Abstract
Transcriptional fusions between the phage lambda promotor pR and ATP synthase genes, atp, on plasmid pBR322 were constructed in order to study the effects upon growth and physiology of Escherichia coli of induced overproduction of H+-ATPase subunits. Constitutive overproduction of the complete enzyme had earlier been found to result in decreased growth rate and cytological defects. When a 15-fold overproduction of subunit a alone, or together with subunit c, or with all other ATP synthase subunits was suddenly induced, the following effects were observed. Inhibition of growth and protein synthesis within 10 min of induction, which effect was suppressed by N,N'-dicyclohexylcarbodiimide, also when the chromosomal atp genes coding for the Fo subunits a, b and c were deleted. Partial collapse of the membrane potential delta psi at 4-6 min after induction paralleled by inhibition of thiomethylgalactoside and guanosine transport. Respiration and alpha-methylglucoside transport was not affected. The partial collapse of delta psi, and the specific inhibition of proton-driven transport systems is taken to show that the subunit a has--when suddenly overproduced and inserted into the membrane--a protonophoric activity. It is suggested that this protonophoric activity of subunit a is related to the function of this subunit in the Fo sector in H+-ATPases.
Full text
PDF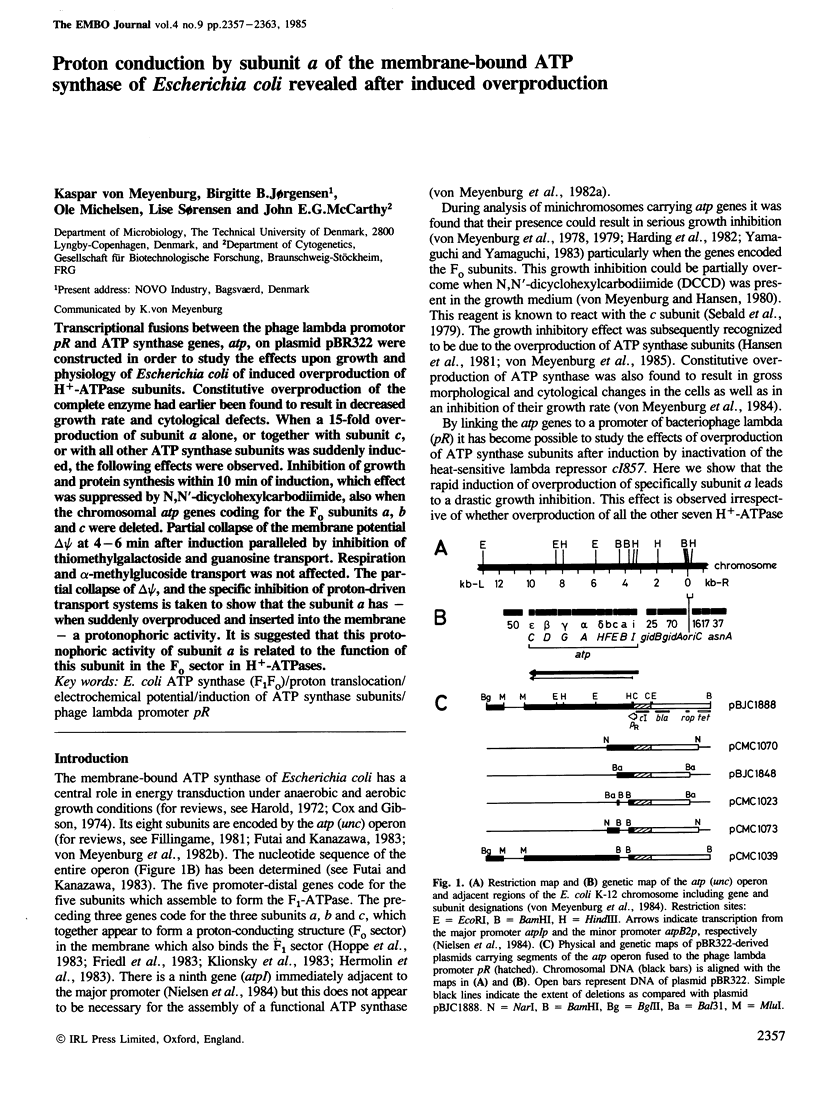



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Are growth rates of Escherichia coli in batch cultures limited by respiration? J Bacteriol. 1980 Oct;144(1):114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Aris J. P., Simoni R. D. Cross-linking and labeling of the Escherichia coli F1F0-ATP synthase reveal a compact hydrophilic portion of F0 close to an F1 catalytic subunit. J Biol Chem. 1983 Dec 10;258(23):14599–14609. [PubMed] [Google Scholar]
- Azzi A., Casey R. P., Nałecz M. J. The effect of N,N'-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta. 1984 Dec 17;768(3-4):209–226. doi: 10.1016/0304-4173(84)90017-x. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. Studies on electron transport and energy-linked reactions using mutants of Escherichia coli. Biochim Biophys Acta. 1974 Apr 30;346(1):1–25. doi: 10.1016/0304-4173(74)90010-x. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Identification of the dicyclohexylcarbodiimide-reactive protein component of the adenosine 5'-triphosphate energy-transducing system of Escherichia coli. J Bacteriol. 1975 Nov;124(2):870–883. doi: 10.1128/jb.124.2.870-883.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
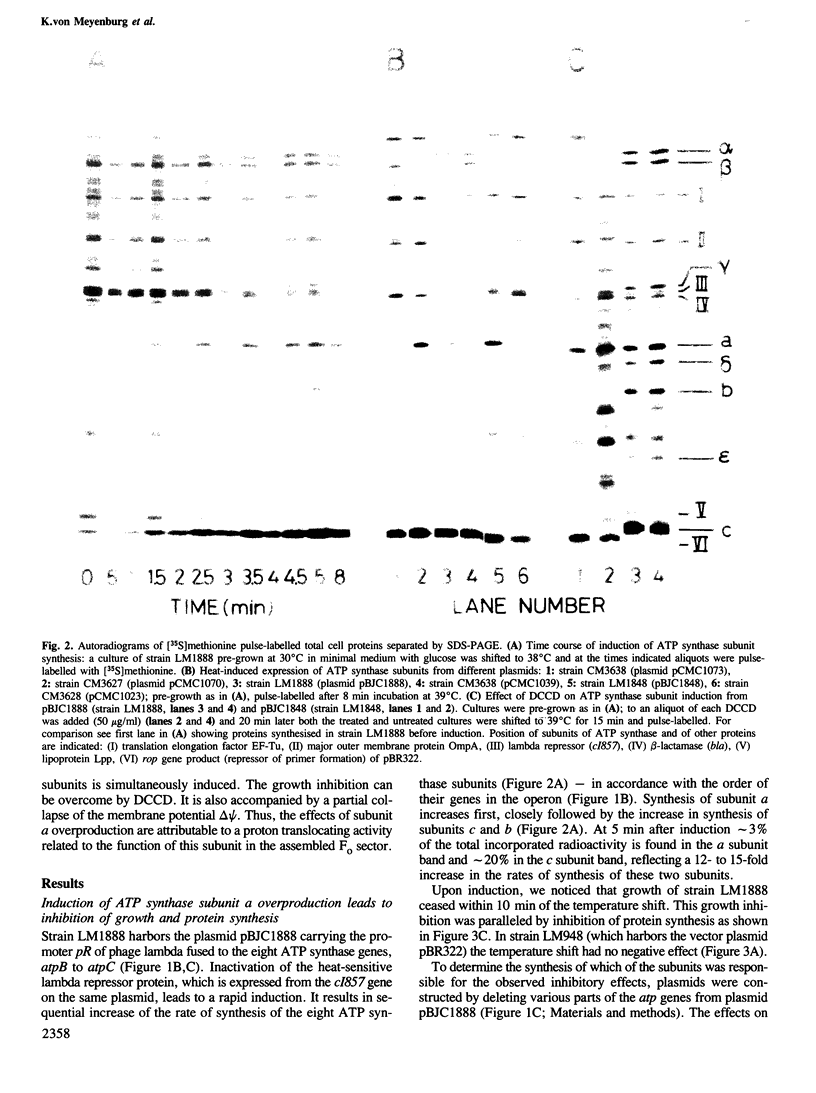
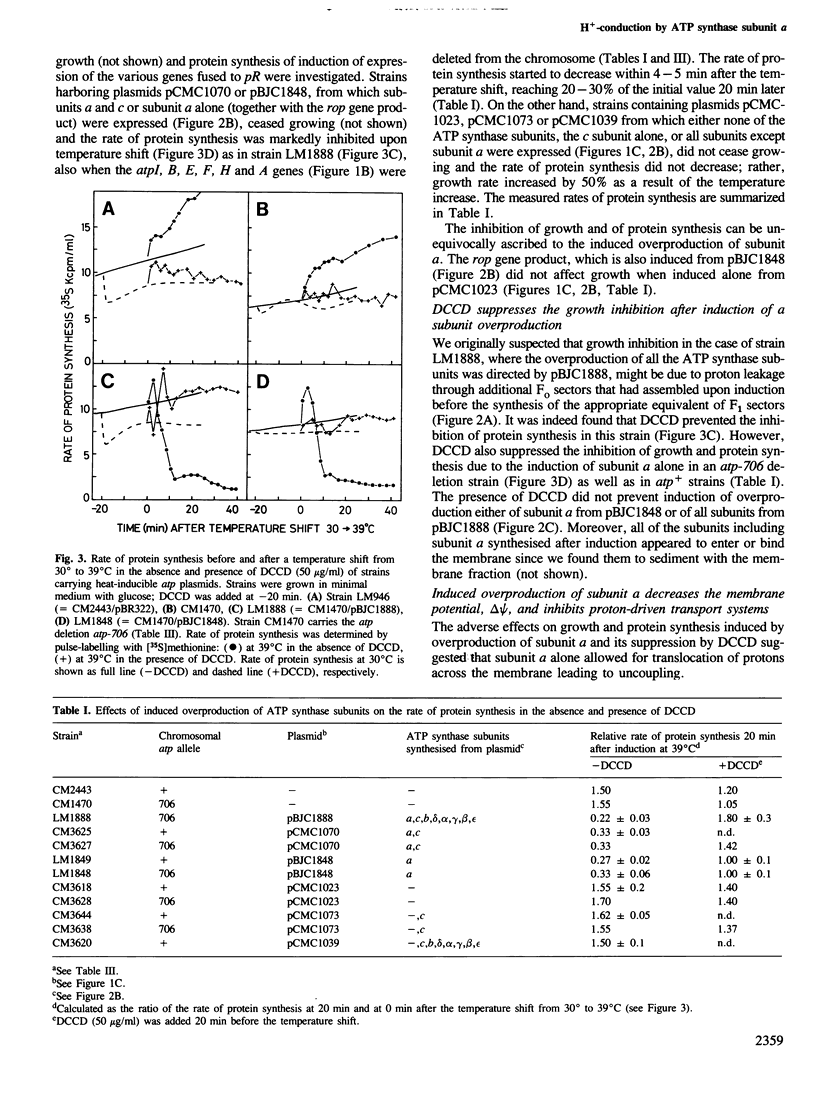
- Friedl P., Hoppe J., Gunsalus R. P., Michelsen O., von Meyenburg K., Schairer H. U. Membrane integration and function of the three F0 subunits of the ATP synthase of Escherichia coli K12. EMBO J. 1983;2(1):99–103. doi: 10.1002/j.1460-2075.1983.tb01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the region encoding the alpha-subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 May 11;9(9):2187–2194. doi: 10.1093/nar/9.9.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Nielsen J., Riise E., von Meyenburg K. The genes for the eight subunits of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;183(3):463–472. doi: 10.1007/BF00268766. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Cleary J. M., Smith D. W., Michon J. J., Brusilow W. S., Zyskind J. W. Chromosomal replication origins (oriC) of Enterobacter aerogenes and Klebsiella pneumoniae are functional in Escherichia coli. J Bacteriol. 1982 Dec;152(3):983–993. doi: 10.1128/jb.152.3.983-993.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermolin J., Gallant J., Fillingame R. H. Topology, organization, and function of the psi subunit in the F0 sector of the H+-ATPase of Escherichia coli. J Biol Chem. 1983 Dec 10;258(23):14550–14555. [PubMed] [Google Scholar]
- Hirota N., Matsuura S., Mochizuki N., Mutoh N., Imae Y. Use of lipophilic cation-permeable mutants for measurement of transmembrane electrical potential in metabolizing cells of Escherichia coli. J Bacteriol. 1981 Nov;148(2):399–405. doi: 10.1128/jb.148.2.399-405.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Friedl P., Schairer H. U., Sebald W., von Meyenburg K., Jørgensen B. B. The topology of the proton translocating F0 component of the ATP synthase from E. coli K12: studies with proteases. EMBO J. 1983;2(1):105–110. doi: 10.1002/j.1460-2075.1983.tb01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Schairer H. U., Sebald W. The proteolipid of a mutant ATPase from Escherichia coli defective in H+-conduction contains a glycine instead of the carbodiimide-reactive aspartyl residue. FEBS Lett. 1980 Jan 1;109(1):107–111. doi: 10.1016/0014-5793(80)81321-4. [DOI] [PubMed] [Google Scholar]
- Jans D. A., Fimmel A. L., Hatch L., Gibson F., Cox G. B. An additional acidic residue in the membrane portion of the b-subunit of the energy-transducing adenosine triphosphatase of Escherichia coli affects both assembly and function. Biochem J. 1984 Jul 1;221(1):43–51. doi: 10.1042/bj2210043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Kiyasu T., Noumi T., Futai M. Overproduction of subunit a of the F0 component of proton-translocating ATPase inhibits growth of Escherichia coli cells. J Bacteriol. 1984 Apr;158(1):300–306. doi: 10.1128/jb.158.1.300-306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. Assembly of a functional F0 of the proton-translocating ATPase of Escherichia coli. J Biol Chem. 1983 Aug 25;258(16):10136–10143. [PubMed] [Google Scholar]
- Lötscher H. R., deJong C., Capaldi R. A. Modification of the F0 portion of the H+-translocating adenosinetriphosphatase complex of Escherichia coli by the water-soluble carbodiimide 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide and effect on the proton channeling function. Biochemistry. 1984 Aug 28;23(18):4128–4134. doi: 10.1021/bi00313a018. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell. 1980 Jun;20(2):507–517. doi: 10.1016/0092-8674(80)90637-6. [DOI] [PubMed] [Google Scholar]
- McCarthy J. E., Ferguson S. J., Kell D. B. Estimation with an ion-selective electrode of the membrane potential in cells of Paracoccus denitrificans from the uptake of the butyltriphenylphosphonium cation during aerobic and anaerobic respiration. Biochem J. 1981 Apr 15;196(1):311–321. doi: 10.1042/bj1960311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A., Mygind B., Nicolaisen A., Pihl N. J. Nucleoside transport in cells and membrane vesicles from Escherichia coli K12. J Biol Chem. 1979 May 25;254(10):3730–3737. [PubMed] [Google Scholar]
- Nielsen J., Hansen F. G., Hoppe J., Friedl P., von Meyenburg K. The nucleotide sequence of the atp genes coding for the F0 subunits a, b, c and the F1 subunit delta of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;184(1):33–39. doi: 10.1007/BF00271191. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Jørgensen B. B., van Meyenburg K. V., Hansen F. G. The promoters of the atp operon of Escherichia coli K12. Mol Gen Genet. 1984;193(1):64–71. doi: 10.1007/BF00327415. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. All three subunits are required for the reconstitution of an active proton channel (F0) of Escherichia coli ATP synthase (F1F0). EMBO J. 1985 Feb;4(2):515–518. doi: 10.1002/j.1460-2075.1985.tb03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. Subunit b of the membrane moiety (F0) of ATP synthase (F1F0) from Escherichia coli is indispensable for H+ translocation and binding of the water-soluble F1 moiety. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7279–7283. doi: 10.1073/pnas.81.23.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W., Friedl P., Schairer H. U., Hoppe J. Structure and genetics of the H+-conducting F0 portion of the ATP synthase. Ann N Y Acad Sci. 1982;402:28–44. doi: 10.1111/j.1749-6632.1982.tb25730.x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi J. I., Matsuura S., Imae Y. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):891–897. doi: 10.1128/jb.144.3.891-897.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens K., Schneider E., Herkenhoff B., Schmid R., Altendorf K. Chemical modification of the F0 part of the ATP synthase (F1F0) from Escherichia coli. Effects on proton conduction and F1 binding. Eur J Biochem. 1984 Feb 1;138(3):617–622. doi: 10.1111/j.1432-1033.1984.tb07959.x. [DOI] [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yamaguchi M. Copy number mutations (Cop-) of the plasmid containing the replication origin (oriC) of the Escherichia coli chromosome: lethal effect of the cop region cloned onto a high-copy-number vector on host cells. J Bacteriol. 1983 Jan;153(1):550–554. doi: 10.1128/jb.153.1.550-554.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg Kaspar Transport-limited growth rates in a mutant of Escherichia coli. J Bacteriol. 1971 Sep;107(3):878–888. doi: 10.1128/jb.107.3.878-888.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Nielsin L. D., Riise E. Origin of replication, oriC, or the Escherichia coli chromosome on specialized transducing phages lambda asn. Mol Gen Genet. 1978 Apr 17;160(3):287–295. doi: 10.1007/BF00332972. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Riise E., Bergmans H. E., Meijer M., Messer W. Origin of replication, oriC, of the Escherichia coli K12 chromosome: genetic mapping and minichromosome replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):121–128. doi: 10.1101/sqb.1979.043.01.018. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., Nielsen J., Hansen F. G., Michelsen O. The membrane bound ATP synthase of Escherichia coli: a review of structural and functional analyses of the atp operon. Tokai J Exp Clin Med. 1982;7 (Suppl):23–31. [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., Nielsen J., Hansen F. G. Promoters of the atp operon coding for the membrane-bound ATP synthase of Escherichia coli mapped by Tn10 insertion mutations. Mol Gen Genet. 1982;188(2):240–248. doi: 10.1007/BF00332682. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]