Abstract
Aims
The present study to address one of the mechanisms in preeclampsia, examined whether levels of oxidative stress, human serum albumin, and endothelial function correlate in pregnant women and whether human serum albumin reduces levels of superoxide produced by NADPH oxidase activation in the human vascular smooth muscle cells.
Materials and methods
Pregnant women with (Preeclampsia group, n = 33) and without preeclampsia (Normal group, n = 37) were recruited to determine levels of reactive oxygen species (serum diacron-reactive oxygen metabolite [d-ROM]), and the flow-mediated dilation (FMD). Human coronary arterial smooth muscle cells or omental arteries were subjected to evaluate isometric force recordings, levels of superoxide, western immunoblotting, and immunohistochemistry. The superoxide scavenging assay was also performed in a cell-free system.
Key findings
Women in the preeclampsia group demonstrated lower FMD and higher serum d-ROM values than those in the normal group. There were the inverse correlations between serum levels of d-ROM and the degree of FMD and between serum levels of albumin and those of d-ROM. D-glucose reduced the levcromakalim-induced dilation of human omental arteries, and it increased levels of superoxide and the recruitment of the NADPH oxidase subunit p47phox in human coronary arterial smooth muscle cells. Human serum albumin (0.05 to 0.5 g/dL) prevented these alterations whereas it exerted no superoxide scavenging effect.
Significance
Serum albumin relates to oxidative stress inversely, but to the endothelial function positively, in pregnant women. Human serum albumin appears to reduce oxidative stress via NADPH oxidase inhibition in the human vascular smooth muscle, indicating that the serum level may be a critical determinant of vascular oxidative stress in some human diseases.
Keywords: Medicine, Reproductive medicine, Systems biology, Cell biology, Endocrinology, Metabolism, Cardiology, Pathology, Physiology
1. Introduction
The systemic vascular dysfunction, which involves the imbalance between constrictors and dilators in the maternal vasculature, hyperresponsiveness to constrictor stimuli, reduced endothelium-dependent dilation, and vascular oxidative stress, is one of the critical pathological mechanisms of preeclampsia [1, 2]. Of these, oxidative stress has been known to be responsible for the morbidity associated with preeclampsia [1, 2]. Hypoalbuminemia is not uncommon in women with the disease state whereas macroalbumiuria is a predictor of adverse pregnancy outcomes in the population [3]. Previous biochemical studies demonstrated that serum albumin plays a role as an antioxidant scavenging carbon-centered free radicals [4, 5]. However, whether levels of oxidative stress, human serum albumin, and endothelial function correlate in pregnant women including those with preeclampsia, remains unclear.
Superoxide is a primary source of several reactive oxygen species, and NADPH oxidase is the most critical system for superoxide production in vascular pathology [6]. The vast majority of cardiovascular diseases including hypertension, hyperglycemia, heart failure and arteriosclerosis involve the NADPH oxidase activation of vascular tissues in the etiology [7, 8, 9, 10]. A clinical study indicated that the albumin supplementation enhances the plasma antioxidant capacity via the reduction in levels of plasma protein carbonyls [11]. However, whether albumin is a superoxide antagonist has been unknown.
The present study was designed to examine whether levels of oxidative stress, human serum albumin, and endothelial function correlate in pregnant women including preeclampsia, whether human serum albumin reduces levels of superoxide produced by NADPH oxidase activation in the human vascular smooth muscle cells and whether human serum albumin exerts the effects independently of the superoxide scavenging. Therefore, results of the current study indicate the role of human serum albumin as an antioxidant in pregnant women in vivo, and in the human vascular tissues in vitro.
2. Materials and methods
The research committee at Aichi Medical University (Aichi, Japan) approved this study (No. 12–010 and 13–037), which was conducted following the Declaration of Helsinki. The clinical protocol of this study was registered at UMIN Clinical Trials Registry (number UMIN000024382; http://www.umin.ac.jp/ctr/). The written informed consent was obtained from each patient enrolled in this study.
2.1. Clinical evaluation
Thirty-three pregnant women with preeclampsia (Preeclampsia group) and 37 with uncomplicated pregnancies (Normal group) were recruited. Preeclampsia was defined according to criteria of the Japan Society for the Study of Hypertension in Pregnancy, as follows: systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg after the gestation of 20 weeks, and proteinuria (≥ 300 mg protein/24 h) [12]. None of the participants with preeclampsia had hemolysis, elevated liver enzymes, low platelet syndrome, chronic kidney disease, and antihypertensives. All women with uncomplicated pregnancies had an ordinary course of gestation, as well as full-term delivery. None of the participants consumed alcohol or caffeine, or had a history of thyroid disease, hypertension, diabetes mellitus, hyperlipidemia, or liver disease, and were not taking any medications known to influence lipoprotein metabolism. The sampled blood at AM 8:00 from each subject was used for collection of biochemical data including levels of serum albumin measured by a modified bromocresol purple assay, as well as the spectrophotometric measurement at 505 nm for the levels of a serum marker of non-selective reactive oxygen species, diacron-reactive oxygen metabolite (d-ROM) using the Free Radical Analytical System 4 (FRAS, Diacron, Italy) with a commercial kit (FRAS, Diacron, Italy) [2, 13]. All blood samples were analyzed immediately. The concentrations of d-ROM were expressed as Carratelli units (CARR U).
The flow-mediated dilation (FMD) of the brachial artery was evaluated to determine the in vivo endothelial function employing a high-resolution Doppler ultrasound with a 10 MHz linear array transducer (Voluson E8, GE Healthcare, Zipf, Austria) as follows [14, 15]. A 5-cm length of the brachial artery was imaged in a longitudinal section above the antecubital fossa, and baseline pictures of brachial artery diameter were recorded and stored electronically. A distal occluding forearm cuff placed just below the antecubital fossa was inflated 50 mmHg above systolic pressure for five min whereas images of brachial artery were continuously recorded and stored for the evaluation. The brachial artery scan images were acquired for ≥ 10 seconds before cuff release, and the data collection continued for 2 min after the release. The percent FMD of the brachial artery was calculated by the following equation: % FMD = 100 × ([the diameter one minute after the cuff release − the diameter of control condition at rest]/the diameter of control condition at rest) [14, 15].
2.2. In vitro experiments using the human omental artery [10, 16, 17] and cultured human coronary arterial smooth muscle cells
The part of greater omentum was obtained from patients (n = 17, 50 to 74 year of ages), who were scheduled for the elective gastric surgery, and without heart disease as well as coronary risk factors including diabetes mellitus, hypertension, hypercholesterolemia and smoking habit. The omentum was put in ice-cold modified Krebs-Ringer bicarbonate solution (control solution, pH 7.4) immediately after the resection. The endothelium of each arterial ring (3 mm in length) from the omental artery (0.5–1.0 mm in diameter) was removed using a 26 G needle with the rough surface to avoid the involvement of endothelium-derived factors. The removal of endothelium was confirmed by the absence of vasodilation induced by bradykinin (10−6 mol/L). All experiments were performed in the presence of D-glucose (5.5 mmol/L) in the control condition. Each arterial ring was connected to an isometric force transducer and suspended in an organ chamber filled with 10 ml control solution (37 °C, pH 7.4) bubbled with 95% O2 and 5% CO2. The ring was gradually stretched to the optimal point of its length-tension curve as determined by the contraction to a prostaglandin H2/thromboxane receptor agonist U46619 (10−7 mol/L). In most of the rings, the optimal resting tension achieved approximately at 1.0 g. Sixty min after the incubation in the absence or the presence of L-glucose (20 mmol/L), D-glucose (20 mmol/L), a superoxide antagonist Tiron (10 mmol/L), a selective ATP-sensitive K+ channel antagonist glibenclamide (10−6 mol/L), human serum albumin (BENESIS or Albuminar 5% I.V. Injection™ [CLS Behring], 0.5 g/dL) or bovine serum albumin (0.5 g/dL), U46619 (10−7 mol/L) was added to the chambers to contract arterial rings. The degree of reduction in a critical thiol group (Cys-34) of human serum albumin may affect the results in the current in vitro experiments if human serum albumin has a scavenging capacity toward oxygen-derived free radicals. Therefore, the present study employed two types of human serum albumin with the different reduction rate of Cys-34 including human serum albumin BENESIS (about 50%) and human serum albumin CLS Behring (about 10%) [18]. During submaximal contraction, which reached plateau about 15 min after the addition of U46619, concentration-response curves to an ATP-sensitive K+ channel opener levcromakalim (10−8 to 3 × 10−6 mol/L) were obtained cumulatively with the interval of about 6 min. Each group of studies shown in the same graph was evaluated in parallel using omental arterial rings from the same patient. The relaxation was expressed as a percentage of the maximal relaxation in response to papaverine (3 × 10−4 mol/L). One drop of Antifoam A for each chamber (10 mL) was employed to suppress foaming during experiments with albumin by bubbling with 95% O2 and 5% CO2. Antifoam A did not solely alter the inhibitory effect of D-glucose (20 mmol/L) in this experimental condition.
Human coronary arterial smooth muscle cells (Cat No. KS-4209, Kurabo Industries Ltd., Osaka, Japan) were grown in HuMedia-SG2 medium (Kurabo Industries Ltd., Osaka, Japan) at 37 °C in the humid air with 5% carbon dioxide. The medium was exchanged every two to three days after the onset of cell outgrowth. After three to four passages, human coronary arterial smooth muscle cells were seeded in the 100 mm culture dishes or four-well glass chamber slides (BD Biosciences, Bedford, MA, USA) for the further experiments.
An oxidative fluorescent dye hydroethidine was used for semiquantitative evaluation of superoxide in situ [10]. Hydroethidine (7 mg) was diluted with N,N-dimethylacetamide (1 mL). The incubation of human coronary arterial smooth muscle cells was made at 37 °C and 5% carbon dioxide in the room air using the modified Krebs-Ringer solution (control solution) of the following composition (mmol/L): NaCl 119, KCl 4.7, CaCl2 2.5, MgSO4 1.17, KH2PO4 1.18, NaHCO3 25, and glucose 5.5. In some experiments, D-glucose (20 mmol/L), L-glucose (20 mmol/L), Tiron (10 mmol/L), a NADPH oxidase inhibitor gp91ds-tat (10−6 mol/L), a scrambled, non-inhibitory version of gp91ds-tat, sgp91ds-tat (10−6 mol/L), human serum albumin (Albumin 5% I.V.‐ BENESIS™ [BENESIS], 0.05 to 0.5 g/dL), methyl-β-cyclodextrin (10 mmol/L), the glycoprotein gp60 antibody (20 μg/mL; HHV-6 gp60/110, cat. # sc-58156; Santa Cruz Biotechnology, Inc, Dallas, TX) or the combination was added to the control solution. After the 60 min’ incubation with combinations of above compounds, hydroethidine (2 × 10−6 mol/L) in phosphate-buffered saline (pH 7.4) was applied to each slide chamber [10]. Hoechst 33258 (1 μg/mL) was simultaneously applied to stain nuclei of smooth muscle cells. Cells were incubated in a light-protected chamber at 37 °C for 20 min. Images of cellular fluorescence were acquired using a microscope fitted with BZ-II analyzer software (Model BZ-9000 Generation II, Keyence, Osaka, Japan). Settings were identical for the acquisition of images from all of the cells. The total ethidium bromide fluorescence determined in each specimen was standardized as the ratio using the control fluorescence in arterial slices in the sole presence of Tiron (10 mmol/L) [10].
The cultured human coronary arterial smooth muscle cells in 100 mm culture dishes were incubated in the modified Krebs-Ringer bicarbonate solution (37 °C, pH 7.4) containing D-glucose (5.5 mmol/L) inflated 5% CO2 in the room air. The cells were incubated with L-glucose (20 mmol/L), D-glucose (20 mmol/L), D-glucose (20 mmol/L) in combination with human serum albumin (BENESIS, 0.5 g/dL) for 60 min. After the incubation, cells were washed twice with cold PBS and then suspended in 500 μL of 10 mmol/L Tris-HCl (pH 7.6) containing 1 × protease inhibitor cocktail. The cells were homogenized on ice for the 30 seconds and then sonicated for another 30 seconds on the ice. The homogenized cell suspensions were then centrifuged at 500 × g for 15 min at 4 °C. After centrifugation, supernatants were further ultracentrifuged at 100,000 × g for 60 min at 4 °C, and the clear supernatants were used as the cytosolic protein fractions. The remaining pellets were the crude membrane fraction and dissolved in RIPA buffer (50 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 × protease inhibitor cocktail) [19]. The protein concentrations in the cytosolic, as well as membrane fractions, were estimated by the BCA protein assay (Thermo Fisher Scientific Inc., Rockford, IL, USA). The same amount of protein was separated by SDS–PAGE and transferred to PVDF membranes (Immobilon-P, EMD Millipore Corp, Billerica, MA, USA). These membranes were probed with antibodies against NOX2 (cat. # GTX63960, 1:500 dilution; GeneTex Inc., Irvine, CA), p47-phox (cat. # 07–497,1:500 dilution; Millipore Inc.), rac1 (cat. #, 1:500 dilution; Abcam plc, Cambridge, UK) and Na+/K+-ATPase (cat. # 3010, 1:500 dilution; Cell Signaling Technology Inc., Danvers MA), followed by peroxidase-conjugated anti-rabbit or anti-mouse IgG (1:5000; Zymed Laboratories, San Francisco, CA, USA). Proteins were visualized using ECL prime western blotting detection reagent (GE Healthcare, Buckinghamshire, UK) [20]. The results were quantified according to the expression level of Na+/K+-ATPase using the Image Analyzer System (ImageQuant LAS4000, GE Healthcare UK Ltd., Pollards Wood, UK).
Human coronary arterial smooth muscle cells were seeded in four-well glass chamber slides were incubated in the modified Krebs-Ringer bicarbonate solution (37 °C, pH 7.4) containing D-glucose (5.5 mmol/L) inflated 5% CO2 in the room air. The cells were incubated with L-glucose (20 mmol/L), D-glucose (20 mmol/L), or D-glucose (20 mmol/L) in combination with human serum albumin (BENESIS, 0.5 g/dL) for 60 min, and after that quickly fixed for 15 min in 4% paraformaldehyde. For immunohistochemical determination of target molecules, the fixed cells were exposed to phosphate-buffered saline with 3% bovine serum albumin in combination with 0.05% Triton X–100 at 24 °C for 60 min. After that, the cells were incubated for 120 min with the anti-p47phox antibody (cat. # sc-7660, 1:100; Santa Cruz Biotechnology, Inc, Dallas, TX). Detection was performed by incubation for 60 min with specific donkey anti-goat secondary antibody conjugated with fluorescein (1:500; Thermo Fisher Scientific Inc.). Finally, 4,6-dianidina-2-phenylindole (DAPI, 1 μg/mL; Thermo Fisher Scientific Inc.) was applied for 5 min to stain all nuclei. The fluorescence images were acquired using a microscope fitted with BZ-II analyzer software (Model BZ-9000 Generation II, Keyence, Osaka, Japan). Settings were identical for the acquisition of images from all of the cells. The negative control did not show any nonspecific staining.
2.3. Studies using a cell-free superoxide generating system
The superoxide production rate in the reaction between xanthine and xanthine oxidase was continuously monitored by a spectrophotometer (GENESYS 10S Bio, Thermo Scientific, Waltham, MA) as previously described with modification [17, 21]. The enzymatic reaction was commenced by adding a 10 μl portion of xanthine oxidase to HEPES (25 mmol/L)-buffered normal saline (90 μl), which was incubated at 25 °C in the cuvette placed on the stage of the spectrophotometer. Each composition of the final reaction mixture at pH 7.4 was HEPES (25 mmol/L), NaCl (127 mmol/L), ferricytochrome c (5 × 10−5 mol/L), xanthine (2 × 10−4 mol/L) and xanthine oxidase (0.1 U/mL). Human serum albumin (1.8 g/dL, BENESIS) or superoxide dismutase (SOD, 1500 U/ml) was also added to the mixture as required (at the final concentrations). Before each batch of experiments, the enzymatic activity of xanthine oxidase was determined by the uric acid generation for the first one minute at 25 °C using an extinction coefficient at 295 nm (EmM = 11). Superoxide production rate was calculated by the increase of reduced form of ferricytochrome c (EmM = 21 at 550 nm) for the initial 12 seconds in comparison with that in the presence of SOD.
2.4. Drugs
The following pharmacological agents were used: Antifoam A, bovine serum albumin, bradykinin, dimethyl sulfoxide, ferricytochrome-c (from bovine heart), glibenclamide, methyl-β-cyclodextrin, papaverine, superoxide dismutase (from bovine erythrocytes), Tiron, Triton X–100, U46619 and xanthine (Sigma Aldrich Inc., St. Louis, MO), N,N-dimethylacetamide, Hoechst 33258 and xanthine oxidase (from buttermilk) (Nacalai Tesque, Kyoto, Japan), hydroethidine (Polyscience Inc., Warrington, PA), gp91ds-tat (AnaSpec, Inc., Fremont, CA) and sgp91ds-tat (Genemed Synthesis Inc., San Antonio, TX), and human serum albumin (Albumin 5% I.V.‐ BENESIS™ Japan Blood Products Organization, Tokyo, Japan [BENESIS]; Albuminar 5% I.V. Injection™ CLS Behring Albumin, CLS Behring K.K., Tokyo, Japan [CLS Behring]). Drugs were dissolved in distilled water such that volumes of < 60 mL are added to the perfusion system. The stock solution of Tiron was prepared in dimethyl sulfoxide, and the highest concentration of dimethyl sulfoxide was 1.74 × 10−6 mol/L. Our previous studies confirmed that this vehicle does not affect vasomotor function in our experimental condition [22]. The concentrations of drugs are expressed as the final molar concentration.
2.5. Statistical analysis
The data are expressed as means ± SD, and n refers to the number of patients from which each data was taken. The power calculation was done using Sample Power 3.0™ (IBM Japan Inc., Tokyo, Japan). In the current study, a sample size of 33 gave 80% power to the detected d-ROM change of 130CARR U at a significance level of 0.05 (SD = 185), 98% power to the detected serum albumin change of 0.3 g/dL at a significance level of 0.05 (SD = 0.3), and 93% power to the detected FMD change of 3.0% at a significance level of 0.05 (SD = 3.5). Statistical analysis using PASW Statistics 18™ (IBM Japan Inc., Tokyo, Japan) was performed by the χ2 test, linear regression analysis evaluating Spearman’s rank correlation coefficient or repeated-measures analysis of variance followed by Scheffe’s test. Differences were considered to be statistically significant when P is < 0.05.
3. Results
3.1. Clinical evaluation
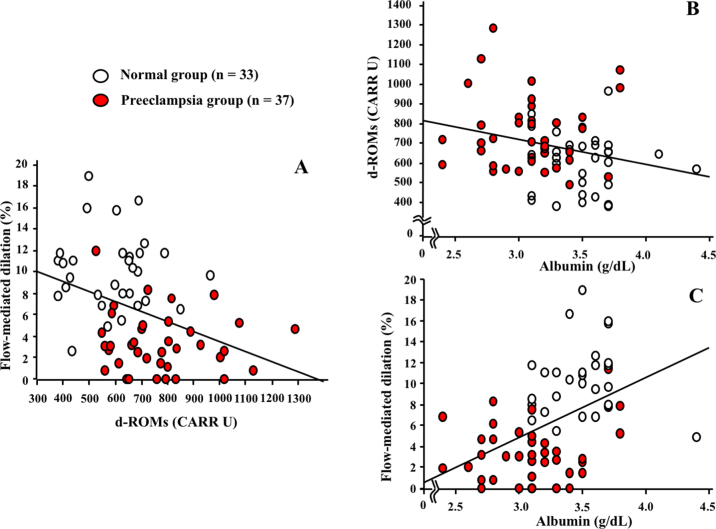
The participants in the preeclampsia group demonstrated significantly higher systolic, as well as diastolic, blood pressure whereas they showed lower FMD values than women in the normal group (Table 1). The gestational age at the data sampling in the preeclampsia group was lower than that in the normal group, whereas the difference was due to early admission for the treatment of preeclampsia and the planned or emergency Caesarean section (Table 1). Also, subjects in the preeclampsia group represented higher levels of serum d-ROM and lower levels of serum total protein as well as albumin than women in the normal group (Table 2). There was an inverse correlation between serum levels of d-ROM and the degree of FMD (R = -0.385, P < 0.05; Fig. 1A), and between serum levels of albumin and those of d-ROM (R = -0.26, P < 0.05; Fig. 1B). In contrast, there was a positive relationship between serum levels of albumin and the degree of FMD (R = 0.469, P < 0.05; Fig. 1C) in these subjects.
Table 1.
Participants’ Characteristics of pregnant women in the Clinical evaluation of oxidative stress and endothelial function.
| Normal group (n = 33) | Preeclampsia group (n = 37) | P value | |
|---|---|---|---|
| Age (years) | 33.5 ± 3.8 | 32.0 ± 5.2 | 0.19 |
| Parity | 0.57 ± 0.61 | 0.33 ± 0.57 | 0.08 |
| BMI (kg/m2) | 23.7 ± 3.2 | 25.0 ± 4.0 | 0.15 |
| Gestational age at the data sampling (week) |
37.9 ± 1.4 | 35.7 ± 3.3 | 0.0007 |
| Systolic Blood Pressure (mmHg) | 113.2 ± 8.5 | 147.1 ± 18.8 | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 70.3 ± 7.0 | 92.4 ± 9.9 | <0.0001 |
| Heart Rate (/min) | 81.5 ± 9.4 | 79.7 ± 13.6 | 0.53 |
| FMD (%) | 10.1 ± 3.5 | 3.4 ± 2.7 | <0.0001 |
BMI: Body mass index; FMD: flow-mediated dilation, Data are expressed as mean ± SD.
Table 2.
Biochemical data, as well as levels of serum diacron-reactive oxygen metabolite (d-ROM) in the clinical evaluation of oxidative stress and endothelial function in the pregnant women.
| Normal group (n = 33) | Preeclampsia group (n = 37) | P value | |
|---|---|---|---|
| d-ROM (CARR U) | 596 ± 141 | 761 ± 185 | <0.0001 |
| TP (g/dL) | 6.3 ± 0.6 | 6.0 ± 0.6 | 0.03 |
| Albumin (g/dL) | 3.5 ± 0.3 | 3.1 ± 0.3 | <0.0001 |
| Creatinine (mg/dL) | 0.5 ± 0.1 | 0.6 ± 0.1 | <0.0001 |
| Uric Acid (mg/dL) | 4.0 ± 0.8 | 6.4 ± 1.7 | <0.0001 |
| TC (mg/dL) | 244 ± 74 | 275 ± 60 | 0.09 |
| TG (mg/dL) | 208 ± 43 | 309 ± 167 | 0.09 |
| HDL (mg/dL) | 86 ± 13 | 80 ± 24 | 0.53 |
| LDL (mg/dL) | 163 ± 49 | 146 ± 53 | 0.43 |
| FBS (mg/dL) | 77 ± 7 | 77 ± 17 | 0.94 |
TP: total protein; TC: total cholesterol; TG: triglyceride; HDL: high density lipoprotein; LDL: low density lipoprotein, Data are expressed as mean ± SD.
Fig. 1.
(A) The inverse correlation (R = -0.385, P < 0.05) between serum levels of derivatives of reactive oxygen metabolites (d-ROMs) and the degree of flow mediated-dilation (FMD) in the women with preeclampsia (Preeclampsia group) and those without complicated pregnancies (Normal group). (B) The inverse relation (R = -0.26, P < 0.05) between serum levels of albumin and those of d-ROM in the women enrolled in this study. (C) The positive relationship (R = 0.469, P < 0.05) between serum levels of albumin and the degree of FMD in the enrolled subjects. Open circles and closed circles represent healthy women with uncomplicated pregnancies and preeclamptic women, respectively.
3.2. In vitro experiments using the human omental artery and cultured human coronary arterial smooth muscle cells
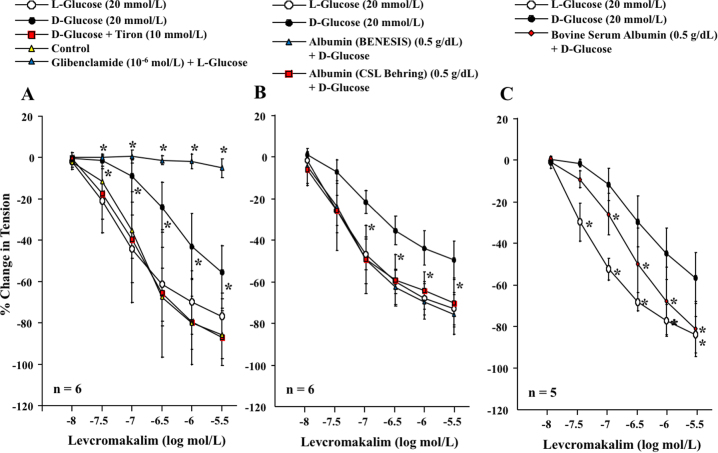
During submaximal contraction to U46619 (10−7 mol/L), levcromakalim (10−8 to 3 × 10−6 mol/L) similarly induced concentration-dependent relaxation in the human omental artery without endothelium treated with D-glucose (5.5 mmol/L) (the control group) or D-glucose (5.5 mmol/L) in combination with L-glucose (20 mmol/L) (the L-glucose group to adjust the osmolality) (Fig. 2A). Glibenclamide (10−6 mol/L) completely abolished the relaxation of arteries in the L-glucose group (Fig. 2A). The addition of D-glucose (20 mmol/L) to the control condition (the D-glucose group) inhibited the dilation induced by levcromakalim, whereas human serum albumin (BENESIS and Behring) and bovine serum albumin (0.5 g/dL), similarly to Tiron (10 mmol/L), restored the dilation inhibited by the addition of D-glucose (20 mmol/L) (Fig. 2B and C).
Fig. 2.
(A-C) Levcromakalim-induced dilation of human omental arteries without endothelium in the absence or the presence of L-glucose, D-glucose, Tiron, glibenclamide, human serum albumin (BENESIS or CSL Behring), bovine serum albumin or the combination. *: P < 0.05 vs. control or L-glucose.
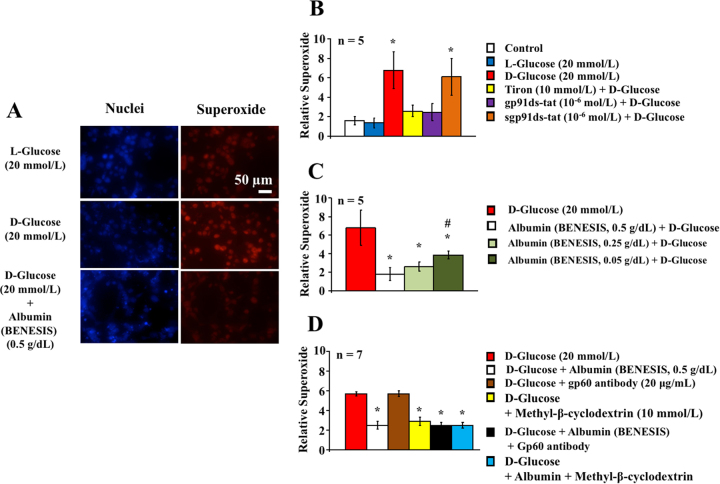
The incubation of human coronary arterial smooth muscle cells for 60 min using the modified Krebs-Ringer solution containing D-glucose (5.5 mmol/L) in combination with D-glucose (20 mmol/L), but not L-glucose (20 mmol/L), increased intracellular levels of superoxide (Fig. 3A and 3B). Gp91ds-tat (10−6 mol/L) and Tiron (10 mmol/L), but not sgp91ds-tat (10−6 mol/L), decreased the levels to the control condition (Fig. 3B). Human serum albumin (BENESIS, 0.05 to 0.5 g/dL) concentration-dependently reduced levels of superoxide produced by D-glucose (20 mmol/L, Figs. 3A and 3C), whereas the caveolae washout by methyl-β-cyclodextrin (10 mmol/L) and human serum albumin (BENESIS, 0.5 g/dL), but not the glycoprotein gp60 antibody (20 μg/ml), similarly attenuated the superoxide levels enhanced by D-glucose (20 mmol/L, Fig. 3D).
Fig. 3.
(A) Representative images of in situ superoxide production. Blue or red fluorescence indicates nuclei and superoxide in the human coronary arterial smooth muscle cells, respectively. (B-D) Relative superoxide production in the human coronary arterial smooth muscle cells treated with the addition of L-glucose, D-glucose, D-glucose in combination with Tiron, gp91ds-tat, sgp91ds-tat, human serum albumin (BENESIS, 0.05 to 0.5 g/dL), methyl-β-cyclodextrin, gp60 antibody (20 μg/mL) or the combination for 60 min. (B) *: P < 0.05 vs. control. (C) *: P < 0.05 vs. D-glucose, and #: P < 0.05 vs. albumin (0.5 g/dL). (D) *: P < 0.05 vs. D-glucose.
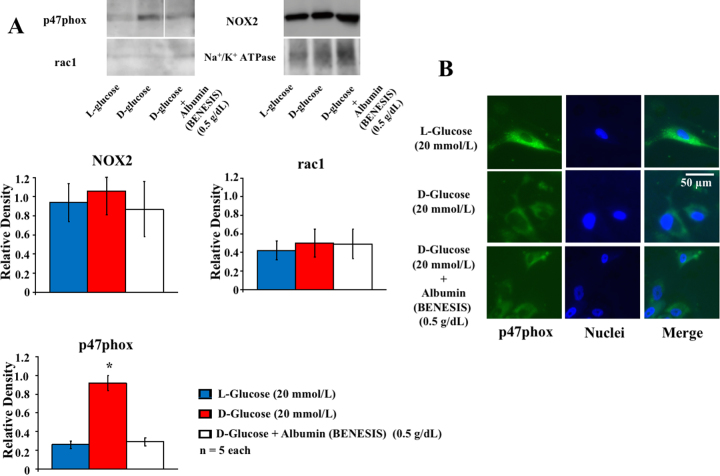
The incubation of human coronary arterial smooth muscle cells for 60 min using the modified Krebs-Ringer solution containing D-glucose (5.5 mmol/L) in combination with D-glucose (20 mmol/L), but not L-glucose (20 mmol/L), enhanced relative expression of a NADPH oxidase subunit p47phox in the membrane protein fraction of human coronary arterial smooth muscle cells, whereas the addition of D-glucose did not affect the relative protein expression of other NADPH oxidase subunits rac1 and NOX2 (Fig. 4A). Human serum albumin (BENESIS, 0.5 g/dL) in combination with D-glucose completely inhibited the membrane recruitment of p47phox (Fig. 4A).
Fig. 4.
(A) Relative protein expressions of NADPH oxidase subunits, p47phox, rac-1, NOX2, and Na+/K+ ATPase in the membrane fraction from human coronary arterial smooth muscle cells 60 min after addition of L-, D-glucose (20 mmol/L) or D-glucose in combination with human serum albumin (BENESIS, 0.5 g/dL). *: P < 0.05 vs. L-glucose. Note that p47phox images split were taken from the same picture in the Supplementary Figure, where the full, non-adjusted images are available. (B) Immunofluorescent image analysis in the human coronary arterial smooth muscle cells 60 min after addition of L-, D-Glucose or D-Glucose in combination with human serum albumin (BENESIS, 0.5 g/dL) from five separate experiments. P47phox (green) was localized to the cytoplasmic region whereas it migrates to the cell periphery after incubation with D-glucose.
In the immunohistochemical analysis, p47phox was localized at the cytoplasmic region close to nuclei after the incubation of human coronary arterial smooth muscle cells for 60 min with L-glucose (20 mmol/L) whereas it migrates to the cell periphery after incubation with D-glucose (20 mmol/L) (Fig. 4B). Human serum albumin (BENESIS, 0.5 g/dL) in combination with D-glucose inhibited the migration of p47phox to the cellular membrane (Fig. 4B).
3.3. Studies using a cell-free superoxide generating system
The addition of xanthine oxidase (0.004 U/mL) to the reaction mixture containing ferricytochrome-c (5 × 10−5 mol/L) increased the absorbance linearly at 550 nm. Removal of xanthine (10−4 mol/L) from the reaction mixture as well as the addition of allopurinol (2 × 10−4 mol/L) abolished the increase in the optical density. The higher concentration of human serum albumin (BENESIS 1.8 g/dL) did not alter the superoxide production rate (saline: 47.3 ± 5.6 [× 10−6 mol/L/min], human serum albumin: 48.8 ± 3.2 [× 10−6 mol/L/min], n = 5 each) from the reaction between xanthine and xanthine oxidase in this cell-free system.
4. Discussion and conclusions
4.1. The role of serum albumin in the clinical evaluation
The participants in the preeclampsia group showed lower values of FMD, which indicate the in vivo endothelial function in humans, than those in women of the normal group. The result is consistent with a recent consensus that the systemic vascular dysfunction including reduced endothelium-dependent dilation, co-exists with preeclampsia [1, 2]. Also, as previous studies documented, subjects in the preeclampsia group showed higher levels of serum d-ROM, which is a marker of reactive oxygen species, suggesting higher serum oxidative stress in the population [1, 2, 13]. Women in the preeclampsia group showed significantly lower levels of serum albumin than those in the normal group. The current study is first to demonstrate that the levels linearly correlate with the degree of oxidative stress (d-ROM) inversely and the endothelial function (FMD) positively in pregnant women and that FMD, serum albumin, and d-ROM are discriminative regarding the normal and preeclampsia groups. Therefore, preservation of the serum albumin level may suppress vascular oxidative stress and prevent endothelial dysfunction in the pregnant. Indeed, following previous clinical studies may support the conclusion in the current study. The albumin supplementation for 3 days enhanced the plasma antioxidant capacity in patients with acute lung injury [11] and albuminuria, which induces a decrease in levels of serum albumin, independently predicted cardiovascular disease incidence and death [23].
4.2. The role of serum albumin in the in vitro studies using human vascular smooth muscle cells
In the next step, in vitro studies evaluating the human vascular smooth muscle cell function were performed to disclose the role of albumin in the vascular oxidative stress. The concentrations of human serum albumin, namely up to 0.5 g/dL, were selected in the in vitro experiments as human serum albumin less than those appeared to make the difference of oxidative stress in the current clinical study. An oxidative stress model using the acute high D-glucose application, which induces superoxide production via the activation of NADPH oxidase, was employed to this purpose as our previous study built and confirmed [10]. A superoxide antagonist and bovine serum albumin, as well as two types of human serum albumin with the different percentage of Cys-34 reduction rate, similarly recovered ATP-sensitive K+ channel opener-induced dilation reduced by the high D-glucose application in the human omental arteries without endothelium. These results in the organ chamber experiments indicate that human serum albumin with any degree of the Cys-34 reduction, helps preserve human vascular smooth muscle function adversely affected by increased levels of superoxide and that the beneficial effect is not unique to human serum albumin but also to the albumin originated from other species [10, 18]. However, previous studies demonstrated that albumin and Tiron are Ca2+-binding molecules, and thus, they may augment the vasodilation by the reduction of extracellular Ca2+ levels [24, 25]. Therefore, one might be cautious to draw the definite conclusion that human serum albumin prevents oxidative stress within the vascular smooth muscle. For these reasons, the following experiments using human coronary arterial smooth muscle cells were conducted to further confirm the conclusion.
The high D-glucose application to human coronary arterial smooth muscle cells enhanced superoxide production and human serum albumin in a concentration-dependent fashion or a competitive inhibitor of the NADPH oxidase cytosolic subunit p47phox assembly (gp91ds-tat) abolished the increase similarly [26]. These results suggest that human serum albumin reduces levels of superoxide in the human vascular smooth muscle cells via the inhibition of p47phox subunit of NADPH oxidase. The idea is further supported by the results of the present study that human serum albumin inhibited the membrane protein expression as well as the membrane recruitment of p47phox since the cytosolic subunit needs to move toward cell membrane upon the activation of NADPH oxidase [6, 26]. It is also critical to note that human serum albumin seems to be capable of reducing oxidative stress occurred in the coronary vascular tissues.
How human serum albumin impairs the function of NADPH oxidase in the human vascular smooth muscle cells has to be mentioned. In the current study, the caveolae-disrupting agent methyl-β-cyclodextrin completely inhibited increased production of superoxide in human coronary arterial smooth muscle cells, indicating the crucial role of caveolae in the activation of NADPH oxidase [27]. It is most likely that human serum albumin acts on NADPH oxidase existed at caveolae, which is a lipid raft of cellular membrane. ATP-sensitive K+ channels are localized in caveolae in the arterial smooth muscle cells, emphasizing the crucial role of caveolae in the anti-oxidant effect of human serum albumin [28]. However, it is unlikely that human serum albumin affects the enzyme via endocytosis since the antibody of a glycoprotein gp60, which is a possible albumin-transporter via caveolae, did not alter the effect of human serum albumin on the level of superoxide [29, 30]. Why the gp60 blocking did not alter superoxide production in the present study is still unclear, and further studies will require verifying the mechanistic insight.
4.3. Effects of human serum albumin in a cell-free system
Three times higher concentration of human serum albumin (1.8 g/dL) than that in the experiments with vascular smooth muscle cells did not alter the superoxide production rate in the cell-free system established by previous studies [17, 21]. Therefore, human serum albumin appears to reduce oxidative stress independently of the superoxide scavenging. The molecular interaction between albumin and D-glucose also has to be considered in this study. The incubation of human serum albumin and D-glucose at the molar ratio of 1:15 at 22 °C requires three to four weeks to accomplish the glycation of the Lys-195 residue of the albumin molecule [31]. D-glucose (15–50 mmol/L) needs 20 to 42 days to make the complete transformation of the equal parts of human serum albumin to the glycated albumin in pH 7.4, 37 °C whereas the reaction happens linearly in the non-enzymatic fashion [32, 33]. These results indicate the negligible role of glycated albumin, as well as the molecular interaction between albumin and D-glucose in the study results since only 0.01% of human serum albumin seems to receive the glycation in the current study.
4.4. Limitations
The current study has several limitations as follows. The clinical study in a single medical institution had a small sample size with the different gestational age between groups although the statistical analysis has proved its adequate power. Therefore, future large-scale multicenter clinical studies will require to verify the conclusion of the current study. The in vitro studies indicate that the acute administration of commercially available human albumin preserves or restores the normal redox status of human blood vessels in some disease conditions. However, whether the supplemental albumin in patients with preeclampsia is potentially critical to prevent future cardiovascular diseases remains unclear although the link between the disease state and the cardiovascular risk after pregnancy has been reported [34].
5. Conclusions
In conclusion, human serum albumin relates to oxidative stress inversely, but to the endothelial function positively, in pregnant women including those with preeclampsia. Human serum albumin appears to reduce levels of superoxide via NADPH oxidase inhibition independently of the superoxide scavenging in the human vascular smooth muscle. The current study indicates the role of human serum albumin as an antioxidant in the clinical setting about the endothelial function in vivo, and in the in vitro conditions regarding changes in the vascular smooth muscle cell function relevant to superoxide production. The serum levels of human albumin may be a critical determinant of vascular oxidative stress in some human diseases.
Declarations
Author contribution statement
Hiroyuki Kinoshita, Kazushi Watanabe: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Toshiharu Azma, Guo-Gang Feng, Takahiko Akahori, Hisaki Hayashi: Performed the experiments.
Motohiko Sato, Yoshihiro Fujiwara, Akihiko Wakatsuki: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (KAKENHI Grant no. 16K20118).
Competing interest statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at UMIN Clinical Trials Registry under the registration number UMIN000024382.
Acknowledgements
The authors thank Jiazheng Li, M.D and Yoshitaka Yasuda, M.D., Department of Anesthesiology, Aichi Medical University School of Medicine for their technical assistance regarding this study.
Appendix A. Supplementary data
The following are Supplementary data to this article:
The full, non-adjusted images of protein expressions of rac-1, p47phox, NOX2, and Na+/K+ ATPase in the membrane fraction from human coronary arterial smooth muscle cells 60 min after addition of L-, D-glucose (20 mmol/L) or D-glucose in combination with human serum albumin (BENESIS, 0.5 g/dL) are shown. Note each part surrounded by the red line, which indicates that the image was used in Figure 4A.
References
- 1.Goulopoulou S., Davidge S.T. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mole. Med. 2015;21:88–97. doi: 10.1016/j.molmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe K., Mori T., Iwasaki A., Kimura C., Matsushita H., Shinohara K., Wakatsuki A. Increased oxygen free radical production during pregnancy may impair vascular reactivity in preeclamptic women. Hypertension Res. 2013;36:356–360. doi: 10.1038/hr.2012.208. [DOI] [PubMed] [Google Scholar]
- 3.Jayaballa M., Sood S., Alahakoon I., Padmanabhan S., Cheung N.W., Lee V. Microalbuminuria is a predictor of adverse pregnancy outcomes including preeclampsia. Pregnancy Hypertens. 2015;5:303–307. doi: 10.1016/j.preghy.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Soriani M., Pietraforte D., Minetti M. Antioxidant potential of anaerobic human plasma: Role of serum albumin and thiols as scavengers of carbon radials. Arch. Biochem. Biophys. 1994;312:180–188. doi: 10.1006/abbi.1994.1297. [DOI] [PubMed] [Google Scholar]
- 5.Candiano G., Petretto A., Bruschi M., Santucci L., Dimuccio V., Prunotto M., Gusmano R., Urbani A., Ghiggeri G.M. The oxido-redox potential of albumin: Methodological approach and relevance to human diseases. J. Proteomics. 2009;73:188–195. doi: 10.1016/j.jprot.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Bedard K., Krause K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Touyz R.M., Chen X., Tabet F., Yao G., He G., Quinn M.T., Pagano P.J., Schiffrin E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries regulation by angiotensin II. Circ. Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 8.Dworakowski R., Walker S., Momin A., Desai J., El-Gamel A., Wendler O., Kearney M.T., Shah A.M. Reduced nicotinamide adenine dinucleotide phosphate oxidase-derived superoxide and vascular endothelial dysfunction in human heart failure. J. Am. Coll. Cardiol. 2008;51:1349–1356. doi: 10.1016/j.jacc.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Guzik T.J., Chen W., Gongora M.C., Guzik B., Lob H.E., Mangalat D., Hoch N., Dikalov S., Rudzinski P., Kapelak B., Sadowski J., Harrison D.G. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita H., Matsuda N., Kaba H., Hatakeyama N., Azma T., Nakahata K., Kuroda Y., Tange K., Iranami H., Hatano Y. Roles of phosphatidylinositol 3-kinase-Akt and NADPH oxidase in adenosine 5′-triphosphate-sensitive K+ channel function impaired by high glucose in the human artery. Hypertension. 2008;52:507–513. doi: 10.1161/HYPERTENSIONAHA.108.118216. [DOI] [PubMed] [Google Scholar]
- 11.Quinlan G.J., Mumby S., Martin G.S., Bernard G.R., Gutteridge J.M.C., Evans T.W. Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit. Care Med. 2004;32:755–759. doi: 10.1097/01.ccm.0000114574.18641.5d. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe K., Naruse K., Tanaka K., Metoki H., Suzuki Y. Outline of definition and classification of pregnancy induced hypertension (PIH) Hypertens. Res. Pregnancy. 2013;1:3–4. [Google Scholar]
- 13.Ebisawa S., Kashima Y., Miyashita Y., Yamazaki S., Abe N., Saigusa T., Miura T., Motoki H., Izawa A., Uichi Ikeda U. Impact of endovascular therapy on oxidative stress in patients with peripheral artery disease. Circ. J. 2014;78:1445–1450. doi: 10.1253/circj.cj-13-1341. [DOI] [PubMed] [Google Scholar]
- 14.Guthikonda S., Sinkey C.A., Haynes W.G. What is the most appropriate methodology for detection of conduit artery endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2007;27:1172–1176. doi: 10.1161/ATVBAHA.106.131011. [DOI] [PubMed] [Google Scholar]
- 15.Harris R.A., Nishiyama S.K., Wray D.W., Richardson R.S. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinoshita H., Azma T., Nakahata K., Iranami H., Kimoto Y., Dojo M., Yuge O., Hatano Y. Inhibitory effect of high concentration of glucose on relaxations to activation of ATP-sensitive K+ channels in human omental artery. Arterioscler. Thromb. Vasc. Biol. 2004;24:2290–2295. doi: 10.1161/01.ATV.0000148006.78179.c7. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita H., Azma T., Iranami H., Nakahata K., Kimoto Y., Dojo M., Yuge O., Hatano Y. Synthetic peroxisome proliferator-activated receptor-(agonists restore impaired vasorelaxation via ATP-sensitive K+ channels by high glucose. J. Pharmacol. Exp. Ther. 2006;318:312–318. doi: 10.1124/jpet.106.100958. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T., Terada T., Arikawa H., Kizaki K., Terawaki H., Imai H., Itoh Y., Era S. Quantitation of oxidative modifications of commercial human albumin for clinical use: Thiol oxidation and carbonylation. Biol. Pharm. Bull. 2016;39:401–408. doi: 10.1248/bpb.b15-00843. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier-Rouvière C., Vignal E., Mériane M., Roux P., Montcourier P., Fort P. RhoG GTPase Controls a Pathway That Independently Activates Rac1 and Cdc42Hs. Mol. Biol. Cell. 1998;9:1379–1394. doi: 10.1091/mbc.9.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan J.H., Feng G.G., Huang L., Tsunekawa K., Honda T., Katano Y., Hirooka Y., Goto H., Kandatsu N., Ando K., Fujiwara Y., Koide T., Okada S., Ishikawa N. Role of naofen in apoptosis of hepatocytes induced by lipopolysaccharide through mitochondrial signaling in rats. Hepatol. Res. 2012;42:696–705. doi: 10.1111/j.1872-034X.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- 21.Azma T., Saeki N., Yuge O. Cytosolic Ca2+ movements of endothelial cells exposed to reactive oxygen intermediates: Role of hydroxyl radical-mediated redox alteration of cell-membrane Ca2+ channels. Br. J. Pharmacol. 1999;126:1462–1470. doi: 10.1038/sj.bjp.0702438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haba M., Kinoshita H., Matsuda N., Azma T., Hama-Tomioka K., Hatakeyama N., Yamazaki M., Hatano Y. Beneficial effect of propofol on arterial adenosine triphosphate-sensitive K+ channel function impaired by thromboxane. Anesthesiology. 2009;111:279–286. doi: 10.1097/ALN.0b013e3181a918a0. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka F., Komi R., Makita S., Onoda T., Tanno K., Ohsawa M., Itai K., Sakata K., Omama S., Yoshida Y., Ogasawara K., Ishibashi Y., Kuribayashi T., Okayama A., Nakamura M., Iwate-Kenco Study Group Low-grade albuminuria and incidence of cardiovascular disease and all-cause mortality in nondiabetic and normotensive individuals. J. Hypertension. 2016;34:506–512. doi: 10.1097/HJH.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh M., Wang H.D., McNeill J.R. Tiron exerts effects unrelated to its role as a scavenger of superoxide anion: effects on calcium binding and vascular responses. Can. J. Physiol. Pharmacol. 2002;80:755–760. doi: 10.1139/y02-106. [DOI] [PubMed] [Google Scholar]
- 25.Guillaume Y.C., Guinchard C., Berthelot A. Affinity chromatography study of magnesium and calcium binding to human serum albumin: pH and temperature variations. Talanta. 2000;53:561–569. doi: 10.1016/s0039-9140(00)00536-1. [DOI] [PubMed] [Google Scholar]
- 26.Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., Pagano P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular 02− and systolic blood pressure in mice. Circ. Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 27.Soe N.N., Sowden M., Baskaran P., Smolock E.M., Kim Y., Nigro P., Berk B.C. Cyclophilin A is required for angiotensin II-induced p47phox translocation to caveolae in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2013;33:2147–2153. doi: 10.1161/ATVBAHA.113.301894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson L.J., Hayabuchi Y., Standen N.B., Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ. Res. 2004;95:1012–1028. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 29.Schnitzer J.E. Gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am. J. Physiol. 1992;262:H246–H254. doi: 10.1152/ajpheart.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- 30.Malik A.B. Targeting endothelial cell surface receptors: Novel mechanisms of microvascular endothelial barrier transport. J. Med. Sci. 2009;2:13–17. [Google Scholar]
- 31.Wang Y., Yu H., Shi X., Luo Z., Lin D., Huang M. Structural mechanism of ring-opening reaction of glucose by human serum albumin. J. Biol. Chem. 2013;288:15980–15987. doi: 10.1074/jbc.M113.467027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esposito C., Gerlach H., Brett J., Stern D., Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J. Exp. Med. 1989;170:1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnaby O.S., Cerny R.L., Clarke W., Hage D.S. Comparison of modification sites formed on human serum albumin at various stages of glycation. Clin Chim Acta. 2011;412:277–285. doi: 10.1016/j.cca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behrens I., Basit S., Lykke J.A., Ranthe M.F., Wohlfahrt J., Bundgaard H., Melbye M., Boyd H.A. Association between hypertensive disorders of pregnancy and later risk of cardiomyopathy. JAMA. 2016;315:1026–1033. doi: 10.1001/jama.2016.1869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full, non-adjusted images of protein expressions of rac-1, p47phox, NOX2, and Na+/K+ ATPase in the membrane fraction from human coronary arterial smooth muscle cells 60 min after addition of L-, D-glucose (20 mmol/L) or D-glucose in combination with human serum albumin (BENESIS, 0.5 g/dL) are shown. Note each part surrounded by the red line, which indicates that the image was used in Figure 4A.