717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macaluso M, Cinti C, Russo G, Russo A, Giordano A. pRb2/p130-E2F4/5-HDAC1-SUV39H1-p300 and pRb2/p130-E2F4/5-HDAC1-SUV39H1-DNMT1 multimolecular complexes mediate the transcription of estrogen receptor-alpha in breast cancer. Oncogene. 2003;22(23):3511–7. doi: 10.1038/sj.onc.1206578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.