Abstract
We have constructed two sets of clones in which one to six head-to-tail connected DNA copies of the potato spindle tuber viroid (PSTV) RNA genome were inserted into the plasmid pSP62- PL downstream of the promoter for SP6 RNA polymerase. In vitro transcription of these constructs with the promoter-specific SP6 RNA polymerase yielded the corresponding oligomeric single-stranded linear PSTV RNA molecules of (+) and (−) polarity. Except for short vector-derived terminal sequences these in vitro synthesized PSTV RNA forms are equivalent to the RNA intermediates of the PSTV replication cycle which are present in vivo only in extremely low concentrations. From each DNA template molecule up to 600 RNA copies could be transcribed in vitro and yields > 100 µg were obtained. When mechanically inoculated to tomato seedlings the PSTV (+) RNA oligomers were as infectious as the natural PSTV (+) RNA monomers. Surprisingly, the corresponding oligomeric PSTV (−) RNAs were ˜104-fold less infectious. However, when these (−) RNAs were partially protected prior to inoculation by mixing or hybridizing them with non-infectious (+) RNA fragments or by `capping' their 5' terminus, an increase in the number of infections was observed. The in vitro synthesis of infectious RNA from cloned cDNA means that, in principle, it should be possible to develop vector systems from pathogens with RNA genomes.
Keywords: replicative intermediates, RNA transcription, RNA vector, viroid cloning, viroid infectivity
Full text
PDF
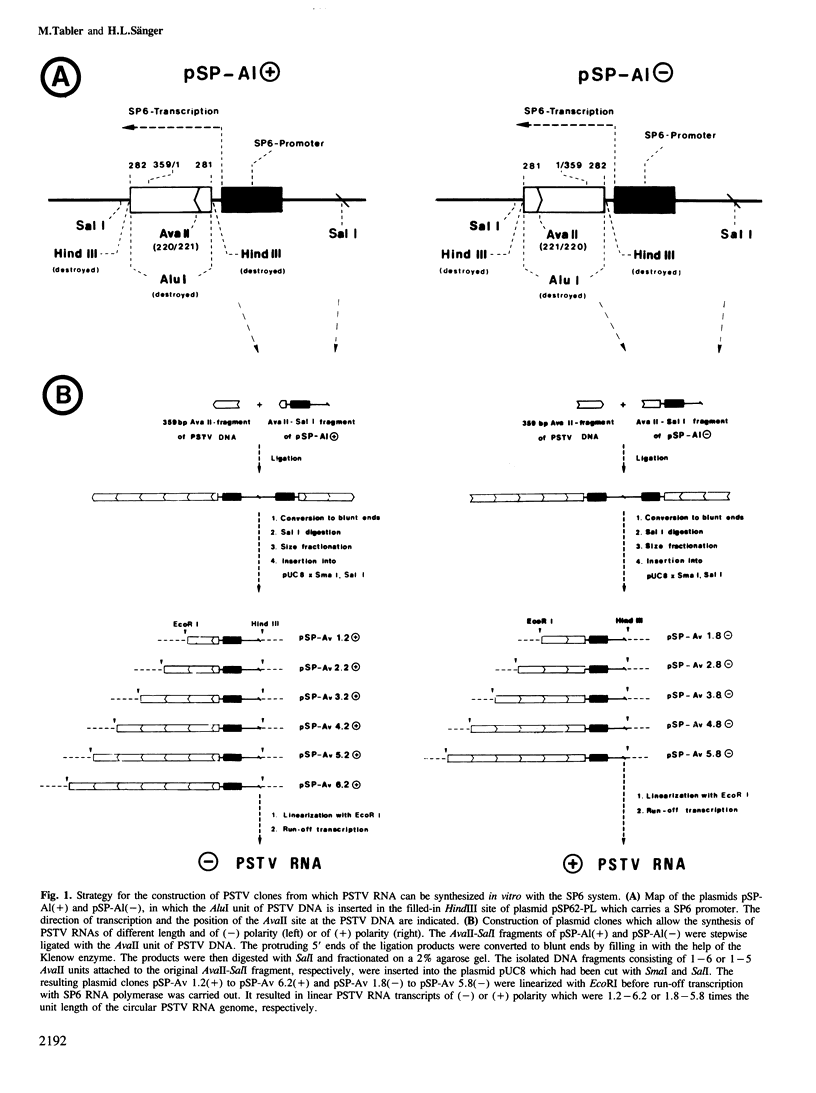







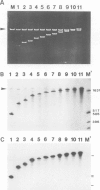
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D., Dickson E. Longer-than-unit-length viroid minus strands are present in RNA from infected plants. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6381–6385. doi: 10.1073/pnas.78.10.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler E. T., Chamberlin M. J. Bacteriophage SP6-specific RNA polymerase. I. Isolation and characterization of the enzyme. J Biol Chem. 1982 May 25;257(10):5772–5778. [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress D. E., Kiefer M. C., Owens R. A. Construction of infectious potato spindle tuber viroid cDNA clones. Nucleic Acids Res. 1983 Oct 11;11(19):6821–6835. doi: 10.1093/nar/11.19.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Portraits of viruses: the viroid. Intervirology. 1984;22(1):1–16. doi: 10.1159/000149528. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids. Adv Virus Res. 1983;28:241–283. doi: 10.1016/s0065-3527(08)60725-3. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Hadidi A., Cress D. E., Diener T. O. Nuclear DNA from uninfected or potato spindle tuber viroid-infected tomato plants contains no detectable sequences complementary to cloned double-stranded viroid cDNA. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6932–6935. doi: 10.1073/pnas.78.11.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. A revised replication cycle for viroids: the role of longer than unit length RNA in viroid replication. Mol Gen Genet. 1984;196(3):421–428. doi: 10.1007/BF00436189. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Butler E. T., Roulland D., Chamberlin M. J. Bacteriophage SP6-specific RNA polymerase. II. Mapping of SP6 DNA and selective in vitro transcription. J Biol Chem. 1982 May 25;257(10):5779–5788. [PubMed] [Google Scholar]
- Keller W. The RNA lariat: a new ring to the splicing of mRNA precursors. Cell. 1984 Dec;39(3 Pt 2):423–425. doi: 10.1016/0092-8674(84)90449-5. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Kütemeier G. Size fractionation of DNA fragments ranging from 20 to 30 000 base pairs by liquid/liquid chromatography. Eur J Biochem. 1982 Nov;128(1):231–238. doi: 10.1111/j.1432-1033.1982.tb06956.x. [DOI] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y. Hop stunt viroid: molecular cloning and nucleotide sequence of the complete cDNA copy. Nucleic Acids Res. 1983 Sep 24;11(18):6185–6197. doi: 10.1093/nar/11.18.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. RNA intermediates in potato spindle tuber viroid replication. Proc Natl Acad Sci U S A. 1982 Jan;79(1):113–117. doi: 10.1073/pnas.79.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde W., Sänger H. L. Detection of complementary RNA intermediates of viroid replication by Northern blot hybridization. Biosci Rep. 1981 Apr;1(4):327–336. doi: 10.1007/BF01114872. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Haas B., Raam K., Hofmann H., Sänger H. L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985 Sep;4(9):2181–2190. doi: 10.1002/j.1460-2075.1985.tb03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiesmacher E., Mühlbach H. P., Schnölzer M., Haas B., Sänger H. L. Oligomeric forms of potato spindle tuber viroid (PSTV) and of its complementary RNA are present in nuclei isolated from viroid-infected potato cells. Biosci Rep. 1983 Aug;3(8):767–774. doi: 10.1007/BF01120988. [DOI] [PubMed] [Google Scholar]
- Spiesmacher E., Mühlbach H. P., Tabler M., Sänger H. L. Synthesis of (+) and (-) RNA molecules of potato spindle tuber viroid (PSTV) in isolated nuclei and its impairment by transcription inhibitors. Biosci Rep. 1985 Mar;5(3):251–265. doi: 10.1007/BF01119595. [DOI] [PubMed] [Google Scholar]
- Tabler M., Schnölzer M., Sänger H. L. Molecular cloning of potato spindle tuber viroid (PSTV) cDNA synthesized by enzymatic elongation of PSTV-specific DNA primers: a general strategy for viroid cloning. Biosci Rep. 1985 Feb;5(2):143–158. doi: 10.1007/BF01117061. [DOI] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Cloned single- and double-stranded DNA copies of potato spindle tuber viroid (PSTV) RNA and co-inoculated subgenomic DNA fragments are infectious. EMBO J. 1984 Dec 20;3(13):3055–3062. doi: 10.1002/j.1460-2075.1984.tb02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]