Abstract
Despite tremendous advances in the field of regenerative medicine, it still remains challenging to repair the osteochondral interface and full-thickness articular cartilage defects. This inefficiency largely originates from the lack of appropriate tissue engineered artificial matrices that can replace the damaged regions and promote tissue regeneration. Hydrogels are emerging as a promising class of biomaterials for both soft and hard tissue regeneration. Many critical properties of hydrogels, such as mechanical stiffness, elasticity, water content, bioactivity, and degradation, can be rationally designed and conveniently tuned by proper selection of the material and chemistry. Particularly, advances in the development of cell-laden hydrogels have opened up new possibilities for cell therapy. In this article, we describe the problems encountered in this field and review recent progress in designing cell-hydrogel hybrid constructs for promoting the reestablishment of osteochondral/cartilage tissues. Our focus centers on the effects of hydrogel type, cell type, and growth factor delivery on achieving efficient chondrogenesis and osteogenesis. We give our perspective on developing next-generation matrices with improved physical and biological properties for osteochondral/cartilage tissue engineering. We also highlight recent advances in biomanufacturing technologies (e.g. molding, bioprinting, and assembly) for fabrication of hydrogel-based osteochondral and cartilage constructs with complex compositions and microarchitectures to mimic their native counterparts.
Graphical abstract
1. Introduction
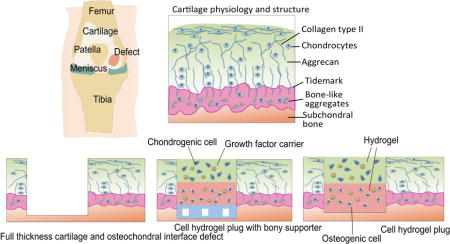
Osteochondral interface defects commonly involve lesions of both articular (hyaline) cartilage and underlying subchondral bone that are caused by trauma, disease, or aging. Different from the vast majority of other tissues, cartilage is basically avascular and low in cellularity in nature [1]. Cartilage thus lacks the ability to self-heal due to the absence of abundant nutrients and proper progenitor cells. When a cartilage defect is left untreated, however, the joint irrevocably and progressively deteriorates, leading to osteoarthritis and eventually, disabilities [2]. Cartilage-related tissue defects and diseases are the most common cause of disability, representing around 6% of disabled people of 30 years and older [3–5]. Current treatment strategies for osteochondral interface and full-thickness cartilage defects include microfracture (marrow stimulation) [6–8], autologous chondrocyte implantation [9–12], and osteochondral autografts and allografts [13–16], among others. Despite their common uses in the clinic, notable limitations and drawbacks still exist. The microfracture treatment drills tiny holes that penetrate the cartilage and the subchondral bone to bring in blood flow and bone marrow from surrounding tissues. Induced cartilage and bone regeneration/remodeling are expected due to the introduction of stem cells and biomolecules at the defects. However, it may lead to the formation of fibrocartilage that has inferior biofunctions compared to articular cartilage [17–19]. The autologous chondrocyte implantation strategy has been used clinically to regenerate articular cartilage for two decades with satisfactory surgical outcome to certain extent. Nevertheless, there are still drawbacks including shortage of chondrocyte source, long chondrocyte harvesting time, difficulty of chondrocyte solution fixation, periosteal hypertrophy and ablation [20], as well as low effectiveness for aged patients [21]. It should also be noted that autologous chondrocyte implantation is incapable to repair osteochondral interface and full-thickness cartilage, which require simultaneous restoration of the subchondral bone. Allografts suffer from limited tissue supply, immunorejection, insufficient integration, low cell viability due to graft storage, and possibility of disease spread. Autografts not only lack integration and tissue source, but also require additional surgery and result in donor site morbidity [1, 17, 22, 23].
To address these limitations, osteochondral and cartilage tissue engineering (OTE and CTE) have been proposed to advance new and more effective treatments. There are two main approaches for regeneration of deficient osteochondral interface and full-thickness cartilage using tissue engineering approaches. One is to develop artificial cartilage constructs to mimic the architectural features, mechanical properties, and thus biological functions of native cartilage tissues. By combining specially designed biopolymers and advanced manufacturing technology, generation of three-dimensional (3D) tissue constructs with similar mechanical stiffness to articular cartilage has been demonstrated [24]. However, it still remains a challenge to mimic the unique biofunctions of articular cartilage and osteochondral interface tissues that are highly complex in composition and zonal structures (Fig. 1). The other tissue engineering approach emphasizes more on regenerative medicine. The essential concept is to deliver appropriate biomaterials as artificial extracellular matrix (ECM) to facilitate cell growth, proliferation, and differentiation at the sites of defects, leaving regeneration of the articular cartilage and subchondral bone to the native biological processes involving interactions among cells and biomolecules (e.g. growth factors). In these cases, the matrix material does not need to be as mechanically strong as the native tissues, since it only serves as a temporary 3D microenvironment for the chondrogenic or osteogenic progenitor cells to generate real cartilage and bone tissues. In this review, we will focus on hydrogel-based tissue engineering approaches, which have gained increasing popularity during the past few years.
Fig. 1.
Tissue engineering strategy for treatment of osteochondral interface and full-thickness cartilage defects with cell-laden hydrogel constructs.
Hydrogels are versatile and appealing biomaterials for tissue engineering and cell therapy applications, due to their unique combination of properties similar to natural ECMs, such as high water content, biodegradability, porosity, and biocompatibility [25]. The composition, structure, mechanical properties, and biochemical properties of hydrogels are conveniently tunable to suit for various desired biomedical applications [26]. As to cartilage and osteochondral engineering, hydrogels can serve as an active matrix to control cell morphology, proliferation, and differentiation [27–30]. Moreover, cell-laden hydrogels, or cell-hydrogel hybrid constructs, can be manufactured by advanced techniques with patient-customized geometries and compositions. As a result, it is widely accepted that hydrogels combining both cells and growth factors have great potentials to address the challenge of regenerating osteochondral interface and full-thickness cartilage (Fig. 1). Over the past decade, a variety of tissue-engineered cell-laden hydrogel systems have been developed for OTE and CTE applications with remarkable successes as fundamental studies [29, 30].
In this review, we will focus on the recent advances of hydrogel design, cell source selection, and growth factor delivery. We then envision further development of the next-generation engineered osteochondral/cartilage constructs composed of hydrogel/inorganic particles/stem cells with improved mechanical properties and biological functions, which promise breakthroughs in clinic practices. Finally, we highlight the development of advanced manufacturing technologies of osteochondral and cartilage constructs with complex gradient composition and zonal structure that have the potential to mimic the native tissues.
2. Designing hydrogels for reconstruction of osteochondral interface and cartilage
Hydrogels, composed of highly hydrated, ECM-mimicking polymeric networks, have attracted strong attention for applications in tissue engineering and regenerative medicine [26,31, 32]. To date, various types of hydrogels derived from different natural or synthetic polymers or their hybrids, have been used for reconstruction of deficient osteochondral interface or articular cartilage tissues [33–35] (summarized in Table 1). Hydrogels based on natural polymers, including polysaccharides (alginate, agarose, chitosan, hyaluronic acid (HA), and gellan gum) and proteins (collagen, gelatin, and fibroin), have been extensively documented [36–55]. The use of a variety of hydrogels based on synthetic polymers, e.g. poly(ethylene glycol) (PEG), polymer oligo(poly(ethylene glycol) fumarate) (OPF), polyvinyl alcohol (PVA), poly(N,N-dimethylacrylamide) (PDMAAm), and methoxy poly(ethylene glycol)-poly(ε-caprolactone) (MPEG–PCL) [53, 56–73], have also been widely reported. Hybrid hydrogels can further be fabricated from combinations of natural and synthetic polymers, providing more possibilities in selection over different compositions. In general, suitable hydrogel candidates for OTE and CTE applications should ideally be able to support cell growth/proliferation, maintain phenotypes of chondrocytes/osteoblasts, and promote chondrogenic/osteogenic differentiation of stem cells for recapitulation of osteochondral interface or cartilage tissues. Among the large variety of hydrogels reported for biomedicine applications, here we review recent progresses of investigations on hydrogels showing promise for repair of osteochondral and cartilage tissues.
Table 1.
Hydrogels, cells, and growth factors used for OTE and CTE
| Hydrogel type | Application (OTE/CTE) | Cell type | Growth factor | Crosslinking/gelation method | Stiff bone layer (Yes/No) | In vivo model | Ref. |
|---|---|---|---|---|---|---|---|
| Alginate | OTE CTE | Chondrocytes MSCs IPSCs | TGF-β3 | Physical (ionic interaction) | Yes | Rabbit medial femoral condyle | [36–40] |
| Agarose | CTE | Chondrocytes MSCs | TGF-β3 | Physical (temperature change) | No | —— | [41, 42] |
| Collagen | OTE CTE | MSCs Chondrocytes | TGF-β1-3 BMP 2-7, FGF-1 |
Chemical | Yes | Sheep medial femoral condyle | [45, 46] |
| Chitosan | CTE | Chondrocytes MSC | TGF-β1 | Chemical | No | —— | [50, 51] |
| Gellan gum | CTE | Chondrocytes | —— | Physical (ionic interaction and temperature change) | No | Mouse subcutaneous | [44] |
| Gelatin | OTE | MSCs | BMP-4 | Chemical | No | Rabbit medial femoral condyle | [52] |
| Fibroin | CTE | Chondrocytes | —— | Physical (ionic interaction) | No | —— | [48, 49] |
| Hyaluronic acid | CTE | Chondrocytes MSCs | TGF-β | Chemical (UV photo- polymerization) | No | —— | [54, 55] |
| PVA | OTE | Osteoblasts Chondrocytes | —— | Chemical (UV photo- polymerization) | No | —— | [65–70] |
| PEG | CTE OTE | MSCs Chondrocytes ESCs | TGF-β3 | Chemical (UV photo- polymerization) | Yes | Rabbit medial femoral condyle | [58–64] |
| OPF | OTE | MSCs | TGF-β1 | Chemical | No | Rabbit medial femoral condyle | [71, 72] |
| PDMAAm | OTE | Chondrocytes | —— | Chemical | No | Rabbit medial femoral condyle | [73] |
| PEG–PCL | CTE | Chondrocytes | —— | Physical (temperature change) | No | Mouse subcutaneous | [196] |
| GelMA | CTE | Chondrocytes | TGF-β1 | Chemical (UV photo- polymerization) | No | —— | [26, 213, 217] |
| —— | OTE | PBMCs | —— | —— | No | Medial femoral condyle | [197] |
(a) PEG: Poly(ethylene glycol); (b) PVA: Polyvinyl alcohol; (c) OPF: Oligo(poly(ethylene glycol) fumarate); (d) PDMAAm: Poly(N,N-dimethylacrylamide); (e) PEG–PCL: Poly(ethylene glycol)–poly(ε -caprolactone); (f) GelMA: Gelatin methacryloy; (g) BMP: Bone morphogenetic protein.
2.1 Typical hydrogels of natural polymers and their chemically modified derivatives
Hydrogels fabricated from natural polymers generally possess good biocompatibility, biodegradability, low immunoresponse, and bioactive motifs encoded in their structures[37–46], and are thus promising biomaterials for OTE and CTE applications. Their derivatives have tunable biodegradability, mechanical properties, and specific biofunctions with chemical modification of functional groups, specific ligands, and macromolecules. This part reviews the recent advances in the development of representative hydrogels using natural polysaccharides (alginate, agarose, chitosan, HA, and gellan gum) and proteins (collagen, gelatin, and fibroin), and their derivatives, with emphases on their resources, properties, crosslinking mechanisms, as well as advantages and disadvantages for repair of cartilage and osteochondral tissue defects.
2.1.1 Alginate hydrogels and their derivatives
Alginate is one of naturally occurring polysaccharide polymers typically obtained from brown seaweed and various bacteria. One unique property of alginate is the ability to be physically crosslinked by divalent cations such as Ca2+ at room temperature [11, 37, 38, 74–76], which makes it very useful in various biofabrication techniques, including molding, spraying, and 3D bioprinting [77–80]. The resulting physical hydrogels have good biocompatibility, low toxicity, and relatively low cost [36–39, 81–89]. Alginate hydrogels have been shown to support growth and proliferation of encapsulated chondrocytes, as well as to maintain their chondrogenic phonotype. After in vitro culturing of chondrocytes for 21–28 days [36–38], collagen type II and aggrecan formed along with enhanced cartilage gene expressions. Alginate hydrogels were also used to deliver bone progenitor cells including mesenchymal stem cells (MSCs) for bone regeneration [84, 85]. Encapsulated MSCs could produce their own collagenous ECM that was well integrated with the host tissue.
Despite these successes, however, alginate hydrogels have some limitations for tissue engineering applications. First, physically crosslinked alginate hydrogels lack long-term stability and can gradually lose their initial mechanical strengths in physiological environment within a relatively short timeframe, which neccessitates additional crosslinking mechanisms to further stiffen the network structures [90]. Second, alginate inherently has low mammalian cell adhesiveness and cellular interaction ability; as a result, the introduction of cell adhesion peptide motifs is generally implemented to better support cell functions [76, 91].
Chemically modified alginate derivatives have been studied to improve the mechanical properties, selective solubility, and cell adhesiveness for tissue engineering purpose. By ester bond formation of long alkyl chains including dodecyl and octadecyl to the alginate backbone, amphiphilic alginate derivatives have been produced. Aqueous solutions of such alginate derivatives showed good rheological properties and could be physically crosslinked to form hydrogels, which would be useful for cartilage regeneration [74, 75]. Phenolic hydroxyl modified alginate (Alg-Ph) hydrogels have been developed through a horseradish peroxidase (HRP)-catalyzed oxidative crosslinking reaction with H2O2 as an electron acceptor [92]. The phosphorylation, cell spreading, and osteogenic differentiation of preosteoblasts encapsulated in these alginate hydrogels could be further controlled by RGD nanopatterning. Modified alginate hydrogels thus hold great potential as a cell delivery matrix for repairing bone and cartilage.
The same group also applied chemical modifications to tune the toughness and chemical stability of alginate hydrogels without compromising their excellent biocompatibility [87, 88]. Cellular adhesion on alginate hydrogels can be increased by increasing the HRP concentration during crosslinking or by chemical ligation with RGD sequences via the carbodiimide chemistry [74, 93].
2.1.2 Agarose hydrogels and their derivatives
Agarose is a type of polysaccharide composed of alternating sequences of 1,3-linked β-D-galactose and 1,4-linked 3,6-anhydro-α-L-galactose. Agarose aqueous solutions form thermally reversible physical hydrogels at 17–40 °C, which become soluble at temperatures over 65 °C [94]. At body temperature, agarose hydrogels are stable and normally deemed as an inert biomaterial, because it lacks native ligands that allow cell-material interactions. However, many studies demonstrated that agarose hydrogels promote chondrocyte phenotype maintenance for cartilage regeneration [95–99]. Importantly, the temperature-responsive gelation capability of agarose makes it possible to design injectable cell-laden hydrogels for minimally invasive treatment of cartilage and osteochondral defects [29, 100].
In addition, agarose hydrogels can support chondrogenic differentiation and in vitro cartilaginous tissue formation of encapsulated stem cells such as MSCs [42, 95, 101–103]. However, ECM generation from MSCs encapsulated in agarose hydrogels was significantly less than that from chondrocytes at day 70 under similar conditions [42]. Moreover, after in vitro chondrogenic culture and maturation for 8–10 weeks, the MSC-laden agarose hydrogel cartilage constructs had lower mechanical stiffness than those laden with chondrocytes [42, 104]. Therefore, further studies are required to optimize conditions to induce chondrogenesis of MSC-laden hydrogels for cartilage regeneration.
In addition to excellent biocompatibility to support chondrogenesis of chondrocytes and MSCs, agarose hydrogels also have good biomechanical properties (e.g., stiffness and viscoelasticity) matching those of cartilage tissues. Therefore they are deemed promising biomaterials for cartilage regeneration. However, for bone tissue engineering applications especially in load-bearing conditions, better mechanical properties of agarose hydrogels are required. Hybrid agarose hydrogels incorporated with inorganic nanomaterials might be interesting alternatives towards improvement of mechanical properties. For example, agarose hydrogels loaded with bioceramic particles not only had highly improved mechanical stiffness but also supported osteogenesis [105, 106].
Chemically modified agarose hydrogels have been studied to improve their cellular affinity and mechanical properties for extensive tissue engineering applications. For example, glycine-arginine-glycine-aspartic acid-serine (GRGDS) oligopeptide-modified 0.5 wt% agarose hydrogels were produced by immobilization of the adhesive fibronectin peptide fragment using a focused laser [41]. Such hydrogels exhibited improved cell adhesion and guided migration abilities that would be beneficial to tissue engineering applications.
Another agarose derivative was generated by carboxylation, a general method for transformation of secondary structures of helical polysaccharides [107]. Through changing the degree of carboxylation, the modulus can be tuned independent of the prepolymer concentration.
2.1.3 Chitosan hydrogels and their derivatives
Chitosan is derived from chitin, the second most abundant natural biopolymer [108] from renewable sources including shell of shellfish, crustacean shells, insect cuticles, mushrooms envelopes, and the wastes of the seafood industry [109, 110]. Chitosan has good biocompatibility and biodegradability, and is thus an attracting candidate material for tissue engineering applications. Chitosan hydrogels prepared by enzymatic crosslinking can support the proliferation of chondrocytes and MSCs, maintain the chondrogenic phenotype and morphology, and boost the deposition of cartilaginous ECM in vitro [50, 51].
Long-term in vitro culture and in vivo subcutaneous implantation of MSC-laden chitosan hydrogels have been carried out to examine the their capacity to support chondrogenesis and hypertrophy of MSCs [101]. After 8 weeks of in vitro chondrogenesis, a chondrogenic phenotype in MSCs was well maintained and robust chondrogenesis was found in chitosan hydrogel constructs with high levels of accumulated aggrecan and collagen type II deposition. The cells were found to deposit a large amount of cartilage ECM and facilitated neither vascularization nor endochondral ossification in vivo [101].
From these results, it is suggested that chitosan is a suitable and promising biomaterial for articular cartilage regeneration. However, chitosan has poor solubility in water under physiological conditions, which limits their extensive utilization in tissue engineering [111]. As a polysaccharide, chitosan also has relatively low cellular adhesiveness for adhesion, proliferation, and ECM formation of certain cell types involved in OTE and CTE such as chondrocytes [112, 113]. Allergenic reactions of chitosan may be an issue for its clinical translation. One prior study has been conducted to evaluate the safety of chitosan biomaterials [114]. Since chitosan can be derived from shellfish or shrimp, this study selected patients who were allergic to shellfish/shrimp to test the allergic responses to chitosan. No participant had a positive skin prick testing result to chitosan or experienced an adverse reaction during bandage challenges. However, more investigations are needed to further elucidate this issue.
Chitosan derivatives have been produced through chemical modification to introduce specific functional groups, ligands, macromolecular side chains, or crosslinking sites to tune the solubility, gelation property, cell affinity, and specific biological properties for repair of cartilage and osteochondral defects [115]. Trimethyl chitosan (TMC) has been developed for an enhanced solubility over a broader range of pH via the reaction of methyl iodide with the amino groups [116]. RGD-chitosan derivatives were generated by reaction with 2-iminothiolane via disulfide bond linkage with the aid of dimethyl sulfoxide [117]. These chitosan derivatives exhibited improved ability of adhesion and proliferation for chondrocytes. Lactose-conjugated chitosan derivatives were developed by reductive N-alkylation for improved chondrocyte adhesion, aggregation and proliferation, as well as aggrecan and collagen type II formation [118–120].
2.1.4 HA hydrogels and their derivatives
HA, as a linear polysaccharide consisting of 250–25,000 repeating disaccharide units [94], is the most abundant component in the cartilage and an important aggrecan component organizing cartilage ECM into resilient structures. Therefore, HA-based hydrogels are one of the most promising naturally derived biomaterials for OTE and CTE applications. HA involves in some key cellular processes of chondrocytes, such as morphogenesis, proliferation, and inflammation [121]. And HA has stimulatory effects on chondrocyte metabolism in vitro [122]. HA could also significantly increase the synthesis of chondroitin-6-sulphate, collagen type II, glycosaminoglycan, hydroxyproline, and DNA. Due to the unique effects of HA on cellular behavior of chondrocytes, much effort has been made to develop chondrocyte-laden HA hydrogels for regeneration of cartilage tissues [55, 123–125].
HA hydrogels were demonstrated to support early differentiation of MSCs down to chondrogenic linage and enhance cartilage tissue formation in vitro and in vivo [126–133]. MSC-laden HA hydrogels were developed to generate engineered cartilage constructs with high mechanical properties by fostering chondrogenic differentiation and ECM production. After in vitro maturation for 9 weeks, HA hydrogel constructs with high-density (60 × 106/mL) of encapsulated MSC reached a compressive modulus of 1 MPa under dynamic culture conditions (compared with 0.3–0.4 MPa under static culture conditions) [127]. The dynamic culture condition (orbital shaking) ensured that optimal nutrient access was delivered, which resulted in improved formation of aggrecan and collagen in the dynamically cultured cartilage constructs based on HA hydrogels, 30% and 29% greater than the amounts of aggrecan and collagen formed in the statically cultured controls. In this case, dynamically cultured samples exhibited much higher compressive modulus. In this case, dynamically cultured samples exhibited much higher compressive modulus. In vivo studies have demonstrated that MSC-laden HA hydrogels could promote neocartilage formation with increasing collagen type II and aggrecan production [126, 131, 132].
Chemical modifications of HA can be achieved via reactions of the carboxylic groups with various hydroxyl- or amine-bearing motifs to form derivatives with improved biocompatibility and controlled biodegradability [94]. HA can also be chemically modified with photocrosslinkable functional groups such as methacrylate and glycidyl methacrylate, which enable feasible crosslinking of the resulting HA derivative via exposure to visible or low-energy ultraviolet light. The photocrosslinkable HA derivatives can be used to formulate injectable cell-laden hydrogels for repair of irregular cartilage and osteochondral tissue defects with enhanced mechanical properties [134, 135]. Compressive moduli of photocrosslinked HA-MA hydrogel could range from 3 to 146 kPa by tailoring the molecular weight and concentration [136]. Through chemical modification, a variety of HA-based hydrogels can be fabricated towards tunable biodegradability and improved mechanical properties, as well as better photocrosslinking ability.
2.1.5 Gellan Gum hydrogels and their derivatives
Gellan gum is a linear anionic polysaccharide consisting of tetrasaccharide repeating units with one carboxyl side group per four saccharide units [137]. Gellan gum shows thermally reversible gelation processes to form physical hydrogels. With temperature decreasing, gellan gum molecules perform a rapid random-coil to double-helix conformational transition with further intermolecular aggregation of the helices, leading to the formation of junction zones and therefore a three dimensional network via complexation with cations and hydrogen bonding in water [138–141]. During the gelation process, the carboxylic groups in glucuronic acid units serve as the ionic complexation sites to bind the polysaccharide chains to each other via divalent cations, resulting in much stronger interactions than monovalent binding [142]. Substantial studies have shown that gellan gum hydrogels possess good biocompatibility, biodegradability, and injectability for OTE and CTE applications, and they can be easily produced by gelation via temperature change and presence of cations without harsh chemical reagents [44, 137, 140, 143–145].
MSC-laden gellan gum hydrogels were studied for in vitro chondrogenesis. After 42-day culture in chondrogenic medium, cartilaginous ECM components of aggrecan and collagen type II were produced with the confirmation of chondrocytic gene expression [146]. Gellan gum hydrogels incorporated with bioglass/bioceramic particles or bone-forming growth factors were shown to support encapsulation, proliferation, and osteogenesis of preosteoblasts and MSCs for bone tissue regeneration [147–149]. Although gellan gum hydrogels are promising for cartilage and bone tissue regeneration, the three key problems may hinder their clinical translations. First, the gelation temperature (>42 °C) is higher than the physiological temperature, which may result in compromised cell viability during the gelation process [139, 143, 150]. Second, Young’s modulus of physically crosslinked gellan gum hydrogels is typically below 5 kPa, which is much lower compared with that of native cartilage tissues (typically over 60 kPa) [139, 151]. Third, gellan gum hydrogels are readily to get loss of their stability and mechanical properties in vivo because of the exchange of divalent cations with monovalent ions presenting in higher concentrations in physiological environment [139].
To decrease the gelation temperature and improve the initial mechanical properties and in vivo stability of gellan gum hydrogels, chemically modified gellan gum derivatives have been developed through various methods. For example, reducing the molecular weight of gellan gum via oxidation can decrease the gelling temperature. It was reported that the gelation temperature of gellan gum decreased to the range of 37 °C to 22 °C by increasing the NaIO4 oxidant dosage or oxidation period [152]. The results of 150-day in vitro culture demonstrated that the modified injectable chondrocyte-laden gellan gum hydrogel with low gelation temperature well maintained the chondrocyte phenotype with high viability and promoted cartilage ECM formation in a long-term scale. In addition to oxidation, gelation temperature can also be tuned by changing proportion of low/high acyl gellan gum and the concentration of covalent cations to optimize cell encapsulation and injectability [153].
Chemical modification of gellan gum has also been demonstrated by the incorporation of methacrylate groups for improved structural stability and mechanical properties with retaining the biocompatibility [139, 154–156]. A Young’s modulus of 148 kPa was achieved for methacrylated gellan gum hydrogels [139]. The in vitro and in vivo studies showed that such hydrogels supported cell encapsulation with good viability and cytocompatibility [155, 156]. Chemically modified gellan gum hydrogels have shown decreased gelation temperature, improved mechanical properties, and better in vivo stability that are crucial as injectable biomaterials for OTE and CTE applications.
2.1.6 Collagen hydrogels and collagen derivative hydrogels
Collagen is the most abundant structural protein component of ECM. Around 90% of the dry weight of articular cartilage is collagen type II. The crosslinked collagen network contains about 70% water and provides the mechanical strength and shape of cartilages during articulation [94]. On the other hand, collagen type I is the most abundant ECM in bone tissues [157]. Collagen hydrogels can be produced by crosslinking via photo-polymerization of UV irradiation, dehydrothermal treatment, or via chemical crosslinking by reacting with aldehydes, carbodiimides, isocyanates, genipin, and transglutaminase, among others [94]. Collagen type I hydrogels have been demonstrated to exert certain immunoisolation effects on the encapsulated chondrocytes, and thus enhance in vitro chondrogenesis and cartilage ECM formation during the culture over 28 days [46]. The isolation and protection, coming from formed ECM and hydrogel, effectively controlled the adverse immunogenicity of seeded chondrocytes and helped to reduce the immunogenicity of the engineered cartilage.
Moreover, it has been demonstrated that collagen type I hydrogels supported growth/adhesion and chondrogenic differentiation of MSCs for in vitro fabrication of engineered osteochondral constructs [158]. Collagen type I hydrogels could be combined with bioceramic scaffolds to prepare hybrid constructs for repairing osteochondral interface defects. In vivo studies indicated that collagen type I hydrogels support cartilaginous integration and cartilage formation during post-implantation of 1 year [158]. In vitro and in vivo results demonstrated that chondrocytes-laden collagen type II hydrogel constructs supported proliferation and chondrogenesis of MSCs [159, 160]. Studies have been done to compare the regulation effects of collagen type II hydrogels on the chondrogenic differentiation and in vitro chondrogenesis of MSCs with alginate and collagen type I hydrogels [161]. The results indicated that in the absence of transforming growth factor (TGF)-β1, collagen type II hydrogels were able to induce and maintain MSC chondrogenic differentiation. All cartilage-related genes were upregulated by collagen, particularly collagen type II.
Collagen hydrogels have excellent biological properties and therefore few studies have been performed on chemical modification of collagen. However, to solve the issues related to the relatively low mechanical properties, collagen hydrogels that were chemically modified with synthetic polymers have been reported [162, 163]. Collagen hydrogels modified with chitosan showed significantly increased strength and elasticity by 100% and 20%, respectively, compared to unmodified collagen hydrogels [163]. Moreover, the introduction of photocrosslinkable groups can also improve strength but retain the ability of supporting chondrocytes proliferation and new cartilage formation [164, 165]. These studies shed some light on ways to avoid the poor mechanical properties of collagen-based hydrogels that might hinder their extensive applications in tissue engineering.
2.1.7 Gelatin hydrogels and their derivatives
Gelatin is a hydrolysis product from collagen, the major ECM component in most tissues. It consists of a number of arginine-glycine-aspartic acid (RGD) sequences promoting cell adhesion, and the matrix metalloproteinase (MMP) target sequences facilitating cell remodeling [26]. Gelatin solution could form physically crosslinked hydrogels through self-gelation at low temperatures (< 30 °C) [166] or chemically crosslinked hydrogels via chemical reactions [26]. Because physically crosslinked gelatin hydrogels are not stable at the temperatures used for in vitro culture of mammalian cells and in vivo implantation [166], chemical crosslinking of gelatin hydrogels is usually preferred for tissue engineering applications. Chemically crosslinked gelatin hydrogels are much more stable and strong, but the toxicity of the harsh crosslinking agents can limit their applications for encapsulating live cells. Recent studies suggested that gelatin-based hydrogels crosslinked by click chemistry (tetrazine and norbornene click pairs) were cell-friendly to support attachment and spreading of MSCs [167]. Subcutaneous implantation in mice revealed a minimal inflammatory response and sustained in vivo biodegradation after infiltration by the hosting cells. Unmodified gelatin has a gelation temperature not suitable for in vitro culture of mammalian cells or in vivo implantation, and is rarely used for OTE and CTE.
Grafting methacryloyl substituent groups is an efficient modification method to make gelatin chemically crosslinkable by photopolymerization. Such photocrosslinking polymerization can proceed at mild conditions (e.g., neutral pH, room temperature, in water-based solutions), and also allows for spatial and temporal control of the gelation process and hydrogel properties. This makes it possible to microfabricate cell-laden hydrogels based on gelatin derivatives for engineering tissue constructs. The methacryloyl-modified gelatin (GelMA) retains most of the functional amino acid motifs (e.g. RGD motifs) so that it inherits the excellent cell adhesive properties of gelatin.
GelMA hydrogels are biodegradable, biocompatible, non-immunogenic, highly tunable, and easy-to-microfabricate [168]. Therefore, GelMA has gained increasing attention as a new family of biomaterials for cell delivery and tissue engineering applications. For example, GelMA and equine chondrocytes were employed to fabricate cell-laden hydrogel constructs by 3D bioprinting [169]. Chondrocytes encapsulated in 10 wt% GelMA hydrogel exhibited a good viability of 80–90% on day 1. Cartilaginous tissue matrices of aggrecan and collagen type II were formed after a 4-week in vitro culture. Alternatively, MSC-laden GelMA hydrogels (8 wt%) were cultured in the chondrogenic medium for 6 weeks to assess the chondrogenesis ability. Abundant aggrecan and collagen type II depositions were found in the hydrogel constructs, which indicated that the GelMA hydrogels supported proliferation and chondrogenic differentiation of MSCs for OTE and CTE purposes [169–171].
In addition to GelMA, phenolic hydroxyl group-modified gelatin was also developed for improved injectability, in situ gellability, chemical stability, and crosslinkability under mild conditions [166]. This gelatin derivative was produced through the aqueous-phase carbodiimide activation chemistry and can be crosslinked via an enzymatic peroxidase-catalyzed reaction within 10 s to form stable hydrogels. Moreover, the gelation time could be further decreased by increasing the phenolic hydroxyl (Ph) groups and peroxidase concentration. The encapsulated cells showed a highly viability of about 95% and proliferated as well as those seeded on unmodified gelatin. The subcutaneous rodent injection test demonstrated successful in situ gelation and prolonged in vivo stability of this gelatin-based hydrogel. Histological studies indicated that the surrounding tissues did not have necrosis, while a layer of thin fibrous capsules were observed on implanted gel surface. [172].
Chemically modified gelatin hydrogels are biodegradable, highly tunable, and easy-to-microfabricate. However, there are some aspects to be further improved for better chondrogenesis and mimicking the functions of cartilage tissues. First, viability of chondrocytes/MSCs in modified gelatin hydrogels should be further increased to produce more connective cartilage extracellular matrix. Second, the mechanical properties should be further enhanced to be able to withstand the high-load-bearing conditions of articular cartilage. Third, the rheological properties (e.g. viscosity) of modified gelatin hydrogels prepolymer solutions should be carefully adjusted to adapt with the micro/nanofabrication process by advanced manufacturing technologies (e.g. 3D bioprinting). To achieve these goals, it would be an effective strategy to improve some key characteristics of modified gelatin hydrogels by incorporating other functional constituents (e.g. natural biopolymers, synthetic biopolymers, inorganic nanoparticles) for different applications [173].
2.1.8 Silk fibroin hydrogels and their derivatives
Natural silk, composed of silk fibroin protein core with sericin protein coating, is produced by the silkworm (Bombyx mori) cocoons. Silk fibroin has become a new biomaterial for tissue engineering applications due to its robust mechanical properties, excellent biocompatibility, slow degradability, and abundant supply source. Fibroin hydrogels can be produced through a variety of mechanisms involving a change in fibroin conformation from an amorphous random coil to organized crystalline β-sheet structures [174]. The gelation process is controlled by protein concentration, temperature, pH, and salt/ion concentration. Gelation methods for fibroin solution include sonication, lyophilization, as well as treatments by acids, dehydrating agents, and ions [175]. Because cartilage regrowth typically requires extended time, hydrogels with long-term stability and mechanical integrity would be advantageous for this purpose. Through optimization of fibrinogen concentration, calcium ion concentration, and pH value, a stable fibroin hydrogel was prepared to provide sufficient time for embedded human chondrocytes to form neocartilage [49]. In vitro culture of chondrocyte/MSC-laden fibroin hydrogels produced abundant native cartilage-like ECM of aggrecan and collagen type II, which suggested that fibroin could be a promising biomaterial for cartilage regeneration [174, 176]. Taking the advantage of fibroin for minimal invasiveness tissue engineering applications, its injectable forms have recently been developed by sodium dodecyl sulfate-induced rapid gelation and vortex-induced gelation [177, 178].
Although fibroin hydrogels have been reported to support chondrocyte proliferation and chondrogenesis for CTE [94], it is desirable to tailor the interactions between chondrocytes/MSCs and fibroin for improved chondrogenesis and osteogenesis by chemical modifications with cell binding domains and growth factors [179–181]. Arg-Gly-Asp-Ser (RGDS)-modified fibroin demonstrated the ability to enhance mRNA expression levels of integrin α5β1, and aggrecan at 12 h after seeding [181]. It also suggested that RGDS induced moderate chondrocyte adhesion to fibroin well maintained the chondrogenic phenotype and facilitated chondrogenesis. Fibroin was also modified using the diazonium-coupling chemistry to control protein structure and overall hydrophilicity to direct encapsulated MSCs towards enhanced osteogenic differentiation [179]. These results indicated that MSCs exhibited different growth rates and morphologies on hydrophobic and hydrophilic fibroin derivatives, although all the fibroin derivatives supported osteogenic differentiation and osteogenesis of seeded MSCs, as confirmed by the expression of osteogenic biomarkers when subjected to osteogenic stimuli. Such chemically modified fibroin hydrogels have the ability to effectively interact with chondrocytes and MSCs due to the immobilized cell binding domains and growth factors, so that they can maintain the chondrogenic/osteogenic phenotype without extra growth factors.
2.2 Typical synthetic (composite) polymer-based hydrogels
Although hydrogels based on naturally derived polymers show excellent biocompatibility for chondrogenic cells growth, proliferation, and phenotype maintaining, the low mechanical properties and uncontrolled degradation often limit their applications in OTE and CTE. Hydrogels based on synthetic polymers, on the other hand, exhibit highly tunable biodegradability, biocompatibility, mechanical properties, and biochemical characteristics, due to the convenience to tune their chemical structure and molecular composition. Moreover, composite hydrogels, consisting of two or more natural/synthetic biopolymers, combine the biocompatibility, biodegradability, and tunable mechanical strength, so that they are appealing for osteochondral and cartilage tissue regeneration.
PVA and modified PVA hydrogels with a modulus of 1 to 5 MPa have been developed to repair cartilage tissues [65–70]. However, PVA is non-biodegradable so that it can only be used as permanent cartilage implant. Further study is still required to improve the biofunctions of PVA hydrogels mimicking natural cartilage. PEG-based hydrogels support adhesion and proliferation of chondrocytes, MSCs, and ESCs [53, 58–64]. With addition of calcium minerals or organic growth factors, MSCs and ESCs encapsulated in PEG hydrogel could differentiate to osteogenic or chondrogenic linages [58]. It has been reported that the mechanical loading and materials stiffness regulated the differentiation of MSCs embedded in PEG hydrogels [62, 182]. Surgical options for cartilage resurfacing may be significantly improved by advances and application of biomaterials that direct tissue repair.
PEG diacrylate (PEGDA)-based hydrogels have been widely studied for cartilage regeneration. For example, the in vitro assessment, preclinical study in a caprine model and a pilot clinical study on PEGDA hydrogels were reported [183]. The results indicated that cartilage ECM was deposited in the hydrogel, adjacent cartilage tissue growth was facilitated by MSCs, and significantly more cartilage tissue formation was found compared to the control group with microfracture treatment. Another composite hydrogel based on PEGDA-fibrinogen (commercially known as GelrinC™ for cartilage repair) has been shown to support enhanced chondrogenesis of MSCs with minimizing hypertrophy [184], which suggested that composite hydrogels can be designed for improved ability to regenerate cartilage tissues.
The higher loading and stiffness resulted to osteogenic differentiation, whereas lower loading and stiffness led to chondrogenic differentiation. Layered OPF hydrogels have been prepared for controlled biodegradability and cartilage/none formation ability for osteochondral interface reconstruction [182]. In vivo results showed that hyaline cartilage formed in the cartilage layer with a zonal structure and hypertrophic cartilage formed in the bone layer (subchondral region), where bone generation was eventually observed with the hydrogel partially degraded. A PDMMAm-based double-network hydrogel was shown to promote in vivo hyaline cartilage regeneration in a large osteochondral defect model [73]. An injectable MPEG-PCL copolymer hydrogel has been fabricated and subcutaneous in vivo study indicated that such hydrogel could form an interconnected microporous structure to support chondrocytes growth and proliferation, as well as hyaline cartilage formation [35]. Hydrogels derived from synthesized polymers exhibit tunable properties and have promising potentials for OTE and OCE applications.
Thermosensitive chitosan-pluronic (CP) hydrogels were synthesized by grafting pluronic onto chitosan. In vitro study indicated that the CP hydrogels were injectable and supported chondrocyte growth [50]. MSC-laden fibrin/PLGA hydrogels were developed for treatment of full-thickness cartilage defects [185]. The gel degraded in 12 weeks in vivo. The cell-hydrogel constructs generated cartilage-like tissue matrices of collagen type II and aggrecan. The osteochondral interface tissue was fully reconstructed in 12 weeks. Immunohistochemical and aggrecan staining results confirmed the formation of hyaline cartilage. OPF-gelatin composite hydrogels with encapsulated MSCs also showed a big promise for osteochondral tissue regeneration [71, 186, 187]. Cartilage-related gene expressions of collagen type II and aggrecan increased by 161 fold and 221 fold, respectively, after in vitro culture for 14 days. At 12 weeks of implantation, the composite hydrogels were partially degraded and cartilage/subchondral tissue formed without persistent inflammation. The cartilage showed a zonal structure and the subchondral region contained hypertrophic cartilage and bone-like tissues. Further pre-clinical and clinical studies are still required to evaluate the treatment efficiency of cell-laden composite hydrogels for functional restoration of osteochondral and cartilage tissues.
2.3 Effects of crosslinking mechanisms on the properties of hydrogels
Depending on the unique characteristics and/or the presence of specific functional groups, hydrogels made from natural polymers (including their derivatives) and synthetic polymers can be fabricated via different crosslinking mechanisms. In general, there are two main mechanisms, i.e. physical crosslinking and chemical (covalent) crosslinking, which result in hydrogels with distinct structures and properties. Physical crosslinking is normally induced by change of environmental factors including temperature, pH, force, concentration, and ions. Typical methods to induce physical crosslinking include cooling, lyophilization, and presence of acids, dehydrating agents, and cation exchange [94, 138, 166, 175]. Many naturally derived polymers can form hydrogels via physical crosslinking, which generally involves changes of molecular conformations of the polymer chains to undergo phase transition and induce intermolecular aggregation. This resulted in the formation of junction zones through bridges between polymeric chains or interactions between charged components (ions or groups) to form a reversible, semi-stable network [138–140, 188].
On the other hand, chemically modified natural polymers and synthetic polymers can be covalently crosslinked to form hydrogels via a specific reaction mechanism. The crosslinking reaction normally involves two or more functional groups presented on different polymeric chains to form new covalent chemical bonds and thus generate a permanent stable network [188]. To induce chemical crosslinking, typical methods include UV irradiation, dehydrothermal treatment, and the addition of crosslinkers [94]. In most cases, physically crosslinked hydrogels are mechanically weaker and less stable in physiological conditions compared to chemically crosslinked hydrogels. By controlling the density of chemical crosslinking sites, the mechanical properties and biodegradation rates of chemical crosslinked hydrogels are highly tunable [33, 88, 139, 140, 152, 165, 180, 188]. However, it should be careful to select proper crosslinkers and crosslinking reactions used for chemical gelation to avoid toxic effects to cells and tissues [188].
2.4 Tailoring biodegradability of hydrogels for OTE and CTE
Manipulating the biodegradability of hydrogels to match the rate of cell growth and tissue repair is an important topic for rational design of hydrogel matrices for cartilage and osteochondral tissue regeneration [189]. It is generally accepted that overly rapid degradation may lead to the reduced retention of ECM proteins, whereas hydrogels that degrade too slowly can hinder cell remodeling and thus tissue formation [190]. Previous studies have demonstrated that chondrocyte/MSC-laden hydrogels with a balanced biodegradation rate were able to promote neocartilage/bone tissue formation and achieve higher mechanical properties after long-term culture [189–195].
The importance of hydrogel degradation rate was investigated by using alginate-based hydrogels [193]. In vitro results showed that partially oxidized alginate hydrogels degraded much faster (disappeared within 9 days) than hydrogels made from pristine alginate (stable for at least 1 month), when crosslinked with calcium ions under otherwise similar conditions. Subcutaneous implantation of such hydrogels seeded with chondrocytes in the dorsal region of mice revealed that oxidized alginate samples degraded and generated abundant of connective cartilage ECM, but non-oxidized alginate samples led to small islands of cartilage ECM surrounded by significant amounts of residual alginate.
MSC-laden methacrylated caprolactone HA (MeCLHA) hydrogels were fabricated and characterized to tailor the biodegradation rate of HA to investigate the influences on in vitro neocartilage formation [190]. It was found the faster degradation of MeCLHA hydrogels increased pore sizes of the matrices and generated void spaces to allow enhanced deposition of newly formed ECM proteins. In vitro culture results after 56 days showed that the mechanical strengths were higher for hydrogels with matched degradation rates with ECM deposition than those that degraded too fast. In addition, PEG-PVA and PEG-oligo(lactic acid) (LA) composite hydrogels were developed with tuned degradation profiles [189, 194]. Their results suggested that incorporation of degradable component to PEG hydrogels facilitated generation of cartilage-like tissues.
To promote mineralized bone tissue formation, hydrogels with tailored degradation rates were synthesized by copolymerizing a degradable macromer, PEG-LA endcapped with methacrylate groups (PEG-LA-DM), with a nondegradable macromer, PEG dimethacrylate (PEGDM) [191]. It was discovered that the copolymers composed of 100:0, 83:17, 67:33, and 50:50 wt% of PEGDM and PEG-LA-DM, showed weight losses of 0, 17, 33, and 50% over the time of 25 days in osteoblast complete medium at 37 °C. Proliferation, alkaline phosphatase, and mineralized bone mineral production of encapsulated osteoblasts were facilitated by increasing PEG-LADM content and corresponding degradation.
PEG-genipin hydrogel blocks crosslinked with 8 mM, 17.6 mM, or 35.2 mM genipin were implanted into osteochondral defects made in the trochlea of mice [195]. It was found that the degradation was reduced with increasing the concentration of genipin. Almost fully degradation occurred at 8 mM, intermediate degradation at 17.6 mM, and minimal degradation at 35.2 mM over the implantation time of 5 weeks. The results showed that the higher degradation of PEG-genipin hydrogel enhanced in vivo osteochondral tissue regeneration. Overall, these results highlighted the vital significance of the degradation profile in dictating cellular behavior and tissue regeneration both in vitro and in vivo. It is necessary for future studies to optimize hydrogel formulations and achieve the best degradation rates designed for specific applications involving different targeting tissues.
3. Chondrogenesis or osteogenesis of various types of cells encapsulated in hydrogels
Tissue engineering and cell therapy have been combined to repair cartilage and osteochondral defects. Although a variety of hydrogels have been developed and used as artificial ECM for cell delivery and 3D culture, only limited cell types such as chondrocytes, preosteoblasts/osteoblasts, MSCs, induced pluripotent stem cells (iPSCs), ESCs, and peripheral blood mononuclear cells (PBMSCs), have the potential of chondrogenic and/or osteogenic differentiation to regenerate cartilage and osteochondral tissues (Table 1) [183, 196–201]. Autologous chondrocyte implantation has been successfully used as a clinical method to treat cartilage defects. However, it is very challenging for orthopedic surgeons to directly fix a chondrocyte graft in a focal cartilage site with complex shape [183]. Therefore, tissue engineers and biomaterials scientists have proposed to deliver chondrocytes with hydrogels to overcome this challenge.
3.1 Chondrogenesis of chondrocytes encapsulated in hydrogels
To date, chondrocytes have been embedded in a variety types of hydrogels. In vitro studies have demonstrated that chondrocytes could spread and proliferate well in 3D hydrogel matrices, showing enhanced expressions of cartilage-related proteins/genes with well-maintained cell morphology and phenotype [38, 39, 42, 45, 48, 49, 196]. However, hydrogel matrices made from different polymers behaved differently in directing chondrogenesis of chondrocytes. As discussed above, both chitosan (Fig. 2A) and agarose hydrogels were found to support long-term survival and retain morphology when cultured in vitro [50]. During in vitro maturation, chondrogenesis took place with the formation of cartilage ECM, including aggrecan and collagen type II that were homogenously distributed throughout the hydrogels. For example, abundant cartilage matrix of aggrecan and collagen type II/VI formed in chondrocyte-laden in oligo(lactic acid)-b-PEG-b-oligo(lactic acid) (PEG-LA) hydrogels cultured for 28 days (Fig. 2B) [194]. Cartilaginous ECM-modified chitosan hydrogels enhanced cellular condensation and chondrogenesis of embedded chondrocytes to promote cartilage regeneration, which was attributed to the integrin α10 binding to collagen type II and thus improvement in cell-matrix adhesion (Fig. 2C) [51]. In vivo evaluations further indicated that chondrocytes encapsulated in hydrogels could regenerate hyaline cartilage tissues with structure remodeling [44, 73, 183, 196]. Although chondrocyte-based therapy shows promising potentials in regenerating cartilage tissues, notable limitations exist. First, chondrocyte harvest involves removing healthy cartilage tissues from non-weight-bearing areas and in vitro culturing for a long time of 3 to 5 weeks [9–11]. Due to the low number of chondrocytes, cartilage defects cannot self-heal so that the donor site will become morbid. Second, autologous chondrocytes therapy is inefficient for the elderly because of the low proliferation capacity of primary chondrocytes derived from aged patients [21].
Fig. 2.
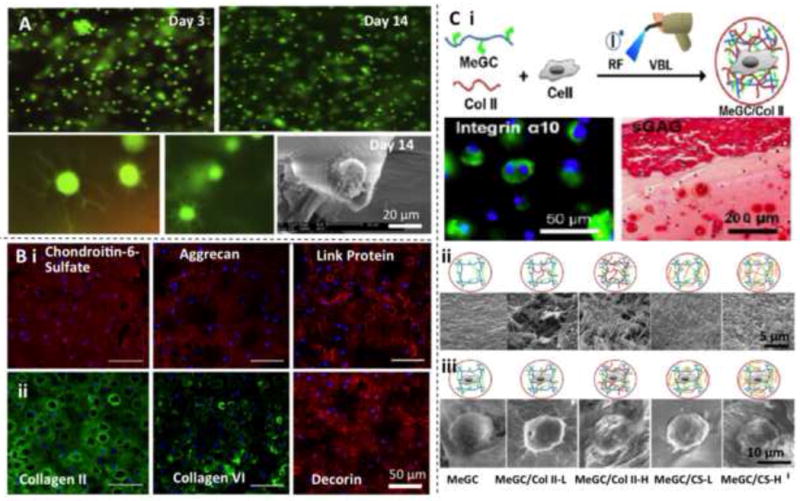
Chondrocytes cultured in various hydrogels for OTE and CTE. (A) Fluorescence microscopy images showing the chondrocyte morphology inside chitosan-based hydrogels after 3 and 14 days in culture. The upper panels show low-magnification views and lower panels display the close-ups. Scanning electron microscopy (SEM) image shows the morphology of a single chondrocyte. Reproduced with permission [50] Copyright © 2009 Elsevier B.V. (B) Cartilage matrix generation of chondrocytes encapsulated in PEG-LA hydrogels (28 days). (i) Proteoglycan deposition: Chondroitin-6-sulfate (red), aggrecan (red), link protein (red), and ell nuclei (blue). (ii) Collagen deposition: collagen II (green), collagen VI (green), decorin (red), and cell nuclei (blue). Reproduced with permission [194] Copyright © 2011 The Association of Bone and Joint Surgeons. (C) Chondrocytes encapsulated in cartilaginous ECM-modified chitosan hydrogels (MeGC = methacrylated glycol chitosan; RF = riboflavin; VBL = visible blue light). (i) Chondrocyte encapsulation and expression of cartilage-related proteins. (ii) Interior microstructure of various chitosan based hydrogels. (iii) Cell adhesion onto the hydrogels. Reproduced with permission [51] Copyright © 2014 American Chemical Society.
3.2 Osteogenesis of preosteoblasts and osteoblasts encapsulated in hydrogels
Preosteoblasts and osteoblasts, as bone progenitor cells, have stable osteogenic phenotype and have been widely studied to regenerate bone tissues [202, 203], including subchondral bones. It has been reported that preosteoblasts can differentiate to osteoblasts in vitro and enhance bone formation in vivo [83, 204]. Osteoblasts were encapsulated in PEG-based hydrogels and in vitro studies indicated that bone-related gene expression increased with gel degradation [205]. Apatite deposition and matrix mineralization increased with increasing concentration of the methacrylate groups. Osteoblasts embedded in PEG-based hydrogels modified with RGD peptides exhibited improved attachment, spreading, and cytoskeletal organization [206].
3.3 Chondrogenesis and osteogenesis of stem cells encapsulated in hydrogels
Alternatively, stem cells have the capacity to differentiate into various tissue-forming cells including chondrogenic and osteogenic lineages for cartilage and bone regeneration, respectively. Therapies combining stem cells and hydrogels are emerging for OTE and CTE. The most commonly studied stem cells include MSCs, iPSCs, ESCs, and PBMSCs. MSCs may come from a variety of sources, such as bone morrow, adipose tissue, muscle, periodontal ligament, lung, liver, spleen, thymus, amnion, placenta, umbilical cord blood, and corneal stroma [207–209]. MSCs may proliferate without differentiation for up to 40 generations [209]. Extensive studies have revealed that the therapeutic efficiency of MSCs largely replies on their capacity to work as a trophic factor generator. MSCs can also interact closely with local biochemical stimuli to generate a number of growth factors providing multiple biofunctions for tissue restoration [207]. MSCs derived from birth-associated neonatal tissues including umbilical cord, placenta, amnion, and cord blood have better proliferative capacity, higher availability, bigger life span, and higher differentiation potential compared to those obtained from distinct adult mature tissues of adipose, muscle, and bone [208]. ESCs are normally isolated from the inner tissues of early embryos so that they are pluripotent and have the potential to differentiate down to almost all cell lineages in the human body [199, 207]. However, the pluripotency makes it difficult to control the differentiation [210]. In addition, ESCs face potential immune rejection and involve ethical issues [200]. More recently, iPSCs were obtained from somatic cells including fibroblasts and exhibit similar pluripotency with ESCs in terms of multiple differentiation, thus finding increasingly widespread applications in regenerative medicine [200, 201, 211, 212].
MSCs have become the most broadly used stem cells in regenerative medicine because they have abundant cell sources and low immunogenicity, no ethical concerns, and minimal teratoma risk [207]. MSCs encapsulated in various hydrogels have been tested to target reconstruction of cartilage and osteochondral tissues [34, 42, 47, 53, 54, 62, 63, 71, 182]. Similarly, it has been well documented that MSCs can spread and proliferate in hydrogels. More importantly, in the presence of chondrogenic and osteogenic cues, MSCs embedded in hydrogels can differentiate into chondrocytes and osteoblasts to produce cartilaginous/bony ECMs in vitro and form cartilage/bone tissues in vivo [52, 198, 213]. Different hydrogels exhibited different ability to support chondrogenesis and osteogenesis. The cartilaginous and osseous ECMs formed after an 8-week culture of MSCs in alginate, chitosan, and fibroin hydrogels as confirmed by histological and immunohistochemical staining (Fig. 3) [101]. Alginate and chitosan hydrogels generated more cartilage ECM than the fibroin hydrogel. MSCs encapsulated in collagen hydrogels encouraged in vitro formation of osteochondral interface tissues with a zonal structure consisting of a pure cartilage layer, a calcified cartilage layer, and a subchondral bone layer [47, 214]. The results suggested that MSC-laden hydrogels would be promising biomaterials for osteochondral interface regeneration. iPSCs had the ability to differentiate into chondrocytes in alginate hydrogels and regenerate cartilage tissues in vivo [197]. ESCs encapsulated in PEG hydrogels could differentiate into chondrogenic cells and produce neocartilage ECM [215]. In addition to the aforementioned stem cells, PBMCs were recently reported to possess similar chondrogenic differentiation and cartilage generation ability compared with MSCs [216]. PBMCs could be readily extracted from peripheral blood with minimally invasion. Their application potential requires further studies. Although stem cells have the intrinsic nature to differentiate down to chondrogenic and osteogenic lineages, growth factors are required to trigger the differentiation. In the next section we will discuss the effects of growth factors delivered by different methods on the chondrogenic/osteogenic differentiation and cartilage/bone formation of stem cells encapsulated in hydrogels.
Fig. 3.
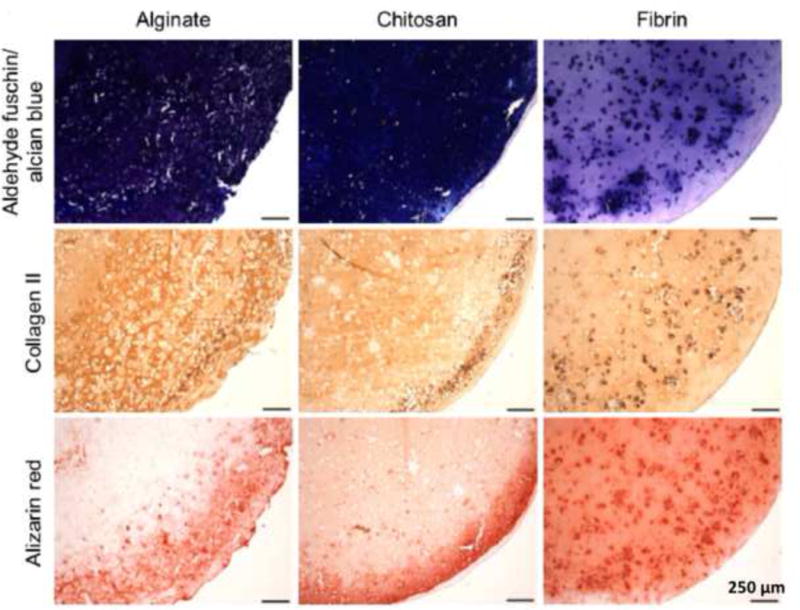
MSCs cultured in different hydrogels for OTE and CTE. Histological and immunohistochemical staining results showing the cartilaginous and osseous ECM formation after an 8-week culture of MSCs in alginate, chitosan, and fibrin hydrogels. Constructs are stained for aggrecan (Alcian blue), collagen type II, and calcium (Alizarin red). Reproduced with the permission [101] Copyright © 2015 Elsevier.
3.4 Co-culture of chondrocytes and stem cells in hydrogels and spatial control of layered constructs for chondrogenesis and osteochondrogenesis
Co-culture of chondrocytes and MSCs in hydrogels have been investigated for improved chondrogenesis and osteogenesis. Chondrocytes and MSCs were encapsulated with different population ratios (1:0, 1:1, 1:2, 1:3, and 1:4) in PCL-PEG composite hydrogels, which were cultured in chondrogenic medium and implanted in rabbits in a full-thickness articular cartilage defect model (Fig. 4A) [196]. In vitro results at 4 weeks indicated that co-culture of articular chondrocytes and MSCs facilitated expression of chondrogenic phenotype and production of cartilaginous ECM. In addition, chondrocytes promoted chondrogenesis of MSCs, while MSCs boosted cell proliferation. In vivo results at 8 weeks demonstrated that co-culture at a chondrocytes:MSCs ratio of 1:4 induced the optimal cartilage regeneration.
Fig. 4.
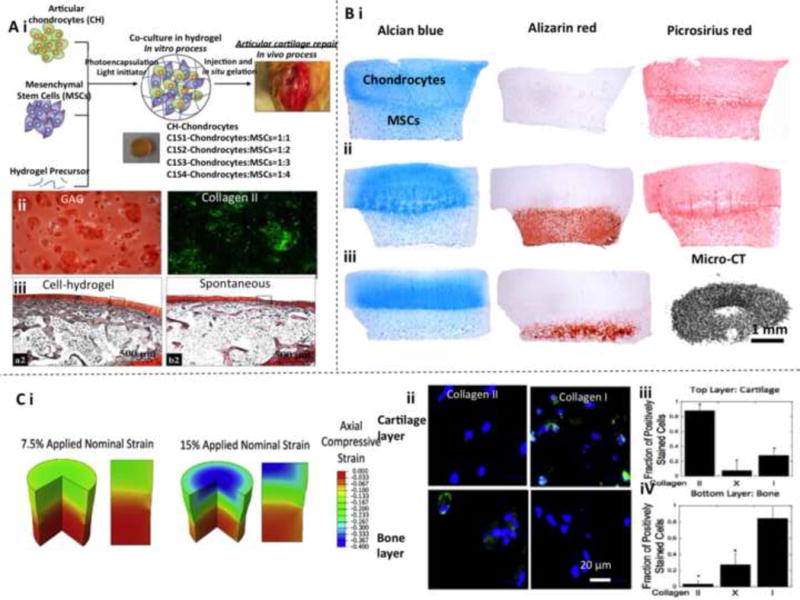
Chondrogenesis and osteogenesis of chondrocytes and MSCs in multi-layered hydrogel osteochondral constructs. (A) Co-culture of chondrocytes and MSCs in PCL-PEG composite hydrogels. (i) Schematic of 3D encapsulation. (ii) In vitro cartilage-related biomarker expression (aggrecan stained by Safranin O) at 4 weeks. (iii) Histological results showing in vivo cartilage formation. Reproduced with permission [196] Copyright © 2013 John Wiley & Sons, Inc. (B) A structured bilayered co-culture of chondrocytes and MSCs in agarose hydrogels for chondrogenesis and endochondral ossification. Alcian blue and Alizarin red staining are used to characterize cartilage ECM formation. Alizarin red staining and micro-computed tomography (micro-CT) scanning are employed to examine bone ECM formation. (i) After a 49-day culture in chondrogenic medium. (ii) After a 21-day culture in chondrogenic medium and a 28-day culture in hypertrophic medium with β -glycerophosphate supplement. (iii) After a 21-day culture in chondrogenic medium and a 28-day subcutaneous implantation in nude mice. Reproduced with permission [218] Copyright © 2013 Elsevier. (C) Mechanical loading regulated MSCs differentiation that were encapsulated in layered PEG hydrogel for controlled chondrogenesis and osteogenesis. (i) Finite elemental modeling results for multi-layered hydrogel constructs under compression conditions. Negative values mean compressive strain. (ii) Expression of cartilage and bone biomarkers in hydrogel layers with different mechanical loading. Green indicates collagen II or I, and blue indicates nuclei. (iii, iv) Quantitative study of chondrogenic- and osteogenic-differentiated cells induced by mechanical property change. Reproduced with permission [182] Copyright © 2015 Elsevier.
A structured bilayered co-culture of chondrocytes and MSCs in agarose hydrogels was developed for osteochondral tissue repair via chondrogenesis and endochondral ossification (Fig. 4B) [218]. In the design, the top layer of the bilayered agarose hydrogel construct was seeded with chondrocytes (termed as the cartilage layer), while the bottom layer was seeded with MSCs (termed as the bone layer). This bi-layered chondrocyte/MSC-laden hydrogel co-culture system was able to promote chondrogenesis in the cartilage layer and facilitated the chondrogenic phenotype that was lost in monolayer expansion of chondrocytes. Moreover, the bilayered co-culture was found to hinder in vitro hypertrophy and mineralization in the bone layer. However, the subcutaneous implantation results suggested that endochondral ossification took place in the bone layer to form an osteochondral tissue, which could be induced by the osteogenic molecules. Such chondrocyte/MSC-laden bi-layered hydrogel constructs hold a great potential for effective regeneration of osteochondral tissue and full-thickness cartilage defects.
A MSC-laden multi-layer PEG hydrogel system with spatial mechanical and biochemical cues was developed for OTE and CTE (Fig. 4C) [182]. This system was composed of a soft chondroitin sulfate hydrogel cartilage layer with a low RGD concentration, a stiff bone layer with a high RGD concentration, and an intermediate layer between them. The stiffness variation generated high strains, low strains, and moderate strains in the three layers, respectively. Importantly, the incorporation of biomolecules and variations in hydrogel stiffness had very limited effects on directing MSC differentiation, whereas mechanical stimulation was the critical factor. High mechanical load induced osteogenic differentiation in the bone layer and low mechanical load induced chondrogenic differentiation in the cartilage layer. This study revealed that a dynamic spatial mechanical environment was able to direct MSC differentiation for osteochondral tissue regeneration [182].
Layered hydrogels laden with multiple types of cells (e.g. chondrocytes and MSCs) have shown the ability to promote osteochondrogenesis and form osteochondral tissues with zonal structure in vivo. Therefore such functional hydrogels are promising for repair of full thickness cartilage and osteochondral tissue defects. If combined with biochemical stimulation, more effective regeneration would be possible to achieve.
3.5 Endochondral ossification of stem cells encapsulated in hydrogel for osteochondral tissue regeneration
MSCs and osteochondral progenitor cells primed by chondrogenesis have been shown to become hypertrophic and undergo endochondral ossification when implanted in vivo [218]. Modulation of this endochondral phenotype may be attractive to engineer cartilaginous and osseous phase of osteochondral constructs. Anti-angiogenic factor chondromodulin-1 has been shown to be able to stabilize the chondrocyte phenotype of osteochondral progenitor cells and support chondrogenesis, but suppress chondrocyte hypertrophy and endochondral ossification [218]. These findings indicated that chondrogenic cell-laden hydrogels were still promising for cartilage tissue repair when inhibitors of endochondral ossification were combined. On the other hand, taking advantages of endochondral ossification, MSC-laden hydrogels can be used to regenerate bone tissues or the osseous phase of osteochondral tissues [218, 219]. A bilayered osteochondral construct of agarose hydrogel seeded with MSCs was produced with the bottom layer for regenerating subchondral bone via endochondral ossification [218]. The success was confirmed by results from in vivo subcutaneous implantation in nude mice.
The three naturally derived hydrogels of alginate, chitosan, and fibroin showed various endochondral ossification of MSCs in vivo [101]. Alginate and fibroin hydrogels facilitated vascularization and endochondral ossification, while the chitosan hydrogel promoted neither vascularization nor endochondral ossification, but produced the greatest amount of cartilage ECM of aggrecan. Cells in the alginate hydrogels produced more bone mineral and supported greater bone formation in the central region. At this point, MSC-laden chitosan hydrogels appeared more appropriate for cartilage regeneration and MSC-laden alginate hydrogel seemed more suitable for endochondral bone tissue engineering applications.
4. Effects of growth factors and delivery methods on chondrogenesis and osteogenesis of cell-laden hydrogels
4.1 Common growth factors and hormones for chondrogenesis and osteogenesis
Native ECMs can sequester specific biomolecules to stimulate cell growth, proliferation, and differentiation, which are referred to as growth factors. Growth factors and hormones are also highly related to the repair of damaged tissues. The idea to use growth factors and hormones to promote chondrogenic and osteogenic tissue regeneration is thus intuitive. TGFs, insulin-like growth factors (IGFs), BMPs, and dexamethasone are among the most extensively used growth factors and hormones to stimulate chondrogenic or osteogenic differentiation of stem cells.
4.1.1 TGFs
TGFs are a family of polypeptides that can affect cell behaviors including growth, proliferation and differentiation [220–223]. There are two types of TGFs, namely, the α and β forms, which have unique amino acid sequences and different interactions with receptors [220, 224, 225]. The TGF-β family has been demonstrated to be effective to induce chondrogenic differentiation of stem cells [132, 220]. TGF-β1 and TGF-β3 have both been extensively used for chondrogenic differentiation and chondrogenic phenotype maintenance of MSCs for cartilage and osteochondral tissue regeneration, and worked well for different types of hydrogels and MSCs from various sources [47, 71, 197, 216, 226, 227]. However, their effects may be not exactly the same. It has been reported that TGF-β1 resulted in significant increases in cartilage-related gene expression in comparison of moderate effects of TGF-β3 for chondrogenic differentiation of MSCs. TGF-β1 promoted cellular adhesion molecule expression and facilitated cellular condensation, whereas TGF-β3 increased cellular proliferation. Moreover, TGF-β1 and TGF-β3 influenced different stages of chondrogenic differentiation of MSCs. As a result, the combined use of both TGF-β1 and TGF-β3 may be more effective for chondroenesis of MSCs. More studies are required to understand this possibility. Specific examples regarding the effects of TGF-β1 and TGF-β3 on chondrogenesis of MSCs will be described in section 4.2 along with introduction of delivery methods of growth factors.
4.1.2 Insulin-like growth factors (IGFs)
IGFs are single-chain polypeptides that have amino-acid sequences close to insulin [228]. There are two types of IGFs: IGF-1 is mainly secreted by the liver stimulated by growth hormone and regulates growth of adults. IGF-2 is deemed to play an important role in fetal growth [229]. IGF-1 has been widely studied for cartilage repair, since it has effects on cartilage homeostasis, proteoglycan synthesis balancing and breakdown. Overexpression of human IGF-1 by transplanted articular chondrocytes encapsulated in alginate hydrogels has been studied for enhancing the repair of full-thickness cartilage and osteochondral defects in rabbits [230]. The results indicated that IGF-1 improved articular cartilage regeneration and accelerated the formation of subchondral bone at both time points 14 weeks. Addition of IGF-1 in the hydrogel constructs was also able to improve chondrogenesis in vitro and in vivo [231–233]. IGFs have shown the ability to effectively facilitate cartilage regeneration by chondrocytes. More studies are required to understand the effects of TGFs on the cellular behavior and chondrogenic differentiation of stem cells.
4.1.3 BMPs
Urist first discovered that the active compound responsible for bone regeneration was a family of proteins and named these as BMPs [234, 235]. BMPs are recognized for their ability to induce ectopic bone and cartilage formation, a process that mimics embryonic endochondral bone formation [220]. Approximately 20 family members of BMPs (BMP-1-18, BMP-3b, and BMP-8b) have been identified to date [234, 236–241]. Specifically, BMP-1, 5, 9, 13 and 14 exhibit functions in cartilage formation and chondrogenic differentiation of MSCs; BMP-3, 4 and 8 play a key role in bone formation; and BMP-2, 7 induce osteogenic/chondrogenic differentiation and bone/cartilage formation. Among all, the most extensively used ones for bone and cartilage regeneration are BMP-2, 7. Implantation of 0.5–115 μg of partially purified recombinant human BMP-2 resulted in cartilage by day 7 and bone formation by day 14 [241]. Studies have shown that BMP-2 was effective for promoting osteogenic differentiation and osteogenesis of MSC-laden or cell-free hydrogels towards in vitro and in vivo, respectively [242–248]. Some studies suggested that BMP-2 had the ability to facilitate cartilage formation by chondrocytes and MSCs for treatment of cartilage and osteochondral defects [45, 249, 250]. BMP-7 was used in cell-laden hydrogel constructs for promoting chondrogenesis and cartilage ECM formation [251]. Extensive research has shown that BMPs are effective growth factors to regulate both cartilage and bone formation. Regeneration of full-thickness cartilage and osteochondral tissues is a complex process that involves biofunction restoration of zonal cartilage and subchondral bone. Further studies are required to understand how the BMPs affect chondrognesis and osteogenesis of chondrogenic and osteogenic progenitor cells under various conditions (e.g. co-culture of chondrocytes and MSCs, in the presence of other types of growth factors).
4.1.4 Dexamethasone
Dexamethasone is a type of adrenocortical hormones and has been demonstrated to promote chondrogenic and osteogenic differentiation of stem cells [252–254]. Dexamethasone has been added in culture medium or covalently bonded onto hydrogel network to induce osteogenic differentiation of MSCs into osteoblasts for improved osteogenesis [255, 256]. It has been reported that dexamethasone can also enhance chondrogenic differentiation of MSCs and ESCs as well as cartilage-related protein formation in the presence of TGF-β [257–259], whereas another study indicated that the addition of dexamethasone had suppressive effects on aggrecan synthesis and accumulation [260]. Dexamethasone is commonly used as a supplement with TGFs or BMPs to further promote cell proliferation and maximize the chondrogenic or osteogenic induction effect for optimal chondrogenesis or osteogenesis.
4.1.5 Newly identified growth factors and bioactive species
There are also significant on-going efforts to identify new growth factors that show enhanced stimulation effects of chondrogenic differentiation and osteogenic differentiation of stem cells. For example, it has been reported that a small molecule known as kartogenin promoted selective chondrocyte differentiation by regulating the transcription factor core-binding factor subunit (CBFb)-RUNX1 transcriptional pathway [261]. In addition, some inorganic nano-/microparticles demonstrated certain bioactivity to function as “growth factors” to trigger osteogenic differentiation of stem cells for bone regeneration [213], which will be discussed in Section 5.
4.2 Delivery methods of growth factors
In general, there are five methods for growth factor delivery: by freeform in medium, by physical blending in hydrogel, by covalent bonding to hydrogel, by microsphere carriers, and by gene delivery. Examples of each delivery methods are illustrated below.
4.2.1 Freeform in medium
The effect of TGF-β3 on chondrogenic differentiation of MSCs has been evaluated in vitro [216]. After 14 days, a larger population of MSCs cultured in TGF-β3-containing medium differentiated into chondrocytes than the control group, as indicated by increased aggrecan and collagen type II expressions. TGF-β3 and dexamethasone were applied for the chondrogenic and osteogenic pre-differentiation of MSCs to generate OPF hydrogel osteochondral constructs [216]. In vitro results indicated the cartilage layer exhibited increased expression of cartilage-related gene/protein biomarkers, while the subchondral layer presented enhanced expression of bone-related biomarkers. It was also found that MSCs that underwent 7 days of chondrogenic pre-differentiation closely resembled the phenotype of hyaline cartilage when combined with osteogenic cells in a bilayer hydrogel composite. An in vitro co-culture approach was developed to make multilayer osteochondral structures using a two-chamber well [198]. This approach can simultaneously provide chondrogenic and osteogenic stimulation with inducers of TGF-β3 and dexamethasone to MSCs encapsulated in different regions of the constructs. It can be concluded that growth factor delivery by freeform in medium is an efficient way to culture engineered cell-laden hydrogel cartilage/osteochondral constructs in vitro. However, frequent dosing is required to maintain the growth factor concentration and bioactivity in the medium, which is not optimal for long-term culture and maturation of engineered tissue constructs. Also, this delivery method is not applicable for in vivo chondrogenesis and osteogenesis.
4.2.2 Physical blending in hydrogel
Encapsulating growth factors in hydrogels was proved to be an easy and effective way to achieve release in a sustaining manner. BMP-2 was directly added in MSC-laden hydrogels composed of chitosan-lactide-fibrinogen to stimulate osteogenic differentiation [262]. Release study and characterization of bone-related biomarker expression indicated that the BMP-2 release could sustain for 4 weeks to induce osteogenic differentiation with increasing alkaline phosphate activity and mineralization. In vivo study demonstrated that BMP-2 containing hydrogels prompted neo-osteogenesis by increasing osteoprogenitor localization in the defect site [263]. Growth factor delivery by encapsulation in hydrogels does not require multi-time dosage and may be able to sustain the release for up to weeks. This is an advantage for long-term culture of engineered cartilage/osteochondral constructs. In addition, it is applicable for in vivo chondrogenesis and osteogenesis. However, the amount of growth factor released from hydrogels will significantly reduce with time, which may limit the efficiency of the chondrogenic and osteogenic induction.
4.2.3 Covalent bonding to hydrogels
In addition to simply entrapping the growth factor in the hydrogels, TGF-β1 was immobilized to a thiol-ene PEG hydrogel by covalent bonding to increase proliferation of encapsulated chondrocytes and cartilage ECM production (Fig. 5B) [264]. The results indicated that TGF-β1 distributed homogenously throughout the PEG hydrogel to significantly increase the proliferation rate of chondrocytes and production of aggrecan and collagen type II over 28 days, at levels exceeding those for chondrocytes in hydrogels where TGF-β1 was dosed in the culture medium. Small-molecule chemical functional groups of t-butyl and phosphate were also tethered in PEG hydrogels and demonstrated that such molecule groups can work as localized growth factors to induce osteogenic differentiation of encapsulated MSCs [265]. These PEG hydrogels with covalently bonded TGF-β1 and t-butyl/phosphate functional groups inspired the design of other hydrogels immobilized with growth factors for a variety of tissue engineering applications. This growth factor delivery method uses a lower total dosage while still promotes high levels of cell proliferation and ECM production, and can be used for in vivo cartilage regeneration.
Fig. 5.
Hybrid hydrogel composites with inorganic particles for OTE and CTE. (A) MSCs encapsulation in silicate-hydrogel nanocomposites. Reproduced with permission [213] Copyright © 2015 American Chemical Society. (B) Osteogenic differentiation of bone morrow derived MSCs induced by nanosilicate platelets. Reproduced with permission [270] Copyright © 2013 John Wiley & Sons, Inc. (C) Hydrogel composites with microsilicate particles as osteogenic inducers. (i) Optical photomicrographs of human dermal fibroblasts (HDFs) cultured on silicate-incorporated hydrogels. (ii) ALP activity of MSCs cultured with different ionic extracts from inorganic particles for 7 days. Reproduced with permission [273] Copyright © 2013 Elsevier. (D) 3D printing of free-standing GelMA-nanosilicate composite hydrogel constructs. Reproduced with permission [213] Copyright © 2015 American Chemical Society.
4.2.4 By microspheres
Localized growth factor release is another practical strategy for culturing osteochondral constructs with controlled cell phenotypes. To this end, chondrogenic and osteogenic growth factors can be loaded into polylactic acid (PLA) microcarriers and embedded in different zones of the constructs [266, 267]. Enhanced bone formation and induced defect bridging at low BMP-2 doses were also found. In one study, TGF-β1/IGF-1 and BMP-2 were loaded in gelatin microspheres and then encapsulated in MSC-laden multilayered hydrogels to induce chondrogenic and osteogenic differentiation for repair of osteochondral defects [233]. In vivo results showed that controlled BMP-2 release sustained for 4 to 6 weeks to promote subchondral bone formation. IGF-1 release did not improve cartilage regeneration. The controlled localized growth factor delivery can induce chondrogenic and osteogenic differentiation of MSCs in the cartilage zone and subchondral bone zone, respectively. As a result, no predifferentiation or additional growth factors in the medium are required. As another embodiment, TGF-β1 and BMP-2 were loaded in PLGA microspheres and encapsulated these microspheres in separated layers of the MSC-laden hydrogels to induce chondrogenic and osteogenic differentiation. In vivo results showed that zonal osteochondral tissues were formed. Controlled release of TGF-β1 led to improved cartilage production and preserved cartilage integrity from 12 weeks up to 24 weeks. It can thus be concluded that microsphere encapsulation of growth factors is a useful strategy to control the release rate and increase delivery efficiency.
4.2.5 By gene delivery
Incorporating therapeutic genes into biomaterials is a relatively new growth factors delivery method to facilitate tissue regeneration. For example, a gene delivery platform of TGF-β3 and BMP-2 in alginate hydrogels has been developed for cartilage and osteochondral tissue [268]. The results indicated that sustained overexpression of the transgenes were achieved with nano-hydroxyapatite (nHAp) plasmid DNA (pDNA) encoding. It was found that gene delivery of TGF-β3 and BMP-2 led to a significant increase in aggrecan and collagen production. Co-delivery of genes encoding TGF-β3 and BMP-2 generated more collagen type II deposition compared with delivery of TGF-β3 or BMP-2 only. Gene delivery of TGF-β3 and BMP-2 is also an efficient method to promote transfection of MSCs and direct their chondrogenic or osteogenic phenotype for OTE and OTE applications. Another study reported the spatial control of cell gene expression by short interfering RNA (siRNA) gradients in hydrogels [269]. Their results demonstrated that siRNA could be presented in a sustained manner to encapsulated cells. This platform may be used to produce gradients of cell function and engineered tissue properties for regenerating complex tissues and tissue interfaces including cartilage and osteochondral tissues.
5. Hydrogel/inorganic particles/stem cell hybrid composites as promising biomaterials for OTE and CTE
Hydrogels possess excellent biocompatibility and biodegradability, and may be formed into desired shapes for OTE and CTE applications. However, many hydrogels suffer from challenges related with insufficient mechanical stiffness, osteoconductivity, osteoinductivity, injectability, or printability. Inorganic particles-incorporated hybrid hydrogel composites are emerging as functional biomaterials for repair of osteochondral and cartilage defects. The most appealing inorganic particles are phosphate and silicate minerals, as well as bioactive glasses due to their outstanding osteoconductivity and osteoinductivity [149, 213, 270–275].
5.1 Functionalized hydrogels with inorganic particles: osteoconductivity and osteoinductivity
Tissue engineered osteochondral constructs of agarose/alginate-hydroxyapatite (HAp, Ca10(PO4)6(OH)2) and alginate-bioglass (BG) hydrogel-based hybrid composites were cultured with chondrocytes and osteoblasts for osteochondral tissue repair application [274, 276]. It was found that hypertrophic chondrocytes produced higher matrix (proteoglycan and collagen type II) deposition and mineralization in HAp particles-incorporated agarose hydrogels. Moreover, the addition of HAp particles remarkably enhanced the compressive and shear mechanical properties of the hydrogels. The highest mechanical properties and mineralization were found in agarose hydrogels with 3% micro-HAp particles. The zonal structured osteochondral matrix of cartilage, calcified cartilage and subchondral bone were produced by co-culture of chondrocytes and osteoblasts in BG-agarose hybrid hydrogel composites. These results demonstrated that the biomimetic of agarose/alginate-HA/BG hydrogel based hybrid composites are highly promising for osteochondral tissue regeneration.
Studies revealed the effects of a synthetic silicate nanoplatelet named Laponite (Na+0.7((Mg5.5Li0.3)Si8O (OH)−0.7, 20–30 nm in diameter) on the osteogenic differentiation of MSCs, as well as its influence in the osteoinductivity, modulus, injectability, and printability of hydrogels [213, 270–272, 277]. These nanosilicates are cytocompatible and can strongly interact with the MSCs under the clathrin-mediated internalization pathway (Fig. 5A,B) [213, 270]. The nanosilicates showed the capability to trigger osteogenic differentiation of MSCs without addition of any external osteoinductive factors, as proven by an overexpression of osteogenic-related markers (RUNX2, osteopontin and osteocalcin), as well as increased alkaline phosphatase activity and bone matrix (collagen type I and calcium) deposition. The advantage of using these nanosilicates for osteogenic differentiation is that they work as localized inducers trapped in hydrogels and are delivered in a single dose.
Wollastonite (CaSiO3) microparticles (100–150 μm) were used to fabricate composite alginate hydrogels (Fig. 5C) [273]. This hybrid hydrogel was bioactive, biocompatible, and osteoinductive. The addition of wollastonite micoparticles significantly improved and bone-like apatite deposition ability of the alginate hydrogel, and decreased the gelling time. Moreover, the composite hydrogel maintained normal cell growth and stimulated the osteogenic differentiation of MSCs. HAp nano/microparticles (200 nm vs. 25 μm) were added into agarose hydrogels for regeneration of osteochondral interface and calcified cartilage. In vitro studies indicated that hypertrophic chondrocytes presented higher ALP activity in the presence of HAp. The HAp microparticles led to formation of more aggrecan, collagen, and calcified aggregates compared with nano-HA particles.
5.2 Mechanical properties of inorganic particles-incorporated hybrid hydrogels
In addition to providing the osteoconductivity and osteoinductivity, inorganic particles can also enhance the mechanical properties and change the microstructures of hydrogel matrices. For example, the incorporation of Laponite nanoparticles in 5 wt% GelMA hydrogels resulted in significantly improved mechanical stiffness [213]. With the addition of 0.5, 1, and 2 wt% Laponite nanoparticles, the Young’s modulus of the hybrid hydrogels increased from 3.3 ± 0.4 kPa to 4.7 ± 0.9, 8.9 ± 2.1, and 12.9 ± 1.3 kPa, respectively. In addition, the elastomeric properties were also highly enhanced. Cyclic compression testing results showed greater than 6-fold increase in energy absorbed by the hybrid hydrogel that is highly elastic under high compressive strains. In another study, when wollastonite microparticles were incorporated at 5 wt%, the resulting hybrid alginate hydrogel had a compressive strength of 50.67 kPa (much higher than that of pure alginate hydrogel) with 4% addition of D-gluconic acid δ-lactone [273]. Young’s modulus of agarose hydrogel increased from 2.9 to 4.3 kPa with an addition of 6% HAp microparticles [274]. After in vitro culture with chondrocytes for 14 days, the modulus lifted up to over 30 kPa. A study showed that when reinforced with 25 wt% bioactive glass microparticles, the resulting gellan gum hybrid hydrogel had an increased Young’s modulus of 1000 kPa, much higher than that of pristine gellan gum hydrogels [149].
5.3 Injectability and printability of inorganic particles-incorporated hybrid hydrogels
In addition to the influences of inorganic nanoparticles on properties of hydrogels such as osteoconductivity, osteoinductivity, and mechanics, another major advantage of inorganic particles-incorporated hybrid hydrogels is that they might behave altered rheological properties, such as shear-thinning behaviors, for improved injectability and printability, which is desirable for biomanufacturing of engineered cartilage and osteochondral constructs with complex shapes and geometry. For example, the Laponite nanoparticles promoted strong interactions with gelatin based polymers to form self-assembled structures that can dynamically form and break, resulting in shear-thinning behaviors [213, 277, 278]. The prepolymer solution of 5 wt% GelMA was liquid at room temperature to body temperature, while the addition of 2 wt% Laponite significantly increased the viscosity of the solution to form a physical hydrogel that could flow under shear force. It is believed that the unique platelet shape and heterogeneous surface charge distribution on the Laponite nanoparticles led to the shear-thinning property. This nanoparticle-hydrogel composite system can work as a bioink for advanced 3D printing techniques to fabricate tissue-engineered, free-standing constructs (Fig. 5D). Wollastonite-alginate hybrid hydrogels exhibited good injectability resulting from the in situ gelling capability induced by the calcium ions released from wollastonite microparticles with the addition of D-gluconic acid δ-lactone [273]. The gelling time was adjustable from 30 s to 10 min by varying the addition of wollastonite particles and D-gluconic acid δ -lactone. These studies suggested that due to the outstanding biocompatibility, osteoconductivity, osteoinductivity, mechanical property, injectability, and printability, hydrogel/inorganic particle/stem cell hybrid composites are promising systems as the next-generation tissue engineered biomaterials for repair of osteochondral and full-thickness cartilage defects.
6. Advanced manufacturing techniques for engineering osteochondral and cartilaginous hydrogel constructs
Osteochondral and cartilage tissues possess complex gradient compositions and zonal structures as shown in Fig. 1. One of the remaining grand challenges in OTE and CTE is to fabricate osteochondral/cartilage constructs with biomimetic highly organized layered architectures, graded chemical/biomolecule compositions, and complex anatomically shapes. In combination with hydrogel biomaterials, the emerging advanced manufacturing techniques including microfluidic biofabrication, molding, bioprinting, and assembly, are promising to address this problem.
6.1 Microfluidic fabrication of hydrogel osteochondral constructs with material/cell gradient
Microfluidic biofabrication can easily produce complex hydrogels with composition, microstructure, and cell gradients in centimeter length scale [279]. The gradient in hydrogels was generated by a passive-pump-induced forward flow followed by an evaporation-induced backward flow of the prepolymer solutions before photocrosslinking. This simple and versatile method can produce multi-layered hydrogel constructs with gradient concentrations of encapsulated chondrogenic/osteogenic cells and growth factors to restore the complex structures and biofunctions of osteochondral and cartilage tissues. However, microfluidic biofabrication still has some limitations. It can readily manufacture cell-laden engineered tissue constructs with simple geometries and small dimensions, but finds difficulties in producing anatomical 3D shapes with complex structures.
6.2 3D molding of anatomically shaped osteochondral and cartilage constructs
A 3D molding technique was developed to fabricate tissue-engineered large-scale osteochondral constructs (typical size over 2 cm) of alginate hydrogel with anatomical shapes [280]. The fabrication process involved 3D medical imaging of femoral condyle and tibial plateau, modeling, two-part reverse mold design/fabrication, and MSC/chondrocyte-laden alginate/agarose hydrogel construct molding. In vitro and in vivo results suggested that the constructs supported chondrogenesis and osteogenesis in the cartilage and bone layer, respectively. This manufacturing technology of 3D molding was successful in making MSC/chondrocyte-laden anatomically shaped alginate/agarose hydrogel osteochondral constructs, which can be transferred to other cell-hydrogel systems for extensive tissue engineering applications. It should be noted, however, that this method may not be appropriate for fabricating cell/composition/structure gradient tissue constructs.
6.3 3D bioprinting of complex-shape and gradient osteochondral and cartilage constructs
3D bioprinting is deemed to be a promising advanced manufacturing technology that can generate well-organized 3D tissue constructs with complex shapes and gradient composition/structure via a multilayered deposition process of bioinks and cells. 3D bioprinted MSC-laden silk fibroin-gelatin hydrogel constructs could be in situ crosslinked by tyrosinase or sonication [281]. The encapsulated MSCs maintained good viabilities during in vitro cell culture over 30 days. Cultured in chondrogenic/osteogenic medium, MSCs further differentiated into chondrocytes/osteoblasts. Studies demonstrated fabrication of cartilage tissues with complex geometries by 3D bioprinting with alginate-based, cell-laden bioinks [36]. The embedded chondrocytes in bioprinted alginate hydrogels exhibited a good long-time viability for the growth of cartilage tissue.
Osteochondral constructs of MSC-laden GelMA-based hydrogel were fabricated by combining the 3D bioprinting and microcarrier technology [266]. High cell density and viability were achieved in the printed constructs. Furthermore, microcarrier encapsulation increased the stiffness of the hydrogel constructs, promoted cell adhesion, osteogenic differentiation, and bone matrix deposition by MSCs. A new multi-material 3D bioprinting technique has been developed [282] to allow active and efficient mixing of complex fluids at the microscale for direct printing of gradient constructs (Fig. 6A). This technique has the capability to continuously mix complex liquids with a high efficiency to manufacture 3D architectures with controlled local compositions and properties. It thus holds a great potential to generate tissue-engineered hydrogel-based zonal osteochondral and cartilage constructs. Normally the thickness of 3D printed hydrogel tissue constructs is limited because of insufficient structural support. A newly reported bioprinting system can print cell-laden hydrogels with other stronger biopolymers providing structural integrity (Fig. 6B) [283]. Such a 3D bioprinting platform can fabricate human-scale tissue constructs of calvarial bone, mandible, cartilage and skeletal muscle. This technique may manufacture engineered clinically useful tissues/organs that combine recapitulated biofunctions and structural stability.
Fig. 6.
3D printing and assembling of cell-laden hydrogel constructs for OTE and CTE. (A) Microscale mixing and 3D printing for fabrication of gradient constructs. Reproduced with permission [282] Copyright © 2015 National Academy of Science. (B) 3D bioprinting of human-scale tissue constructs with structural integrity. Reproduced with permission [283] Copyright © 2016 Nature America, Inc. (C) A 3D puzzle assembly strategy for fabrication of large engineered hydrogel based cartilage and osteochondral tissue constructs. Reproduced with permission [286] Copyright © 2016 Elsevier.
3D bioprinting is a powerful technology for manufacturing cell-laden tissue constructs with complex shapes and gradient compositions/structures. It involves selection of bioinks, cells, growth/differentiation factors, and technical challenges related to the sensitivities of living cells and tissues [284]. Some challenges still exist that might limit the extensive clinical applications of 3D bioprinting for tissue regeneration. First, due to the intrinsic properties of hydrogels, 3D bioprinted tissue constructs commonly lack proper mechanical strengths and structural integrity that are required for maintaining their shapes and withstanding external stress after implantation [285]. Second, the relatively low resolution of printed biomaterials and cells makes it hard to fabricate biomimetic constructs with fine structures at micro/nano scales. Third, the processing time of 3D bioprinting may be lengthy for manufacturing clinically relevant products, which may result in reduced cell viability.
6.4 3D assembly of large-scale cartilage constructs
Difficulties associated with scaling-up is one of the most challenging problems that might hinder the clinical utilization of engineered tissue constructs. Nutrients and other chemical factors are required to transport timely and efficiently throughout the constructs to promote sufficient cell/tissue growth. This is difficult for engineered large-scale cartilage and osteochondral tissues. Moreover, the single-block large hydrogels normally lose their original shapes after swelling in physiological conditions that would decrease their ability to withstand biomechanical loads. A novel 3D puzzle assembling technology was developed recently to manufacture engineered large-scale cartilage tissue constructs of agarose hydrogels with improved mechanical and biochemical properties (Fig. 6C) [286]. The constructs were composed of individually cultured, chondrocyte-laden interlocking smaller puzzle-shaped subunits. A 4-fold greater Young’s modulus was achieved compared with the large one-block constructs. The assembled constructs sustained large deformation under 40–50% compressive strain before failure and supported long-term in vitro maturation. Results of implantation in nude mice indicated that the constructs were biocompatible and fused well in vivo. The study opened up a new effective strategy to engineer large-scale tissue constructs. It would be possible to be combined with other techniques to manufacture structures with more complex shapes, compositions, and cell types.
7. Challenges of cell-laden hydrogel strategies for OCE and OTE
The basic biology of articular cartilage has been reviewed towards its complex structure, composition, and function [287]. As pointed out in this review referring to the literature [288–298], the 2–4 mm thick articular cartilage consists of a dense ECM with a spatial distribution of greatly specialized chondrocytes. The articular cartilage has a very complicated functional zonal structure with different cell density/morphology/organization, ECM composition/distribution, and biomechanical functions for each zone. Full thickness articular cartilage defects generally involve throughout the four zones, while osteochondral defects are involved the subchondral bone as well. Given the complexity of articular cartilage in composition, structure, and functions, there are still some key challenges to be addressed for regeneration of cartilage and osteochondral tissues.
First, formation of cartilaginous neotissues with sufficient collagen type II, proteoglycan, and other ECM components, is essential to reconstruct the cartilage and osteochondral tissues with restored functions. However, this is still difficult to accomplish with tissue engineering methods including cell-laden hydrogel strategy. A large number of chondrogenic cells are required to produce enough cartilage ECM. However, chondrocytes proliferate very slowly and over the long expansion period they are very easy to lose their phenotype and de-differentiate into fibroblasts [299]. MSC-laden engineered cartilage or osteochondral constructs may readily become hypertrophy via endochondral ossification resulting in the production of more bone-like tissues [101, 219, 300–302]. Significant efforts have been made to solve this problem. It was found that angiogenic factors including vascular endothelial growth factor (VEGF) plays a key role in endochondral ossification [303]. Excitingly, the presence of anti-angiogenic factors including chondromoduli-1, thrombospondin 1, Parathyroid hormone-related protein, gremlin-1, and frizzled-related protein were able to stabilize the chondrocyte phenotype by supporting chondrogenesis and inhibit chondrocyte hypertrophy and endochondral ossification [303–308]. In addition, co-culture of MSCs and chondrocytes reduced endochondral ossification to promote cartilage ECM formation [218, 309–311], since chondrocytes contain some chondrogenic hypertrophy inhibitory factors.
Second, it remains a challenge to regenerate cartilage and osteochondral tissues with fully restored zonal composition, structure, and functions. The physiological conditions in articular area are very complex with repeated high-loading biomechanical motion and a low oxygen atmosphere. It would be helpful to develop new biomimetic engineered cartilage tissues by combining advanced manufacturing technologies and dynamic in vitro culture mechanisms (e.g. bioreactors) that mimic the native microenvironment of articular cartilage.
Third, it is changeling to match the degradation of hydrogels with the growth of cartilage and osteochondral tissues. The degradation of hydrogels has an important effect on the tissue regeneration in vitro and in vivo. Previous studies on various hydrogels for repair of cartilage and osteochondral tissues have proved the critical role of matrices degradation rate on the function of encapsulated cells [190]. Further studies are required to find out the best degradation parameters of hydrogels for OTE and CTE.
Hydrogels fabricated from natural biopolymers and their derivatives have promising biocompatibility, biodegradability, and relatively low cost. Synthetic hydrogels allow precise control over their mechanical and biological properties [30]. These hydrogels can be injectable product, pre-formed single block, spatial functional layered constructs, 3D molded/assembled constructs and 3D printed constructs. However only relatively simple hydrogels have been used clinically for cartilage repair. Commercially available hydrogel biomaterials for CTE [312] include collagen, fibroin, and HA. PEG [183, 313] and PEG-fibroin [184, 314] hydrogels have progressed to clinical testing. There are still some barriers to the clinical translation of the more complex hydrogel based constructs. It requires special formulations to make complex hydrogel constructs with advanced biofabrication technologies. For example, appropriate bioinks are essential for 3D bioprinting. To increase the viscosity, alginate is normally incorporated into the bioinks [79, 80, 173, 266], whereas alginate inherently has low cell adhesiveness and cellular interaction ability [76, 91], which decreases the biocompatibility of the 3D printed hydrogels. And manufacture of complex hydrogel constructs takes relatively long time and the cost is much higher than simple products. To facilitate clinical translation of biofabricated complex tissue constructs, better biocompatible hydrogels are required and the manufacturing time/cost should be significantly reduced.
8. Conclusions and outlook
We have reviewed recent progresses in designing and preparing cell-laden hydrogel biomaterials for OTE and CTE applications, in terms of hydrogel types, cell sources, growth factor delivery, and advanced biofabrication technologies. During the past decade, a rich variety of hydrogels have been developed from naturally derived polymers, chemically modified natural polymers, synthetic polymers, and their combinations for regeneration of cartilage and osteochondral tissues. Some hydrogel systems were found to support the growth, spreading, and proliferation of chondrogenic/osteogenic cells, and are capable to maintain the cell morphology/phenotype. Naturally derived hydrogels are more biocompatible for higher cell viability, while chemically modified and synthetic hydrogels in general have widely tunable mechanical properties and biodegradability that are crucial for effective cartilage regeneration and clinical translation. Rationally designed composite hydrogels could thus combine the advantages of natural, modified and synthesized polymers.
Chondrocytes are successful for minor focal cartilage restoration and also promising for repair of full-thickness cartilage and osteochondral defects. However, more studies are required to address the issues of limited supply of chondrocytes from young donors and long expansion time with the potential to de-differentiate to fibroblasts. Stem cells with the capacity of chondrogenic and osteogenic differentiation are more promising due to their abundant supply sources. A variety of types of stem cells have been investigated for osteochondral and cartilage tissue engineering applications. Different types of stem cells encapsulated in hydrogels were shown to differentiate into chondrocytes or osteoblasts induced by growth factors (e.g., the family of TGF or BMP) and to promote chondrogenesis and osteogenesis in vitro and in vivo. However, engineered cartilage/osteochondral constructs seeded with stem cells may readily become hypertrophic and undergo endochondral ossification, which hinders the formation of effective functional chondrogenesis and osteochondrogenesis.
Growth factor delivery by microcarrier, covalent bonding to hydrogel network, and gene delivery are appealing strategies for localized and controlled release of differentiation inductive agents. New small-molecule bioactive factors (e.g. kartogenin [261], t-butyl methacrylate [265]) have also been developed to induce chondrogenic or osteogenic differentiation of stem cells. Advanced manufacturing techniques including microfluidic biofabrication, molding, bioprinting, and assembly have been developed to generate cell-laden hydrogel constructs with gradient composition, organized zonal architecture, and atomical geometry that mimic native osteochondral and cartilage tissues. However, it remains a great challenge to regenerate cartilage and osteochondral tissues with fully restored zonal composition, structure, and functions.
Development of hydrogels with controllable biodegradability that match the growth rate of cartilage and bone is of critical importance. It is still challenging to find out the best degradation rate of hydrogels for regeneration of different types of tissues. Due to the outstanding biocompatibility, mechanical property, osteoinductivity, injectability, and printability, hydrogel/inorganic particles/stem cell hybrid composites have attracted increasing attentions as promising tissue engineered biomaterials for repair of osteochondral and full thickness cartilage defects.
Statement of significance.
Despite tremendous advances in the field of regenerative medicine, it still remains challenging to repair the osteochondral interface and full-thickness articular cartilage defects. This inefficiency largely originates from the lack of appropriate tissue-engineered biomaterials that replace the damaged regions and promote tissue regeneration. Cell-laden hydrogel systems have been emerging as a promising tissue-engineering platform to address this issue. In this article, we describe the fundamental problems encountered in this field and review recent progress in designing cell-hydrogel constructs for promoting the reestablishment of osteochondral/cartilage tissues. Our focus centers on the effects of hydrogel composition, cell type, and growth factor delivery on achieving efficient chondrogenesis and osteogenesis. We give our perspective on developing next-generation hydrogel/inorganic particle/stem cell hybrid composites with improved physical and biological properties for osteochondral/cartilage tissue engineering. We also highlight recent advances in biomanufacturing and bioengineering technologies (e.g. 3D bioprinting) for fabrication of hydrogel-based osteochondral and cartilage constructs.
Acknowledgments
The authors acknowledge funding from the National Science Foundation (EFRI-1240443), IMMODGEL (602694), and the National Institutes of Health (EB012597, AR057837, DE021468, HL099073, AI105024, AR063745). J.Y. would like to thank the Australian Endeavour Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Sun J, Hoemann CD, Lascau-Coman V, Ouyang W, McKee MD, Shive MS, Buschmann MD. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res. 2009;27(11):1432–1438. doi: 10.1002/jor.20905. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 4.C. Centers for Disease, Prevention. National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions–United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56(1):4–7. [PubMed] [Google Scholar]
- 5.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthri Rheum. 1998;41(8):1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 7.Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 8.Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26(17):3617–3629. doi: 10.1016/j.biomaterials.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220–2233. doi: 10.2106/JBJS.J.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117–24. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 11.Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90(5):597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 12.Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, Steinwachs M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36(11):2091–2099. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- 13.Glenn RE, Jr, McCarty EC, Potter HG, Juliao SF, Gordon JD, Spindler KP. Comparison of fresh osteochondral autografts and allografts: a canine model. Am J Sports Med. 2006;34(7):1084–1093. doi: 10.1177/0363546505284846. [DOI] [PubMed] [Google Scholar]
- 14.Benazzo F, Cadossi M, Cavani F, Fini M, Giavaresi G, Setti S, Cadossi R, Giardino R. Cartilage repair with osteochondral autografts in sheep: effect of biophysical stimulation with pulsed electromagnetic fields. J Orthop Res. 2008;26(5):631–642. doi: 10.1002/jor.20530. [DOI] [PubMed] [Google Scholar]
- 15.Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91(12):2818–2826. doi: 10.2106/JBJS.I.00398. [DOI] [PubMed] [Google Scholar]
- 16.Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94(12):1105–1110. doi: 10.2106/JBJS.J.02010. [DOI] [PubMed] [Google Scholar]
- 17.Galperin A, Oldinski RA, Florczyk SJ, Bryers JD, Zhang M, Ratner BD. Integrated bi-layered scaffold for osteochondral tissue engineering. Adv Healthc Mater. 2013;2(6):872–883. doi: 10.1002/adhm.201200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26(8):583–589. doi: 10.1177/107110070502600801. [DOI] [PubMed] [Google Scholar]
- 19.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Sudkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr Cartil. 2006;14(11):1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Tuan RS. A second-generation autologous chondrocyte implantation approach to the treatment of focal articular cartilage defects. Arthri Res Ther. 2007;9(5):109. doi: 10.1186/ar2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannoni P, Pagano A, Maggi E, Arbico R, Randazzo N, Grandizio M, Cancedda R, Dozin B. Autologous chondrocyte implantation (ACI) for aged patients: development of the proper cell expansion conditions for possible therapeutic applications. Osteoarthr Cartil. 2005;13(7):589–600. doi: 10.1016/j.joca.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;(402):21–37. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Bal BS, Rahaman MN, Jayabalan P, Kuroki K, Cockrell MK, Yao JQ, Cook JL. In vivo outcomes of tissue-engineered osteochondral grafts. J Biomed Mater Res B Appl Biomater. 2010;93(1):164–174. doi: 10.1002/jbm.b.31571. [DOI] [PubMed] [Google Scholar]
- 24.Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6(2):162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 25.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv Mater. 2006;18(11):1345–1360. [Google Scholar]
- 26.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuernberger S, Cyran N, Albrecht C, Redl H, Vecsei V, Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32(4):1032–1040. doi: 10.1016/j.biomaterials.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 28.Dehne T, Karlsson C, Ringe J, Sittinger M, Lindahl A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res Ther. 2009;11(5):R133–R133. doi: 10.1186/ar2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amini AA, Nair LS. Injectable hydrogels for bone and cartilage repair. Biomed Mater. 2012;7(2):024105. doi: 10.1088/1748-6041/7/2/024105. [DOI] [PubMed] [Google Scholar]
- 30.Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev. 2011;17(4):281–99. doi: 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geckil H, Xu F, Zhang X, Moon S, Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine. 2010;5(3):469–484. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A. 2010;16(8):2675–2685. doi: 10.1089/ten.tea.2009.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD. Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009;5(6):1956–1965. doi: 10.1016/j.actbio.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 35.Kwon JS, Yoon SM, Kwon DY, Kim DY, Tai GZ, Jin LM, Song B, Lee B, Kim JH, Han DK, Min BH, Kim MS. Injectable in situ-forming hydrogel for cartilage tissue engineering. J Mater Chem B. 2013;1(26):3314–3321. doi: 10.1039/c3tb20105h. [DOI] [PubMed] [Google Scholar]
- 36.Markstedt K, Mantas A, Tournier I, Martinez Avila H, Hagg D, Gatenholm P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules. 2015;16(5):1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 37.Tritz J, Rahouadj R, de Isla N, Charif N, Pinzano A, Mainard D, Bensoussan D, Netter P, Stoltz J-F, Benkirane-Jessel N, Huselstein C. Designing a three-dimensional alginate hydrogel by spraying method for cartilage tissue engineering. Soft Matter. 2010;6(20):5165–5174. [Google Scholar]
- 38.Zeng L, Yao Y, Wang DA, Chen X. Effect of microcavitary alginate hydrogel with different pore sizes on chondrocyte culture for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;34:168–175. doi: 10.1016/j.msec.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Chung JY, Song M, Ha CW, Kim JA, Lee CH, Park YB. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res Ther. 2014;5(2):39. doi: 10.1186/scrt427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schutz K, Despang F, Lode A, Gelinsky M. Cell-laden biphasic scaffolds with anisotropic structure for the regeneration of osteochondral tissue. J Tissue Eng Regen Med. 2016;10(5):404–417. doi: 10.1002/term.1879. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater. 2004;3(4):249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 42.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr Cartil. 2006;14(2):179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Scholz B, Kinzelmann C, Benz K, Mollenhauer J, Wurst H, Schlosshauer B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur Cell Mater. 2010;20:24–36. doi: 10.22203/ecm.v020a03. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira JT, Santos TC, Martins L, Picciochi R, Marques AP, Castro AG, Neves NM, Mano JF, Reis RL. Gellan gum injectable hydrogels for cartilage tissue engineering applications: in vitro studies and preliminary in vivo evaluation. Tissue Eng Part A. 2010;16(1):343–353. doi: 10.1089/ten.TEA.2009.0117. [DOI] [PubMed] [Google Scholar]
- 45.Yamaoka H, Asato H, Ogasawara T, Nishizawa S, Takahashi T, Nakatsuka T, Koshima I, Nakamura K, Kawaguchi H, Chung UI, Takato T, Hoshi K. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. J Biomed Mater Res A. 2006;78(1):1–11. doi: 10.1002/jbm.a.30655. [DOI] [PubMed] [Google Scholar]
- 46.Yuan T, Zhang L, Li K, Fan H, Fan Y, Liang J, Zhang X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2014;102(2):337–344. doi: 10.1002/jbm.b.33011. [DOI] [PubMed] [Google Scholar]
- 47.Cheng HW, Luk KD, Cheung KM, Chan BP. In vitro generation of an osteochondral interface from mesenchymal stem cell-collagen microspheres. Biomaterials. 2011;32(6):1526–1535. doi: 10.1016/j.biomaterials.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 48.Chao PH, Yodmuang S, Wang X, Sun L, Kaplan DL, Vunjak-Novakovic G. Silk hydrogel for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;95(1):84–90. doi: 10.1002/jbm.b.31686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eyrich D, Brandl F, Appel B, Wiese H, Maier G, Wenzel M, Staudenmaier R, Goepferich A, Blunk T. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28(1):55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 50.Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, Feijen J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2544–2551. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Choi B, Kim S, Lin B, Wu BM, Lee M. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl Mater Interfaces. 2014;6(22):20110–20121. doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 52.Mazaki T, Shiozaki Y, Yamane K, Yoshida A, Nakamura M, Yoshida Y, Zhou D, Kitajima T, Tanaka M, Ito Y, Ozaki T, Matsukawa A. A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci Rep. 2014;4:4457. doi: 10.1038/srep04457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan P, Pan Z, Cao L, He Y, Wang H, Qu Z, Dong J, Ding J. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J Biomed Mater Res A. 2014;102(1):180–192. doi: 10.1002/jbm.a.34683. [DOI] [PubMed] [Google Scholar]
- 54.Snyder TN, Madhavan K, Intrator M, Dregalla RC, Park D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng. 2014;8:10. doi: 10.1186/1754-1611-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2011;32(34):8771–8782. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohamad N, Mohd Amin MC, Pandey M, Ahmad N, Rajab NF. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: accelerated burn wound healing in an animal model. Carbohydr Polym. 2014;114:312–320. doi: 10.1016/j.carbpol.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 57.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10(3–4):515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 58.Munoz-Pinto DJ, McMahon RE, Kanzelberger MA, Jimenez-Vergara AC, Grunlan MA, Hahn MS. Inorganic-organic hybrid scaffolds for osteochondral regeneration. J Biomed Mater Res A. 2010;94(1):112–121. doi: 10.1002/jbm.a.32695. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, Chen J, Tao J, Hu C, Chen L, Zhao H, Xu G, Heng BC, Ouyang HW. The promotion of osteochondral repair by combined intra-articular injection of parathyroid hormone-related protein and implantation of a bi-layer collagen-silk scaffold. Biomaterials. 2013;34(25):6046–6057. doi: 10.1016/j.biomaterials.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 60.Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. doi: 10.1038/srep03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen QT, Hwang Y, Chen AC, Varghese S, Sah RL. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials. 2012;33(28):6682–90. doi: 10.1016/j.biomaterials.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu SQ, Tian Q, Hedrick JL, Po Hui JH, Ee PL, Yang YY. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials. 2010;31(28):7298–7307. doi: 10.1016/j.biomaterials.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Klein TJ, Rizzi SC, Schrobback K, Reichert JC, Jeon JE, Crawford RW, Hutmacher DW. Long-term effects of hydrogel properties on human chondrocyte behavior. Soft Matter. 2010;6(20):5175–5183. [Google Scholar]
- 64.Liu SQ, Tay R, Khan M, Rachel Ee PL, Hedrick JL, Yang YY. Synthetic hydrogels for controlled stem cell differentiation. Soft Matter. 2010;6(1):67–81. [Google Scholar]
- 65.Leone G, Volpato MD, Nelli N, Lamponi S, Boanini E, Bigi A, Magnani A. Continuous multilayered composite hydrogel as osteochondral substitute. J Biomed Mater Res A. 2015;103(8):2521–2530. doi: 10.1002/jbm.a.35389. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Wang Z, Xu C, Li Y, Gao J, Wang W, Liu Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. Journal of Materials Chemistry. 2011;21(28):10399–10406. [Google Scholar]
- 67.Bonakdar S, Emami SH, Shokrgozar MA, Farhadi A, Ahmadi SAH, Amanzadeh A. Preparation and characterization of polyvinyl alcohol hydrogels crosslinked by biodegradable polyurethane for tissue engineering of cartilage. Mater Sci Eng C. 2010;30(4):636–643. [Google Scholar]
- 68.Kobayashi M, Chang YS, Oka M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials. 2005;26(16):3243–3248. doi: 10.1016/j.biomaterials.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 69.Ma R, Xiong D, Miao F, Zhang J, Peng Y. Novel PVP/PVA hydrogels for articular cartilage replacement. Mater Sci Eng C. 2009;29(6):1979–1983. [Google Scholar]
- 70.Grant C, Twigg P, Egan A, Moody A, Smith A, Eagland D, Crowther N, Britland S. Poly(vinyl alcohol) hydrogel as a biocompatible viscoelastic mimetic for articular cartilage. Biotechnol Prog. 2006;22(5):1400–1406. doi: 10.1021/bp060181u. [DOI] [PubMed] [Google Scholar]
- 71.Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, Tabata Y, Kasper FK, Mikos AG, Jansen JA. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6(1):39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75(1):156–167. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 73.Yasuda K, Kitamura N, Gong JP, Arakaki K, Kwon HJ, Onodera S, Chen YM, Kurokawa T, Kanaya F, Ohmiya Y, Osada Y. A novel double-network hydrogel induces spontaneous articular cartilage regeneration in vivo in a large osteochondral defect. Macromol Biosci. 2009;9(4):307–316. doi: 10.1002/mabi.200800223. [DOI] [PubMed] [Google Scholar]
- 74.Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pelletier PHS, Payan E, Marchal P, Choplin L, Dellacherie E. Amphiphilic derivatives of sodium alginate and hyaluronate for cartilage repair: Rheological properties. J Biomed Mater Res A. 2000;54(1):102–108. doi: 10.1002/1097-4636(200101)54:1<102::aid-jbm12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto T, Suzuki Y, Tanihara M, Kakimaru Y, Suzuki K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials. 2004;25(7–8):1407–1414. doi: 10.1016/j.biomaterials.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 77.Ivanovska J, Zehnder T, Lennert P, Sarker B, Boccaccini AR, Hartmann A, Schneider-Stock R, Detsch R. Biofabrication of 3D Alginate-Based Hydrogel for Cancer Research: Comparison of Cell Spreading, Viability, and Adhesion Characteristics of Colorectal HCT116 Tumor Cells. Tissue Eng Part C Method. 2016;22(7):708–715. doi: 10.1089/ten.TEC.2015.0452. [DOI] [PubMed] [Google Scholar]
- 78.Schütz K, Placht A-M, Paul B, Brüggemeier S, Gelinsky M, Lode A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.2058. [DOI] [PubMed] [Google Scholar]
- 79.Dreher R, Starly B. Biofabrication of Multimaterial Three-Dimensional Constructs Embedded With Patterned Alginate Strands Encapsulating PC12 Neural Cell Lines. Nanotech Engineer Med. 2015;6(2):021004. [Google Scholar]
- 80.Ruitong X, Zhengyi Z, Wenxuan C, Yong H, Douglas BC. Freeform drop-on-demand laser printing of 3D alginate and cellular constructs. Biofabrication. 2015;7(4):045011. doi: 10.1088/1758-5090/7/4/045011. [DOI] [PubMed] [Google Scholar]
- 81.Tellechea A, Silva EA, Min J, Leal EC, Auster ME, Pradhan-Nabzdyk L, Shih W, Mooney DJ, Veves A. Alginate and DNA Gels Are Suitable Delivery Systems for Diabetic Wound Healing. Int J Low Extrem Wounds. 2015;14(2):146–153. doi: 10.1177/1534734615580018. [DOI] [PubMed] [Google Scholar]
- 82.Chan G, Mooney DJ. Ca(2+) released from calcium alginate gels can promote inflammatory responses in vitro and in vivo. Acta Biomater. 2013;9(12):9281–9291. doi: 10.1016/j.actbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shea LD, Wang D, Franceschi RT, Mooney DJ. Engineered bone development from a pre-osteoblast cell line on three-dimensional scaffolds. Tissue Eng. 2000;6(6):605–617. doi: 10.1089/10763270050199550. [DOI] [PubMed] [Google Scholar]
- 84.Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials. 2007;28(30):4409–4417. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 85.Fonseca KB, Gomes DB, Lee K, Santos SG, Sousa A, Silva EA, Mooney DJ, Granja PL, Barrias CC. Injectable MMP-sensitive alginate hydrogels as hMSC delivery systems. Biomacromolecules. 2014;15(1):380–390. doi: 10.1021/bm4016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verbeke CS, Mooney DJ. Injectable, Pore-Forming Hydrogels for In Vivo Enrichment of Immature Dendritic Cells. Adv Healthc Mater. 2015;4(17):2677–2687. doi: 10.1002/adhm.201500618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Darnell MC, Sun JY, Mehta M, Johnson C, Arany PR, Suo Z, Mooney DJ. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials. 2013;34(33):8042–8048. doi: 10.1016/j.biomaterials.2013.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Desai RM, Koshy ST, Hilderbrand SA, Mooney DJ, Joshi NS. Versatile click alginate hydrogels crosslinked via tetrazine-norbornene chemistry. Biomaterials. 2015;50:30–37. doi: 10.1016/j.biomaterials.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 89.Zeng Q, Han Y, Li H, Chang J. Bioglass/alginate composite hydrogel beads as cell carriers for bone regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(1):42–51. doi: 10.1002/jbm.b.32978. [DOI] [PubMed] [Google Scholar]
- 90.Vallée F, Müller C, Durand A, Schimchowitsch S, Dellacherie E, Kelche C, Cassel JC, Leonard M. Synthesis and rheological properties of hydrogels based on amphiphilic alginate-amide derivatives. Carbohydr Res. 2009;344(2):223–228. doi: 10.1016/j.carres.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 91.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive Alginate Hydrogels for Bone Tissue Engineering. J Den Res. 2001;80(11):2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 92.Sakai S, Hirose K, Moriyama K, Kawakami K. Control of cellular adhesiveness in an alginate-based hydrogel by varying peroxidase and H2O2 concentrations during gelation. Acta Biomaterialia. 2010;6(4):1446–1452. doi: 10.1016/j.actbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Lee KY, Kong HJ, Mooney DJ. Quantifying Interactions between Cell Receptors and Adhesion Ligand-Modified Polymers in Solution. Macromol Biosci. 2008;8(2):140–145. doi: 10.1002/mabi.200700169. [DOI] [PubMed] [Google Scholar]
- 94.Zhao W, Jin X, Cong Y, Liu Y, Fu J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J Chem Technol Biotechnol. 2013;88(3):327–339. [Google Scholar]
- 95.Awad HA, Quinn Wickham M, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25(16):3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 96.Lima EG, Tan AR, Tai T, Bian L, Ateshian GA, Cook JL, Hung CT. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J Biomech. 2008;41(15):3253–3259. doi: 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, Hung CT. Dynamic Mechanical Loading Enhances Functional Properties of Tissue-Engineered Cartilage Using Mature Canine Chondrocytes. Tissue Eng Part A. 2009;16(5):1781–1790. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of Seeding Density and Dynamic Deformational Loading on the Developing Structure/Function Relationships of Chondrocyte-Seeded Agarose Hydrogels. Ann Biomed Eng. 2002;30(8):1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 99.Kelly T-AN, Ng KW, Wang CCB, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39(8):1489–1497. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 100.Román J, Cabañas MV, Peña J, Doadrio JC, Vallet-Regí M. An optimized β-tricalcium phosphate and agarose scaffold fabrication technique. J Biomed Mater Res A. 2008;84A(1):99–107. doi: 10.1002/jbm.a.31394. [DOI] [PubMed] [Google Scholar]
- 101.Sheehy EJ, Mesallati T, Vinardell T, Kelly DJ. Engineering cartilage or endochondral bone: A comparison of different naturally derived hydrogels. Acta Biomater. 2015;13:245–253. doi: 10.1016/j.actbio.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 102.Sheehy EJ, Buckley CT, Kelly DJ. Chondrocytes and bone marrow-derived mesenchymal stem cells undergoing chondrogenesis in agarose hydrogels of solid and channelled architectures respond differentially to dynamic culture conditions. J Tissue Eng and Regen Med. 2011;5(9):747–758. doi: 10.1002/term.385. [DOI] [PubMed] [Google Scholar]
- 103.Charles Huang CY, Reuben PM, D’Ippolito G, Schiller PC, Cheung HS. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Bio. 2004;278A(1):428–436. doi: 10.1002/ar.a.20010. [DOI] [PubMed] [Google Scholar]
- 104.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential Maturation and Structure–Function Relationships in Mesenchymal Stem Cell- and Chondrocyte-Seeded Hydrogels. Tissue Eng Part A. 2009;15(5):1041–1052. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Watanabe J, Kashii M, Hirao M, Oka K, Sugamoto K, Yoshikawa H, Akashi M. Quick-forming hydroxyapatite/agarose gel composites induce bone regeneration. J Biomed Mater Res A. 2007;83(3):845–852. doi: 10.1002/jbm.a.31435. [DOI] [PubMed] [Google Scholar]
- 106.Puértolas JA, Vadillo JL, Sánchez-Salcedo S, Nieto A, Gómez-Barrena E, Vallet-Regí M. Compression behaviour of biphasic calcium phosphate and biphasic calcium phosphate–agarose scaffolds for bone regeneration. Acta Biomater. 2011;7(2):841–847. doi: 10.1016/j.actbio.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 107.Forget A, Christensen J, Lüdeke S, Kohler E, Tobias S, Matloubi M, Thomann R, Shastri VP. Polysaccharide hydrogels with tunable stiffness and provasculogenic properties via α-helix to β-sheet switch in secondary structure. PNAS. 2013;110(32):12887–12892. doi: 10.1073/pnas.1222880110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rinaudo M. Chitin and chitosan: Properties and applications. Prog Polym Sci. 2006;31(7):603–632. [Google Scholar]
- 109.Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49(4):780–792. [Google Scholar]
- 110.Kim I-Y, Seo S-J, Moon H-S, Yoo M-K, Park I-Y, Kim B-C, Cho C-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26(1):1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 111.Riva R, Ragelle H, des Rieux A, Duhem N, Jérôme C, Préat V. Chitosan and Chitosan Derivatives in Drug Delivery and Tissue Engineering. In: Jayakumar R, Prabaharan M, Muzzarelli ARA, editors. Chitosan for Biomaterials II. Springer Berlin Heidelberg; Berlin, Heidelberg: 2011. pp. 19–44. [Google Scholar]
- 112.Yamane S, Iwasaki N, Majima T, Funakoshi T, Masuko T, Harada K, Minami A, Monde K, Nishimura S-i. Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterials. 2005;26(6):611–619. doi: 10.1016/j.biomaterials.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 113.Hsu S-h, Whu SW, Hsieh S-C, Tsai C-L, Chen DC, Tan T-S. Evaluation of Chitosan-alginate-hyaluronate Complexes Modified by an RGD-containing Protein as Tissue-engineering Scaffolds for Cartilage Regeneration. Artif Organs. 2004;28(8):693–703. doi: 10.1111/j.1525-1594.2004.00046.x. [DOI] [PubMed] [Google Scholar]
- 114.Waibel KH, Haney B, Moore M, Whisman B, Gomez R. Safety of Chitosan Bandages in Shellfish Allergic Patients. Military Medicine. 2011;176(10):1153–1156. doi: 10.7205/milmed-d-11-00150. [DOI] [PubMed] [Google Scholar]
- 115.Liu X, Ma L, Mao Z, Gao C. Chitosan-Based Biomaterials for Tissue Repair and Regeneration. In: Jayakumar R, Prabaharan M, Muzzarelli ARA, editors. Chitosan for Biomaterials II. Springer Berlin Heidelberg; Berlin, Heidelberg: 2011. pp. 81–127. [Google Scholar]
- 116.Mourya VK, Inamdar NN. Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mater Med. 2008;20(5):1057–1079. doi: 10.1007/s10856-008-3659-z. [DOI] [PubMed] [Google Scholar]
- 117.Masuko T, Iwasaki N, Yamane S, Funakoshi T, Majima T, Minami A, Ohsuga N, Ohta T, Nishimura S-I. Chitosan–RGDSGGC conjugate as a scaffold material for musculoskeletal tissue engineering. Biomaterials. 2005;26(26):5339–5347. doi: 10.1016/j.biomaterials.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 118.Donati I, Stredanska S, Silvestrini G, Vetere A, Marcon P, Marsich E, Mozetic P, Gamini A, Paoletti S, Vittur F. The aggregation of pig articular chondrocyte and synthesis of extracellular matrix by a lactose-modified chitosan. Biomaterials. 2005;26(9):987–998. doi: 10.1016/j.biomaterials.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 119.Colnot C, Sidhu SS, Poirier F, Balmain N. Cellular and subcellular distribution of galectin-3 in the epiphyseal cartilage and bone of fetal and neonatal mice. Cell Mol Biol (Noisy-le-grand) 1999;45(8):1191–1202. [PubMed] [Google Scholar]
- 120.Tan H, Lao L, Wu J, Gong Y, Gao C. Biomimetic modification of chitosan with covalently grafted lactose and blended heparin for improvement of in vitro cellular interaction. Polym Adv Technol. 2008;19(1):15–23. [Google Scholar]
- 121.Knudson CB. Hyaluronan and CD44: Strategic players for cell–matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res C Embryo Today. 2003;69(2):174–196. doi: 10.1002/bdrc.10013. [DOI] [PubMed] [Google Scholar]
- 122.Akmal M, Singh A, Anand A, Kesani A, Aslam N, Goodship A, Bentley G. The effects of hyaluronic acid on articular chondrocytes. J Bone Joint Surg Br. 2005;87-B(8):1143–1149. doi: 10.1302/0301-620X.87B8.15083. [DOI] [PubMed] [Google Scholar]
- 123.Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res A. 2006;77(3):518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barbucci R, Lamponi S, Borzacchiello A, Ambrosio L, Fini M, Torricelli P, Giardino R. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials. 2002;23(23):4503–4513. doi: 10.1016/s0142-9612(02)00194-1. [DOI] [PubMed] [Google Scholar]
- 125.Kang JY, Chung CW, Sung J-H, Park B-S, Choi J-Y, Lee SJ, Choi B-C, Shim C-K, Chung S-J, Kim D-D. Novel porous matrix of hyaluronic acid for the three-dimensional culture of chondrocytes. Int J Pharm. 2009;369(1–2):114–120. doi: 10.1016/j.ijpharm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 126.Aulin C, Bergman K, Jensen-Waern M, Hedenqvist P, Hilborn J, Engstrand T. In situ cross-linkable hyaluronan hydrogel enhances chondrogenesis. J Tissue Eng Regen Med. 2011;5(8):188–196. doi: 10.1002/term.415. [DOI] [PubMed] [Google Scholar]
- 127.Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, Mauck RL. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012;8(8):3027–3034. doi: 10.1016/j.actbio.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Erickson IE, Kestle SR, Zellars KH, Dodge GR, Burdick JA, Mauck RL. Improved cartilage repair via in vitro pre-maturation of MSC-seeded hyaluronic acid hydrogels. Biomed Mater. 2012;7(2):024110. doi: 10.1088/1748-6041/7/2/024110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15(2):243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2012;18(7–8):715–24. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Unterman SA, Gibson M, Lee JH, Crist J, Chansakul T, Yang EC, Elisseeff JH. Hyaluronic acid-binding scaffold for articular cartilage repair. Tissue Eng Part A. 2012;18(23–24):2497–2506. doi: 10.1089/ten.tea.2011.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32(27):6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Toh WS, Lee EH, Guo XM, Chan JK, Yeow CH, Choo AB, Cao T. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31(27):6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 134.Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable Hyaluronan as a Scaffold for Articular Cartilage Repair. Ann Biomed Eng. 2004;32(3):391–397. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 135.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled Degradation and Mechanical Behavior of Photopolymerized Hyaluronic Acid Networks. Biomacromolecules. 2005;6(1):386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fenn SL, Oldinski RA. Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair. J Biomed Mater Res B Appl Biomater. 2016;104(6):1229–1236. doi: 10.1002/jbm.b.33476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oliveira JT, Martins L, Picciochi R, Malafaya PB, Sousa RA, Neves NM, Mano JF, Reis RL. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J Biomed Mater Res Part A. 2010;93(3):852–863. doi: 10.1002/jbm.a.32574. [DOI] [PubMed] [Google Scholar]
- 138.Emako Miyoshi TT, Nishinari Katsuyoshi. Rheological and thermal studies of gel-sol transition in Gellan gumaqueous solutions. Carbohydrate Polymers. 1996;30(2–3):109–119. [Google Scholar]
- 139.Coutinho DF, Sant SV, Shin H, Oliveira JT, Gomes ME, Neves NM, Khademhosseini A, Reis RL. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials. 2010;31(29):7494–7502. doi: 10.1016/j.biomaterials.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Du H, Hamilton P, Reilly M, Ravi N. Injectable in situ Physically and Chemically Crosslinkable Gellan Hydrogel. Macromol Biosci. 2012;12(7):952–961. doi: 10.1002/mabi.201100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsukawa S, Huang Z, Watanabe T. Structural change of polymer chains of gellan monitored by circular dichroism. In: Nishinari K, editor. Physical Chemistry and Industrial Application of Gellan Gum. Springer Berlin Heidelberg; Berlin, Heidelberg: 1999. pp. 92–97. [Google Scholar]
- 142.Nitta Y, Takahashi R, Nishinari K. Viscoelasticity and Phase Separation of Aqueous Na-Type Gellan Solution. Biomacromolecules. 2010;11(1):187–191. doi: 10.1021/bm901063k. [DOI] [PubMed] [Google Scholar]
- 143.Oliveira JT, Santos TC, Martins L, Silva MA, Marques AP, Castro AG, Neves NM, Reis RL. Performance of new gellan gum hydrogels combined with human articular chondrocytes for cartilage regeneration when subcutaneously implanted in nude mice. J Tissue Eng Regen Med. 2009;3(7):493–500. doi: 10.1002/term.184. [DOI] [PubMed] [Google Scholar]
- 144.Smith AM, Shelton R, Perrie Y, Harris JJ. An Initial Evaluation of Gellan Gum as a Material for Tissue Engineering Applications. J Biomater Appl. 2007;22(3):241–254. doi: 10.1177/0885328207076522. [DOI] [PubMed] [Google Scholar]
- 145.Ogawa E, Takahashi R, Yajima H, Nishinari K. Effects of molar mass on the coil to helix transition of sodium-type gellan gums in aqueous solutions. Food Hydrocoll. 2006;20(2–3):378–385. [Google Scholar]
- 146.Fan J, Gong Y, Ren L, Varshney RR, Cai D, Wang DA. In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater. 2010;6(3):1178–1185. doi: 10.1016/j.actbio.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 147.Douglas TEL, Krawczyk G, Pamula E, Declercq HA, Schaubroeck D, Bucko MM, Balcaen L, Van Der Voort P, Bliznuk V, van den Vreken NMF, Dash M, Detsch R, Boccaccini AR, Vanhaecke F, Cornelissen M, Dubruel P. Generation of composites for bone tissue-engineering applications consisting of gellan gum hydrogels mineralized with calcium and magnesium phosphate phases by enzymatic means. J Tissue Eng Regen Med. 2016;10(11):938–954. doi: 10.1002/term.1875. [DOI] [PubMed] [Google Scholar]
- 148.Timothy ELD, Wojciech P, Elzbieta P, Jana L, David S, Sander CGL, Gilles B, Lieve B, Rainer D, Heidi D, Katarzyna CK, Agnieszka D, Vincent MJIC, Frank V, Ria C, Tom C, Aldo RB, Peter D. Injectable self-gelling composites for bone tissue engineering based on gellan gum hydrogel enriched with different bioglasses. Biomed Mater. 2014;9(4):045014. doi: 10.1088/1748-6041/9/4/045014. [DOI] [PubMed] [Google Scholar]
- 149.Gantar A, da Silva LP, Oliveira JM, Marques AP, Correlo VM, Novak S, Reis RL. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;43:27–36. doi: 10.1016/j.msec.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 150.Tang Y, Sun J, Fan H, Zhang X. An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation. Carbohydr Polym. 2012;88(1):46–53. [Google Scholar]
- 151.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15(4):499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 152.Gong Y, Wang C, Lai RC, Su K, Zhang F, Wang D-a. An improved injectable polysaccharide hydrogel: modified gellan gum for long-term cartilage regenerationin vitro. Jo Mater Chem. 2009;19(14):1968–1977. [Google Scholar]
- 153.Lee H, Fisher S, Kallos MS, Hunter CJ. Optimizing gelling parameters of gellan gum for fibrocartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2011;98(2):238–245. doi: 10.1002/jbm.b.31845. [DOI] [PubMed] [Google Scholar]
- 154.Silva-Correia J, Gloria A, Oliveira MB, Mano JF, Oliveira JM, Ambrosio L, Reis RL. Rheological and mechanical properties of acellular and cell-laden methacrylated gellan gum hydrogels. J Biomed Mater Res A. 2013;101(12):3438–3446. doi: 10.1002/jbm.a.34650. [DOI] [PubMed] [Google Scholar]
- 155.Shin H, Olsen BD, Khademhosseini A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials. 2012;33(11):3143–3152. doi: 10.1016/j.biomaterials.2011.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Silva-Correia J, Zavan B, Vindigni V, Silva TH, Oliveira JM, Abatangelo G, Reis RL. Biocompatibility Evaluation of Ionic- and Photo-Crosslinked Methacrylated Gellan Gum Hydrogels: In Vitro and In Vivo Study. Adv Healthc Mater. 2013;2(4):568–575. doi: 10.1002/adhm.201200256. [DOI] [PubMed] [Google Scholar]
- 157.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marquass B, Somerson JS, Hepp P, Aigner T, Schwan S, Bader A, Josten C, Zscharnack M, Schulz RM. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J Orthop Res. 2010;28(12):1586–1599. doi: 10.1002/jor.21173. [DOI] [PubMed] [Google Scholar]
- 159.Pulkkinen HJ, Tiitu V, Valonen P, Jurvelin JS, Lammi MJ, Kiviranta I. Engineering of cartilage in recombinant human type II collagen gel in nude mouse model in vivo. Osteoarthr Cartil. 2010;18(8):1077–1087. doi: 10.1016/j.joca.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 160.Ren X, Wang F, Chen C, Gong X, Yin L, Yang L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet Disord. 2016;17(1):1–10. doi: 10.1186/s12891-016-1130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93(6):1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 162.Suri S, Schmidt CE. Photopatterned collagen–hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 2009;5(7):2385–2397. doi: 10.1016/j.actbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 163.Rafat M, Li F, Fagerholm P, Lagali NS, Watsky MA, Munger R, Matsuura T, Griffith M. PEG-stabilized carbodiimide crosslinked collagen–chitosan hydrogels for corneal tissue engineering. Biomaterials. 2008;29(29):3960–3972. doi: 10.1016/j.biomaterials.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 164.Guoping Chen TS. Takashi Ushida, Naoyuki Ochiai, Tetsuya Tateishi, Tissue Engineering of Cartilage Using a Hybrid Scaffold of Synthetic Polymer and Collagen. Tissue Eng. 2004;10(3–4):323–330. doi: 10.1089/107632704323061681. [DOI] [PubMed] [Google Scholar]
- 165.Shinichi Ibusuki AP, Ranka Milan P, Halbesma Gertjan J, Randolph Mark A, Redmond Robert W, Kochevar Irene E, Gill Thomas J. Engineering Cartilage in a Photochemically Crosslinked Collagen Gel. J Knee Surg. 2009;22(1):72–81. doi: 10.1055/s-0030-1247729. [DOI] [PubMed] [Google Scholar]
- 166.Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials. 2009;30(20):3371–3377. doi: 10.1016/j.biomaterials.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 167.Koshy ST, Desai RM, Joly P, Li J, Bagrodia RK, Lewin SA, Joshi NS, Mooney DJ. Click-Crosslinked Injectable Gelatin Hydrogels. Adv Healthc Mater. 2016;5(5):541–547. doi: 10.1002/adhm.201500757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Loessner D, Meinert C, Kaemmerer E, Martine LC, Yue K, Levett PA, Klein TJ, Melchels FPW, Khademhosseini A, Hutmacher DW. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat Protocols. 2016;11(4):727–746. doi: 10.1038/nprot.2016.037. [DOI] [PubMed] [Google Scholar]
- 169.Schuurman W, Levett PA, Pot MW, van Weeren PR, Dhert WJA, Hutmacher DW, Melchels FPW, Klein TJ, Malda J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs. Macromol Biosci. 2013;13(5):551–561. doi: 10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 170.Vivian HMM, Ferry PWM, Jetze V, Wouter JAD, Debby G, Jos M. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication. 2016;8(3):035003. doi: 10.1088/1758-5090/8/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Visser J, Levett PA, te Moller NCR, Besems J, Boere KWM, van Rijen MHP, de Grauw JC, Dhert WJA, van Weeren PR, Malda J. Crosslinkable Hydrogels Derived from Cartilage, Meniscus, and Tendon Tissue. Tissue Eng Part A. 2015;21(7–8):1195–1206. doi: 10.1089/ten.tea.2014.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.O KC, Ravin AG, Levin LS, Usala A-L, Klitzman B. Long- and short-term effects of biological hydrogels on capsule microvascular density around implants in rats. J Biomed Mater Res A. 2001;58(3):313–318. doi: 10.1002/1097-4636(2001)58:3<313::aid-jbm1023>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 173.Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yodmuang S, McNamara SL, Nover AB, Mandal BB, Agarwal M, Kelly T-AN, Chao P-hG, Hung C, Kaplan DL, Vunjak-Novakovic G. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015;11:27–36. doi: 10.1016/j.actbio.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4):457–470. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 176.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29(8):1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wu X, Hou J, Li M, Wang J, Kaplan DL, Lu S. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin. Acta Biomater. 2012;8(6):2185–2192. doi: 10.1016/j.actbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 178.Yucel T, Cebe P, Kaplan DL. Vortex-Induced Injectable Silk Fibroin Hydrogels. Biophys J. 2009;97(7):2044–2050. doi: 10.1016/j.bpj.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Murphy AR, John PS, Kaplan DL. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials. 2008;29(19):2829–2838. doi: 10.1016/j.biomaterials.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Murphy AR, Kaplan DL. Biomedical applications of chemically-modified silk fibroin. J Mater Chem. 2009;19(36):6443–6450. doi: 10.1039/b905802h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kambe Y, Yamamoto K, Kojima K, Tamada Y, Tomita N. Effects of RGDS sequence genetically interfused in the silk fibroin light chain protein on chondrocyte adhesion and cartilage synthesis. Biomaterials. 2010;31(29):7503–7511. doi: 10.1016/j.biomaterials.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 182.Steinmetz NJ, Aisenbrey EA, Westbrook KK, Qi HJ, Bryant SJ. Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering. Acta Biomater. 2015;21:142–153. doi: 10.1016/j.actbio.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 183.Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B, Coburn J, Hui AY, Marcus N, Gold GE, Elisseeff JH. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5(167):167ra6. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goldshmid R, Cohen S, Shachaf Y, Kupershmit I, Sarig-Nadir O, Seliktar D, Wechsler R. Steric Interference of Adhesion Supports In-Vitro Chondrogenesis of Mesenchymal Stem Cells on Hydrogels for Cartilage Repair. Sci Rep. 2015;5:12607. doi: 10.1038/srep12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang W, Li B, Yang J, Xin L, Li Y, Yin H, Qi Y, Jiang Y, Ouyang H, Gao C. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials. 2010;31(34):8964–8973. doi: 10.1016/j.biomaterials.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 186.Kim K, Lam J, Lu S, Spicer PP, Lueckgen A, Tabata Y, Wong ME, Jansen JA, Mikos AG, Kasper FK. Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J Control Release. 2013;168(2):166–178. doi: 10.1016/j.jconrel.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28(21):3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 189.Martens PJ, Bryant SJ, Anseth KS. Tailoring the Degradation of Hydrogels Formed from Multivinyl Poly(ethylene glycol) and Poly(vinyl alcohol) Macromers for Cartilage Tissue Engineering. Biomacromolecules. 2003;4(2):283–292. doi: 10.1021/bm025666v. [DOI] [PubMed] [Google Scholar]
- 190.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30(26):4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Danielle ARD, Benoit SW, Anseth Kristi S. Manipulations in Hydrogel Degradation Behavior Enhance Osteoblast Function and Mineralized Tissue Formation. Tissue Eng. 2006;12(6):1663–1673. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 192.Holloway JL, Ma H, Rai R, Burdick JA. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J Control Release. 2014;191:63–70. doi: 10.1016/j.jconrel.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. Biotechnol Prog. 2001;17(5):945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 194.Roberts JJ, Nicodemus GD, Greenwald EC, Bryant SJ. Degradation Improves Tissue Formation in (Un)Loaded Chondrocyte-laden Hydrogels. Clin Orthop Relat Res. 2011;469(10):2725–2734. doi: 10.1007/s11999-011-1823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mario Ferretti KGM, Kobayashi Kenji, Defail Alicia J, Chu Constance R. Controlled in Vivo Degradation of Genipin Crosslinked Polyethylene Glycol Hydrogels within Osteochondral Defects. Tissue Eng. 2006;12(9):2657–2663. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 196.Ko CY, Ku KL, Yang SR, Lin TY, Peng S, Peng YS, Cheng MH, Chu IM. In vitro and in vivo co-culture of chondrocytes and bone marrow stem cells in photocrosslinked PCL-PEG-PCL hydrogels enhances cartilage formation. J Tissue Eng Regen Med. 2016;10(10):485–496. doi: 10.1002/term.1846. [DOI] [PubMed] [Google Scholar]
- 197.Ko JY, Kim KI, Park S, Im GI. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials. 2014;35(11):3571–3581. doi: 10.1016/j.biomaterials.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 198.Chen K, Ng KS, Ravi S, Goh JC, Toh SL. In vitro generation of whole osteochondral constructs using rabbit bone marrow stromal cells, employing a two-chambered co-culture well design. J Tissue Eng Regen Med. 2016;10(4):294–304. doi: 10.1002/term.1716. [DOI] [PubMed] [Google Scholar]
- 199.Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444(7117):364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 200.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 201.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 202.Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol Lett. 2009;31(12):1817–1824. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 203.Zouani OF, Chollet C, Guillotin B, Durrieu MC. Differentiation of pre-osteoblast cells on poly(ethylene terephthalate) grafted with RGD and/or BMPs mimetic peptides. Biomaterials. 2010;31(32):8245–8253. doi: 10.1016/j.biomaterials.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 204.Bergeron E, Marquis ME, Chretien I, Faucheux N. Differentiation of preosteoblasts using a delivery system with BMPs and bioactive glass microspheres. J Mater Sci Mater Med. 2007;18(2):255–263. doi: 10.1007/s10856-006-0687-4. [DOI] [PubMed] [Google Scholar]
- 205.Benoit DS, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12(6):1663–1673. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 206.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23(22):4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 207.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34(6):747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Krampera M, Pizzolo G, Aprili G, Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39(4):678–683. doi: 10.1016/j.bone.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 210.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 211.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 212.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 213.Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, Kaunas R, Gaharwar AK. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano. 2015;9(3):3109–3118. doi: 10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- 214.Lu CH, Yeh TS, Yeh CL, Fang YH, Sung LY, Lin SY, Yen TC, Chang YH, Hu YC. Regenerating cartilages by engineered ASCs: prolonged TGF-beta3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol Ther. 2014;22(1):186–195. doi: 10.1038/mt.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12(9):2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 216.Lam J, Lu S, Meretoja VV, Tabata Y, Mikos AG, Kasper FK. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater. 2014;10(3):1112–1123. doi: 10.1016/j.actbio.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Visser J, Gawlitta D, Benders KE, Toma SM, Pouran B, van Weeren PR, Dhert WJ, Malda J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials. 2015;37:174–182. doi: 10.1016/j.biomaterials.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 218.Sheehy EJ, Vinardell T, Buckley CT, Kelly DJ. Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater. 2013;9(3):5484–5492. doi: 10.1016/j.actbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 219.Sheehy EJ, Mesallati T, Kelly L, Vinardell T, Buckley CT, Kelly DJ. Tissue Engineering Whole Bones Through Endochondral Ossification: Regenerating the Distal Phalanx. BioRes Open Access. 2015;4(1):229–241. doi: 10.1089/biores.2015.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19(1 Suppl):1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 221.Roberts AB, Frolik CA, Anzano MA, Sporn MB. Transforming growth factors from neoplastic and nonneoplastic tissues. Fed Proc. 1983;42(9):2621–2626. [PubMed] [Google Scholar]
- 222.Miller DA, Pelton RW, Derynck R, Moses HL. Transforming growth factor-beta. A family of growth regulatory peptides. Ann N Y Acad Sci. 1990;593:208–117. doi: 10.1111/j.1749-6632.1990.tb16113.x. [DOI] [PubMed] [Google Scholar]
- 223.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19(1):103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 224.Farbman AI, Buchholz JA. Transforming growth factor-alpha and other growth factors stimulate cell division in olfactory epithelium in vitro. J Neurobiol. 1996;30(2):267–280. doi: 10.1002/(SICI)1097-4695(199606)30:2<267::AID-NEU8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 225.Derynck R, Roberts AB, Winkler ME, Chen EY, Goeddel DV. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- 226.Guo X, Park H, Liu G, Liu W, Cao Y, Tabata Y, Kasper FK, Mikos AG. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009;30(14):2741–2752. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lu CH, Yeh TS, Yeh CL, Fang YHD, Sung LY, Lin SY, Yen TC, Chang YH, Hu YC. Regenerating Cartilages by Engineered ASCs: Prolonged TGF-[beta]3/BMP-6 Expression Improved Articular Cartilage Formation and Restored Zonal Structure. Mol Ther. 2014;22(1):186–195. doi: 10.1038/mt.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Coelho MJ, Cabral AT, Fernande MH. Human bone cell cultures in biocompatibility testing. Part I: osteoblastic differentiation of serially passaged human bone marrow cells cultured in alpha-MEM and in DMEM. Biomaterials. 2000;21(11):1087–1094. doi: 10.1016/s0142-9612(99)00284-7. [DOI] [PubMed] [Google Scholar]
- 229.Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr Cartil. 2006;14(5):403–412. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 230.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12(15):1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 231.Gooch TBKJ, Courter DL, Sieminski AL, Bursac PM, Vunjak-Novakovic G, Freed LE. IGF-I and Mechanical Environment Interact to Modulate Engineered Cartilage Development. Biochem Biophys Res Commun. 2001;286(5):909–915. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 232.Elisseeff J, McIntosh W, Fu K, Blunk T, Langer R. Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19(6):1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 233.Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJ, Tabata Y, Wong ME, Jansen JA, Mikos AG, Kasper FK. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials. 2014;35(31):8829–8839. doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2(1):1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 235.Urist MR. Bone: Formation by Autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 236.Xiao YT, Xiang LX, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Commun. 2007;362(3):550–553. doi: 10.1016/j.bbrc.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 237.Rosen V. BMP and BMP Inhibitors in Bone. Ann N Y Acad Sci. 2006;1068(1):19–25. doi: 10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- 238.Wozney JM, Rosen V. Bone Morphogenetic Protein and Bone Morphogenetic Protein Gene Family in Bone Formation and Repair. Clin Orthop Relat Res. 1998;346:26–37. [PubMed] [Google Scholar]
- 239.Vicki Rosen RST. The BMP proteins in bone formation and repair. Trends Genet. 1992;8(3):97–102. doi: 10.1016/0168-9525(92)90197-c. [DOI] [PubMed] [Google Scholar]
- 240.Wozney JM. Bone Morphogenetic Proteins. Prog Growth Factor Res. 1989;1(4):267–280. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- 241.Wang EA, Rosen V, D’Alessandro JS, Bauduy M, Cordes P, Harada T, Israel DI, Hewick RM, Kerns KM, LaPan P. Recombinant human bone morphogenetic protein induces bone formation. PNAS. 1990;87(6):2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Diab T, Pritchard EM, Uhrig BA, Boerckel JD, Kaplan DL, Guldberg RE. A silk hydrogel-based delivery system of bone morphogenetic protein for the treatment of large bone defects. J Mech Behav Biomed Mater. 2012;11:123–131. doi: 10.1016/j.jmbbm.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Park KH, Kim H, Moon S, Na K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108(6):530–537. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 244.Boerckel JD, Kolambkar YM, Dupont KM, Uhrig BA, Phelps EA, Stevens HY, García AJ, Guldberg RE. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32(22):5241–5251. doi: 10.1016/j.biomaterials.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chung YI, Ahn KM, Jeon SH, Lee SY, Lee JH, Tae G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle–hydrogel complex. J Control Release. 2007;121(1–2):91–99. doi: 10.1016/j.jconrel.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 246.Ben-David D, Srouji S, Shapira-Schweitzer K, Kossover O, Ivanir E, Kuhn G, Müller R, Seliktar D, Livne E. Low dose BMP-2 treatment for bone repair using a PEGylated fibrinogen hydrogel matrix. Biomaterials. 2013;34(12):2902–2910. doi: 10.1016/j.biomaterials.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 247.Kempen DHR, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, Dhert WJA, Yaszemski MJ. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29(22):3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yoshitake Takahashi MY, Yamada Keisuke, Kawakami Osamu, Tabata Yasuhiko. Skull Bone Regeneration in Nonhuman Primates by Controlled Release of Bone Morphogenetic Protein-2 from a Biodegradable Hydrogel. Tissue Eng. 2007;13(2):293–300. doi: 10.1089/ten.2006.0088. [DOI] [PubMed] [Google Scholar]
- 249.Park Y, Sugimoto M, Watrin A, Chiquet M, Hunziker EB. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthr Cartil. 2005;13(6):527–536. doi: 10.1016/j.joca.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 250.Sellers RS, Peluso D, Morris EA. The Effect of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) on the Healing of Full-Thickness Defects of Articular Cartilage. J Bone Joint Surg Am. 1997;79(10):1452–1463. doi: 10.2106/00004623-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 251.Gokce A, Yilmaz I, Bircan R, Tonbul M, Gokay NS, Gokce C. Synergistic Effect of TGF-β1 And BMP–7 on Chondrogenesis and Extracellular Matrix Synthesis: An In Vitro Study. Open Orthop J. 2012;6:406–413. doi: 10.2174/1874325001206010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tanaka H, Murphy CL, Murphy C, Kimura M, Kawai S, Polak JM. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J Cell Biochem. 2004;93(3):454–462. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- 253.Coelho MJ, Fernandes MH. Human bone cell cultures in biocompatibility testing. Part II: effect of ascorbic acid, beta-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials. 2000;21(11):1095–1102. doi: 10.1016/s0142-9612(99)00192-1. [DOI] [PubMed] [Google Scholar]
- 254.Morsczeck C, Moehl C, Gotz W, Heredia A, Schaffer TE, Eckstein N, Sippel C, Hoffmann KH. In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol Int. 2005;29(7):567–575. doi: 10.1016/j.cellbi.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 255.Temenoff JS, Park H, Jabbari E, Sheffield TL, LeBaron RG, Ambrose CG, Mikos AG. In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A. 2004;70A(2):235–244. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 256.Nuttelman CR, Tripodi MC, Anseth KS. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. J Biomed Mater Res A. 2006;76(1):183–195. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- 257.Na K, Kim S, Woo DG, Sun BK, Yang HN, Chung HM, Park KH. Combination material delivery of dexamethasone and growth factor in hydrogel blended with hyaluronic acid constructs for neocartilage formation. J Biomed Mater Res A. 2007;83(3):779–786. doi: 10.1002/jbm.a.31374. [DOI] [PubMed] [Google Scholar]
- 258.Florine EM, Miller RE, Porter RM, Evans CH, Kurz B, Grodzinsky AJ. Effects of Dexamethasone on Mesenchymal Stromal Cell Chondrogenesis and Aggrecanase Activity: Comparison of Agarose and Self-Assembling Peptide Scaffolds. Cartilage. 2013;4(1):63–74. doi: 10.1177/1947603512455196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tanaka H, Murphy CL, Murphy C, Kimura M, Kawai S, Polak JM. Chondrogenic differentiation of murine embryonic stem cells: Effects of culture conditions and dexamethasone. J Cell Biochem. 2004;93(3):454–462. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- 260.Hani Y-DCH, Awad A, Gimble Jeffrey M, Guilak Farshid. Effects of Transforming Growth Factor β1 and Dexamethasone on the Growth and Chondrogenic Differentiation of Adipose-Derived Stromal Cells. Tissue Eng. 2004;9(6):1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 261.Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X, Schultz PG. A stem cell-based approach to cartilage repair. Science. 2012;336(6082):717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- 262.Kim S, Bedigrew K, Guda T, Maloney WJ, Park S, Wenke JC, Yang YP. Novel osteoinductive photo-cross-linkable chitosan-lactide-fibrinogen hydrogels enhance bone regeneration in critical size segmental bone defects. Acta Biomater. 2014;10(12):5021–5033. doi: 10.1016/j.actbio.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shekaran A, Garcia JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, Garcia AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35(21):5453–5461. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sridhar BV, Doyle NR, Randolph MA, Anseth KS. Covalently tethered TGF-β1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production. J Biomed Mater Res A. 2014;102(12):4464–4472. doi: 10.1002/jbm.a.35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7(10):816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Levato R, Visser J, Planell JA, Engel E, Malda J, Mateos-Timoneda MA. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication. 2014;6(3):035020. doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- 267.Reyes R, Delgado A, Sanchez E, Fernandez A, Hernandez A, Evora C. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFbeta1 from a bilayered alginate-PLGA scaffold. J Tissue Eng Regen Med. 2014;8(7):521–533. doi: 10.1002/term.1549. [DOI] [PubMed] [Google Scholar]
- 268.Gonzalez-Fernandez T, Tierney EG, Cunniffe GM, O’Brien FJ, Kelly DJ. Gene Delivery of TGF-β3 and BMP2 in an MSC-Laden Alginate Hydrogel for Articular Cartilage and Endochondral Bone Tissue Engineering. Tissue Eng Part A. 2016;22(9–10):776–787. doi: 10.1089/ten.TEA.2015.0576. [DOI] [PubMed] [Google Scholar]
- 269.Hill MC, Nguyen MK, Jeon O, Alsberg E. Spatial Control of Cell Gene Expression by siRNA Gradients in Biodegradable Hydrogels. Adv Healthc Mater. 2015;4(5):714–722. doi: 10.1002/adhm.201400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gaharwar AK, Mihaila SM, Swami A, Patel A, Sant S, Reis RL, Marques AP, Gomes ME, Khademhosseini A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv Mater. 2013;25(24):3329–3336. doi: 10.1002/adma.201300584. [DOI] [PubMed] [Google Scholar]
- 271.Mihaila SM, Gaharwar AK, Reis RL, Khademhosseini A, Marques AP, Gomes ME. The osteogenic differentiation of SSEA-4 sub-population of human adipose derived stem cells using silicate nanoplatelets. Biomaterials. 2014;35(33):9087–9099. doi: 10.1016/j.biomaterials.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 272.Gaharwar AK, Mukundan S, Karaca E, Dolatshahi-Pirouz A, Patel A, Rangarajan K, Mihaila SM, Iviglia G, Zhang H, Khademhosseini A. Nanoclay-enriched poly(varepsilon-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2014;20(15–16):2088–2101. doi: 10.1089/ten.tea.2013.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Han Y, Zeng Q, Li H, Chang J. The calcium silicate/alginate composite: preparation and evaluation of its behavior as bioactive injectable hydrogels. Acta Biomater. 2013;9(11):9107–9117. doi: 10.1016/j.actbio.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 274.Khanarian NT, Haney NM, Burga RA, Lu HH. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials. 2012;33(21):5247–5258. doi: 10.1016/j.biomaterials.2012.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Su WT, Chou WL, Chou CM. Osteoblastic differentiation of stem cells from human exfoliated deciduous teeth induced by thermosensitive hydrogels with strontium phosphate. Mater Sci Eng C Mater Biol Appl. 2015;52:46–53. doi: 10.1016/j.msec.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 276.Jiang J, Tang A, Ateshian GA, Guo XE, Hung CT, Lu HH. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng. 2010;38(6):2183–2196. doi: 10.1007/s10439-010-0038-y. [DOI] [PubMed] [Google Scholar]
- 277.Paul A, Manoharan V, Krafft D, Assmann A, Uquillas JA, Shin SR, Hasan A, Hussain MA, Memic A, Gaharwar AK, Khademhosseini A. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J Mater Chem B. 2016;4(20):3544–3554. doi: 10.1039/C5TB02745D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gaharwar AK, Avery RK, Assmann A, Paul A, McKinley GH, Khademhosseini A, Olsen BD. Shear-Thinning Nanocomposite Hydrogels for the Treatment of Hemorrhage. ACS Nano. 2014;8(10):9833–9842. doi: 10.1021/nn503719n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. Engineering graded tissue interfaces. PNAS. 2008;105(34):12170–12175. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mesallati EST, Vinardell T, Buckley CT, Kelly DJ. Tissue engineering scaled-up, anatomically shaped osteochondral constructs for joint resurfacing. Eur Cell Mater. 2015;30:163–186. doi: 10.22203/ecm.v030a12. [DOI] [PubMed] [Google Scholar]
- 281.Das S, Pati F, Choi YJ, Rijal G, Shim JH, Kim SW, Ray AR, Cho DW, Ghosh S. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015;11:233–246. doi: 10.1016/j.actbio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 282.Ober TJ, Foresti D, Lewis JA. Active mixing of complex fluids at the microscale. PNAS. 2015;112(40):12293–12298. doi: 10.1073/pnas.1509224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotech. 2016;34(3):312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 284.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotech. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 285.Seol YJ, Kang HW, Lee SJ, Atala A, Yoo JJ. Bioprinting technology and its applications. Eur J Cardiothorac Surg. 2014:1–7. doi: 10.1093/ejcts/ezu148. [DOI] [PubMed] [Google Scholar]
- 286.Nover AB, Jones BK, Yu WT, Donovan DS, Podolnick JD, Cook JL, Ateshian GA, Hung CT. A puzzle assembly strategy for fabrication of large engineered cartilage tissue constructs. J Biomechan. 2016;49(5):668–677. doi: 10.1016/j.jbiomech.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sophia Fox AJ, Bedi A, Rodeo SA. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health: A Multidisciplinary Approach. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Alford JW, Cole BJ. Cartilage Restoration, Part 1: Basic Science, Historical Perspective, Patient Evaluation, and Treatment Options. Am J Sports Med. 2005;33(2):295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 289.Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205(2):551–558. doi: 10.1148/radiology.205.2.9356644. [DOI] [PubMed] [Google Scholar]
- 290.Buckwalter JAM, Van C, Ratcliffe Anthony. Restoration of Injured or Degenerated Articular Cartilage. J Am Acad Orthop Surg. 1994;4(2):192–201. doi: 10.5435/00124635-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 291.R L, Buckwalter JA, Hunziker EB. Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy. Raven Press; Nwe York, NY: 1990. [Google Scholar]
- 292.W M, Eyre DR, Wu JJ. Articular cartilage collagen: an irreplaceable framework? Eur Cell Mater. 2006;12:57–63. doi: 10.22203/ecm.v012a07. [DOI] [PubMed] [Google Scholar]
- 293.M V, Guilak F. The mechanical environment of the chondrocyte: a biphasic finite element model of cell—matrix interactions in articularcartilage. J Biomech. 2000;33:1663–1673. [PubMed] [Google Scholar]
- 294.M A. Physiochemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Cambridge University Press; Kent, United Kingdom: 1979. pp. 215–290. [Google Scholar]
- 295.R A, Mow VC, Poole AR. Cartilage and diarthrodial joints asparadigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 296.Muir H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. BioEssays. 1995;17(12):1039–1048. doi: 10.1002/bies.950171208. [DOI] [PubMed] [Google Scholar]
- 297.Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: Ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res. 1987;5(4):509–522. doi: 10.1002/jor.1100050406. [DOI] [PubMed] [Google Scholar]
- 298.Wojtys E, Wilson M, Buckwalter K, Braunstein E, Martel W. Magnetic resonance imaging of knee hyaline cartilage and intraarticular pathology. AM J Sports Med. 1987;15(5):455–463. doi: 10.1177/036354658701500505. [DOI] [PubMed] [Google Scholar]
- 299.Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol Adv. 2013;31(5):706–721. doi: 10.1016/j.biotechadv.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 300.Yang W, Yang F, Wang Y, Both SK, Jansen JA. In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta Biomater. 2013;9(1):4505–4512. doi: 10.1016/j.actbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 301.Klinger P, Surmann-Schmitt C, Brem M, Swoboda B, Distler JH, Carl HD, von der Mark K, Hennig FF, Gelse K. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthr Rheum. 2011;63(9):2721–2731. doi: 10.1002/art.30335. [DOI] [PubMed] [Google Scholar]
- 302.Visser J, Gawlitta D, Benders KEM, Toma SMH, Pouran B, van Weeren PR, Dhert WJA, Malda J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials. 2015;37:174–182. doi: 10.1016/j.biomaterials.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 303.Nagai T, Sato M, Kutsuna T, Kokubo M, Ebihara G, Ohta N, Mochida J. Intravenous administration of anti-vascular endothelial growth factor humanized monoclonal antibody bevacizumab improves articular cartilage repair. Arthr Res Ther. 2010;12(5):1–10. doi: 10.1186/ar3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bénazet J-D, Bischofberger M, Tiecke E, Gonçalves A, Martin JF, Zuniga A, Naef F, Zeller R. A Self-Regulatory System of Interlinked Signaling Feedback Loops Controls Mouse Limb Patterning. Science. 2009;323(5917):1050–1053. doi: 10.1126/science.1168755. [DOI] [PubMed] [Google Scholar]
- 305.Peter VNB, Weiguang Z, Yogendra PK, Frederick JB, Andre-Jean L, Mary Beth G, Tripti G, Gary SS, Jane BL, Barry SK. The Wnt Antagonist Secreted Frizzled-Related Protein-1 Is a Negative Regulator of Trabecular Bone Formation in Adult Mice. Mol Endocrinol. 2004;18(5):1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 306.Shapiro F, Flynn E, Calicchio ML. Molecular differentiation in epiphyseal and physeal cartilage. Prominent role for gremlin in maintaining hypertrophic chondrocytes in epiphyseal cartilage. Biochem Biophys Res Commun. 2009;390(3):570–576. doi: 10.1016/j.bbrc.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 307.Shukunami C, Oshima Y, Hiraki Y. Chondromodulin-I and tenomodulin: A new class of tissue-specific angiogenesis inhibitors found in hypovascular connective tissues. Biochem Biophys Res Commun. 2005;333(2):299–307. doi: 10.1016/j.bbrc.2005.05.133. [DOI] [PubMed] [Google Scholar]
- 308.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of Rate of Cartilage Differentiation by Indian Hedgehog and PTH-Related Protein. Science. 1996;273(5275):613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 309.Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of Human Mesenchymal Stem Cells and Articular Chondrocytes Reduces Hypertrophy and Enhances Functional Properties of Engineered Cartilage. Tissue Eng Part A. 2010;17(7–8):1137–1145. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Cooke ME, Allon AA, Cheng T, Kuo AC, Kim HT, Vail TP, Marcucio RS, Schneider RA, Lotz JC, Alliston T. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr and Cartil. 2011;19(10):1210–1218. doi: 10.1016/j.joca.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone–related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthr Rheum. 2010;62(9):2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 312.Sadtler K, Singh A, Wolf MT, Wang X, Pardoll DM, Elisseeff JH. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nature Rev Mater. 2016;1:16040. [Google Scholar]
- 313.Strehin I, Nahas Z, Arora K, Nguyen T, Elisseeff J. A versatile pH sensitive chondroitin sulfate–PEG tissue adhesive and hydrogel. Biomaterials. 2010;31(10):2788–2797. doi: 10.1016/j.biomaterials.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26(15):2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]


