LETTER TO THE EDITOR
A single subanesthetic intravenous (IV) dose of ketamine leads to rapid anti-obsessional effects in obsessive-compulsive disorder (OCD) patients with near-constant intrusive obsessions,1 but these effects usually do not persist.1–3 We tested whether a brief course of exposure-based cognitive behavioral therapy (CBT) could extend ketamine’s effects in a two week pilot open trial and if this effect was maintained (without additional treatment) two weeks later. Our rationale was: 1) ketamine is reported to enhance plasticity and extinction learning in rodents,4–6 and 2) enhanced extinction learning may facilitate CBT gains, as reported in trials that combined CBT with medication thought to facilitate extinction learning (e.g. D-cycloserine).7, 8 Mimicking those trials, CBT was abbreviated (i.e. 10 one-hour exposure sessions) but delivered during the putative time interval when ketamine facilitates extinction learning (within 14 days).4
Methods
With IRB approval, ten unmedicated OCD outpatients (aged 18–55) with near-constant intrusive obsessions (>8 hours/day) were recruited (3/2014–3/2015). They provided written informed consent. Participants met DSM-IV and DSM-5 criteria for OCD with at least moderate symptoms (Yale-Brown Obsessive-Compulsive Scale [Y-BOCS9, 10] score ≥16). Exclusion criteria included severe depression (Hamilton Depression Rating Scale [HDRS] >25),11 current CBT, and comorbid psychiatric or medical conditions that made participation unsafe.
In an open-label design, participants received a single 40-minute IV infusion of ketamine (dose=0.5 mg/kg), followed by 10 one-hour exposure sessions delivered over two weeks. The CBT treatment was planned in a 90-minute session the day before the ketamine infusion. All CBT sessions were administered by the same therapist (M.W.) and followed standard procedures.12
At baseline, during the infusion, at 20, 90, 110, 230 minutes post-infusion, patients rated their obsessional severity using the OCD-VAS.1, 13–15 We focused on obsessions because the patients were supine and connected to stationary monitoring equipment during the infusion. At baseline and weekly for four weeks post-ketamine, an independent evaluator, blind to study design, evaluated patients using the Y-BOCS, which appraises obsessive and compulsive symptoms over the prior week. Treatment response was defined a priori as ≥35% Y-BOCS reduction at week 2.16 Y-BOCS outcomes were analyzed using mixed-effects regression to model symptoms as a function of time.
Results
Of the 10 patients who started ketamine, nine completed the infusion. Eight reported a rapid reduction in obsessive severity as measured by the OCD-VAS, which persisted up to 230 minutes post-infusion in seven patients. Eight completed the 10 hours of exposure and the two week follow-up and were included in the Y-BOCS analyses. From baseline to four weeks post-infusion, OCD severity, as measured by the YBOCS, was significantly decreased over time (F=14.36, df=4,28, p<.0001; Figure 1). Compared to baseline, the mean estimated Y-BOCS score was significantly lower at week 2 (difference= −10.75 points, SE=1.44, p<.0001) and at week 4 (difference=−6.88, SE=2.61,p=0.01); there was a trend-level increase between week 2 and 4 (difference=3.63, SE=1.97, p=0.07). At the end of CBT (week 2), 63% of patients demonstrated treatment response (≥35% Y-BOCS reduction). Importantly, individuals varied in their response, with one subject having no benefit, the majority benefitting for up to two weeks, and one no longer meeting criteria for OCD (i.e., achieving minimal symptoms post-infusion that persisted throughout the CBT and for up to 6 months in naturalistic follow-up).
Figure 1. OCD Severity Over Four Weeks (n=8)a.
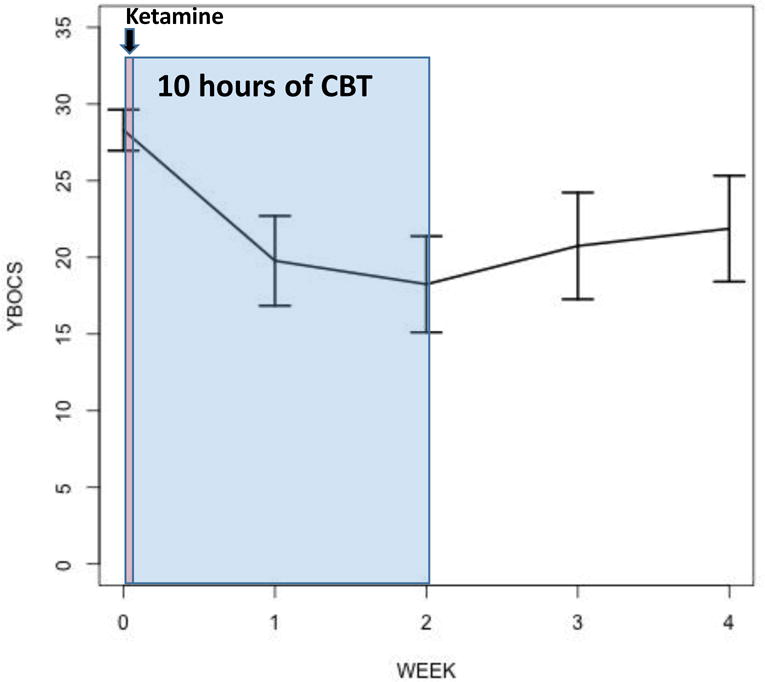
aAfter a single 40-minute infusion of ketamine 0.5mg/kg, 10 hours of exposure sessions were delivered over two weeks. Estimated means from mixed-effects regression are plotted with standard error. Patients had mean illness duration of 20.5 years (with standard deviation [SD] 10.2 years). The mean number of adequate SRI trials was 1.8 (SD 1.4), 80% received at least one prior adequate trail of an SRI, 10% failed at least one prior adequate trial of antipsychotic augmentation, and 60% had failed at least one prior adequate trial of EX/RP. Observed mean YBOCS score with standard deviation were as follows: Baseline: 28.8 (SD 4.6); Week 2: 18.3 (SD 8.9); Week 4: 21.9 (SD 9.8).
Abbreviation: CBT = cognitive-behavioral therapy.
Conclusions/Discussion
These results corroborate prior findings1, 2 that IV ketamine can rapidly reduce obsessions in unmedicated OCD patients and advance the growing literature of enhancing CBT with agents that facilitate extinction learning. 7, 8, 17 Limitations typical of an open-label trial include lack of randomization to a comparison group, which may lead to allocation and ascertainment (response) bias. The data suggest that a brief course of CBT may help some individuals maintain the improvement they experienced from ketamine; however, this needs to be formally tested in a randomized controlled trial to determine whether the improvement seen after two weeks of CBT is due to the addition of CBT, or whether the effects of ketamine persist longer in some than previously described.
Acknowledgments
The authors thank the individuals who generously donated their time to participate in this research study. We thank Roberto Lewis-Fernandez and John Markowitz for helpful comments on the manuscript.
Financial disclosures: Drs. Rodriguez, Wheaton, Steinman, Galfalvy, and Ms. Zwerling and Sonnenfeld, report no additional financial or other relationships relevant to the subject of this manuscript. Dr. Simpson has received royalties from Cambridge University Press and UpToDate, Inc.
Funding support: This study was supported by the National Institutes of Mental Health (K23MH092434 [Dr. Rodriguez], K24MH09155 [Dr. Simpson]), the Black Family Foundation, and the New York State Psychiatric Institute.
Footnotes
Previous Presentation: None
References
- 1.Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013 Nov;38(12):2475–2483. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez CI, Kegeles LS, Flood P, Simpson HB. Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011 Apr;72(4):567–569. doi: 10.4088/JCP.10l06653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch MH, Wasylink S, Landeros-Weisenberger A, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012 Dec 1;72(11):964–970. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duman RS. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety. 2014 Apr;31(4):291–296. doi: 10.1002/da.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012 Jun 1;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014 May 27; doi: 10.1073/pnas.1323920111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: An inhibitory learning approach. Behav Res Ther. 2014 May 9;58C:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann SG. D-cycloserine for treating anxiety disorders: making good exposures better and bad exposures worse. Depress Anxiety. 2014 Mar;31(3):175–177. doi: 10.1002/da.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989 Nov;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 10.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989 Nov;46(11):1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foa EB, Yadin E, Lichner TK. Exposure and Response (Ritual) Prevention for Obsessive Compulsive Disorder: Therapist Guide. Oxford University Press; 2012. [Google Scholar]
- 13.Murphy DL, Mueller EA, Hill JL, Tolliver TJ, Jacobsen FM. Comparative anxiogenic, neuroendocrine, and other physiologic effects of m-chlorophenylpiperazine given intravenously or orally to healthy volunteers. Psychopharmacology (Berl) 1989;98(2):275–282. doi: 10.1007/BF00444705. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg BD, Benjamin J, Martin JD, et al. Delayed obsessive-compulsive disorder symptom exacerbation after a single dose of a serotonin antagonist in fluoxetine-treated but not untreated patients. Psychopharmacology (Berl) 1998 Dec;140(4):434–444. doi: 10.1007/s002130050787. [DOI] [PubMed] [Google Scholar]
- 15.Abrantes AM, Strong DR, Cohn A, et al. Acute changes in obsessions and compulsions following moderate-intensity aerobic exercise among patients with obsessive-compulsive disorder. J Anxiety Disord. 2009 Oct;23(7):923–927. doi: 10.1016/j.janxdis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Tolin DF, Abramowitz JS, Diefenbach GJ. Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown obsessive compulsive scale. J Clin Psychiatry. 2005 Dec;66(12):1549–1557. doi: 10.4088/jcp.v66n1209. [DOI] [PubMed] [Google Scholar]
- 17.Grassi G, Godini L, Grippo A, Piccagliani D, Pallanti S. Enhancing cognitive-behavioral therapy with repetitive transcranial magnetic stimulation in refractory obsessive-compulsive disorder: a case report. Brain Stimul. 2015 Jan-Feb;8(1):160–161. doi: 10.1016/j.brs.2014.10.007. [DOI] [PubMed] [Google Scholar]


