Abstract
Vaccinia virus (VV) can potently activate NK and T cell responses, leading to efficient viral control and generation of long-lasting protective immunity. We have previously shown that granulocytic myeloid derived suppressor cells (g-MDSCs) can suppress the NK cell response to VV infection. It remains unknown what regulates T cell responses to VV infection in vivo. In this study, we first showed that monocytic MDSCs (m-MDSCs), but not g-MDSCs, from VV-infected mice could directly suppress CD4+ and CD8+ T cell activation in vitro. We then demonstrated that defective recruitment of m-MDSCs to the site of VV infection in CCR2−/− mice enhanced VV-specific CD8+ T cell response and that adoptive transfer of m-MDSCs into VV-infected mice suppressed VV-specific CD8+ T cell activation, leading to a delay in viral clearance. Mechanistically, we further showed that T cell suppression by m-MDSCs is mediated by indication of iNOS and production of NO upon VV infection, and that IFN-γ is required for activation of m-MDSCs. Collectively, our results highlight a critical role for m-MDSCs in regulating T cell responses against VV infection and may suggest potential strategies using m-MDSCs to modulate T cell responses during viral infections.
Introduction
Vaccinia virus (VV), the most studied member of the poxvirus family, is the live vaccine responsible for the successful elimination of smallpox worldwide [1]. This success has led to the development of recombinant VV as a vaccine vehicle for infectious diseases and cancer [2, 3]. This unique potency of VV is, in large part, due to its ability to elicit strong and long-lasting protective T cell immunity [4, 5]. Recent studies have also shown that VV can efficiently activate the innate immune system through both TLR-dependent and –independent pathways [6, 7], both of which are critical for CD8+ T cell responses to VV infection in vivo [8, 9]. Furthermore, VV can efficiently activate NK cells and the activated NK cells migrate to the site of infection, contributing to the initial viral control [10–14].
Myeloid-derived suppressor cells (MDSCs), a heterogeneous population of immature myeloid cells, was first shown to play an important role in the regulation of immune responses in cancer patients in that the accumulation of MDSCs at tumor sites suppresses antitumor immunity and promotes tumor growth [15, 16]. Since then, extensive studies have established a critical role for MDSCs in the regulation of T cell responses within the tumor microenvironment [17, 18]. There are two subsets of MDSCs in mice: granulocytic MDSCs (g-MDSCs) are defined by CD11b+Ly6CloLy6G+; whereas monocytic MDSCs (m-MDSCs) have a phenotype of CD11b+Ly6ChiLy6G− [18]. It has recently become clear that these two populations have distinct cellular targets and suppressive capacities [19].
The expansion of MDSCs has also been observed in response to viral infections [20–24]. In a murine model of VV infection, we have recently shown that both g-MDSCs and m-MDSCs accumulated at site of infection and g-MDSCs are critical for the regulation of the NK cell response to VV infection through the production of reactive oxygen species (ROS)[23]. However, it remains unknown with regard to the role of m-MDSCs in immune responses against VV infection in vivo.
In this study, we evaluated whether m-MDSCs could influence T cell responses to VV infection in vivo. We first showed that m-MDSCs, but not g-MDSCs, from VV-infected mice could directly suppress the activation of CD4+ and CD8+ T cells in vitro. We then found that recruitment of m-MDSCs to the site of VV infection is dependent on CCR2 and that defective m-MDSC recruitment in CCR2−/− mice led to enhanced VV-specific CD8+ T cell response. Furthermore, adoptive transfer of m-MDSCs into VV-infected mice significantly suppressed the VV-specific CD8+ T cells and delayed viral clearance, suggesting an important role for m-MDSCs in regulating T cell responses against VV infection. We further demonstrated that induction of inducible nitric oxide synthase (iNOS) and the production of nitric oxide (NO) by m-MDSCs were required for the suppression of T cell responses. Finally, we showed that the suppressive capacity of m-MDSC is dependent on IFN-γ.
Results
m-MDSCs inhibit T cell proliferation in vitro
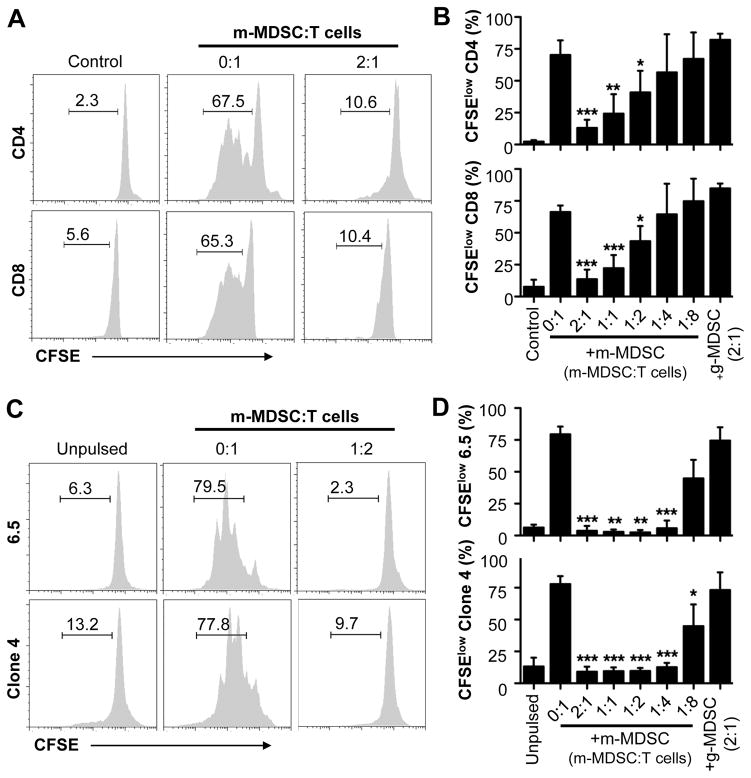
We have shown previously that g-MDSCs, but not m-MDSCs, hampered the NK cell response to VV infection [23]. However, since both m-MDSCs and g-MDSCs accumulated in the peritoneal cavity in response to VV infection intraperitoneally, we hypothesized that m-MDSCs could regulate T cell responses at the site of VV infection. To address this, we utilized a previously described in vitro T-cell co-culture system [9]. We found that addition of m-MDSCs from VV-infected mice to T cell cultures markedly suppressed the proliferation of both CD4+ and CD8+ T cells in response to stimulation with anti-CD3 and anti-CD28 antibodies in a cell dose-dependent manner (Fig. 1A, B). In contrast, no T cell suppression was observed when g-MDSCs (with g-MDSC to T cell ratio of 2:1) were added to the culture (Fig. 1B). To address whether m-MDSCs were able to suppress antigen-specific T cell responses, we used influenza hemagglutinin (HA)-specific CD4+ and CD8+ T cells derived from 6.5 and Clone 4 HA-TCR transgenic mice, respectively. Similarly, addition of m-MDSCs, not g-MDSCs, significantly (p<0.01) inhibited the proliferation of HA-specific CD4+ and CD8+ T cells in response to stimulation with their respective cognate peptides (Fig. 1C, D). These results indicate that m-MDSCs could directly suppress antigen-specific and -non-specific T cell responses in vitro.
Figure 1.
m-MDSCs suppress T cell proliferation in vitro. (A–B) m-MDSCs purified from peritoneal exudates of VV-infected mice were cultured with purified naïve CD4+ (CD4) or CD8+ (CD8) T cells labeled with CFSE at different m-MDSCs to T cell ratios (m-MDSC:T cells), in the presence of 1 μg/ml of anti-CD3 and anti-CD28 antibodies. Some T cells were incubated with medium only (Control). Some T cells were incubated with g-MDSCs at g-MDSC:T cell ratio of 2:1. T cell proliferation was determined 72 h later. (A) FACS plots showing the percentage of CFSElow cells representing proliferating T cells. (B) The mean percentages ± SD of CFSElow cells among total CD4+ or CD8+ T cells are shown (n = 4). (C–D) m-MDSCs were cultured with CFSE-labeled naïve HA-specific CD4+ (6.5) or CD8+ (Clone 4) T cells at different m-MDSCs to T cell ratios (m-MDSC:Tcells), in the presence of class I or class II HA peptide-pulsed CD11c+ DCs. Some 6.5 and Cone 4 T cells were incubated with unpulsed CD11c+ DCs (Unpulsed). Some T cells were incubated with g-MDSCs at g-MDSC:T cell ratio of 2:1. Proliferation was assessed 72 h later. (C) FACS plots showing the percentage of CFSElow cells. (D) The mean percentages ± SD of CFSElow cells among total 6.5 or Clone 4 T cells are shown (n = 3). Asterisks indicate the p value (unpaired student t-test) compared to the 0:1 ratio: * = p <0.05; ** = p <0.01; *** = p <0.001. Data is representative of three independent experiments.
Defective recruitment of m-MDSCs to the site of VV infection in CCR2−/− mice enhances VV-specific CD8+ T cell response
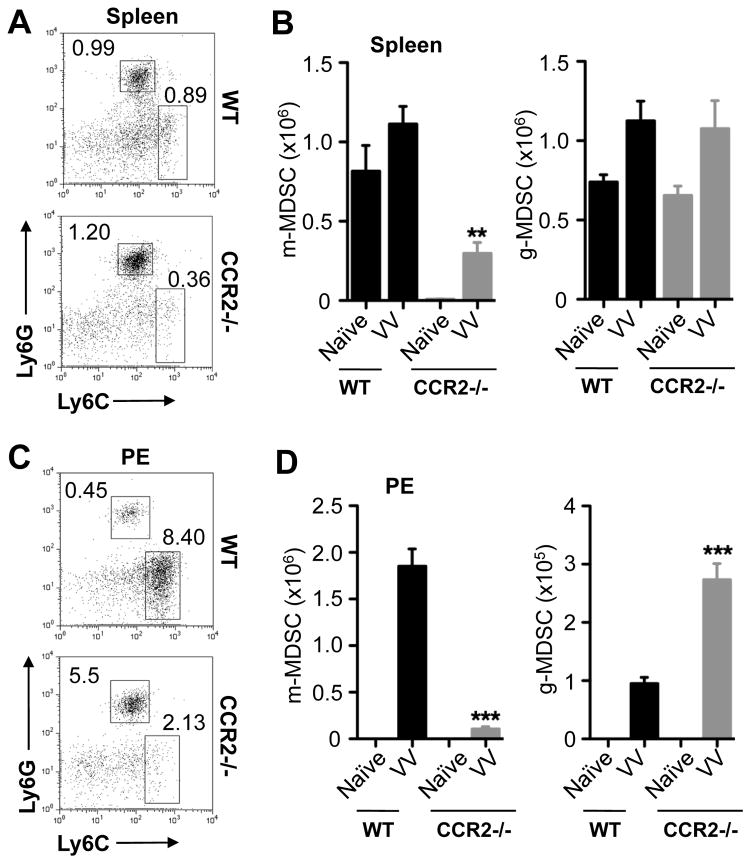
It has been shown that m-MDSCs express CCR2 [25, 26] and that CCR2−/− MDSCs had an impaired migration to the tumor microenvironment [26]. We thus used CCR2−/− mice to evaluate the role of m-MDSCs in CD8+ T cell response to VV infection in vivo. We first determined whether there was a defect in m-MDSC recruitment in CCR2−/− mice in response to VV infection. We found that the percentage (Fig. 2A, C) and absolute number (Fig. 2B, D) of m-MDSCs in the spleen (Fig. 2A, B) and peritoneal exudates (PE, Fig. 2C, D) were markedly lower in VV-infected CCR2−/− mice than the infected wild-type (WT) controls, suggesting that the migration of m-MDSCs to the site of VV infection is severely compromised. However, the recruitment of g-MDSCs was not affected (Fig. 2).
Figure 2.
Impaired migration of m-MDSCs to the site of VV infection in CCR2−/− mice. WT and CCR2−/− mice were infected with 2 × 106 PFU of VV intraperitoneally, or left uninfected (Naïve). 7 days later, cells from spleen (A & B) and peritoneal cavity exudates (PE, C & D) were stained with anti-CD11b, anti-Ly6G and anti-Ly6C. (A & C) FACS plots showing the percentage of m-MDSCs (Ly6ChiLy6G−) and g-MDSCs (Ly6CloLy6G+) among CD11b+ cells. (B & D) The mean cell number ± SD of m-MDSCs or g-MDSCs among total cells is shown (n = 5). Asterisks indicate the p value (unpaired student t-test) compared to the VV-infected WT group: ** = p <0.01; *** = p <0.001. Data is representative of three independent experiments.
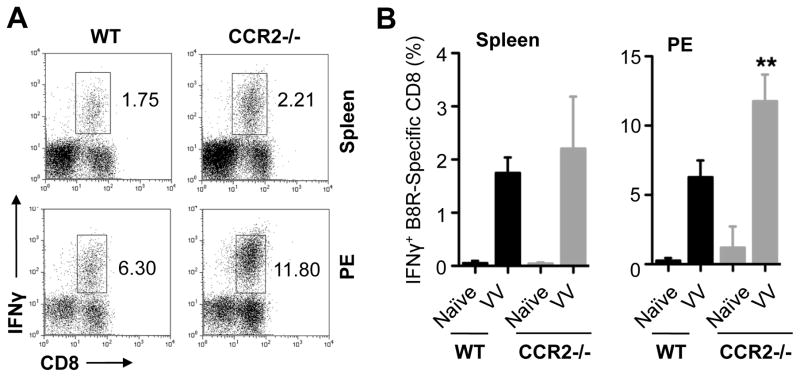
We next investigated whether defective recruitment of m-MDSCs to the site of infection in CCR2−/− mice affected the CD8+ T cell response to VV infection. Using an immunodominant VV-derived epitope peptide, B8R20–27 [27], we assessed the production of IFN-γ by VV-specific CD8+ T cells. We found that there was a slight increase in the percentage of IFN-γ+CD8+ T cells in spleens of infected CCR2−/− mice compared to WT mice (Fig. 3A, B). However, the frequency of IFN-γ+CD8+ T cells in peritoneal cavity was significantly (p < 0.01) increased in infected CCR2−/− mice (Fig. 3B). Collectively, these results indicated that defective migration of m-MDSCs to the site of VV infection enhanced VV-specific CD8 T cell response, suggesting an inhibitory role for m-MDSCs in VV-specific CD8+ T cell response in vivo.
Figure 3.
Enhanced CD8+ T cell response to VV infection in CCR2−/− mice. WT and CCR2−/− mice were infected with 2 × 106 PFU of VV intraperitoneally, or left uninfected (Naïve). 7 days later, cells from spleen and peritoneal cavity exudates (PE) were stimulated with 2 μg/ml B8R20–27 peptide and stained for IFN-γ production by CD8+ T cells intracellularly. (A) FACS plots showing the percentage of IFN-γ+ B8R-Specific CD8+ T cells among total cells. (B) The mean percentage ± SD of IFN-γ+ B8R-Specific CD8+ T cells among total cells is provided (n = 6). Asterisks indicate the p value (unpaired student t-test) compared to the infected (VV) WT group: ** = p <0.01; *** = p <0.001. Data is representative of three independent experiments.
m-MDSCs suppress the CD8+ T cell response to VV infection in vivo
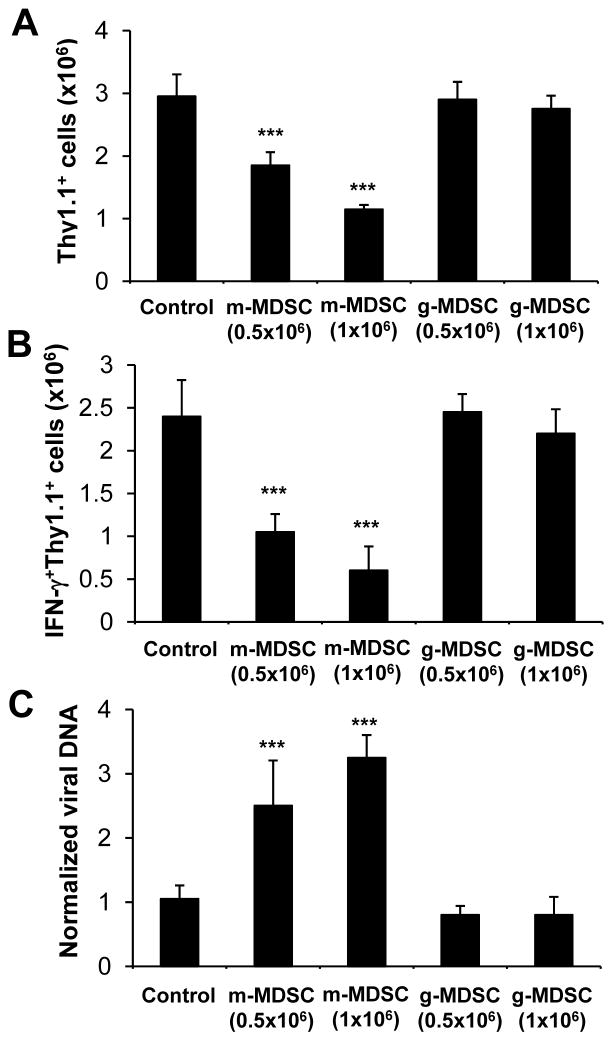
To further support the inhibitory role of m-MDSCs, we asked if adoptive transfer of m-MDSCs suppressed the CD8+ T cell response to VV infection in vivo. We used a previously described model in which CD8+ T cells from Clone 4 HA-TCR transgenic mice were adoptively transferred into congenic mice, followed by infection with VV-HA [28, 29]. 5 days after infection, mice were adoptively transferred with m-MDSCs purified from VV-infected mice. We found that a significant (p <0.001) reduction in the number of total (Fig. 4A) and IFN-γ+ (Fig. 4B) clonotypic HA-specific CD8+ T cells at a dose-dependent fashion, compared to vehicle transferred control mice. By contrast, no reduction in HA-specific CD8+ T cells was observed in mice received g-MDSCs (Fig. 4A, B). The suppression of virus-specific T cell response by m-MDSCs was associated with an increase in VV DNA (Fig. 4C). These results suggest that m-MDSCs can directly suppress VV-specific CD8+ T cell response, leading to a delay in viral clearance in vivo.
Figure 4.
m-MDSCs suppress VV-specific CD8+ T cell response and delay viral clearance in vivo. 104 purified naïve HA-specific CD8+ T cells (Thy1.1+) were adoptively transferred into B10.D2 mice (Thy1.2+), which were subsequently infected with 5 × 106 PFU of a recombinant VV-HA. 5 days later, these mice received m-MDSCs or g-MDSCs at a dose of 0.5 × 106 or 1 × 106 cells purified from another cohort of VV-infected mice or a vehicle control. 2 days later after transfer, splenocytes were analyzed for the presence of total and IFN-γ+ HA-specific CD8+ T cells, as well as for the detection of VV DNA by quantitative real-time PCR. (A–B) The mean cell numbers ± SD of total (A) and IFN-γ+ (B) Thy1.1+CD8+ T cells among total cells are shown (n = 4). (C) Relative viral DNA amount ± SD (n = 4). Samples were normalized to β-actin gene. Asterisks indicate the p value (unpaired student t-test) compared to the control group: *** = p <0.001. Data is representative of three independent experiments.
Induction of iNOS and production of NO by m-MDSCs in response to VV infection are critical for their suppressive activity
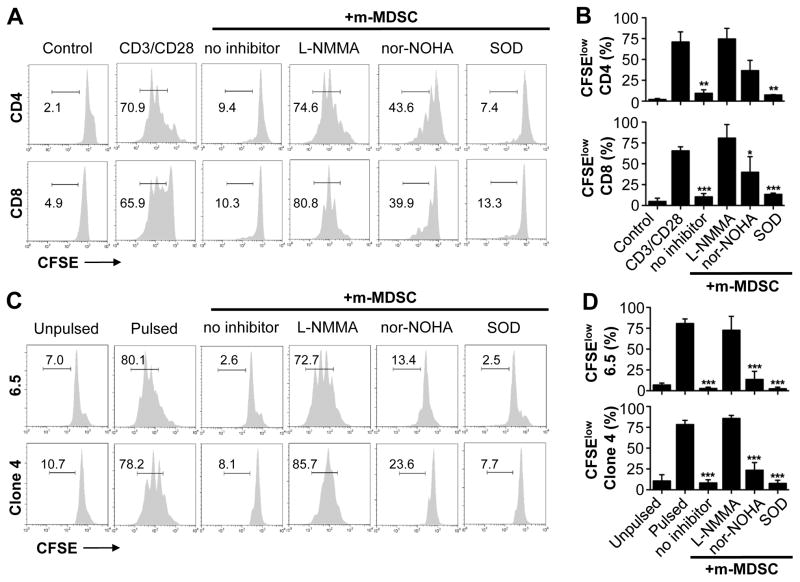
How do m-MDSCs from VV-infected mice suppress T cell responses? The induction of iNOS and the production of NO, the induction of arginase-1 activity, and the production of ROS have been implicated in the suppressive activity mediated by MDSCs on anti-tumor T cell responses [30]. To examine the potential roles of iNOS, arginase-1, and ROS, we added their respective inhibitors to the m-MDSC-T cell co-culture system. These inhibitors include L-NMMA for iNOS, nor-NOHA for arginase-1, SOD for ROS. We found that L-NMMA could completely reverse m-MDSC-mediated suppression on the proliferation of CD4+ and CD8+ T cells, and nor-NOHA could partially reverse the suppression, whereas SOD had no effects on m-MDSC-mediated suppression (Fig. 5). The reversal of suppression on T cells by L-NMMA was associated with a marked reduction of NO in the culture (data not shown), suggesting a role for NO in mediating the suppression. These results indicated that m-MDSC-mediated suppression on T cell activity is mainly mediated by the induction of iNOS.
Figure 5.
Suppression by m-MDSCs is mediated by iNOS. (A–B) m-MDSCs purified from VV-infected mice were co-cultured with naïve CFSE-labeled CD4+ (CD4) or CD8+ (CD8) T cells at a 2:1 m-MDSC to T cell ratio (+m-MDSC), in the presence of 1 μg/ml of anti-CD3 and anti-CD28 antibodies (CD3/CD28). Some T cells were incubated with media only (Control). Where indicated, SOD, L-NMMA, or nor-NOHA was added to the culture. Proliferation was determined 72 h later. (A) FACS plots showing the percentage of CFSElow cells representing proliferating T cells. (B) The mean percentages ± SD of CFSElow cells among total CD4 or CD8 cells are shown (n = 4). (C–D) m-MDSCs were co-cultured with CFSE-labeled naïve HA-specific CD4+ (6.5) or CD8+ (Clone 4) T cells at a 1:2 m-MDSC to T cell ratio (m-MDSC:T cells) in the presence of HA-pulsed CD11c+ DCs (Pulsed). Some 6.5 and Clone 4 cells were incubated with control CD11c+ DCs (Unpulsed). Where indicated, SOD, L-NMMA, or nor-NOHA was added to the culture. Proliferation was assessed 72 h later. (C) FACS plots showing the percentage of CFSElow cells. (D) The mean percentages ± SD of CFSElow cells among total 6.5 or Clone 4 T cells are shown (n = 4). Asterisks indicate the p value (unpaired student t-test) compared to the CD3/CD28 (B) or the pulsed (D) group: * = p <0.05; ** = p <0.01; *** = p <0.001. Data is representative of three independent experiments.
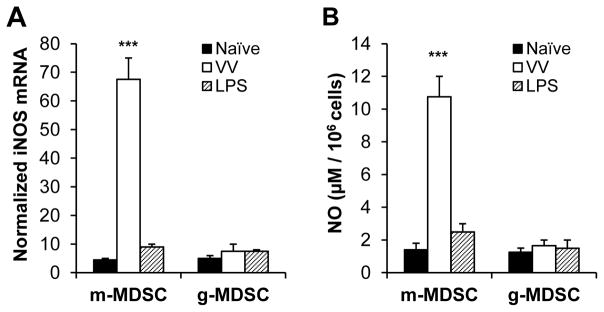
We next examined whether VV infection induced m-MDSCs to upregulate iNOS and produce NO. To address this question, mice were injected with VV or LPS, intraperitoneally. 3 days later, m-MDSCs or g-MDSCs were purified from peritoneal cavity exudates and assayed for iNOS induction and NO production. We found that m-MDSCs from VV-infected, but not LPS-treated, mice had a significant (p <0.001) increase in the expression iNOS (Fig. 6A) and production of NO (Fig. 6B), compared to naïve control. However, no significant induction of iNOS or NO by g-MDSCs was observed (Fig. 6). These results suggest that VV infection preferentially induced m-MDSCs to upregulate iNOS and produce NO.
Figure 6.
Induction of iNOS and production of NO by m-MDSCs upon VV infection. Mice were injected with 2 × 106 PFU of VV, or 10 μg of LPS, intraperitoneally, or left untreated (Naïve). 3 days later, m-MDSCs or g-MDSCs were purified from peritoneal cavity exudates and assayed for iNOS induction and NO production. (A) Total RNA was isolated and the expression of iNOS was determined by real-time quantitative PCR. Relative iNOS mRNA abundance ± SD is shown ((n = 3).). Samples were normalized to β-actin mRNA. (B) Cells were incubated in medium for additional 24 h before determining NO production with Griess’ reagent. The mean NO production ± SD is shown (n = 3). The asterisk indicates the p value (unpaired student t-test) compared to naïve m-MDSCs: *** = p <0.01. Data is representative of two independent experiments.
IFN-γ activates m-MDSC activity
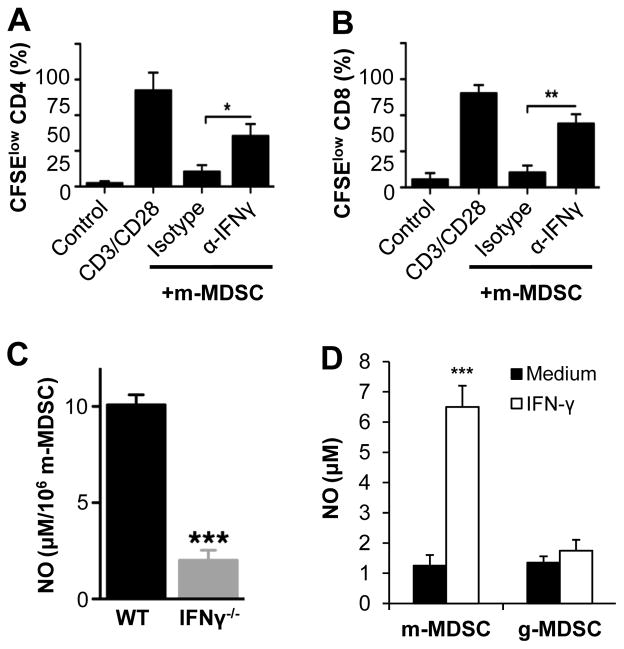
We next investigated what regulates the activity of m-MDSCs in response to VV infection. IFN-γ has been implicated in the activation of m-MDSC within the tumor microenvironment [25]. Since IFN-γ is produced in high quantity during viral infection, we hypothesized that IFN-γ may be required for the activation of m-MDSC elicited upon VV infection. To test this, we cocultured m-MDSC from peritoneal cavity with CD4+ or CD8+ T cells in the presence of a blocking anti-IFN-γ mAb. We showed that the addition of anti-IFN-γ mAb could reverse the suppression of CD4+ and CD8+ T cell proliferation by m-MDSCs (Fig. 7A, B). To address the role of IFN-γ in m-MDSC activity in response to VV infection in vivo, m-MDSC purified from VV-infected WT and IFN-γ−/− mice were measured for NO production. We found that NO production by m-MDSC from IFN-γ−/− mice was significantly (p < 0.001) reduced (Fig. 7C), compared to that from WT mice.
Figure 7.
IFN-γ is required for the activation of m-MDSCs. (A–B) Purified naïve CD4+ (A) or CD8+ (B) T cells were labeled with CFSE and co-cultured at a 2:1 m-MDSC to T cell ratio (+m-MDSC) with purified m-MDSC, in the presence of 1 μg/ml of anti-CD3 and anti-CD28 antibodies (CD3/CD28). Some CD4 and CD8 cells were incubated with media only (Control). Where indicated, 10 μg/ml of a blocking anti-IFN-γ (α-IFNγ), or an isotype control (Isotype), was added to the culture. T cell proliferation was determined 72 h later. The mean percentages ± SD of CFSElow cells among total CD4 or CD8 T cells are shown (n = 3). Asterisks indicate the p value (unpaired student t-test) compared to the isotype control group to show rescue of proliferation: * = p <0.05; ** = p <0.01. (C) WT and IFN-γ−/− mice were infected with 2 × 106 PFU of VV intraperitoneally. 3 days later, m-MDSCs were purified from peritoneal cavity exudates and incubated in medium for 24 h before determining NO production with Griess’ reagent. The mean NO production ± SD by m-MDSCs is shown. The asterisk indicates the p value compared to WT group: *** = p <0.01. (D) m-MDSCs or g-MDSCs harvested bone marrow of naïve mice were cultured in the presence of IFN-γ (40 U/ml) for 24 h and assayed for the production of NO. The mean NO production ± SD is shown. The asterisk indicates the p value (unpaired student t-test) compared to the medium control: *** = p <0.01. Data is representative of three independent experiments.
To further test whether IFN-γ is sufficient to induce NO production, m-MDSCs or g-MDSCs harvested bone marrow of naïve mice were cultured in the presence of IFN-γ for 24 h and assayed for the production of NO. Indeed, we found that addition of IFN-γ significantly (p <0.001) induced the production of NO by m-MDSCs, but not g-MDSCs (Fig. 7D). Taken together, these results support that IFN-γ is required for the suppressive capacities of m-MDSC upon VV infection.
Discussion
In this study, we showed that m-MDSCs from VV-infected mice could directly suppress the activation of CD4+ and CD8+ T cells in vitro. We also found that defective recruitment of m-MDSCs to the site of VV infection in CCR2−/− mice enhanced VV-specific CD8 T cell response. Furthermore, adoptive transfer of m-MDSCs into VV-infected mice significantly suppressed the activation of VV-specific CD8 T cells and delayed viral clearance, suggesting an important role for m-MDSCs in suppressing T cell responses against VV infection. In addition, we demonstrated that m-MDSC-mediated suppression on T cells was mediated by iNOS and NO, and that IFN-γ is required for the suppressive capacity of m-MDSCs.
Studies have shown that VV can efficiently activate the innate immune system through both TLR-dependent and –independent pathways [6, 7], leading to potent activation of T cell responses to VV infection in vivo [8, 9]. VV infection can also efficiently activate NK cells [10–14]. Thus, VV infection is capable of activating both the innate and adaptive immune responses for effective viral control. However, immune responses against viral infections are often tightly regulated to avoid collateral damage and systemic inflammation. We have recently shown that g-MDSCs and ROS are critical for the control of NK cell activation in response to VV infection [23]. We now show that T cell responses to VV infection are negatively regulated by m-MDSCs. This is mediated by the induction of iNOS and production of NO by m-MDSCs upon VV infection.
ROS, iNOS/NO, and arginase-1 have been shown to mediate suppression on anti-tumor T cell responses by MDSCs [30]. In a model of viral infection, here we show that it is m-MDSCs mediated by iNOS/NO that suppress T cell responses, whereas g-MDSCs mediated by ROS regulate the NK cell response [23]. Interestingly, g-MDSCs even at a high dose could not suppress T cell responses both in vitro and vivo. The reason for the differential regulation of NK vs. T cell responses during VV infection by different MDSC subsets in vivo is not entirely unclear, but could be related to the timing for induction of suppressive capacities of MDSC subsets; the kinetics of NK and T cell activation; and the involvement of different signaling pathways for the respective suppression. It has been shown that NO can both directly and indirectly target T cells to modulate their activation. NO can disrupt the binding between TCR and MHC by the nitration of tyrosine residues present in the TCR-CD8 complex, leading to a reduction in T cell responsiveness [31, 32]. Alternatively, NO can suppress IL-2R signaling, resulting in inhibition of T cell activation and proliferation [33]. Future studies are needed to further investigate the mechanisms underlying the suppression by different subsets of MDSCs.
Our observation that migration of m-MDSCs to the site of VV infection is dependent on CCR2 is consistent with a previous report that CCR2 is required for the recruitment of immunosuppressive monocytes in response to MCMV infection [34]. However, studies have shown that migration of monocytes to the respiratory tract upon infection with modified vaccinia Ankara (MVA) is related to CCR1 and CCL2 [35, 36]. The reason for the differences remains unclear, but could be related to the complexity of poxviruses that encode a series of genes responsible for various mechanisms of immune modulation.
Our results that T cell responses to VV infection is regulated by m-MDSCs are consistent with previous observations on the role of m-MDSCs in T cell responses in models of various tumors [25, 37, 38], experimental autoimmune encephalomyelitis (EAE) [39], graft versus host diseases (GVHD) [40], as well as other viral infections [20–24]. In addition, a recent report has shown that modified vaccinia Ankara (MVA)-based vaccine can induce myeloid cell expansion that restricts protective CD8 T cell immunity to SIV [41]. These observations suggest inflammatory signals that drive the activation of m-MDSCs and production of NO during viral infections are probably similar to other chronic inflammatory conditions, such as tumor microenvironment, autoimmunity and allogeneic rejection. What could drive the production of NO by m-MDSCs in response to VV infection? Here we provided evidence that IFN-γ produced during VV infection is critical for NO production and the suppressive function of m-MDSCs both in vitro and in vivo. It has been shown that IFN-γ is crucial for the control of viral infection and tumor growth [42–44] and IFN-γ can iNOS in a STAT1-dependent manner, leading to NO production [45, 46]. Thus, IFN-γ produced by activated T cells during VV infection could contribute to the induction of iNOS and production of NO by m-MDSCs, leading to the control of potentially overwhelming T cell activation in vivo.
In summary, we have provided evidence that m-MDSCs can suppress T cell responses to VV infection. Mechanistically, m-MDSC-mediated suppression on T cells is mediated by iNOS and NO, and that IFN-γ is required for the suppressive capacity of m-MDSCs. Our study may suggest potential strategies using m-MDSCs to modulate T cell activity for potential therapeutic benefits in viral infections.
Materials and methods
Mice
C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD). B10.D2 and CCR2−/− B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The clone 4 influenza hemagglutinin (HA)-TCR transgenic mice that express a TCR recognizing a Kd-restricted HA epitope (518IYSTVASSL526) were provided by Dr. L. Sherman (The Scripps Research Institute, La Jolla, CA) [47]. The 6.5 TCR-HA transgenic mice that express a TCR recognizing an I-Ed–restricted HA epitope (110SFERFEIFPKE120) were provided by Dr. H. von Boehmer (Harvard University, Boston, MA) [48]. These mice were backcrossed onto the Thy1.1+ B10.D2 background. All mice used in this study were between 7 and 10 wk of age. Experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Duke University (Durham, NC).
Antibodies and flow cytometry
Allophycocyanin-conjugated anti–CD11b (clone M1/70), PE-conjugated anti-CD8a (clone 53-6.7), PE-Cy5–conjugated anti-CD4 (clone RM4-5), FITC-conjugated anti-IFN-γ (clone XMG1.2), PE-conjugated anti-CD90.1 (Thy1.1) (clone OX-7), PE-conjugated Ly6G (clone 1A8), PE-Cy5–conjugated anti-CD8a (clone 53-6.7), FITC-conjugated Ly6C (clone AL-21), purified NA/LE anti-Mouse CD3e (clone 145-2C11), purified anti-mouse CD28 (clone 37.51), purified NA/LE anti-mouse IFN-γ (clone R4-6A2), and purified NA/LE rat IgG1κ isotype control (R3-34) were purchased from BD Biosciences (San Diego, CA). FACSCanto (BD Biosciences, San Diego, CA) was used for flow cytometry event collection, which was analyzed using FACSDiva software (BD Biosciences, San Diego, CA).
Vaccinia virus
The Western Reserve strain of VV was purchased from American Type Culture Collection (Manassas, VA). VV was grown in TK-143B cells (American Type Culture Collection) and purified by a 35% sucrose cushion as described [49]. The titer was determined by plaque assay on TK-143B cells, and VV was stored at −80°C until use. For in vivo studies, 2 × 106 PFU of live VV in 0.05 ml 1 mM Tris (pH 9) was injected into mice intraperitoneally. The recombinant VV encoding HA (VV-HA) was previously described and 5 × 106 PFU of live VV-HA was used for in vivo studies [50].
In vitro T cell proliferation and suppression assays
For antigen-specific T cell proliferation and suppression assays, naïve clone 4 CD8+ and 6.5 CD4+ T cells purified from clone 4 and 6.5 HA-TCR transgenic mice, respectively, were first labeled with 3.3 mM carboxy- fluorescein diacetate, succinimidyl ester (CFSE) (Invitrogen, Grand Island, NY). CFSE-labeled T cells (2 × 105) were then cultured in the presence of HA class I [HA518–526 (IYSTVASSL)] or class II [HA110–120 (SFERFEIFPKE)] peptide –pulsed CD11c+ dendritic cells (DCs, 4 × 104) generated from the bone marrow cells as described [13], in 300 μl T cell media (RPMI 1640 supplemented with 5% FBS, 2 mM L-glutamine, 100 IU/ml penicillin, 100 IU/ml streptomycin, 10 mM HEPES buffer, 0.1 mM non-essentials amino-acids, 1 mM sodium pyruvate, and 50 μM 2-ME) for 72 h. In some experiments, m-MDSCs (CD11b+Ly6G−Ly6Chi) purified from peritoneal exudates (PE) of B10.D2 mice infected with 2 × 106 PFU of VV for 72 h, were added to the co-culture at the indicated m-MDSC:T cell ratio.
For polyclonal proliferation assays, CD8+ T cells and CD4+ T cells were purified from naïve B10.D2 mice and labeled with CFSE as described above. CFSE-labeled T cells were then cultured in the presence of 1 μg/ml soluble anti-CD3 and 1 μg/ml soluble anti-CD28, and M-MDSCs in 300 μl T cell media for 72 h.
In some experiments, 200 U/ml bovine superoxide dismutase (SOD) (Sigma-Aldrich, St. Louis, MO), 0.5 mM NG-monomethyl-L-arginine (L-NMMA; Calbiochem, San Diego, CA), or 0.5 mM N(ω)-hydroxy-nor-L-arginine (nor-NOHA; Calbiochem, San Diego, CA) were added to the coculture to inhibit ROS, iNOS and arginase-1 activity as described [25, 51]. Finally, the biological activity of IFN-γ was blocked in to the coculture by adding a final concentration of 10 μg/ml of purified anti-mouse IFN-γ or Rat IgG1κ as isotype control.
Measurement of intracellular IFN-γ production by CD8+ T cells
To measure intracellular levels of IFN-γ, 1 × 106 splenocytes or peritoneal cavity exudates were stimulated with either 2 μg/ml B8R20–27 (TSYKFESV) peptide or the Kd-restricted HA518–526 (IYSTVASSL) peptide for 6 h in the presence of 5 μg/ml brefeldin A at 37°C. After incubation, cells were washed and stained with PE-Cy5-conjugated anti-CD8 or with PE-Cy5-conjugated anti-CD8 and PE-conjugated anti-CD90.1. Cells were then permeabilized using the Cytofix/Cytoperm kit (BD Biosciences, San Diego, CA), subsequently stained with FITC-conjugated anti–IFN-γ and analyzed by flow cytometry.
In vivo suppression assay
Naïve CD8 T cells were purified from clone 4 HA-TCR transgenic mice using anti-CD8 beads according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). Cells were then washed twice in cold HBSS and resuspended at 5 × 104 cells/ml in HBSS. Thereafter, 1 × 104 CD8+ T cells were transferred intravenously into B10.D2 mice, which were subsequently infected intraperitoneally with 5 × 106 PFU of VV-HA. 5 days after infection, these infected mice received m-MDSCs or g-MDSCs purified from another cohort of VV-infected B10.D2 mice. Two days later, splenocytes were analyzed for total and IFN-γ+ clonotypic T cells by intracellular cytokine staining.
Measurement of VV DNA
Total genomic DNA was isolated from the spleen. Real-time quantitative PCR was used to measure VV genomic DNA using primers located in the VV A33R gene. The sequences of the forward and reverse primers for A33R were 5′-TATTACTGACGCCGC TGTTG-3′ and 5′-GTGTTGATGATTCCGCAGTG-3′, respectively. Amounts of VV DNA were normalized to β-actin gene within each sample. Normalized viral DNA value in each sample was calculated as the relative quantity of VV DNA divided by the relative quantity of β-actin gene.
iNOS expression by real-time PCR
Total RNA was isolated from 2×105 sorted m-MDSCs and g-MDSCs using RNAeasy Mini Kit (Qiagen, Hilden, Germany) according to manufacturer. First-strand cDNA was performed using RT kit (Promega, Madison, WI). Quantitative PCR was carried out with primers for iNOS and relative amounts of iNOS mRNA were normalized to β-actin. The sequences of the forward and reverse primers for iNOS were GTTCTCAGCCCAACAATACAAGA and GTGGACGGGTC GATGTCAC; respectively.
NO production
Equal volumes of culture supernatants (50 μl) and Greiss reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% N-1-naphthyl-ethylenediamine dihydrochloride in double-distilled water, Sigma-Aldrich, St. Louis, MO) were mixed together. After 10-min incubation at room temperature, the absorbance at 550 nm was measured using microplate plate reader (Bioteck Instrument, Winooski, VT). Nitrite (NO) concentrations were determined by comparing the absorbance values for the test samples to a standard curve generated by serial dilution of 0.25 mM sodium nitrite [51].
Statistical analysis
A two-sided, unpaired student t-test with 95% confidence bound and Welch’s correction was used for all statistical analysis, which was performed using GraphPad Prism Version 5.0f for Mac OS X (GraphPad Software, San Diego, CA). Significance was assumed at p<0.05.
Supplementary Material
Acknowledgments
We thank Lynn Martinek and Michael Cook from the Cancer Center Facility, Duke University, for excellent technical assistance in cell sorting.
This work was supported by the National Institutes of Health grants CA136934, CA186973 and CA193167 (to Y.Y.). C.F. is supported by a post-doctoral fellowship award from the FRSQ (Fonds de recherche du Québec - Santé).
References
- 1.Fenner F, Henderson D, Arita I, Jezek Z, Ladnyi I. Small-pox and Its Eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- 2.Walsh SR, Dolin R. Vaccinia viruses: vaccines against smallpox and vectors against infectious diseases and tumors. Expert Rev Vaccines. 2011;10:1221–1240. doi: 10.1586/erv.11.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verardi PH, Titong A, Hagen CJ. A vaccinia virus renaissance: new vaccine and immunotherapeutic uses after smallpox eradication. Hum Vaccin Immunother. 2012;8:961–970. doi: 10.4161/hv.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demkowicz WE, Jr, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quigley M, Huang X, Yang Y. Extent of stimulation controls the formation of memory CD8 T cells. J Immunol. 2007;179:5768–5777. doi: 10.4049/jimmunol.179.9.5768. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-{beta} Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez J, Huang X, Yang Y. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc Natl Acad Sci U S A. 2010;107:6442–6447. doi: 10.1073/pnas.0913291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quigley M, Huang X, Yang Y. STAT1 Signaling in CD8 T Cells Is Required for Their Clonal Expansion and Memory Formation Following Viral Infection In Vivo. J Immunol. 2008;180:2158–2164. doi: 10.4049/jimmunol.180.4.2158. [DOI] [PubMed] [Google Scholar]
- 9.Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009;113:2256–2264. doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 11.Natuk RJ, Welsh RM. Accumulation and chemotaxis of natural killer/large granular lymphocytes at sites of virus replication. J Immunol. 1987;138:877–883. [PubMed] [Google Scholar]
- 12.Parker AK, Parker S, Yokoyama WM, Corbett JA, Buller RM. Induction of natural killer cell responses by ectromelia virus controls infection. J Virol. 2007;81:4070–4079. doi: 10.1128/JVI.02061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J Immunol. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- 14.Fang M, Lanier LL, Sigal LJ. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog. 2008;4:e30. doi: 10.1371/journal.ppat.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J Clin Invest. 2006;116:2587–2590. doi: 10.1172/JCI29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 20.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Grone HJ, Platt FM, Zambon M, Cerundolo V. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowen JL, Olson JK. Innate immune CD11b+Gr-1+ cells, suppressor cells, affect the immune response during Theiler’s virus-induced demyelinating disease. J Immunol. 2009;183:6971–6980. doi: 10.4049/jimmunol.0902193. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Huang X, Yang Y. Myeloid-derived suppressor cells regulate natural killer cell response to adenovirus-mediated gene transfer. J Virol. 2012;86:13689–13696. doi: 10.1128/JVI.01595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortin C, Huang X, Yang Y. NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells. J Immunol. 2012;189:1843–1849. doi: 10.4049/jimmunol.1200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. Chronic but Not Acute Virus Infection Induces Sustained Expansion of Myeloid Suppressor Cell Numbers that Inhibit Viral-Specific T Cell Immunity. Immunity. 2013;38:309–321. doi: 10.1016/j.immuni.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 26.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, Merghoub T, Wolchok JD. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–886. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179:8243–8251. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 29.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 34.Daley-Bauer LP, Wynn GM, Mocarski ES. Cytomegalovirus impairs antiviral CD8+ T cell immunity by recruiting inflammatory monocytes. Immunity. 2012;37:122–133. doi: 10.1016/j.immuni.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehmann MH, Kastenmuller W, Kandemir JD, Brandt F, Suezer Y, Sutter G. Modified vaccinia virus ankara triggers chemotaxis of monocytes and early respiratory immigration of leukocytes by induction of CCL2 expression. J Virol. 2009;83:2540–2552. doi: 10.1128/JVI.01884-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehmann MH, Price PJ, Brandmuller C, Sutter G. Modified Vaccinia virus Ankara but not vaccinia virus induces chemokine expression in cells of the monocyte/macrophage lineage. Virol J. 2015;12:21. doi: 10.1186/s12985-015-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM, Casares S, Chen SH, Yang WC, Pan PY. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185:5828–5834. doi: 10.4049/jimmunol.0903636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 40.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sui Y, Hogg A, Wang Y, Frey B, Yu H, Xia Z, Venzon D, McKinnon K, Smedley J, Gathuka M, Klinman D, Keele BF, Langermann S, Liu L, Franchini G, Berzofsky JA. Vaccine-induced myeloid cell population dampens protective immunity to SIV. J Clin Invest. 2014;124:2538–2549. doi: 10.1172/JCI73518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koszinowski UH, Del Val M, Reddehase MJ. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 43.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 44.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 45.Samardzic T, Jankovic V, Stosic-Grujicic S, Trajkovic V. STAT1 is required for iNOS activation, but not IL-6 production in murine fibroblasts. Cytokine. 2001;13:179–182. doi: 10.1006/cyto.2000.0785. [DOI] [PubMed] [Google Scholar]
- 46.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 47.Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 48.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6:e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 51.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.