Abstract
The intestinal epithelium forms a highly dynamic and selective barrier that controls absorption of fluid and solutes while restricting pathogen access to underlying tissues. Barrier properties are achieved by intercellular junctions that include an apical tight junction (TJ) and subjacent adherens junction and desmosomes. The TJ tetraspan claudin proteins form pores between epithelial cells to control paracellular fluid and ion movement. In addition to regulation of barrier function, claudin family members control epithelial homeostasis and are expressed in a spatiotemporal manner in the intestinal and crypt-luminal axis. This delicate balance of physiologic differential claudin protein expression is altered during mucosal inflammation. Inflammatory mediators influence transcriptional regulation, as well as endocytic trafficking, targeting, and retention of claudins in the TJ. Increased expression of intestinal epithelial claudin-1, -2, and -18, with downregulation of claudin-3, -4, -5, -7, -8, and -12, has been observed in intestinal inflammatory disorders. Such changes in claudin proteins modify the epithelial barrier function in addition to influencing epithelial and mucosal homeostasis. An improved understanding of the regulatory mechanisms that control epithelial claudin proteins will provide strategies to strengthen the epithelial barrier function and restore mucosal homeostasis in inflammatory disorders.
Keywords: epithelium, intestine, mucosal barrier, tight junction, claudins, inflammation
Introduction
Epithelial cells form selective barriers that interface distinct internal and external environments. While forming a protective barrier to pathogen access, the epithelium controls select movement of ions, fluid, and solutes. Epithelial barrier function is achieved by a series of intercellular junctions that include an apical tight junction (TJ) and subjacent adherens junction, which are collectively referred to as the apical junctional complex (AJC). Desmosomes and gap junctions, which reside below the AJC, mediate intercellular adhesion and cross talk between adjacent epithelial cells.1,2 In the intestine, a single layer of epithelial cells is organized in densely packed invaginations referred to as crypts (Fig. 1A). Small intestinal epithelial cells (IECs) also line luminal protrusions or villi. The intestinal epithelium is highly dynamic and is actively turned over as progenitor stem cells in the crypt proliferate, differentiate in the crypt–luminal axis, and are shed in a regulated manner into the gut lumen.3 It is remarkable that epithelial barrier properties are not only maintained but are also modified as the enterocytes navigate the crypt-luminal axis. Here, we largely focus on the complexity and function of intestinal epithelial claudins and touch upon recent concepts on how claudins are regulated during epithelial differentiation and in response to mucosal inflammation.
Figure 1.
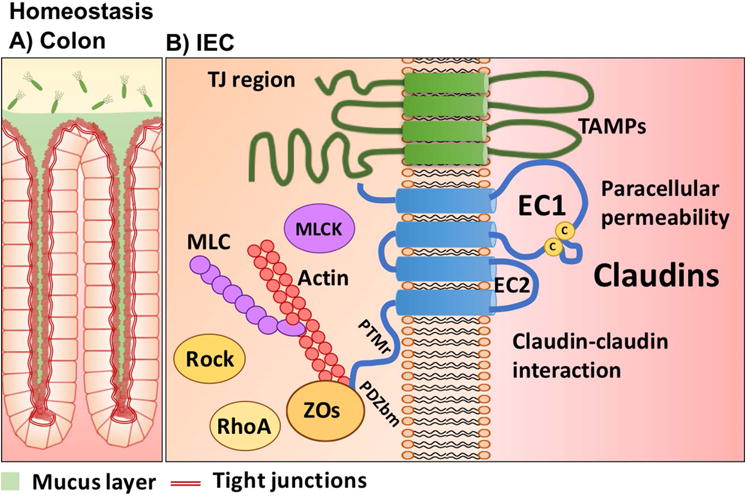
Claudin functional domains and key binding partners that mediate intestinal epithelial homeostasis. (A) In the colon, a single layer of epithelial cells is organized in crypts. (B) Schematic representation of claudin functional domains and their key binding partners in the tight junction. IEC, intestinal epithelial cells; TJ, tight junction; EC, extracellular loop; PTMr, posttranslational modifications region; PDZbm, PDZ binding motif.
Tight junctions
Epithelial TJs reside at the junction between the apical and basolateral plasma membranes and regulate epithelial polarity and vectorial movement of solutes and fluids in the intercellular space (paracellular pathway).4–6 Dynamics of the paracellular pathway are intimately linked with transcellular transport mechanisms that maintain homeostasis across polarized cells.7,8 Such coordination between paracellular and transcellular movement of solutes also serves as a mechanism to save energy while ensuring polarized fluid and solute movement across polarized cells.8,9 In addition to these functions, TJ proteins actively participate in mediating communication between intercellular contacts and cellular homeostasis encompassing cellular proliferation, differentiation, and migration. TJs are visualized as areas of close membrane apposition between cells by transmission electron microscopy and as anastomosing multistrand structures by freeze–fracture electron microscopy.5 The protein-containing strands span the extracellular space between adjoining cells, and properties of the TJ seal have been determined by protein composition of strands and their ultimate organization within the junction. Thus, the TJ strand organization varies in epithelial cells with different permeability properties.10,11 Maintenance of TJ strands requires association of proteins that constitute the strands with submembranous cytoplasmic plaque proteins that serve as scaffolds to link TJ strands to the apical actin cytoskeleton. The contractile properties of this perijunctional actin actoskeleton plays an important role in controlling the physiologic TJ function.
The three main families of TJ transmembrane proteins are claudins, TJ-associated MARVEL proteins (TAMPs), and the cortical thymocyte marker in Xenopus (CTX).12 Occludin, MarvelD3, and tricellulin constitute the TAMP family of proteins. The epithelial CTX family includes JAM-A (junctional adhesion molecule), CAR (coxsackie virus and adenovirus receptor) and CLMP (CXADR-like membrane protein). While the TAMP family of proteins has been implicated in influencing barrier function, proteins such as occludin have important signaling properties that regulate epithelial homeostasis.13 The CTX family protein JAM-A regulates epithelial TJ barrier function by influencing claudin protein expression and actin–myosin dynamics, thereby influencing properties of both the TJ pore and leak paracellular pathways. Permeability properties are further modified at epithelial tricellular contacts that contain additional transmembrane proteins, such as tricellulin and LSR (lipolysis-stimulated lipoprotein receptor).14
Transmembrane proteins associate with subjacent plaque proteins that provide a link to underlying actin filaments. These associations are dynamically regulated by myosin II and affiliated proteins to control TJ function.15 In addition to structural plaque proteins that link transmembrane proteins to the actin cytoskeleton, the TJ submembranous plaque comprises a plethora of regulatory proteins, including small GTPases, kinases/phosphatases, and transcription factors. In fact, over 80 proteins have been identified in the TJ, suggesting that the associated proteins function as signaling hubs that orchestrate a number of cellular events, such as epithelial polarity, homeostasis, and barrier function.16–19
The TJ is a dynamic structure that is actively remodeled during the life span of the epithelium.20–22 Furthermore, the TJ protein composition is modified in response to a number of cues in the intestinal crypt-luminal axis. Such delicate control mechanisms are perturbed in pathologic states, such as inflammation and cancer, that influence epithelial homeostasis and barrier properties.23–26 The paracellular movement of molecules has been suggested to occur by “pore” or “leak” pathways. Pores between cells are structurally formed by claudins that serve as a low-capacity, ion-charge, and size-selective pathway. While the molecular mechanism that form the basis of the leak pathway is less well understood, it has been proposed to be regulated by changes in TJ strand organization, including strand breaks and transient paracellular gaps that allow large molecule non-selective flux.27–31 This latter leak pathway is regulated by transmembrane proteins, as well as by scaffold protein and tension of the perijunctional actin myosin cytoskeleton.
Claudins
The tetraspan claudin family of proteins includes 26 family members in humans. Ultrastructurally, claudins reside in TJ strands and serve to control the charge and size selective properties of the paracellular space, thereby regulating barrier properties. Claudins have been proposed to function as “tight” or sealing claudins or as “leaky” pore-forming claudins. The paracellular pore properties have been attributed to claudin amino acid composition in the first extracellular loop (EC1).32,33 Pore- or channel-forming claudins have selectivity for charged ions and water. Cysteine residues in the EC1 enhance the stability of the protein, while the smaller second extracellular loop (EC2) mediates claudin–claudin interactions within a cell (cis) and in adjoining cells (trans)27,34(Fig. 1B). Tight claudins include claudin-1, -3, -4, -5, -6, -8, -12, -18, and -19,35–41 while leaky claudin-2 and claudin-15 contribute to increased paracellular permeability to sodium and water.42–44 While these distinctions have been proposed, the overall function of a claudin is dependent on complement of other claudins expressed within a TJ. The recently solved claudin-15 crystal structure has provided significant advances in understanding of its higher-ordered organization in TJs and shed light on pore characteristics that mediate ion and fluid movement in the paracellular space.45
Claudin-1 is widely expressed in the intestinal epithelium, is known by its barrier-forming abilities, and has been proposed to have an important role for TJ integrity.35,46 Claudin-3 expression is higher in the colon than in the rest of the gut47 and has been classified as a barrier protein.48 However, converse effects of claudin-3 on barrier function have been reported in lung alveolar epithelia.49 In an analogous manner, the role of claudin-4 in controlling barrier function is dependent on the background claudin family members within a junction. In vitro studies have identified barrier-forming abilities50 of claudin-4 in colonic epithelial cells.51 Claudin-5 enhances barrier function in model IECs37 and plays a pivotal role in promoting barrier properties of endothelial cells in the blood–brain barrier,52 while its importance in controlling in vivo intestinal epithelial barrier function and homeostasis is not clear. There is contrasting evidence showing that claudin-7 can function as an anion pore or cation barrier in porcine and dog kidney cells, which is most likely dependent on the background of other claudins that are expressed within the TJ.38–40 The specific contribution of claudin-7 in controlling the intestinal epithelial paracellular pathway is, however, not well understand. Epithelial-specific downregulation of claudin-7 increases paracellular movement of small organic solutes, suggesting that it contributes to the intestinal barrier of epithelial cells.53 Claudin-8 function has been largely explored in the kidney,54 where aldosterone-induced increase in claudin-8 expression has been linked to increased barrier function and decreased sodium movement into the apical compartment of epithelial cells.55–58 Claudin-259–61 and claudin-1544,62 regulate paracelullar transport of Na+ and water in the intestine. These pore- or channel-forming claudins are widely expressed in the proliferative compartment of the colonic crypt epithelium. Mice with intestinal epithelial loss of claudin-15 have low intraluminal sodium that influences sodium-coupled glucose absorption via the apical sodium glucose co-transporter SGLT.44
The majority of claudins, with a few exceptions, such as claudin-23, contain a PDZ (PSD95, Dlg1, and ZO-1) binding motif that associates with submembrane plaque proteins containing PDZ domains and regulatory molecules, which serve to organize and control TJ structure and ultimately epithelial barrier function.63–66 The most extensively studied cytoplasmic plaque PDZ domain–containing proteins are the zonula occludens (ZO) proteins, which includes ZO-1 and ZO-2. However, the mechanisms that govern preferential association of family members with specific plaque proteins remain less well understood. Freeze–fracture ultrastructural analysis has revealed that claudins reside and organize TJ strands between cells.62 A recent study using super-resolution live-cell imaging elegantly demonstrated the contribution of ZO-1 in stabilizing claudin strands with the actin cytoskeleton.67 Such TJ protein–actin cytoskeleton affiliations control physiologic epithelial barrier properties and are targeted in pathologic states associated with barrier compromise. Posttranslational modifications of claudins have been suggested to influence their association with scaffold proteins in the TJ.68,69
While the plaque protein play an important role in controlling TJ transmembrane protein organization, claudin proteins have an innate ability to self-organize/oligomerize within TJs. Claudin proteins can interact in cis and in trans, and not all claudins are pair compatible. Within a TJ, the complement of claudins ultimately determines the properties of the paracellular pore pathway in epithelial and endothelial cells.70,71 While TJ plaque proteins play important roles in stabilizing and regulating transmembrane claudins, claudin proteins can be targeted to the TJ independent of these associations. In vitro–expressed claudins-1 and -5 lacking the PDZ binding motif localize to TJs.66 However, loss of the entire C-terminal juxtamembrane sequence prevents their association with TJ of epithelial and endothelial cells, suggesting that the amino acid composition in this region contributes to claudin protein–mediated barrier function.66 Interestingly, within a TJ, differential claudin protein associations have been reported in strands that reside in the basal versus apical aspects of the junction. Such organization has been postulated to occur in response to shear stress to which the junction is exposed.72 While such variability in claudin-containing strands of intestinal epithelial TJ have not been explored, one can envision that such organization exists in IECs that are continuously exposed to physical forces associated with epithelial turnover and gut motility.
Amino acids in the claudin X-terminus undergo posttranslational modifications (phosphorylation, palmitoylation, and sumoylation)63–65 that have been proposed to control their subcellular localization, protein levels, and stability.66 Such modification could potentially play an important role in claudin isoform switching in response to environmental cues.
Spatiotemporal expression of intestinal epithelial claudin proteins
The composition of epithelial claudins not only varies spatially along the length of the gastrointestinal tract but also in the crypt-luminal axis as epithelial cells transition from a proliferative to differentiated phenotype (Fig. 2). It is remarkable that such claudin remodeling occurs within a few days during the life span of the intestinal epithelium that maintains and yet modifies its barrier properties.73 However, mechanisms that govern such physiologic remodeling of TJ claudins in differentiating IECs are not well understood.
Figure 2.
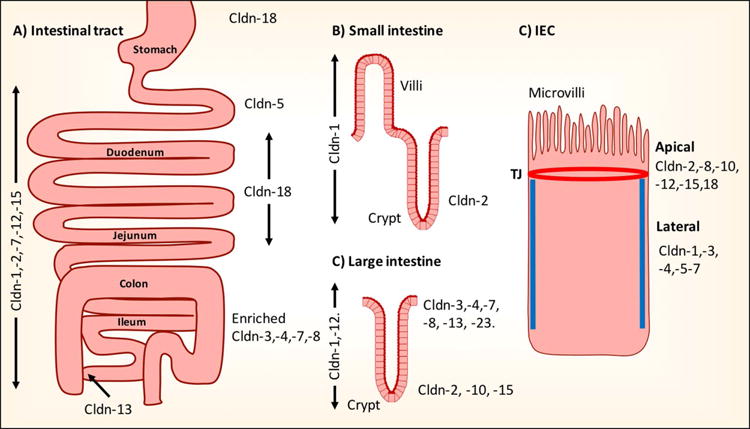
Claudin expression along the intestinal tract. Schematic representation of reported claudin expression. (A) Along the intestinal tract. (B and C) Crypt-luminal axis of small and large intestine. D) Membrane distribution in the TJ or in the lateral membrane of intestinal epithelial cells (C). Cldn, claudin; IECs, intestinal epithelial cells.
A number of studies have investigated the spatiotemporal distribution of claudin proteins in the intestine (Fig. 2A). In humans, claudin-1, -2, -7, -12, and -15 mRNA was detected along the entire intestinal tract axis. While abundant claudin-5 expression was identified in the duodenum, claudin -3, -4, -7, and -8 exhibited increased expression in the distal colonic epithelium.74 In the adult mouse and rat intestine, reverse transcriptase polymerase chain reaction (RT-PCR) analysis identified expression of claudin-1, -2, -3, -4, -5 -7, -8, -9, -10, -11, -12, 13, -14, -15, -17, and -18, while claudin-6, -16, -19, -22, and -24 were not detected. Claudin-2, -3, -7, and -15 mRNA are the most highly expressed in the gut.75–77
Increasing claudin-8 mRNA expression has been reported in distal versus proximal IECs.75,76 The converse has been reported for claudin-15. Peak claudin-2, -5, -7, and -10 mRNA expression has been noted in TJs of epithelial cells in the ileocecal junction. In contrast, intestinal epithelial claudin-18 is expressed in the esophagus, stomach, duodenum, and jejunum,75,78,79 while claudin-13 mRNA was identified in the large intestine/colon, with increased expression in luminal epithelial cells.76
In the crypt-luminal axis, mRNA expression of pore-forming claudin-2, -10, and -15 is restricted in the crypt base, while barrier-forming claudin-3 and claudin-4 were observed to be exclusively expressed in luminal epithelial cells75–77 (Fig. 2B and 2C). In a recent study, we mapped the spatial distribution of claudin mRNA expression in colonic epithelial cells after microdissection of crypt versus luminal epithelial cells. Claudin-3, -4, -7, - 8, and -23 were detected in luminal epithelial cells.80 The contribution of claudin-23 in controlling intestinal epithelial barrier function, however, remains unexplored. Given its spatial expression in luminal IECs,80 claudin-23 likely contributes to the barrier-forming abilities of other claudin protein family members expressed at these sites. In contrast, uniform distribution of claudins1 and claudin-12 was observed in the entire colonic crypt-luminal axis.47,81
The specific organization of claudins in adult IECs differs from that in the developing intestine. In mice, mRNA expression of intestinal epithelial claudin-1, -2, -5, and -8 is lower at 1 and 90 days after birth, while claudin-19 expression is exclusively detected 1–14 days postpartum. At birth, the pore-forming claudin-2 is expressed in the entire intestinal crypt-luminal axis and becomes restricted to the crypt base in the colon 90 days after birth. Increasing levels of intestinal epithelial claudin-3, -4, -7, and -15 have been identified in the maturing intestine after birth.75
Besides the above spatial organization, at the cellular level claudin proteins can be either targeted exclusively to the TJ or also reside in the lateral membrane of polarized epithelial cells (Fig. 2D). While claudin- 2, -8, -10, -12, -15, and -18 proteins have restricted TJ localization, claudin-1, -3, -4, -5, and -7 are also targeted to the lateral plasma membrane (Fig. 2C).82 Lateral expression of claudin-7 in IECs has been proposed to have signaling properties that influence maintenance of extracellular matrix interactions, homeostasis,83 and differentiation.84 The functional role of lateral membrane–associated claudins is not well understood. This pool of claudins could function as a reservoir for TJ-associated claudins during dynamic remodeling of the junctional protein complex. This possibility has been proposed in studies that have utilized fluorescence recovery after photobleaching (FRAP) to study remodeling of intestinal epithelial junctional claudin proteins.21,85 Furthermore, lateral membrane–associated claudin proteins could also have signaling function that controls epithelial homeostasis.
In addition to their pivotal role in controlling barrier function, claudins regulate epithelial homeostasis that encompasses proliferation and differentiation of cells. While many of the above studies have mapped the topographical distribution of claudin proteins in intestinal TJs, the mechanisms by which claudin remodeling is achieved remain less well understood. It is, however, clear that claudin organization in the intestinal tract, as well as in the crypt-luminal axis, responds to intracellular and extracellular cues that control physiologic properties of the epithelia barrier. Dysregulation of claudin proteins has been observed in inflammatory states associated with a leaky epithelial barrier, as well as in neoplastic states.23,25,26,86–91 In these scenarios, claudin proteins have been proposed to actively contribute to disease pathogenesis.
Transcriptional regulation of claudins in intestinal epithelial cells
Claudin remodeling is orchestrated by transcriptional regulation, posttranslational modifications, intracellular trafficking, and stability in the plasma membrane. The differential expression of claudin proteins in the crypt-luminal axis has been linked to epithelial proliferation and differentiation programs.
Limited information is available on the transcriptional programs that control CLDN genes. β-Catenin–TCF/LEF, CDX2, and HNFα transcription factors (TFs) have been implicated in regulating the expression of claudin family members in the intestine. The β-catenin–TCF/LEF signaling pathway plays an important role in controlling intestinal crypt epithelial cell proliferation, and previous studies have identified a link between β-catenin signaling and claudin-1 expression.92 The claudin-1 promoter contains TCF/LEF-binding sites, and decreased claudin-1 expression has been reported after downregulation of β-catenin. Furthermore, increased claudin-1 expression is associated with enhanced cellular proliferation.93 The transcription factor FOXO4 is involved in oxidative stress and insulin signaling, longevity, cell cycle progression, and apoptosis. Downregulation of claudin-1 was noted in epithelial cells after FOXO4 depletion. These observations suggest that claudin-1 expression is linked to intestinal epithelial cell proliferation and plays a role in the regulation of epithelial homeostasis. The intestinal epithelium resides in a low-oxygen environment, and the family of hypoxia-inducible factors (HIFs) control epithelial maintenance and repair.94 HIF-1β depletion in IECs results in barrier defects. Furthermore, hypoxia-response elements have been identified in the claudin-1 promoter, and IEC HIF-1β knockdown decreases claudin-1 mRNA.46 These observations support a unique relationship among HIF TFs, claudin-1, and intestinal epithelial barrier function that is maintained in a relatively hypoxic environment.
The TFs CDX-1 and CDX-2 have been reported to regulate intestinal epithelial development and physiologic epithelial homeostasis.95 The claudin-1 and claudin-2 promoters contain several CDX-2–binding sites, and CDX-2 promoter binding was demonstrated using chromatin immunoprecipitation assays.96 Overexpression of CDX-2 in cell lines SW480 and HCT116 resulted in increased claudin-1 mRNA. Additionally, coexpression of CDX-2 and active β-catenin had synergistic effects in increasing claudin-1 mRNA. Both CDX-1 and CDX-2 activate the claudin-2 promoter in the Caco-2 model (a human IEC line). Interestingly, hepatocyte nuclear factor (HNF) 1α potentiates CDX2-mediated transactivation of claudin-2. This effect is further enhanced in the presence of the TF GATA4.97–100 These findings not only support an interplay between these transcription factors that serve to control claudin mRNA expression but also highlight the complexity of claudin protein regulation in the intestine.
HNF4α is a member of the steroid-receptor family of TFs that localizes exclusively to the nucleus and has been implicated in the regulation of intestinal epithelial barrier function in mice.101 HNF4α binds the claudin-7 and claudin-15 promoters, and in a recent study we observed a relationship between HNF4α and claudin-7 expression in differentiating IECs.84 A gradient of HNF4α expression in the intestinal epithelial crypt-luminal axis was identified, with increased expression in luminal epithelial cells that also express claudin-4, -7, and -23 mRNA. In contrast to HNF4α, the TFs KLF4 and HOPX were restricted to crypt-base IECs that express claudin-2, -5, and -15.80
Intracellular trafficking of claudin proteins
Intracellular trafficking of claudin proteins contributes to the remodeling of TJs in physiologic and pathologic states. Internalization of claudin-based TJ strands has been observed in the rat intestine and in the human fetal hindgut.102,103 Such protein trafficking contributes to the rapid changes in cell adhesion, migration, and extrusion of apoptotic cells in the intestine. Claudin protein endocytosis has been reported during the epithelial-to-mesenchymal transition and in response to inflammatory mediators, oxidative stress, and ischemic injury. Depending on the stimulus, select junctional proteins, including claudins, are internalized from the cell surface through a number of mechanisms that differentially involve clathrin, caveolin, and micropinocytosis pathways.104 Internalized TJ proteins are delivered to early endosomes, followed by their trafficking to recycling endosomes to be targeted back to the TJ or into late endosomes for degradation.
Biochemically, TJ proteins are identified in an immobile stable pool of proteins integrated into the TJ and a mobile unstable pool of proteins peripheral to the TJ.85 Despite the stability of claudin-based TJs in confluent epithelia, these structures undergo steady-state remodeling that involves constant endocytosis and recycling of different claudin proteins.105,106 Interestingly claudin family members exhibit differential dynamics within TJs. For example, claudin-1, -2, and -3 are readily internalized and recycled, whereas claudin-4 shows negligible endocytosis in confluent epithelial monolayers. Such endocytosis/recycling of TJ proteins most likely contributes to homeostatic movements and/or steady-state remodeling of epithelial cell–cell contacts under physiological conditions.
Lessons from mice with genetically modified intestinal epithelial claudins
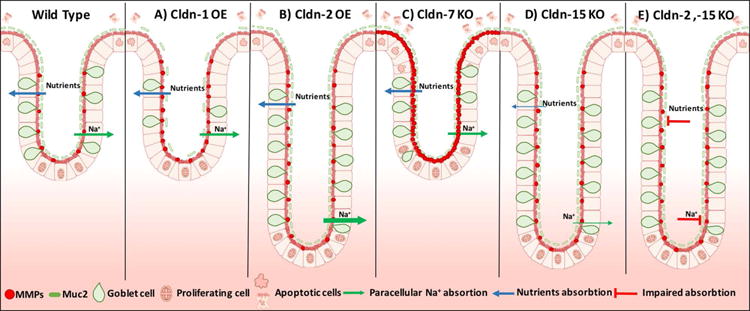
A number of mice with genetically modified expression of claudins have been generated to investigate the functional role of specific claudin family members in controlling epithelial barrier function. Transgenic mice that overexpress claudin-1 (TG) in IECs exhibit upregulation of MMP-9 and ERK that is associated with Notch activation.107 Previous studies have demonstrated a suppressive role of Notch signaling in intestinal epithelial differentiation that is evidenced by decreased goblet cells and mucin-2 (Muc-2) synthesis (Fig. 3A).108 Such observations in claudin-1 TG mice provide insight into a link between claudin-1 and Notch signaling that could serve to control intestinal epithelial homeostasis, differentiation, and mucosal barrier function.
Figure 3.
Phenotypic findings in the intestinal epithelial crypts of claudin transgenic mice. OE, overexpression; KO, knockout. Thickness of the arrows represent level of absorption.
Claudin-2 regulates paracellular movement of Na+, Ca2+, and water in the intestine.42,44,109 Deletion of the claudin-2 gene in mice decreases the transepithelial conductance and paracellular permeability for Na+.109 Specific overexpression of claudin-2 in the intestinal epithelia results in increased length of colonic crypts and an overall enlargement of the intestine that is associated with increased paracellular permeability to the Na+ ion and solute FITC–dextran. Interestingly, claudin-2 also promotes colonic epithelial cell proliferation (Fig. 3B).110,111 This was previously shown in a human lung adenocarcinoma cell line, where claudin-2 nuclear distribution increases epithelial cell proliferation by promoting translocation of ZONAB into the nucleus.112
Claudin-7 global knockout mice have increased intestinal mucosal inflammation during postnatal development that is associated with enhanced epithelial cell proliferation and apoptosis. Additionally, deletion of claudin-7 was associated with a 120-fold increase in intestinal metalloproteinase-3 (MMP-3) mRNA, which was implicated in promoting extracellular matrix degradation and the mucosal immune response.83 In another study, intestinal epithelial–specific knockout of claudin-7 resulted in increased paracellular permeability to solutes (457 and 4000 Da) (Fig. 3C).53
Claudin-15 KO mice exhibit megaintestine, which is associated with nutritional defects as a result of impaired intestinal Na+ and K+ paracellular permeability and decreased glucose absorption.44,113 The molecular basis of these phenotypes is likely related to claudin-15 regulation of epithelial homeostasis (Fig. 3D).113 In keeping with this notion, claudin-15 loss due to a mutation in the transcription factor TCF2 was associated with abnormal intestinal development.114 Furthermore claudin-2 and-15 double-knockout mice have severe malnutrition as a result of defects in intestinal epithelial absorption of glucose, amino acids, and lipids, leading to death after birth (Fig. 3E).115
Inflammation and claudin expression in the intestine
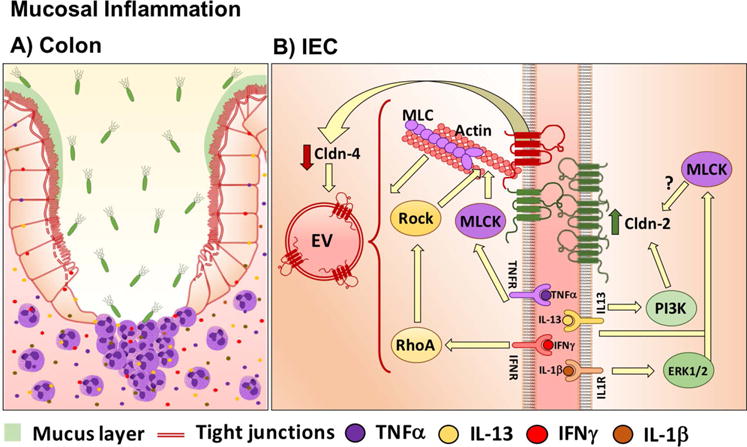
Mucosal inflammation as observed in acute colitis/enteritis and inflammatory bowel disease compromises the epithelial barrier, resulting in the exposure of lamina propria tissue compartments to luminal antigens and microbes that further contribute to the inflammatory response and barrier defects.116,117 The barrier damage occurs in response to inflammatory mediators as well as trafficking of immune cells to sites of inflammation. Several studies have reported differential effects of such inflammatory mediators on TJ proteins (Fig. 4A). Increased expression of claudin-1, -2, and -1825,86,118–120 and downregulation of claudin-3, -4, and -7 was reported in ulcerative colitis.25,118 In Crohn’s disease, upregulation of claudin-1 and claudin-2 and decreased expression of intestinal epithelial claudin-3, -5, -8, and -12 were observed25,26,74,86,121 (Table 1). In response to inflammation, altered claudin protein profiles in the TJ are associated with perturbed paracellular movement of fluid and solutes, which is reflected in overall change in epithelial barrier function. Replacement of barrier-forming claudins with pore/channel-forming claudins ultimately influences ion and fluid movement across cells, which is clinically reflected in disease symptoms, including diarrhea. However, it is important to take into account that changes in the level of a claudin or complement of claudins during an inflammatory response can not only result in change to the barrier properties but can also function in the protective response to host defense. An example of this scenario is claudin-2. In the physiologic state, claudin-2 expression is restricted to proliferative colonic crypt base epithelial cells. During mucosal inflammation, claudin-2 expression is upregulated in cells and its expression extends beyond the crypt-base proliferative cells in the colon. In vitro analyses have identified upregulation of claudin-2 in response to TH1 and TH2 proinflammatory cytokine exposure.122 Transcriptional regulation, intracellular trafficking, and retention of claudin-2 in the TJ influences overall claudin-2 protein levels.42,123,124 The molecular basis of the spatial claudin-2 expression in the crypt-luminal axis, however, is not well understood. Increase in claudin-2 has been observed in the context of decreased claudin-826,54 in Crohn’s disease (Table 1). While claudin-2 has been linked to increased paracellular permeability, it also has protective roles, as has been observed in an experimental model of colitis.125 While the direct mechanistic basis of claudin-2–mediated barrier protection is not understood, indirect mechanisms by which claudin-2 exerts such a protective effect include enhanced synthesis of TGF-β, which suppresses the immune response through inhibition of the inflammatory mediators, such as NF-ƙB and STAT-3.125 Mice lacking STAT6 in the intestinal epithelium are less sensitive to oxazolone-induced colitis, which is also associated with decreased claudin-2 expression and generation of TH2 cytokines in the colon and inflammatory mediators.126
Figure 4.
Influence of inflammatory mediators on intestinal claudin proteins. Cldn, claudin; EV, endocytic vesicle
Table 1.
Claudin expression in the colon during IBD (Crohn’s disease and ulcerative colitis) and experimental colitis murine models.
| Claudin | Function/permeability properties | Experimental colitis in mice | IBD samples | |
|---|---|---|---|---|
| UC | CD | |||
| 1 | Cation barrier35 | ↑120 | ↑86,119 | ↑86 |
|
| ||||
| 2 | Cation pore and paracellular water channel37,43,61 | – | ↑25,86,118 | ↑26,86 |
|
| ||||
| 3 | Cation barrier15 | – | ↓25 | ↓25 |
|
| ||||
| 4 | Cation barrier36 | – | ↓25,86 | – |
|
| ||||
| 5 | Cation barrier37 | – | – | ↓26 |
|
| ||||
| 7 | Anion barrier and pore38,39,40 | – | ↓86 | – |
|
| ||||
| 8 | Cation barrier54 | – | – | ↓26 |
|
| ||||
| 12 | Barrier | – | ↓74 | |
|
| ||||
| 18 | Barrier15 | ↑ TNBS127 | ↑128 | – |
NOTE: UC, ulcerative colitis; CD, Crohn’s disease; TNBS, trinitrobenzenesulfonic acid.
Increased claudin-18 expression has been noted in the intestinal mucosa of ulcerative colitis patients and in mice with trinitrobenzenesulfonic acid (TNBS) colitis.127 Since claudin-18 is predominantly expressed in the stomach, its induction (along with expression of some other markers of gastric epithelium) suggests localized shifts in intestinal stem cell differentiation toward the gastric program in ulcerative colitis patients.127,128 The increase of claudin-18 was also recently demonstrated in epithelial organoid cultures from ulcerative colitis patients128 (Table 1).
Biopsies from individuals with ulcerative colitis have increased epithelial claudin-1 in actively inflamed intestinal mucosa that is accompanied by elevated levels of cleaved Notch and suppression of Muc-2.107 Deletion of the Muc-2 gene in mice increased sensitivity to dextran sodium sulfate (DSS) colitis and delayed tissue repair. However, HIF1β knockout mice with TNBS colitis have decreased epithelial claudin-1 levels, and deletion of the HIF1β gene in Caco-2 cells decreases claudin-1 expression, which is associated with barrier compromise.46 The above studies suggest that inflammation-mediated changes in claudin-1 are context dependent and vary with the nature of injury, time of onset, and duration of the inflammatory response. Changes in the widely expressed claudin-3 in the intestine have been observed in the mucosal tissue of inflammatory bowel disease patients,25 and claudin-7 is decreased in the intestinal epithelium of individuals with ulcerative colitis.83,118
A number of in vitro and in vivo studies have addressed mechanisms by which inflammatory mediators (TNF-α, IFNγ, IL-1, IL-4 IL-6, IL-13, IL-17) modify the claudin composition of TJs in different cell lines100,119,129–134 (Table 2). In response to inflammation, differential changes in expression of select intestinal epithelial claudins influence the overall balance of TJ claudins and, ultimately, barrier function. We recently observed that increased expression of intestinal epithelial claudin-2 in response to IFNγ signaling influences the dynamics of the barrier-forming claudin-4 that are mediated by a competition of claudin-2 and claudin-4 for residence within the epithelial TJ, thereby contributing to barrier compromise.124 The proinflammatory cytokines IFNγ and TNF-α promote endocytosis of select TJ transmembrane proteins, including claudin-1 and claudin-4, from the plasma membrane. The underlying mechanisms that mediate this effect are dependent on the stimulus that induces junctional remodeling. While IFNγ promotes endocytosis of TJ transmembrane proteins, including claudin-1 and occludin, TNF-α exposure promotes endocytosis of occludin via a caveolar-mediated mechanism.135,136 Such endocytic events are further orchestrated by restructuring of the perijunction actin–myosin II. In response to proinflammatory cytokine (IFNγ, TNF-α, IL13, IL-1β) signaling proteins that converge to influence perijunctional actin myosin II dynamics, TJ transmembrane protein restructuring and barrier changes include phosphorylation and activation of myosin light-chain Rho GTPase, with downstream activation of Rho-associated kinase and myosin light-chain kinase.137 These signaling mediators maintain balanced actin–myosin contraction that is important in controlling overall TJ-mediated barrier function (Fig. 4B).22 Additionally, in vitro studies suggest that TNF-α signaling also influences proteins involved in controlling epithelial polarity (atypical PKC–PAR6–PAR3) and the TJ plaque protein ZO1.138
Table 2.
Cytokine effects on claudin expression in IEC lines.
| Cytokine | Claudin expression | Cells | References |
|---|---|---|---|
| TNF-α | ↑ Claudin-1 | IEC-18 | 119 |
| TNF-α | ↑ Claudin-2 | HT-29/B6 | 100 |
| TNF-α | ↓ Claudin-2 | T84 | 134 |
| TNF-α | No change in claudin-2 | T84 | 129 |
| INFγ + TNF-α |
↓ Claudin-2 ↓ Claudin-3 |
T84 | 25 |
| IL-1β |
↑ Claudin-1 ↓ Occludin |
Caco-2 | 132 |
| IL-4 | ↑ Claudin-2 | T84 | 134 |
| IL-6 | ↑ Claudin-2 | Caco-2 | 131 |
| IL-6 | ↑ Claudin-2 | Caco-2 | 133 |
| IL-13 | ↑ Claudin-2 | T84 | 129 |
| IL-13 |
↑ Claudin-2 ↑ Claudin-4 |
T84 | 25 |
| IL-17 |
↑ Claudin-1 mRNA ↑ Claudin-2 mRNA |
T84 | 130 |
Cell lines: HT-29/B6 and Caco-2, human colorectal carcinoma; IEC-18, non transformed small intestine; T84, colorectal carcinoma form lung metastasis.
Conclusions
Dynamic physiologic claudin protein regulation along the intestine, as well as in the crypt-luminal axis, plays a vital role in controlling barrier function and mucosal homeostasis. While the mechanisms that control spatial claudin expression are incompletely understood, epithelial transcriptional control and intracellular trafficking programs linked to cell proliferation and differentiation have been proposed to orchestrate such regional expression programs. Such precise organization of claudin proteins is perturbed in response to mucosal inflammation that compromises the intestinal epithelial barrier function, which further perpetuates the inflammatory response. In addition to playing a pivotal role in controlling epithelial barrier function, recent studies have shed light on claudin protein regulation of cellular programs that govern epithelial homeostasis. Future studies aimed at identifying spatial control of claudin proteins in the gut will not only be important in understanding the pathogenesis of diseases associated with the leaky epithelial barrier but will also provide direction for developing therapies to enhance epithelial barrier function and inhibit mucosal inflammatory responses.
Acknowledgments
This work was supported by NIH grants (RO1DK59888, RO1DK055679) to A. Nusrat and the Crohn’s and Colitis Foundation of America Research Fellowship Award (326912) to M. Quiros.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 3.Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35–60. doi: 10.1146/annurev-pathol-011811-120905. [DOI] [PubMed] [Google Scholar]
- 4.Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14:531–536. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 5.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2:a002907. doi: 10.1101/cshperspect.a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harbor Perspectives in Biology. 2009;1:a002584–a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner JR. Show me the pathway! Regulation of paracellular permeability by Na(+)-glucose cotransport. Adv Drug Deliv Rev. 2000;41:265–281. doi: 10.1016/s0169-409x(00)00046-6. [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran SA, Beyenbach KW, Rajasekaran AK. Interactions of tight junctions with membrane channels and transporters. Biochim Biophys Acta. 2008;1778:757–769. doi: 10.1016/j.bbamem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Kapus A, Szaszi K. Coupling between apical and paracellular transport processes. Biochem Cell Biol. 2006;84:870–880. doi: 10.1139/o06-202. [DOI] [PubMed] [Google Scholar]
- 10.Farquhar MG, Palade GE. Junctional complexes in various epithelia. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 12.Quiros M, Nusrat A. RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex. Semin Cell Dev Biol. 2014;36:194–203. doi: 10.1016/j.semcdb.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Mandell KJ, Parkos CA, Mrsny RJ, Nusrat A. The second loop of occludin is required for suppression of Raf1-induced tumor growth. Oncogene. 2005;24:4412–4420. doi: 10.1038/sj.onc.1208634. [DOI] [PubMed] [Google Scholar]
- 14.Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N. Molecular organization of tricellular tight junctions. Tissue Barriers. 2014;2:e28960. doi: 10.4161/tisb.28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunzel D, Fromm M. Claudins and other tight junction proteins. Compr Physiol. 2012;2:1819–1852. doi: 10.1002/cphy.c110045. [DOI] [PubMed] [Google Scholar]
- 16.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–461. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 17.Itoh M, et al. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Van Itallie CM, Tietgens AJ, Anderson JM. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol Biol Cell. 2016 doi: 10.1091/mbc.E16-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capaldo CT, et al. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell. 2014;25:2710–2719. doi: 10.1091/mbc.E14-02-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. Journal of Oncology. 2010;2010:541957–541911. doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabaries S, Siegel PM. The role of claudins in cancer metastasis. Oncogene. 2016 doi: 10.1038/onc.2016.289. [DOI] [PubMed] [Google Scholar]
- 25.Prasad S, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Laboratory investigation; a journal of technical methods and pathology. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 26.Zeissig S, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. AJP: Cell Physiology. 2002;283:C142–147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 28.Krug SM, et al. Charge-selective claudin channels. Ann N Y Acad Sci. 2012;1257:20–28. doi: 10.1111/j.1749-6632.2012.06555.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Itallie CM, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. Journal of Cell Science. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 30.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. 2014;36:204–212. doi: 10.1016/j.semcdb.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mineta K, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–6128. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Mrsny RJ, et al. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am J Pathol. 2008;172:905–915. doi: 10.2353/ajpath.2008.070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. AJP: Cell Physiology. 2003;284:C1346–1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 35.Furuse M, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. The Journal of Cell Biology. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amasheh S, et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 38.Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl- permeability. Biochem Biophys Res Commun. 2007;357:87–91. doi: 10.1016/j.bbrc.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 39.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005;118:2683–2693. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 40.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem. 2006;281:36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- 41.Konrad M, et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amasheh S. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. Journal of Cell Science. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 43.Rosenthal R, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 44.Tamura A, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki H, et al. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science. 2014;344:304–3071. doi: 10.1126/science.1248571. [DOI] [PubMed] [Google Scholar]
- 46.Saeedi BJ, et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell. 2015;26:2252–2262. doi: 10.1091/mbc.E14-07-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markov AG, Veshnyakova A, Fromm M, Amasheh M, Amasheh S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B. 2010;180:591–598. doi: 10.1007/s00360-009-0440-7. [DOI] [PubMed] [Google Scholar]
- 48.Milatz S, et al. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010;1798:2048–2057. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2011;301:L40–490. doi: 10.1152/ajplung.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou J, Waldegger S, Renigunta A, Yang J. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. 2010 doi: 10.1073/pnas.1009399107/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hering NA, et al. Transforming growth factor-beta, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J Nutr. 2011;141:783–789. doi: 10.3945/jn.110.137588. [DOI] [PubMed] [Google Scholar]
- 52.Ohtsuki S, et al. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;2103:81–86. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka H, et al. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut. 2015;64:1529–38. doi: 10.1136/gutjnl-2014-308419. [DOI] [PubMed] [Google Scholar]
- 54.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem. 2003;278:17350–17359. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 55.Amasheh S, et al. Tight junction proteins as channel formers and barrier builders. Ann N Y Acad Sci. 2009;1165:211–219. doi: 10.1111/j.1749-6632.2009.04439.x. [DOI] [PubMed] [Google Scholar]
- 56.Yu ASL, Angelow S, Schneeberger EE. Claudin-8 Expression in Renal Epithelial Cells Augments the Paracellular Barrier by Replacing Endogenous Claudin-2. Journal of Membrane Biology. 2007;215:147–159. doi: 10.1007/s00232-007-9014-3. [DOI] [PubMed] [Google Scholar]
- 57.Angelow S, Schneeberger EE, Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol. 2007;215:147–1593. doi: 10.1007/s00232-007-9014-3. [DOI] [PubMed] [Google Scholar]
- 58.Angelow S, Kim KJ, Yu AS. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J Physiol. 2006;571:15–26. doi: 10.1113/jphysiol.2005.099135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amasheh S, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 60.Rosenthal R, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. Journal of Cell Science. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 61.Yu ASL, et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. The Journal of General Physiology. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annual Review of Physiology. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 63.Van Itallie CM, et al. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. Journal of Cell Science. 2012;125:4902–4912. doi: 10.1242/jcs.111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. Journal of Cell Science. 2005;118:1427–1436. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 65.Van Itallie CM, Mitic LL, Anderson JM. SUMOylation of claudin-2. Annals of the New York Academy of Sciences. 2012;1258:60–64. doi: 10.1111/j.1749-6632.2012.06541.x. [DOI] [PubMed] [Google Scholar]
- 66.Ruffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. European journal of cell biology. 2004;83:135–144. doi: 10.1078/0171-9335-00366. [DOI] [PubMed] [Google Scholar]
- 67.Van Itallie CM, Tietgens AJ, Anderson JM. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol Biol Cell. 2017;28:524–534. doi: 10.1091/mbc.E16-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dravis C, Henkemeyer M. Ephrin-B reverse signaling controls septation events at the embryonic midline through separate tyrosine phosphorylation-independent signaling avenues. Dev Biol. 2011;355:138–151. doi: 10.1016/j.ydbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Findley MK, Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61:431–4375. doi: 10.1002/iub.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. Journal of Biological Chemistry. 2007;2820:30005–30013. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 71.Piontek J, et al. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 72.Nunes FD, et al. Distinct subdomain organization and molecular composition of a tight junction with adherens junction features. J Cell Sci. 2006;1193:4819–4827. doi: 10.1242/jcs.03233. [DOI] [PubMed] [Google Scholar]
- 73.Lu Z, Ding L, Lu Q, Chen YH. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers. 2013;1:e249788. doi: 10.4161/tisb.24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lameris AL, et al. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48:58–69. doi: 10.3109/00365521.2012.741616. [DOI] [PubMed] [Google Scholar]
- 75.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Fujita H. Differential Expression and Subcellular Localization of Claudin-7, -8, -12, -13, and -15 Along the Mouse Intestine. Journal of Histochemistry and Cytochemistry. 2006;54:933–944. doi: 10.1369/jhc.6A6944.2006. [DOI] [PubMed] [Google Scholar]
- 77.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 78.Niimi T, et al. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol. 2001;21:7380–7390. doi: 10.1128/MCB.21.21.7380-7390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jovov B, et al. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. American journal of physiology Gastrointestinal and liver physiology. 2007;293:G1106–1113. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 80.Lili LN, et al. Claudin-based barrier differentiation in the colonic epithelial crypt niche involves Hopx/Klf4 and Tcf7l2/Hnf4-alpha cascades. Tissue Barriers. 2016;4:e1214038. doi: 10.1080/21688370.2016.1214038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujita H, et al. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2006;54:933–944. doi: 10.1369/jhc.6A6944.2006. [DOI] [PubMed] [Google Scholar]
- 82.Luissint AC, Parkos CA, Nusrat A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology. 2016;151:616–632. doi: 10.1053/j.gastro.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding L, et al. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology. 2012;142:305–315. doi: 10.1053/j.gastro.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farkas AE, et al. HNF4alpha regulates claudin-7 protein expression during intestinal epithelial differentiation. Am J Pathol. 2015;185:2206–2218. doi: 10.1016/j.ajpath.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Laboratory Investigation. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tabaries S, et al. Claudin-2 is selectively enriched in and promotes the formation of breast cancer liver metastases through engagement of integrin complexes. Oncogene. 2010;30:1318–1328. doi: 10.1038/onc.2010.518. [DOI] [PubMed] [Google Scholar]
- 88.Tabariès S, et al. Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes. Molecular and Cellular Biology. 2012;32:2979–2991. doi: 10.1128/MCB.00299-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhawan P, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zwanziger D, et al. The impact of CLAUDIN-1 on follicular thyroid carcinoma aggressiveness. Endocr Relat Cancer. 2015;22:819–830. doi: 10.1530/ERC-14-0502. [DOI] [PubMed] [Google Scholar]
- 91.French AD, et al. PKC and PKA phosphorylation affect the subcellular localization of claudin-1 in melanoma cells. Int J Med Sci. 2009;6:93–101. doi: 10.7150/ijms.6.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miwa N, et al. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12:469–476. doi: 10.3727/096504001108747477. [DOI] [PubMed] [Google Scholar]
- 93.Huang J, et al. Claudin-1 enhances tumor proliferation and metastasis by regulating cell anoikis in gastric cancer. Oncotarget. 2015;6:1652–1665. doi: 10.18632/oncotarget.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–2879. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beck F, Stringer EJ. The role of Cdx genes in the gut and in axial development. Biochem Soc Trans. 2010;38:353–357. doi: 10.1042/BST0380353. [DOI] [PubMed] [Google Scholar]
- 96.Sakaguchi T, et al. Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem. 2002;277:21361–213700. doi: 10.1074/jbc.M110261200. [DOI] [PubMed] [Google Scholar]
- 97.Bhat AA, et al. Caudal homeobox protein Cdx-2 cooperates with Wnt pathway to regulate claudin-1 expression in colon cancer cells. PLoS One. 2012;7:e37174. doi: 10.1371/journal.pone.0037174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang YG, et al. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci Rep. 2015;5:10642. doi: 10.1038/srep10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol. 2005;203:15–269. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- 100.Mankertz J, et al. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009;336:67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- 101.Babeu JP, Boudreau F. Hepatocyte nuclear factor 4-alpha involvement in liver and intestinal inflammatory networks. World J Gastroenterol. 2014;202:22–30. doi: 10.3748/wjg.v20.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Polak-Charcon S, Shoham J, Ben-Shaul Y. Tight junctions in epithelial cells of human fetal hindgut, normal colon, and colon adenocarcinoma. J Natl Cancer Inst. 1980;65:53–62. [PubMed] [Google Scholar]
- 103.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 104.Utech M, Mennigen R, Bruewer M. Endocytosis and recycling of tight junction proteins in inflammation. J Biomed Biotechnol. 2010;2010:484987. doi: 10.1155/2010/484987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. Journal of Cell Science. 2004;117:1247–1257. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- 106.Dukes JD, Whitley P, Chalmers AD. The PIKfyve inhibitor YM201636 blocks the continuous recycling of the tight junction proteins claudin-1 and claudin-2 in MDCK cells. PLoS One. 2012;7:e286599. doi: 10.1371/journal.pone.0028659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pope JL, et al. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63:622–634. doi: 10.1136/gutjnl-2012-304241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Okamoto R, et al. Requirement of Notch activation during regeneration of the intestinal epithelia. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G23–35. doi: 10.1152/ajpgi.90225.2008. [DOI] [PubMed] [Google Scholar]
- 109.Muto S, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A. 2010;107:8011–8016. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dhawan P, et al. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene. 2011;30:3234–3247. doi: 10.1038/onc.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mankertz J, et al. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochemical and Biophysical Research Communications. 2004;314:1001–1007. doi: 10.1016/j.bbrc.2003.12.185. [DOI] [PubMed] [Google Scholar]
- 112.Ikari A, et al. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim Biophys Acta. 2014;18437:2079–2088. doi: 10.1016/j.bbamcr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 113.Tamura A, et al. Megaintestine in claudin-15-deficient mice. Gastroenterology. 2008;134:523–534. doi: 10.1053/j.gastro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 114.Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol. 2007;9:954–960. doi: 10.1038/ncb1621. [DOI] [PubMed] [Google Scholar]
- 115.Wada M, Tamura A, Takahashi N, Tsukita S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology. 2013;144:369–380. doi: 10.1053/j.gastro.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 116.Schmitz H, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 117.Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590:1035–1044. doi: 10.1113/jphysiol.2011.224568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. Journal of Gastroenterology and Hepatology. 2008;23(Suppl 2):S146–150. doi: 10.1111/j.1440-1746.2008.05405.x. [DOI] [PubMed] [Google Scholar]
- 119.Poritz LS, Harris LR, 3rd, Kelly AA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci. 2011;56:2802–2809. doi: 10.1007/s10620-011-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Poritz LS, et al. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 121.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;232:379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 122.Heller F, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 123.Weber CR, et al. Claudin-2-dependent paracellular channels are dynamically gated. Elife. 2015;4:e09906. doi: 10.7554/eLife.09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Capaldo CT, et al. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Molecular Biology of the Cell. 2014;25:2710–2719. doi: 10.1091/mbc.E14-02-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ahmad R, et al. Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunology. 2014:1–14. doi: 10.1038/mi.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosen MJ, et al. STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines. Journal of immunology (Baltimore, Md: 1950) 2013;190:1849–1858. doi: 10.4049/jimmunol.1201373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zwiers A, et al. Increased expression of the tight junction molecule claudin-18 A1 in both experimental colitis and ulcerative colitis. Inflamm Bowel Dis. 2008;14:1652–1659. doi: 10.1002/ibd.20695. [DOI] [PubMed] [Google Scholar]
- 128.Dotti I, et al. Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut. 2016 doi: 10.1136/gutjnl-2016-312609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weber CR, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 131.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. The Journal of biological chemistry. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Al-Sadi R, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One. 2014;9:e85345. doi: 10.1371/journal.pone.0085345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wisner DM, Poritz LS, Harris LR, Green CL. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. The Journal of surgical research. 2008;144:1–7. doi: 10.1016/j.jss.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 135.Utech M, et al. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell. 2007;18:3429–3439. doi: 10.1091/mbc.E07-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mashukova A, Wald FA, Salas PJ. Tumor necrosis factor alpha and inflammation disrupt the polarity complex in intestinal epithelial cells by a posttranslational mechanism. Mol Cell Biol. 2011;31:756–765. doi: 10.1128/MCB.00811-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


