Abstract
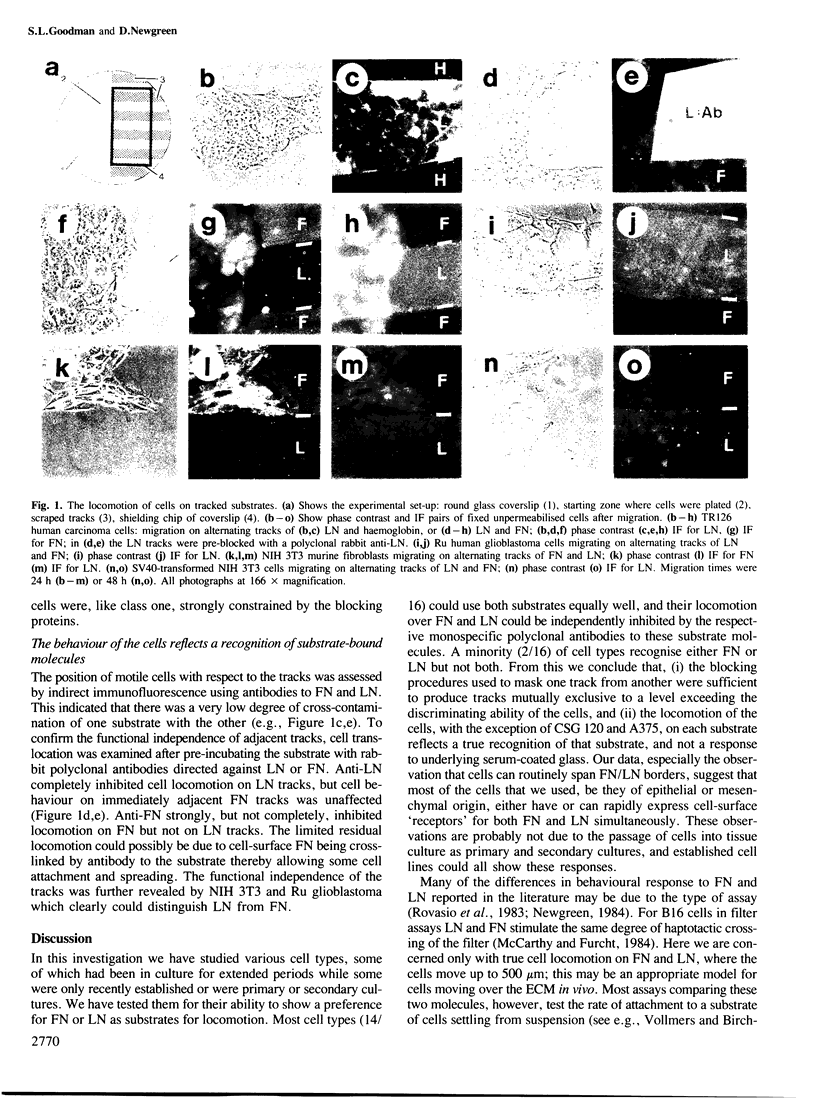
Sixteen cell types from a variety of tissues and from primary and secondary cell cultures and established cell lines were tested for their ability to distinguish between fibronectin and laminin substrates during locomotion in vitro. Laminin and fibronectin were presented to the cells as directly adjacent tracks. Most cells, regardless of origin, showed no preference for one substrate over the other. Only two of the cell types tested showed a strong preference for one or other other substrate molecule. Cells were responding to the local substrate, since antibodies directed against one substrate molecule only interfered with locomotion on tracks coated with that molecule. We conclude that many cells simultaneously express functionally active receptors for fibronectin and laminin, and that differential locomotory response to these two molecules cannot be assumed without experimental confirmation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M. Contact inhibition and malignancy. Nature. 1979 Sep 27;281(5729):259–262. doi: 10.1038/281259a0. [DOI] [PubMed] [Google Scholar]
- Avnur Z., Geiger B. The removal of extracellular fibronectin from areas of cell-substrate contact. Cell. 1981 Jul;25(1):121–132. doi: 10.1016/0092-8674(81)90236-1. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Haptotaxis and the mechanism of cell motility. Nature. 1967 Jan 21;213(5073):256–260. doi: 10.1038/213256a0. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Principles of cell motility: the direction of cell movement and cancer invasion. Nature. 1965 Dec 18;208(5016):1183–1187. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Hök M., Rees D. A., Timpl R. Adhesion, growth, and matrix production by fibroblasts on laminin substrates. J Cell Biol. 1983 Jan;96(1):177–183. doi: 10.1083/jcb.96.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Vollmers H. P., Birchmeier W. Control of cell locomotion: perturbation with an antibody directed against specific glycoproteins. Cell. 1985 Jul;41(3):1029–1038. doi: 10.1016/s0092-8674(85)80083-0. [DOI] [PubMed] [Google Scholar]
- Hogan B. Laminin and epithelial cell attachment. Nature. 1981 Apr 30;290(5809):737–738. doi: 10.1038/290737a0. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E. B., Goodman S. L., Trejdosiewicz L. K. Disruption of the keratin filament network during epithelial cell division. EMBO J. 1982;1(11):1365–1372. doi: 10.1002/j.1460-2075.1982.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. B., Furcht L. T. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984 Apr;98(4):1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen D. Spreading of explants of embryonic chick mesenchymes and epithelia on fibronectin and laminin. Cell Tissue Res. 1984;236(2):265–277. doi: 10.1007/BF00214227. [DOI] [PubMed] [Google Scholar]
- Palotie A., Peltonen L., Risteli L., Risteli J. Effect of the structural components of basement membranes on the attachment of teratocarcinoma-derived endodermal cells. Exp Cell Res. 1983 Mar;144(1):31–37. doi: 10.1016/0014-4827(83)90438-x. [DOI] [PubMed] [Google Scholar]
- Raper J. A., Bastiani M., Goodman C. S. Pathfinding by neuronal growth cones in grasshopper embryos. II. Selective fasciculation onto specific axonal pathways. J Neurosci. 1983 Jan;3(1):31–41. doi: 10.1523/JNEUROSCI.03-01-00031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovasio R. A., Delouvee A., Yamada K. M., Timpl R., Thiery J. P. Neural crest cell migration: requirements for exogenous fibronectin and high cell density. J Cell Biol. 1983 Feb;96(2):462–473. doi: 10.1083/jcb.96.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Lawton J., Dollenmeier P., Ehrismann R., Chiquet M. Guidance of myogenic cell migration by oriented deposits of fibronectin. Dev Biol. 1983 Feb;95(2):497–504. doi: 10.1016/0012-1606(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Gospodarowicz D. Respective roles of laminin and fibronectin in adhesion of human carcinoma and sarcoma cells. Nature. 1981 Jan 22;289(5795):304–306. doi: 10.1038/289304a0. [DOI] [PubMed] [Google Scholar]



