Abstract
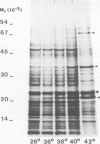
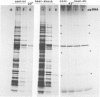
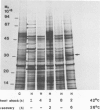
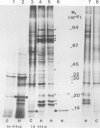
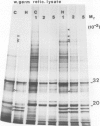
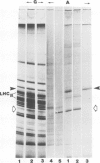
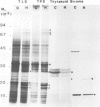
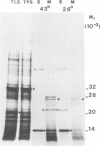
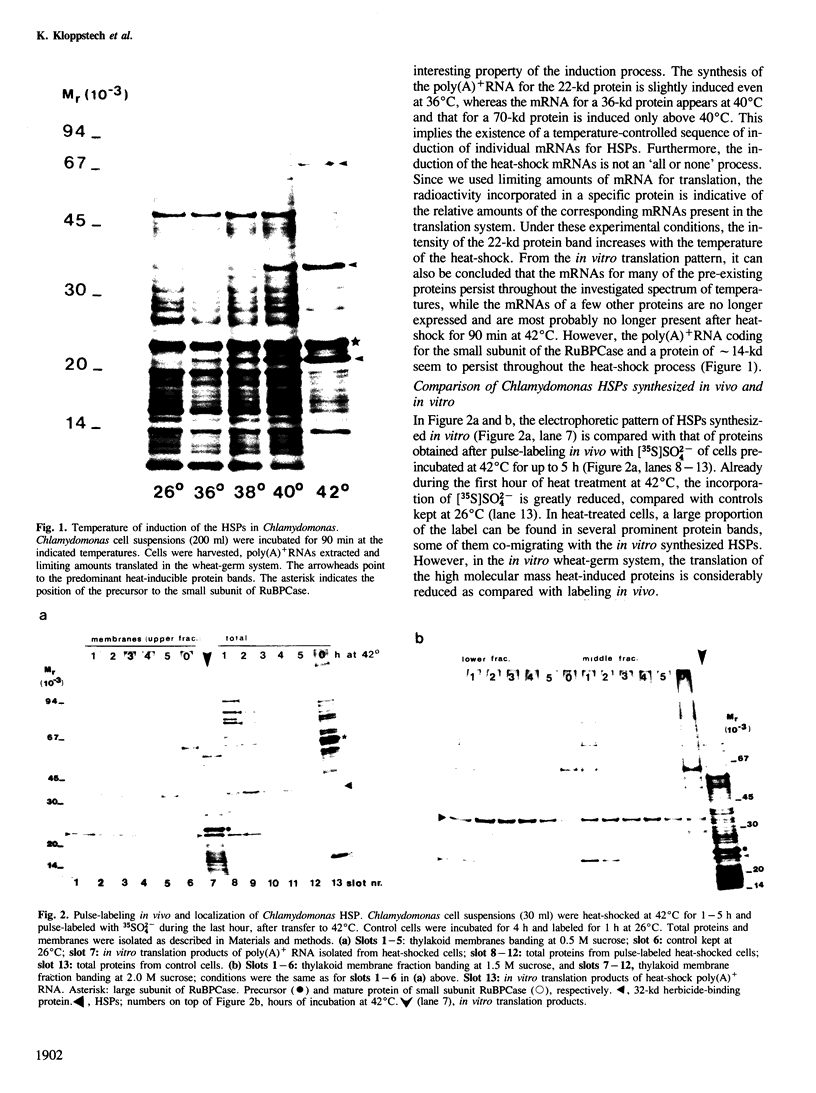
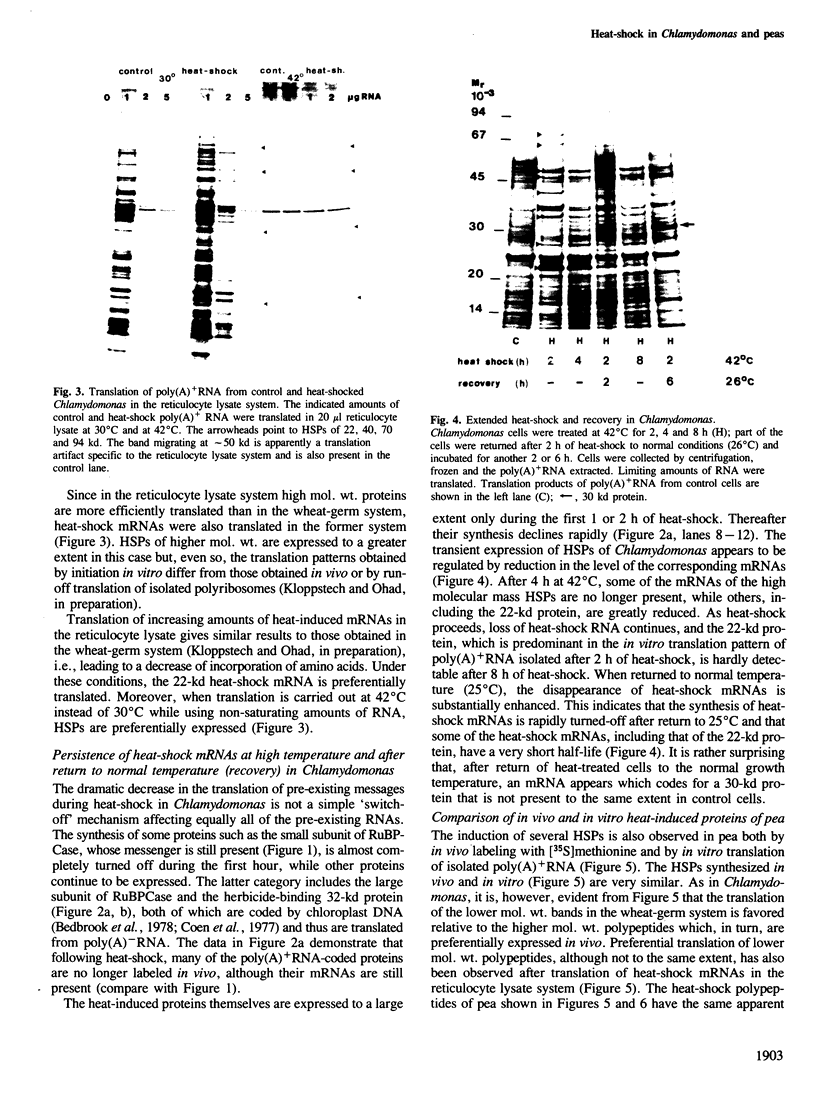
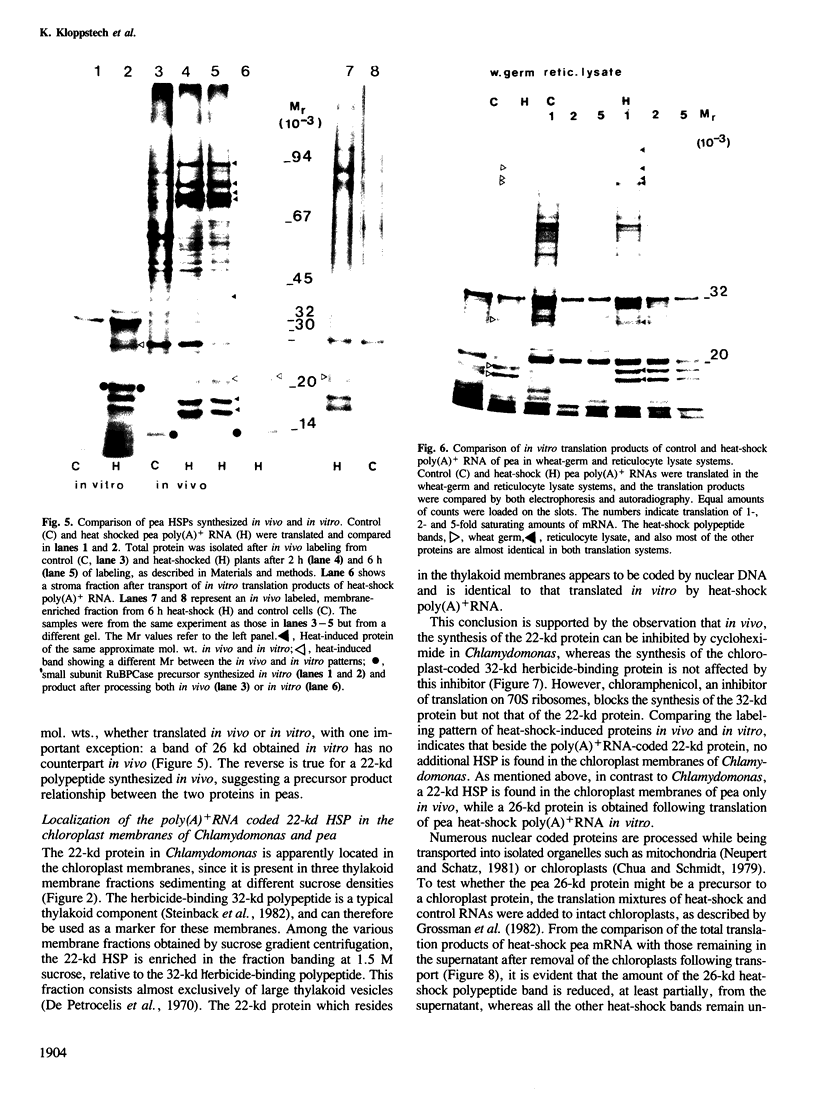
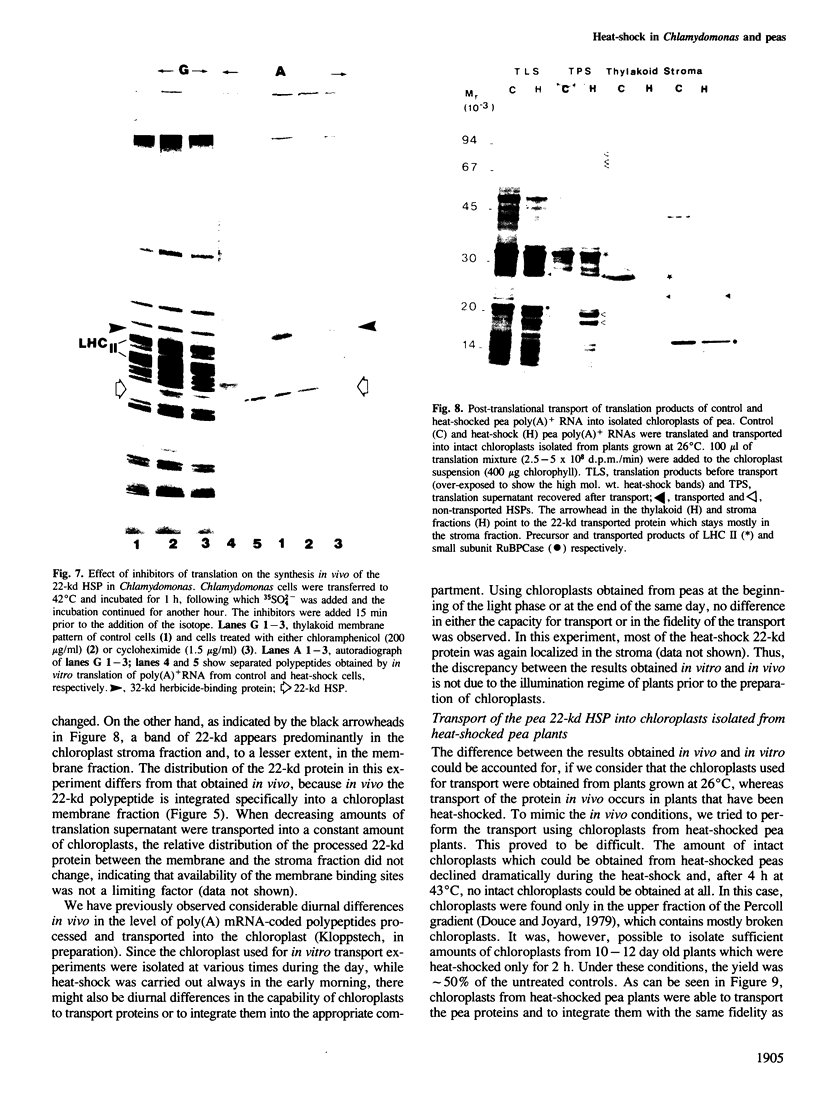
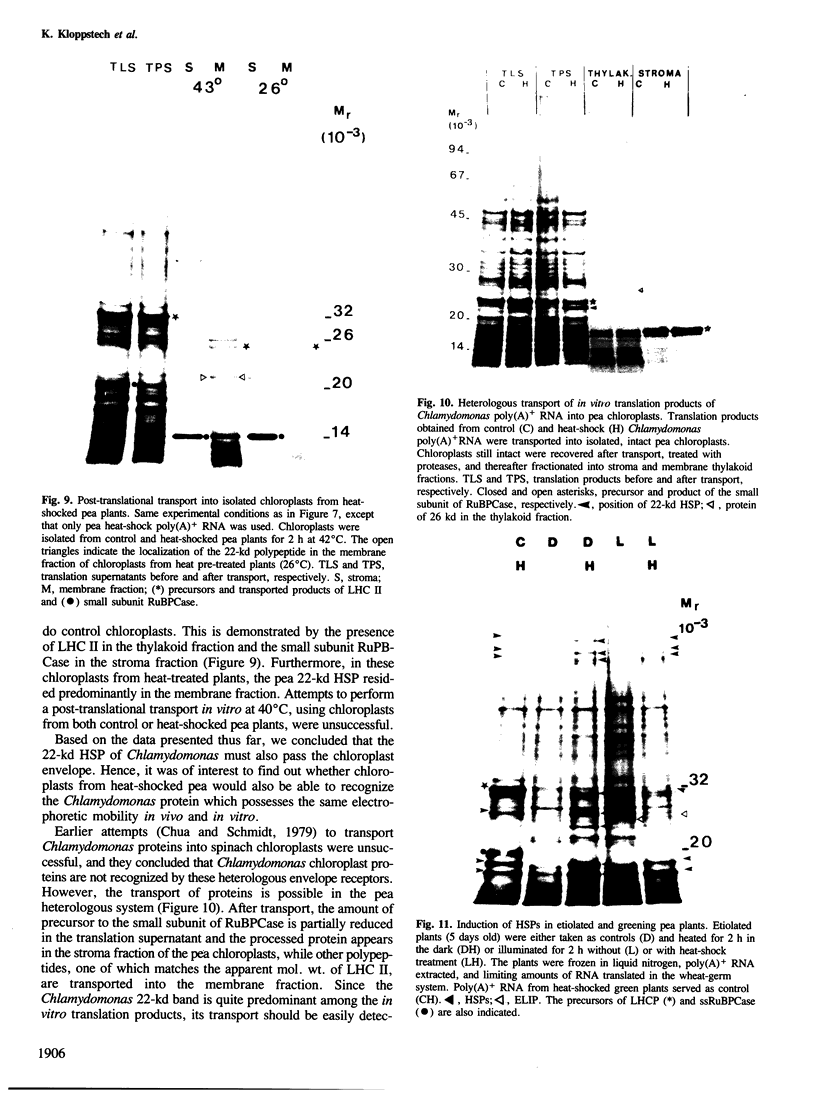
The synthesis, transport and localization of a nuclear coded 22-kd heat-shock protein (HSP) in the chloroplast membranes was studied in pea plants and Chlamydomonas reinhardi. HSPs were detected in both systems by in vivo labeling and in vitro translation of poly(A)+RNA, using the wheat-germ and reticulocyte lysate systems. Heat-shock treatment of pea plants for 2 h at 42-45°C induces the expression of ˜10 nuclear coded proteins, among which several (18 kd, 19 kd, 22 kd) are predominant. A 22-kd protein is synthesized as a 26-kd precursor protein and is localized in a chloroplast membrane fraction in vivo. Following post-translational transport into intact chloroplasts in vitro of the 26-kd precursor, the protein is processed but the resulting 22-kd mature protein is localized in the chloroplast stroma. If, however, the in vitro transport is carried out with chloroplasts from heat-shocked plants, the 22-kd protein is preferentially transported to the chloroplast membrane fraction. In C. reinhardi the synthesis of poly(A)+RNAs coding for several HSPs is progressively and sequentially induced when raising the temperature for 1.5 h from 36°C to 42°C, while that of several preexisting RNAs is reduced. Various pre-existing poly(A)+RNAs endure in the cells at 42°C up to 5 h but are no longer translated in vivo, whereas some poly(A)−RNAs persist and are translated. As in pea, a poly(A)+RNA coded 22-kd HSP is localized in the chloroplast membranes in vivo, although it is translated as a 22-kd protein in vitro. The in vitro translated protein is not transported in isolated pea chloroplast which, however, processes and transports other nuclear coded chloroplast proteins of Chlamydomonas. The poly(A)+RNA coding for the 22-kd HSP appears after 1 h at 36°C. Its synthesis increases with the temperature of incubation up to 42°C, although it decreases after ˜2 h of heat treatment and the already synthesized RNA is rapidly degraded. The degradation is faster upon return of the cells to 26°C. None of the heat-induced proteins is identical to the light-inducible proteins of the chloroplast membranes.
Keywords: Chlamydomonas, pea, chloroplast membranes, heat-shock, in vivo and in vitro protein synthesis, post-translational transport
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Bedbrook J. R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H., Hauptmann R. M. Tissue specificity of the heat-shock response in maize. Plant Physiol. 1984 Jun;75(2):431–441. doi: 10.1104/pp.75.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis B., Siekevitz P., Palade G. E. Changes in chemical composition of thylakoid membranes during greening of the y-1 mutant of Chlamydomonas reinhardi. J Cell Biol. 1970 Mar;44(3):618–634. doi: 10.1083/jcb.44.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Hoober J. K., Marks D. B., Keller B. J., Margulies M. M. Regulation of accumulation of the major thylakoid polypeptides in Chlamydomonas reinhardtii y-1 at 25 degrees C and 38 degrees C. J Cell Biol. 1982 Nov;95(2 Pt 1):552–558. doi: 10.1083/jcb.95.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Hall C., Leung T., Whatley S. The relationship of the rat brain 68 kDa microtubule-associated protein with synaptosomal plasma membranes and with the Drosophila 70 kDa heat-shock protein. Biochem J. 1984 Dec 1;224(2):677–680. doi: 10.1042/bj2240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G., Kloppstech K. A rapidly light-induced chloroplast protein with a high turnover coded for by pea nuclear DNA. Eur J Biochem. 1984 Jan 2;138(1):201–207. doi: 10.1111/j.1432-1033.1984.tb07900.x. [DOI] [PubMed] [Google Scholar]
- Neumann D., Scharf K. D., Nover L. Heat shock induced changes of plant cell ultrastructure and autoradiographic localization of heat shock proteins. Eur J Cell Biol. 1984 Jul;34(2):254–264. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Arntzen C. J. Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J Cell Biol. 1984 Aug;99(2):481–485. doi: 10.1083/jcb.99.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. I. Plastid dedifferentiation in a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):521–552. doi: 10.1083/jcb.35.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. C., Ohad I. Phosphorylation of chlamydomonas reinhardi chloroplast membrane proteins in vivo and in vitro. J Cell Biol. 1982 Jun;93(3):712–718. doi: 10.1083/jcb.93.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pfisterer J., Lachmann P., Kloppstech K. Transport of proteins into chloroplasts. Binding of nuclear-coded chloroplast proteins to the chloroplast envelope. Eur J Biochem. 1982 Aug;126(1):143–148. doi: 10.1111/j.1432-1033.1982.tb06758.x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Raschke E., Nagao R. T. The DNA sequence analysis of soybean heat-shock genes and identification of possible regulatory promoter elements. EMBO J. 1984 Nov;3(11):2491–2497. doi: 10.1002/j.1460-2075.1984.tb02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Pardue M. L., Penman S. Messenger RNA in heat-shocked Drosophila cells. J Mol Biol. 1977 Feb 5;109(4):559–587. doi: 10.1016/s0022-2836(77)80091-0. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez J. M., Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984 Mar;36(3):655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Glover J. F., Tata J. R. Culture shock. Synthesis of heat-shock-like proteins in fresh primary cell cultures. Exp Cell Res. 1984 Oct;154(2):581–590. doi: 10.1016/0014-4827(84)90182-4. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]