Abstract
Salivary glands develop as highly branched structures designed to produce and secrete saliva. Advances in mouse genetics, stem cell biology and regenerative medicine are having a tremendous impact on our understanding of salivary gland organogenesis. Understanding how SMG initiation, branching morphogenesis and cell differentiation occur, as well as defining the progenitor/stem cells and cell and tissue interactions that drive SMG development will help guide regenerative approaches for patients suffering from loss of salivary gland function. This review focuses on recent literature from the past 5 years investigating the regulatory mechanisms driving submandibular gland (SMG) organogenesis.
Keywords: Salivary gland, Development, Organogenesis, Branching Morphogenesis, Progenitor cells, Differentiation, MicroRNAs, Salivary stem cell, Stem cell niche, Microenvironment
1 Introduction
1.1 Function and Anatomy
The three pairs of major salivary glands, the submandibular (SMG), sublingual (SLG) and parotid glands (PG), produce ~90% of saliva secreted into the oral cavity; the other 10% is produced by minor glands distributed throughout the oral mucosa. The major functions of saliva include lubrication of the oral cavity to enable speaking and eating, digestion of food, antimicrobial activity, maintenance of mucosal integrity, and oral homeostasis. A reduction in salivary flow can cause clinical problems that include increased caries, xerostomia, oral infections, and difficulties with mastication, swallowing, and speech (Delli et al., 2014). The reader is directed to recent reviews on the physiological mechanisms of salivary secretion (Ambudkar, 2014; Catalan et al., 2009) as well as the anatomy and function of the salivary glands (Holmberg and Hoffman, 2014; Tucker, 2007). Briefly, secretory acinar cells produce the isotonic primary salivary secretion. The acini are surrounded by myoepithelial cells, which are within a basement membrane. The myoepithelial cells may facilitate saliva secretion by contracting, although this has not been demonstrated experimentally. The primary salivary secretion is modified by ductal cells, which reabsorb ions resulting in hypotonic saliva, supersaturated with calcium and phosphate. The types of ducts, characterized by their morphology and histological appearance, are the intercalated, striated, granular and excretory ducts. The epithelial compartment of the SMG is surrounded by mesenchymal stroma, which also contains immune cells, blood vessels, lymphatic vessels, fibroblasts, and nerves. Innervation is also essential for organogenesis, secretory function, and maintenance of acini, as denervation results in glandular atrophy. Both the sympathetic and parasympathetic branches of the autonomic nervous system innervate SMGs. The reader is referred to recent reviews of innervation of the developing salivary gland for details (Ferreira and Hoffman, 2013; Proctor and Carpenter, 2014).
2 Regulation of Branching Morphogenesis
2.1 SMG initiation
SMG initiation and organogenesis involve complex interactions among multiple stem and/or progenitor cells. By definition, a stem cell is capable of both self-renewal and differentiation into all mature gland cell types. As stem cells differentiate into more committed progenitor cells they lose their ability to self-renew and become restricted to one lineage. However, no single salivary stem cell has been identified and there is currently no clear distinction between salivary stem and progenitor cells. Therefore, we will use the term stem/progenitor in this review. The reader is also directed to recent comprehensive reviews on SMG organogenesis and branching morphogenesis (Knosp et al., 2012; Kwon and Larsen, 2015; Miletich, 2010; Patel and Hoffman, 2014).
A major research focus has been to identify epithelial stem/progenitor cells involved in murine SMG initiation. These stem/progenitors function within a niche or local microenvironment, which includes extracellular matrix (ECM) and mesenchymal, neuronal, and endothelial cells. During fetal development these other cell types may include stem/progenitor cells; therefore, different types of stem/progenitors may influence each other via multidirectional signaling networks involving both secreted factors and physical interactions. At embryonic day 11 (E11) in the mouse, the migrating neural crest cells form a loose mesenchymal aggregate beside a local thickening of the oral epithelium, which forms the SMG epithelial placode. In terms of the developmental origin of the salivary gland epithelium, lineage tracing with an endodermal-specific Sox17-cre suggests that all major salivary glands of the mouse are not endodermal, but likely ectodermal in origin (Rothova et al., 2012). At E12 the endothelial cells form a plexus within the surrounding mesenchyme, which begins to condense. The signals that cause mesenchymal condensation have not been identified. The neural crest also contains neuronal cell bodies that coalesce to form the parasympathetic submandibular ganglion (PSG). Gland initiation involves enlargement of the salivary epithelial placode, and a simultaneous invagination of the oral epithelium into the adjacent mesenchyme. This results in the placode forming a primary endbud on a stalk of oral epithelium. Importantly, the mesenchyme produces fibroblast growth factor 10 (Fgf10) which induces epithelial proliferation via its epithelial receptor FGFR2b (Kwon and Larsen, 2015). In the absence of Fgf10 the SMG epithelium does not develop (Figure 1). The formation of the PSG and subsequent innervation occur in parallel with epithelial development. Recently, the developmental origin of the PSG was identified as peripheral Schwann cell precursors that migrate along the preganglionic nerves and form both the glial cells and the PSG neurons of the glands (Dyachuk et al., 2014; Espinosa-Medina et al., 2014). These neurons then coalesce around the primary epithelial duct to form the PSG, establishing communication with the developing epithelium as it begins branching morphogenesis.
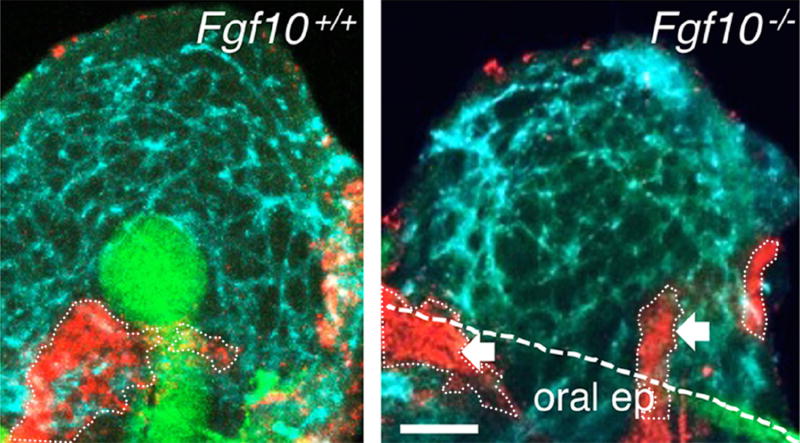
Figure 1. Fgf10 is essential for SMG epithelial development.
Whole mount immunostaining of an E12 wildtype (Fgf10+/+) SMG and Fgf10−/− SMG mesenchyme. SMG development involves the formation of an epithelial endbud and primary duct (E-Cadherin, green) within a condensed mesenchyme, which is surrounded by an endothelial cell plexus (PCAM, cyan blue) and the formation of a PSG (Tubb3, red). White dotted lines outline PSG, white dashed line is the oral epithelium (oral ep); arrows indicate nerves in the adjacent tongue and oral epithelium. Scale bar, 50 μm. From Figure 2B in Knosp et al., 2015.
Recently four Wnt ligands were identified. They are mainly produced by the keratin (K)-5 expressing (K5+) progenitors in the primary salivary gland duct. These Wnts provide the signal to initiate gangliogenesis, in part by inducing neuronal proliferation and cell survival. This finding came unexpectedly from studying a mouse model of increased FGF signaling, in which two Sprouty (Spry) genes were deleted. Sproutys are key intracellular modulators of FGF signaling and act as negative-feedback antagonists (Tang et al., 2011). The genetic deletion of Spry1 and Spry2 (Spry1/2DKO) from the SMG epithelium resulted in a striking loss of the PSG and SMG innervation (Figure 2), resulting in reduced branching morphogenesis (Knosp et al., 2015). During PSG formation it was shown that increasing FGF signaling reduced the expression of the Wnt signals in the epithelium. The K5+ progenitor cells mainly produced these Wnt signals that promoted neuronal survival and proliferation, PSG formation and gland innervation. Inhibiting Wnt signaling or treatment with exogenous Fgfs disrupted PSG formation, the association of the PSG with the epithelium, and organ innervation. Wnt expression in the epithelium was inhibited by FGFR2b/MAPK signaling and promoted via neuregulin ErbB2/3/PI3K-dependent signaling. This provided a new mechanism by which the K5+ progenitor cells regulated their own innervation by integrating both FGFR2b and ErbB signaling to produce Wnts. Moreover, it is the balance of FGFR/ErbB signaling that regulates Wnts, which act on neuronal cells to promote survival and proliferation (Knosp et al., 2015).
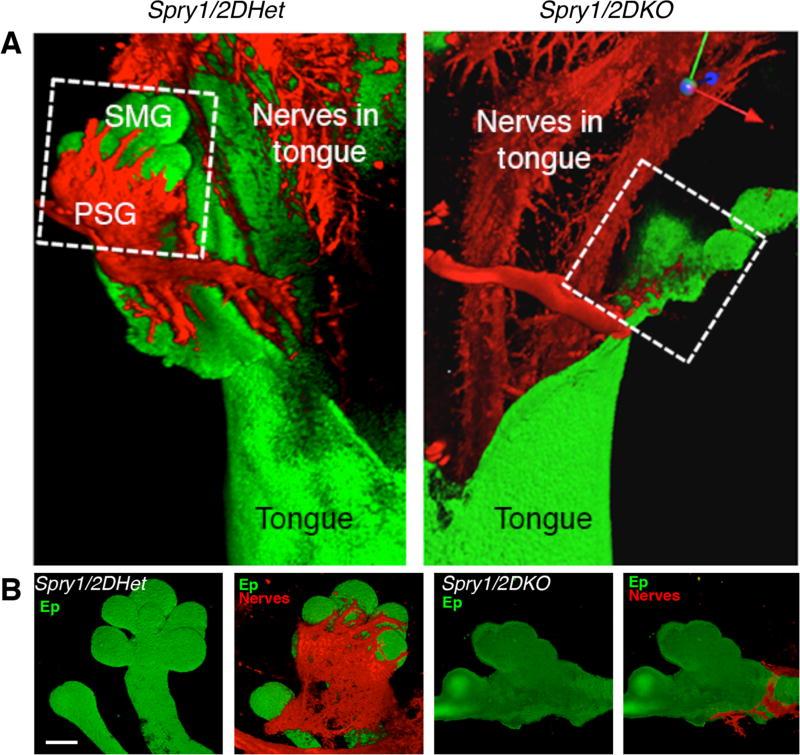
Figure 2. There is loss of the PSG and SMG innervation in the Spry1/2DKO mouse, although the sensory innervation of the tongue is not affected.
(A) Whole mount immunostaining for the epithelium (Ep, E-cadherin) and nerves (Tubb3) in Spry1/2DHet (control) and Spry1/2DKO tongues with SMGs attached. (B) The SMGs (green) and PSG (red) that are in white box are shown separately in the lower panels. Scale bar, 100 μm. From Figure S1A in Knosp et al., 2015.
2.2 Cleft formation
After the formation of a primary duct, initial endbud, and PSG, the endbud begins the process of branching morphogenesis. This involves the processes of clefting, epithelial proliferation, migration, and differentiation, as well as innervation and vascular development. Endbud expansion and clefting with branch-point formation and duct elongation result in 3–5 endbuds forming by E13. Cleft formation requires several interrelated cellular processes, such as proliferation, migration, cell-cell adhesion, cell-ECM adhesion, ECM accumulation, and cellular contraction. The basement membrane separates the epithelium from the surrounding mesenchyme and is a specialized ECM containing laminins, collagen IV, proteoglycans, nidogen, and agrin (Hohenester and Yurchenco, 2013), as well as fibronectin. Accumulation of fibronectin during cleft initiation induces Btdb7, a factor made in the epithelium. Btdb7 induces the expression of Snail2 and suppresses E-cadherin levels (Onodera et al., 2010), which reduces columnar organization and cell-cell adhesion molecules in the cells of the outer epithelial layer, allowing cleft progression.
Contraction of the cytoskeleton also promotes progression of clefts. Rho-associated coiled-coil containing kinase (ROCK) regulates cytoskeletal contraction at discrete stages and inhibition of ROCK leads to ectopic clefting in ex vivo SMG culture. ROCK controls tissue organization and cell polarity via PAR-1b protein. ROCK regulates the transition of initiated clefts to a stabilized state, which is then able to undergo cleft progression, a proliferation-independent process (Daley et al., 2012). Cleft stabilization and progression occur through the stabilization of actin (Ray et al., 2014), where LIM-kinase (LIMK), a regulator of early and late stage cleft formation and initiation, regulates both microfilaments and microtubules. LIMK-dependent regulation of the cytoskeleton controls fibronectin assembly and activation of β1 integrins. Furthermore, the microtubule assembly factor p25 regulates the stabilization and elongation of late-stage progressing clefts. In sum, multiple actin- and microtubule-dependent stabilization steps are controlled by LIMK and are required for cleft progression.
2.3 Migration and ECM proteolysis
Cleft formation is coordinated with cell migration in the endbud. Using a single cell tracking technique, epithelial cell migration was shown to be highest in outer bud cells near the basement membrane, lower in the inner bud cells, and lowest in duct cells (Hsu et al., 2013). Inhibitors of integrin α6β1 and nonmuscle myosin II reduced the peripheral cell motility whereas inhibiting E-cadherin reduced inner bud motility. These findings suggest cell motility in different regions of the endbuds is dependent on different cellular mechanisms.
In addition, highly dynamic remodeling of ECM drives epithelial proliferation during branching morphogenesis. Membrane-type 2 matrix metalloproteinase (MT2-MMP)-dependent collagen IV proteolysis releases small collagen fragments called NC1 domains (Rebustini et al., 2009). These collagen fragments increase MT-2-MMP expression and genes related to proliferation, by binding to b1 integrins and signaling via PI3K and AKT. The epithelium also produces heparin-binding epidermal growth factor (HBEGF), which increases MT2-MMP expression and further release of NC1 domains. Therefore, feedback from multiple protease-dependent pathways increases branching morphogenesis.
Remodeling of the basement membrane also controls local epithelial expansion. The basement membrane at the tip of the E13 endbud becomes perforated with small holes as rapid epithelial expansion occurs (Harunaga et al., 2014). This structure appears as a mesh-like net and allows epithelial expansion while maintaining tissue integrity. The basement membrane is remodeled in a distal direction, appearing to accumulate around the secondary ducts. These local and global dynamics require both protease and myosin II activity, suggesting epithelial expansion requires proteolytic degradation and remodeling via actomyosin contractility during branching morphogenesis. Taken together, cleft initiation, stabilization, and progression are coordinated with cell migration and ECM remodeling during SMG branching morphogenesis.
2.4 Cell Differentiation
Branching morphogenesis is coordinated with the cytodifferentiation of the ductal and acinar compartments of the gland. Recently, it was reported that the transcription factor NFIB (nuclear factor IB) was involved in secretory cell differentiation during SMG development (Mellas et al., 2015). NFIB has clinical importance because a gene fusion of NFIB with MYB (MYB-NFIB) occurs in adenoid cystic carcinoma (ACC) of salivary glands (Stenman, 2013). ACC is the second most common salivary malignancy and more than 80 % of patients with head and neck ACC die 10–15 years after diagnosis. SMG from Nfib−/− mice were hypoplastic at E18, although some branching morphogenesis did occur. The SMGs of Nfib−/− mice showed reduced apicobasal polarity, as measured by disrupted apical ZO-1 staining, had reduced ductal and acinar lumen formation, and appeared disorganized. The terminal tubules did not differentiate into secretory cells, as evidenced by a lack of staining for SMG-C, a proacinar cell secretory marker, and aquaporin 5, a water channel that is present in the apical membranes of acinar and intercalated duct cells. Thus NFIB is required for acinar cell differentiation and is important for lumen formation during mouse SMG development.
During branching morphogenesis, lumen formation occurs at the distal ends of epithelial branches and proximal microlumens coalesce in a distal direction to form a contiguous lumen. The interaction between FGF signaling and the canonical (beta-catenin dependent) and non-canonical Wnt signaling coordinates this processes. Using Axin2LacZ reporter mice to show where Wnt signaling occurs, it was demonstrated that Wnt signaling appeared first in the mesenchyme at E12 and then at E14, switched to the ductal epithelium. Gain- and loss- of- function experiments showed that Wnts exert an inhibitory effect on salivary gland branching morphogenesis. Furthermore, endbuds do not have active Wnt signaling due to FGF-mediated inhibition. Thus, FGF signaling prevents lumenization of epithelial endbuds and slows down the lumenization of presumptive ducts. The mechanism involves reducing Cp2l1, a marker of duct differentiation, by repressing non-canonical Wnt5b expression and signaling via regulating sFRP1, a secreted Wnt inhibitor. Thus FGF signaling maintains undifferentiated endbud cells by inhibiting ductal fate and lumenization (Patel et al., 2011).
Similarly, core binding factor beta (CBFb), a cotranscription factor that forms a heterodimer with Runx transcription factors, was shown to influence postnatal duct differentiation. CBFb is expressed in SMG ducts and conditional deletion of CBFb with a K14-cre decreases the size of the SMG and reduces saliva secretion in adult male mice (Islam et al., 2015). There was a loss of a specific ductal compartment, the granular convoluted tubules (GCT), with reduced expression of genes that are expressed in GCT, such as Klk1, Ngf, and Egf. GCT development is androgen-dependent, but circulating testosterone levels were not affected by CBFb deletion, suggesting that CBFb signaling regulates androgen receptor signaling pathway, not circulating testosterone levels. Therefore, Runx/CBFb-dependent transcription is required for the postnatal development of androgen-dependent GCT in the SMG. Together, these studies illustrate the critical roles transcription factors play in cell differentiation during gland development.
3 SMG Innervation
3.1 Parasympathetic-epithelial communication
Both the parasympathetic and sympathetic branches of the autonomic nervous system richly innervate the adult salivary gland and are critical for saliva secretion. The PSG releases acetylcholine (ACh), which activates the muscarinic receptors 1 and 3 (Chrm1 and Chrm3) to stimulate fluid secretion. A recent review of the interactions between developing nerves and salivary glands provides a comprehensive background (Ferreira and Hoffman, 2013). Parasympathetic innervation occurs along the epithelium during SMG branching morphogenesis and sympathetic innervation occurs along the vasculature. Parasympathetic innervation is required for maintaining epithelial K5+ cells as a pool of undifferentiated progenitor cells for further development (Knox et al., 2010). Keratins are cytoskeletal proteins used as epithelial markers; K5 is used to label progenitor cells and K19 to label ductal cells. Removal of the PSG from the SMG in culture resulted in a striking decrease in the number of K5+ cells. This was dependent on ACh production by the PSG and muscarinic receptor M1 signaling in the K5+ cells. Furthermore, K5+ cell maintenance and differentiation into K19+ ductal cells were dependent on HBEGF/EGFR signaling (Knox et al., 2010). These data highlight the importance of the bi-directional communication between the PSG and the developing epithelium.
A recent advance in understanding parasympathetic-epithelial communication during organogenesis concerns the epithelial production of the neurotrophic factor neurturin (NRTN). NRTN increases PSG function and promotes neuronal survival and directional axon outgrowth. During SMG organogenesis, the PSG axons that extend along the ducts to envelop the endbuds respond to localized neurotropic cues. NRTN promotes innervation by binding to its receptor GFRα2 and the tyrosine kinase coreceptor RET, and signals via Src kinase. Gene targeting of RET, GFRα2, or NRTN in mice results in smaller PSG and reduced innervation of multiple organs. Using isolated PSG from SMGs, it was shown that NRTN reduced neuronal apoptosis and increased axon outgrowth and the expression of genes involved in parasympathetic function (Knox et al., 2013). Furthermore, antibodies blocking NRTN reduced parasympathetic nerve outgrowth and function, which reduced branching morphogenesis in intact SMG culture. The functions of NRTN on SMGs were also investigated after irradiation of the gland. In this case NRTN reduced neuronal apoptosis and restored parasympathetic function, which helped the epithelium to regenerate. The hypothesis that NRTN may improve regeneration after irradiation in adult glands is currently under investigation.
Another report highlighting parasympathetic-epithelial crosstalk showed that parasympathetic innervation regulates ductal tubulogenesis of the epithelium (Nedvetsky et al., 2014). Vasoactive intestinal peptide (VIP) is produced by the nerves and promotes ductal growth and the formation of a contiguous ductal lumen. Isolated SMG epithelia treated with VIP in ex vivo culture formed lumens, which expanded via cAMP/PKA-dependent pathway. The lumen expansion was independent of apoptosis and involved the cystic fibrosis transmembrane conductance channel (CFTR), which is a cAMP-regulated chloride channel. Interestingly, ductal tubulogenesis did not require ACh/M1 signaling. In addition, analysis of the SMGs in neuregulin 1 (Nrg1) null mice, which have depleted innervation of multiple tissues, showed that they had aberrant ductal morphogenesis and reduced branching morphogenesis. A similar problem with ductal morphogenesis was also observed in the CFTR null SMGs. An emerging theme is the importance of multiple neuronal-derived factors on the branching epithelium. ACh, VIP and Nrg1 regulate K5+ progenitor cell function and ductal tubulogenesis during SMG development. It is likely that other neuropeptides and regulatory factors produced by the nerves will influence organogenesis.
3.2 Sympathetic Innervation
The sympathetic nerves also stimulate secretion via the α and β adrenoreceptors, which increase fluid and protein-rich secretions, respectively (Patel and Hoffman, 2014). An autocrine Wnt5a-Ror signaling loop was shown to mediate sympathetic axon branching during SMG innervation (Ryu et al., 2013). Using a conditional knockout approach, the non-canonical Wnt5a was deleted with either Wnt1:cre or tyrosine hydroxylase:cre, the latter of which is specific for sympathetic nerves. Wnt5a, which is produced by the nerves, was involved in the autocrine signaling loop and target innervation. Deletion of Wnt5a reduced the extension and arborization of sympathetic fibers in the gland, but did not affect overall tissue patterning or the proliferation, migration, or differentiation of neuronal progenitors. Less is known about the function of the sympathetic nerves than the parasympathetic nerves during organogenesis. Further studies using genetic ablation or knockdown of genes required for sympathetic innervation may provide insight into the function of these nerves during development.
4 Stem/progenitor cells
4.1 Investigating stem/progenitor cells during development
Identifying stem/progenitor cells present during fetal development and understanding how they function will impact approaches to regenerating adult glands. Epithelial progenitor cells have been isolated from adult murine SMGs using fluorescent-activated cell sorting (FACS) of the cell surface marker Kit and combinations of CD24, CD49f, CD133, and Sca1. These cells have been used to partly regenerate function in irradiated adult murine salivary glands (Lombaert et al., 2008; Nanduri et al., 2011). However, Kit labels a heterogeneous population of cells, and understanding how Kit functions during development and its role in cell-fate decisions and regeneration are important research questions. Kit+ cells are in epithelial endbuds during fetal development and are important for organogenesis. Epithelial Kit expression is upregulated by FGFR2b signaling, which is also essential for gland development (Lombaert et al., 2013). The combined effects of Kit and FGFR2b signaling via separate AKT and MAPK pathways, respectively, amplified expression of transcription factors downstream of FGFR2b signaling, including Sox10, Myc, Etv4 and Etv5. Importantly, the combined signaling increased the number of Kit+ cells in the distal endbuds that also expressed K14 and Sox10. The distinct cell types within the Kit+ population were characterized by their keratin expression. Whereas K5 usually pairs with K14 in other tissues, in SMG endbuds there are distinct populations of Kit+K14+ and Kit+K5+ cells (Figure 3A). K8 was used as a pan-epithelial marker and K19 as a ductal marker. Genetic lineage tracing with a K14-cre confirmed that the K14 cells were multipotent and gave rise to progeny that included K5+ ductal cells, acinar cells and myoepithelial cells (Figure 3B). FACS analysis confirmed the Kit+ cells were heterogeneous, and that there were distinct K14+ and K5+ populations, but a surprisingly large (56%) subset of the Kit+K8+ cells did not express K14, K5 or K19, and these cells localized between the basal layer and duct of the endbud (Figure 3C). Furthermore, SMG with a signaling-defective form of Kit had reduced branching, demonstrating that the expansion of the distal Kit+K14+ progenitors was essential for branching morphogenesis (Lombaert et al., 2013). Surprisingly, the reduction in Kit also caused a loss of K5+ cells in the duct, due to a loss of innervation. Reduced innervation was due to reduced NRTN, which is produced by the Kit+ endbuds. Thus, the distal epithelial progenitor cells secrete neurotrophic factors to stimulate neuronal innervation that maintains proximal epithelial progenitor cells.
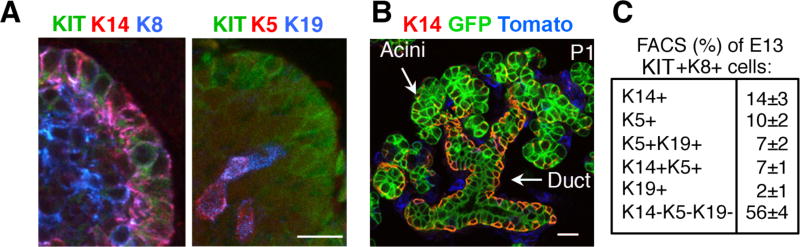
Figure 3. SMG endbuds contain distinct populations of KIT+K14+ and KIT+K5+ progenitors, and the K14+ progenitors are multipotent.
(A) Immunostaining of Kit, K14, K8, and K19 in an E13 endbud. Pink cells co-express K14 and K8 or K5 and K19. 1μm optical sections. (B) Lineage tracing of K14 in a postnatal day 1 SMG from a K14-Cre×RosamTmG mouse. K14 progeny cells (GFP, green) are throughout the gland and label acini, ducts and myoepithelial cells. The image also shows the endogenous K14 (red & yellow) and non-K14 cells (Tomato, blue). 2μm sections. Scale bar, 20μm. (C) FACS analysis to quantitate the subpopulations of epithelial Kit+K8+ cells sorted by K5, K14, and K19 expression from E13 SMGs. Mean ±SEM; n>3 biological samples. From Figure 4 in Lombaert et al., 2013.
Other markers that have been used to label progenitor cells in the developing SMG include Sox2, which is a putative stem cell marker in the sublingual gland. Lineage tracing during gland ontogenesis showed Sox2 progeny in the sublingual ducts and acinar cells of adult glands (Arnold et al., 2011). Sox2 is also expressed in some K5+ cells in the SMG duct early in development (Lombaert et al., 2011), although further studies are required to investigate their role as stem/progenitors in the adult gland. Another progenitor population in the SMG is marked by Ascl3, a transcription factor in the ducts of SMGs. Ascl3+ cells are proliferating progenitors and lineage tracing showed they generate both ductal and acinar cells in the adult. However, genetic ablation showed that gland development occurred normally in the absence of Ascl3 progenitors, which were shown to be a separate population from the K5+ progenitor pool (Arany et al., 2011). We speculate that in the absence of one progenitor population, compensation by another can occur during development.
4.2 Postnatal homeostasis and label retaining cells
A recent report has challenged the current dogma that salivary gland homeostasis in the adult SMG is stem cell-dependent (Aure et al., 2015). In fact, few studies had addressed the extent to which a particular stem/progenitor cell type contributes to adult gland homeostasis. Using an inducible Mist1-Cre, which is acinar cell-specific, they genetically labeled differentiated adult acinar cells and monitored their progeny over a chase period of up to 3 months. Surprisingly, the results showed that acinar cell replacement occurs by acinar cell division, not by the differentiation of an unlabeled cell population from the ducts. Using a Mist1CreER with Rosa26Brainbow2.1 reporter mice to label individual cells and their progeny, Aure et al.,2015 beautifully showed proliferation and clonal expansion of differentiated acinar cells in all three pairs of major salivary glands (Figure 4). Therefore, salivary gland acinar homeostasis is based on self-duplication of differentiated acinar cells. Interestingly, Mist1-cre labeling with a 6 month chase revealed that there was a significant number of single labeled cells in the sublingual gland, and a subpopulation of these cells expresses Sox2. Since these Sox2+ cells appeared quiescent, it was concluded they are not likely involved in gland homeostasis but may play a role during regeneration following injury. An emerging concept is that the stem/progenitor cells involved in homeostasis may be different from those responding to damage of the gland. This is supported by studies on liver regeneration that show that hepatocytes are both maintained and regenerated independently from facultative stem cells (Schaub et al., 2014; Yanger et al., 2014).
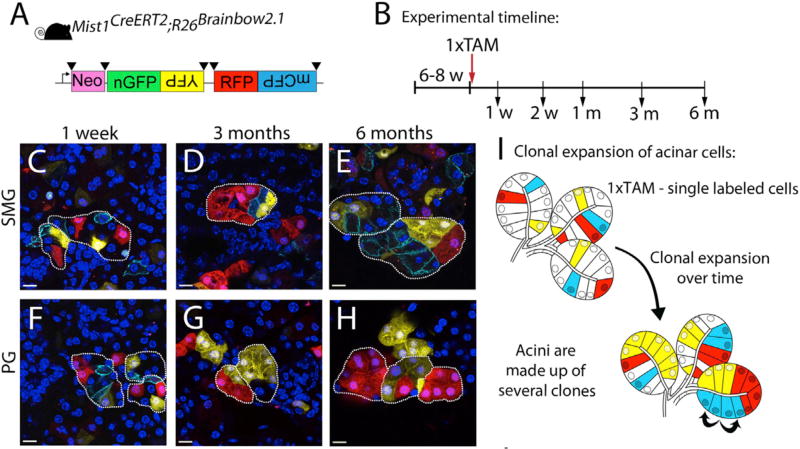
Figure 4. Acinar cell renewal occurs by self-duplication, resulting in clonal expansion of acinar cells in the SMG and PG.
(A) The inducible Mist1-CreERT2 was used in combination with the R26Brainbow2.1 reporter strain (schematic). Black triangles represent LoxP sites. (B) Adult mice (6–8 weeks old) were treated with a single dose of tamoxifen. Tissues were harvested at indicated time points. (C) Labeled cells in the SMG at 1 week following tamoxifen treatment. (D) Clonal expansion of labeled cells in the SMG at 3 months after tamoxifen treatment. (E) Acini comprised of several clones demonstrate tissue turnover 6 months after tamoxifen treatment. (F) Single labeled cells in the PG 1 week following tamoxifen treatment. (G) Clonal expansion of labeled cells in the PG at 3 months. (H) Clonal analysis after a 6-month chase in the PG. (I) Model of acinar cell proliferation and clonal expansion in adult SMG and PG. From Figure 2 in Aure et al., 2015.
In the search for a quiescent stem cell population in the adult gland, a recent study used a doxycycline-inducible histone 2B-green fluorescent protein (H2BGFP) to label K14 ductal cells (Kwak and Ghazizadeh, 2015). The K14 cells were labeled with H2BGFP during in utero gland development and then for 2 weeks after birth, followed by a chase period of up to 12 weeks. A quiescent adult K14 stem cell population was not detected during homeostasis, as the labeled cells had proliferated and lost their labels. A similar experiment labeled all cells in the SMG using a doxycycline-inducible ubiquitous driver, Rosa26. After a 12-week chase, again no evidence was found for a quiescent H2BGFP+ LRC population in an undifferentiated compartment. Staining for K14 (which stains the basal cells in excretory ducts), K19 (which stains granular ducts, striated ducts, and the luminal cells in excretory ducts), and Kit (which stains intercalated ducts), mapped the location of H2BGFP-LCRs to differentiated excretory, striated and intercalated ducts. Since striated ducts have very low cellular turnover, the data suggested that there were actively dividing pools of stem/progenitor cells in the intercalated ducts and the basal layer of excretory ducts, and that these stem/progenitor populations function independently during homeostasis. The data are also consistent with the report mentioned above, which showed that acinar cells do not arise from a quiescent stem cell pool or from ductal cells during homeostasis (Aure et al., 2015).
Another study investigating the identity of LRCs in adult glands used 5-Ethynyl-2'-deoxyuridine (EdU) to label proliferating cells in postnatal day 10 mice and an 8 week chase (Chibly et al., 2014). EdU+ LRCs colocalized with cells expressing the progenitor markers K5, K14, or Kit, further indicating that LRCs are a heterogeneous population of progenitors. The proliferative potential of the LRCs was shown with sphere assays, in which LRCs were stained with the proliferation marker Ki67. These data are consistent with the previous studies showing that the ductal compartment of the gland is proliferative during homeostasis. In addition, LRCs in irradiated mice did not undergo apoptosis following irradiation. This is consistent with the survival of ductal cells in patients whose salivary glands have been irradiated; however, it is the loss of acinar cells after irradiation and their inability to regenerate that presents a challenge.
Overall, these studies highlight that specific progenitor populations may be involved in homeostasis and regeneration depending on the type of damage that has occurred. It will be important in the future to identify the progenitors that are affected by, and that respond to, different types of gland damage, such as irradiation, ductal ligation, partial or full gland extirpation, and genetic ablation of specific cell populations or genetic activation of a signaling pathway
4.3 Regulation of epithelial progenitors: Heparan sulfate and micro RNAs
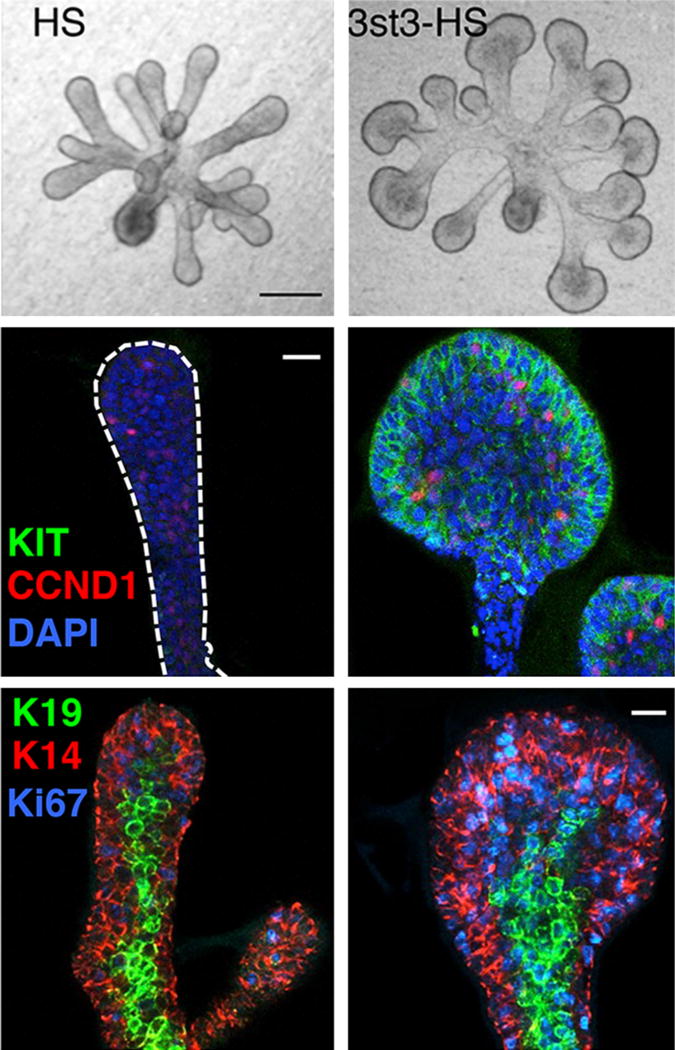
Fgf10 and its receptor FGFR2b are required for both human and mouse SMG development (reviewed by (Patel and Hoffman, 2014). Since FGFR2b signaling is essential for progenitor survival and proliferation during organogenesis, understanding how receptor function is regulated may provide targets for progenitor expansion or gland regeneration. Importantly, FGFR2b signaling requires a heparan sulfate (HS)-containing coreceptor, which is present on the cell surface or in the ECM. HS increases the affinity of Fgf10 for FGFR2b, stabilizing the ternary signaling complex. HS is the most diverse polysaccharide, due to the abundance of sulfate modifications on its sugar chains. The sulfate modifications create specific sulfated epitopes that regulate biological outcomes such as proliferation, duct elongation, and endbud expansion (Patel et al., 2008). One important mechanism that regulates epithelial morphogenesis is the variation in binding affinities that different FGFs have for HS, which affects their diffusion through the ECM, creating morphogenic gradients (Makarenkova et al., 2009). Additionally, specific types of sulfate modifications create specific epitopes that influence signaling in progenitor cells. Surprisingly, analysis of the enzymes that add sulfates to HS in Kit+ progenitor cells showed an enrichment of the HS 3-O-sulfotransferase (Hs3st) family. There are seven Hs3st isoforms in mice and humans; however, they produce the least abundant sulfate modification on HS. The expression of Hs3st3 enzyme isoforms, which make 3-O-sulfated epitopes, were rapidly upregulated in response to Fgf10/FGFR2b signaling. This resulted in an autocrine increase in FGFR2b-dependent MAPK signaling and expression of Hs3st3 genes, Kit, and transcription factor genes that are downstream of FGFR2b signaling. The rapid response to 3-O-sulfated HS (3st3-HS) increased the number of KIT+ and K14+ progenitors, proliferation and endbud morphogenesis (Figure 5). Thus, rapidly modifying 3-O-sulfation provides a cellular mechanism to modulate the biologic response to FGFR2b signaling and control progenitor expansion (Patel et al., 2014). This mechanism also improved the maintenance and expansion of epithelial KIT+FGFR2b+ progenitors in salisphere culture, and biosynthetic 3-O-sulfated-HS may specifically expand these cells for regenerative therapies. These studies suggest that HS may be a useful tool to stimulate FGFR signaling on progenitor cells to control cellular functions.
Figure 5. 3-O-, sulfated-HS (3st3-HS) increases epithelial endbud morphogenesis and proliferation as well as KIT and K14 expression.
Brightfield images of E13 SMG epithelia cultured with FGF10 and either HS or 3st3-HS for 28 hr, top two panels. The epithelia were stained for proliferating cells with CCND1 (red), KIT (green) and nuclei (blue), middle two panels. Lower two panels show K14 (red), K19 (green), and proliferation (Ki67, blue). Images are 2 μm confocal sections. Scale bars, 10 μm. From Figures 2 and 6 in Patel et al., 2014.
MicroRNAs (miRNAs) have emerged as important regulators of stem/progenitor gene expression. miRNAs are small, single-stranded, non-coding RNAs that target the 3’UTR of multiple mRNAs and regulate gene expression post-transcriptionally. miRNAs regulate the activity of canonical signaling cascades such as the Wnt pathway and are implicated in branching morphogenesis. miR-21, a mesenchymal miRNA that downregulates target genes Reck and Pdcd4, enhances branching morphogenesis through the degradation of ECM by activated MMPs (Hayashi et al., 2011). In contrast, the miR-200c family is highly expressed in SMG epithelial endbuds during development and influences epithelial proliferation. mir-200c targets Zeb1, Hs3st1 and Vldlr, thus regulating E-cadherin, HS and very-low density lipoprotein receptor function, respectively. By targeting Vldlr expression and its ligand reelin, miR-200c reduces expression of FGFR-dependent genes and epithelial proliferation (Rebustini et al., 2012). The appeal of using miRNAs to regulate progenitor cell gene expression and function for regenerative purposes is that a single miRNA may influence multiple genes and thus have a broad effect on a specific biological process rather than on a single gene/signaling pathway.
5 Conclusion
SMG organogenesis involves interactions among multiple cell types, which add to the complexity of regulatory mechanisms that drive development. While understanding epithelial-neuronal communication has been very informative in understanding organogenesis, there is still much to learn about endothelial-epithelial interactions and endothelial-neuronal interactions. Recent studies suggest that specific stem/progenitor populations may respond to different types of damage to regenerate the gland and that these may be different from the cells that maintain homeostasis. Understanding how these different stem/progenitor cells respond to changes in their local microenvironments will be important for directing regeneration after damage. Little is known about the mechanisms of quiescence, maintenance and expansion of specific stem/progenitor cells in response to specific damage. These are emerging and exciting areas of salivary gland research that remain to be explored.
Acknowledgments
The authors would like to thank Drs. Vaishali Patel, Isabelle Lombaert, Jennifer Symonds and Wendy Knosp for critical reading of this manuscript. This work was supported by the intramural research program of the NIDCR at NIH.
References
- Ambudkar IS. Cell Calcium. 2014;55:297–305. doi: 10.1016/j.ceca.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany S, Catalan MA, Roztocil E, Ovitt CE. Developmental biology. 2011;353:186–93. doi: 10.1016/j.ydbio.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Cell stem cell. 2011;9:317–29. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure MH, Konieczny SF, Ovitt CE. Dev Cell. 2015;33:231–237. doi: 10.1016/j.devcel.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan MA, Nakamoto T, Melvin JE. The journal of medical investigation : JMI. 2009;56(Suppl):192–6. doi: 10.2152/jmi.56.192. [DOI] [PubMed] [Google Scholar]
- Chibly AM, Querin L, Harris Z, Limesand KH. PLoS One. 2014;9:e107893. doi: 10.1371/journal.pone.0107893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Gervais EM, Centanni SW, Gulfo KM, Nelson DA, Larsen M. Development. 2012;139:411–22. doi: 10.1242/dev.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli K, Spijkervet FK, Kroese FG, Bootsma H, Vissink A. Monogr Oral Sci. 2014;24:109–25. doi: 10.1159/000358792. [DOI] [PubMed] [Google Scholar]
- Dyachuk V, Furlan A, Shahidi MK, Giovenco M, Kaukua N, Konstantinidou C, Pachnis V, Memic F, Marklund U, Muller T, Birchmeier C, Fried K, Ernfors P, Adameyko I. Science. 2014;345:82–7. doi: 10.1126/science.1253281. [DOI] [PubMed] [Google Scholar]
- Espinosa-Medina I, Outin E, Picard CA, Chettouh Z, Dymecki S, Consalez GG, Coppola E, Brunet JF. Science. 2014;345:87–90. doi: 10.1126/science.1253286. [DOI] [PubMed] [Google Scholar]
- Ferreira JN, Hoffman MP. Organogenesis. 2013;9 doi: 10.4161/org.25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harunaga JS, Doyle AD, Yamada KM. Dev Biol. 2014;394:197–205. doi: 10.1016/j.ydbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Koyama N, Azuma Y, Kashimata M. Dev Biol. 2011;352:299–307. doi: 10.1016/j.ydbio.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Yurchenco PD. Cell Adh Migr. 2013;7:56–63. doi: 10.4161/cam.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg KV, Hoffman MP. Monogr Oral Sci. 2014;24:1–13. doi: 10.1159/000358776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JC, Koo H, Harunaga JS, Matsumoto K, Doyle AD, Yamada KM. Dev Dyn. 2013;242:1066–77. doi: 10.1002/dvdy.24000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MN, Itoh S, Yanagita T, Sumiyoshi K, Hayano S, Kuremoto K, Kurosaka H, Honjo T, Kawanabe N, Kamioka H, Sakai T, Ishimaru N, Taniuchi I, Yamashiro T. Dev Dyn. 2015;244:488–96. doi: 10.1002/dvdy.24231. [DOI] [PubMed] [Google Scholar]
- Knosp WM, Knox SM, Hoffman MP. Wiley interdisciplinary reviews. Developmental biology. 2012;1:69–82. doi: 10.1002/wdev.4. [DOI] [PubMed] [Google Scholar]
- Knosp WM, Knox SM, Lombaert IM, Haddox CL, Patel VN, Hoffman MP. Dev Cell. 2015;32:667–677. doi: 10.1016/j.devcel.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, Hoffman MP. Nature communications. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Science. 2010;329:1645–7. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M, Ghazizadeh S. Stem Cells Dev. 2015;24:565–74. doi: 10.1089/scd.2014.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HR, Larsen M. Curr Opin Genet Dev. 2015;32C:47–54. [Google Scholar]
- Lombaert IM, Abrams SR, Li L, Eswarakumar VP, Sethi AJ, Witt RL, Hoffman MP. Stem Cell Reports. 2013;1:1–16. doi: 10.1016/j.stemcr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. PloS one. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Knox SM, Hoffman MP. Oral Dis. 2011;17:445–9. doi: 10.1111/j.1601-0825.2010.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Science signaling. 2009;2:ra55. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellas RE, Kim H, Osinski J, Sadibasic S, Gronostajski RM, Cho M, Baker OJ. J Dent Res. 2015;94:312–9. doi: 10.1177/0022034514559129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich I. Front Oral Biol. 2010;14:1–20. doi: 10.1159/000313703. [DOI] [PubMed] [Google Scholar]
- Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, Coppes RP. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2011;99:367–72. doi: 10.1016/j.radonc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- Nedvetsky PI, Emmerson E, Finley JK, Ettinger A, Cruz-Pacheco N, Prochazka J, Haddox CL, Northrup E, Hodges C, Mostov KE, Hoffman MP, Knox SM. Dev Cell. 2014;30:449–62. doi: 10.1016/j.devcel.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T, Sakai T, Hsu JC, Matsumoto K, Chiorini JA, Yamada KM. Science. 2010;329:562–5. doi: 10.1126/science.1191880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Sharpe PT, Miletich I. Developmental biology. 2011;358:156–67. doi: 10.1016/j.ydbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Patel VN, Hoffman MP. Semin Cell Dev Biol. 2014;25–26:52–60. doi: 10.1016/j.semcdb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Likar KM, Zisman-Rozen S, Cowherd SN, Lassiter KS, Sher I, Yates EA, Turnbull JE, Ron D, Hoffman MP. The Journal of biological chemistry. 2008;283:9308–17. doi: 10.1074/jbc.M709995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Lombaert IM, Cowherd SN, Shworak NW, Xu Y, Liu J, Hoffman MP. Dev Cell. 2014;29:662–73. doi: 10.1016/j.devcel.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH. Monogr Oral Sci. 2014;24:14–29. doi: 10.1159/000358781. [DOI] [PubMed] [Google Scholar]
- Ray S, Fanti JA, Macedo DP, Larsen M. Mol Biol Cell. 2014;25:2393–407. doi: 10.1091/mbc.E14-02-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebustini IT, Hayashi T, Reynolds AD, Dillard ML, Carpenter EM, Hoffman MP. Development. 2012;139:191–202. doi: 10.1242/dev.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, Hoffman MP. Dev Cell. 2009;17:482–93. doi: 10.1016/j.devcel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothova M, Thompson H, Lickert H, Tucker AS. Dev Dyn. 2012;241:1183–91. doi: 10.1002/dvdy.23804. [DOI] [PubMed] [Google Scholar]
- Ryu YK, Collins SE, Ho HY, Zhao H, Kuruvilla R. Dev Biol. 2013;377:79–89. doi: 10.1016/j.ydbio.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub JR, Malato Y, Gormond C, Willenbring H. Cell Rep. 2014;8:933–9. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman G. Head Neck Pathol. 2013;7(Suppl 1):S12–9. doi: 10.1007/s12105-013-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Marshall WF, McMahon M, Metzger RJ, Martin GR. Science. 2011;333:342–5. doi: 10.1126/science.1204831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AS. Semin Cell Dev Biol. 2007;18:237–44. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, Pikarsky E, Stanger BZ. Cell Stem Cell. 2014;15:340–9. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]