Abstract
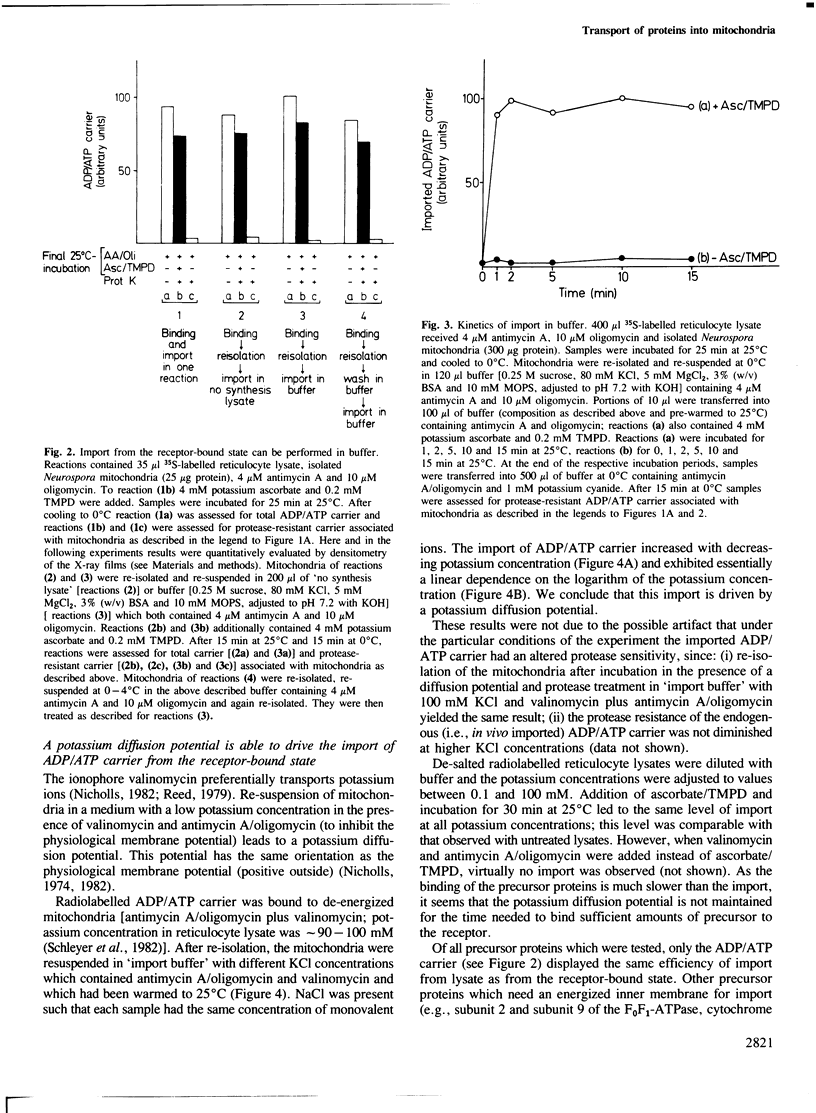
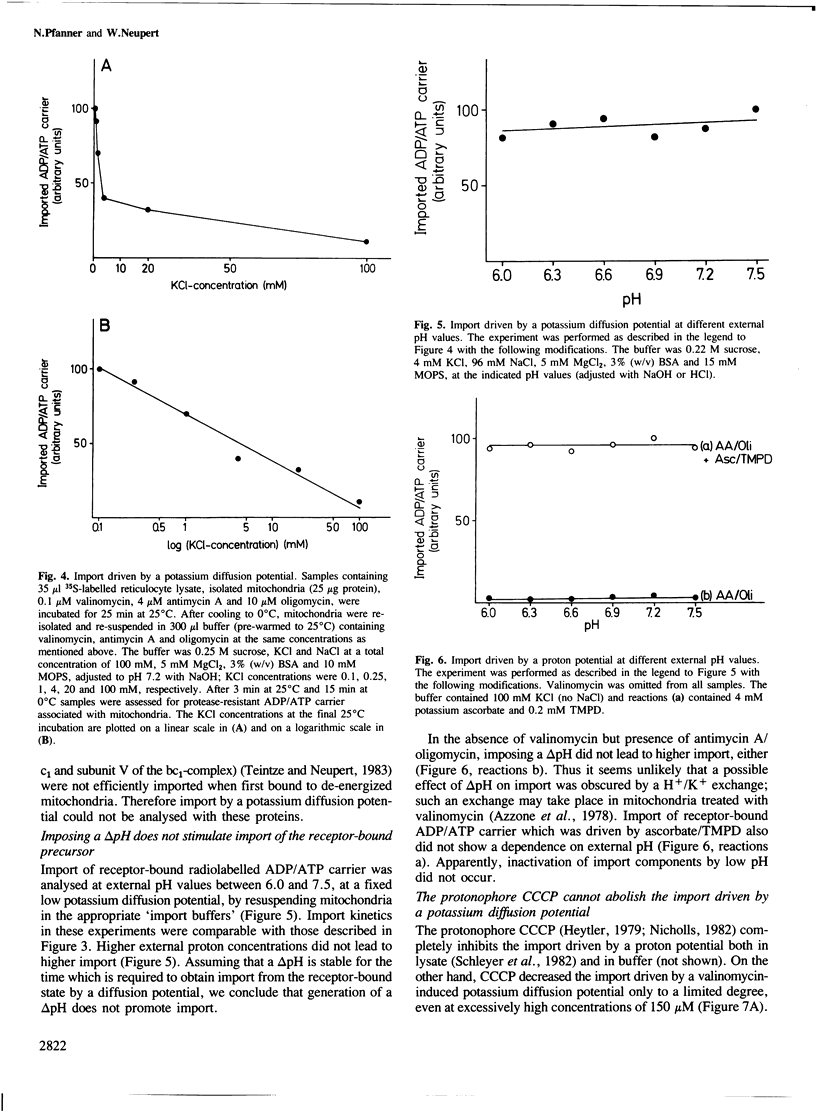
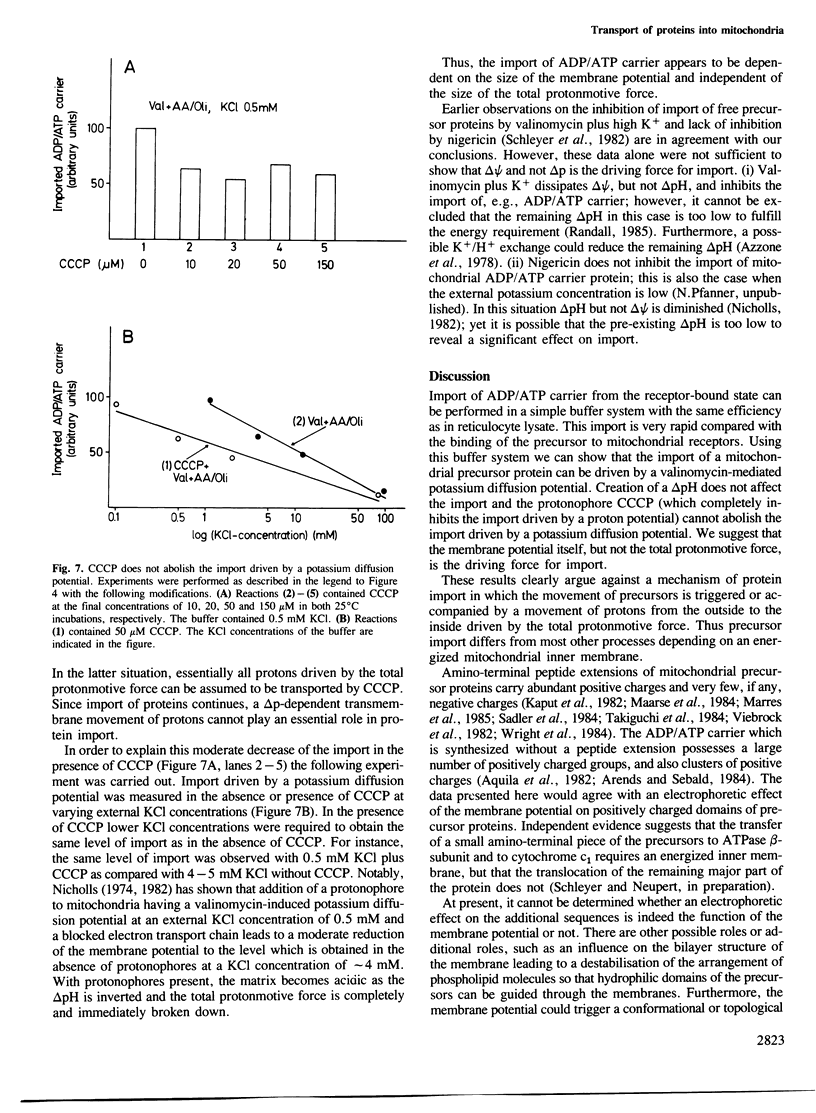
The transfer of cytoplasmically synthesized precursor proteins into or across the inner mitochondrial membrane is dependent on energization of the membrane. To investigate the role of this energy requirement, a buffer system was developed in which efficient import of ADP/ATP carrier into mitochondria from the receptor-bound state occurred. This import was rapid and was dependent on divalent cations, whereas the binding of precursor proteins to the mitochondrial surface was slow and was independent of added divalent cations. Using this buffer system, the import of ADP/ATP carrier could be driven by a valinomycin-induced potassium diffusion potential. The protonophore carbonylcyanide m-chlorophenyl-hydrazone was not able to abolish this import. Imposition of a delta pH did not stimulate the import. We conclude that the membrane potential delta psi itself and not the total protonmotive force delta p is the required energy source.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquila H., Misra D., Eulitz M., Klingenberg M. Complete amino acid sequence of the ADP/ATP carrier from beef heart mitochondria. Hoppe Seylers Z Physiol Chem. 1982 Mar;363(3):345–349. [PubMed] [Google Scholar]
- Arends H., Sebald W. Nucleotide sequence of the cloned mRNA and gene of the ADP/ATP carrier from Neurospora crassa. EMBO J. 1984 Feb;3(2):377–382. doi: 10.1002/j.1460-2075.1984.tb01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzone G. F., Bortolotto F., Zanotti A. Induction of electroneutral exchanges of H+ with K+ in rat liver mitochondria. FEBS Lett. 1978 Dec 1;96(1):135–140. doi: 10.1016/0014-5793(78)81078-3. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Fenton W. A., Rosenberg L. E. Processing of pre-ornithine transcarbamylase requires a zinc-dependent protease localized to the mitochondrial matrix. Biochem Biophys Res Commun. 1982 Mar 15;105(1):1–7. doi: 10.1016/s0006-291x(82)80002-8. [DOI] [PubMed] [Google Scholar]
- Daniels C. J., Bole D. G., Quay S. C., Oxender D. L. Role for membrane potential in the secretion of protein into the periplasm of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5396–5400. doi: 10.1073/pnas.78.9.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Zwizinski C., Ludmerer S., Wickner W. Mechanisms of membrane assembly: effects of energy poisons on the conversion of soluble M13 coliphage procoat to membrane-bound coat protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):827–831. doi: 10.1073/pnas.77.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hennig B., Koehler H., Neupert W. Receptor sites involved in posttranslational transport of apocytochrome c into mitochondria: specificity, affinity, and number of sites. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4963–4967. doi: 10.1073/pnas.80.16.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heytler P. G. Uncouplers of oxidative phosphorylation. Methods Enzymol. 1979;55:462–442. doi: 10.1016/0076-6879(79)55060-5. [DOI] [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Klingenberg M., Aquila H., Riccio P. Isolation of functional membrane proteins related to or identical with the ADP, ATP carrier of mitochondria. Methods Enzymol. 1979;56:407–414. doi: 10.1016/0076-6879(79)56039-x. [DOI] [PubMed] [Google Scholar]
- Kolansky D. M., Conboy J. G., Fenton W. A., Rosenberg L. E. Energy-dependent translocation of the precursor of ornithine transcarbamylase by isolated rat liver mitochondria. J Biol Chem. 1982 Jul 25;257(14):8467–8471. [PubMed] [Google Scholar]
- Maarse A. C., Van Loon A. P., Riezman H., Gregor I., Schatz G., Grivell L. A. Subunit IV of yeast cytochrome c oxidase: cloning and nucleotide sequencing of the gene and partial amino acid sequencing of the mature protein. EMBO J. 1984 Dec 1;3(12):2831–2837. doi: 10.1002/j.1460-2075.1984.tb02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marres C. A., Van Loon A. P., Oudshoorn P., Van Steeg H., Grivell L. A., Slater E. C. Nucleotide sequence analysis of the nuclear gene coding for manganese superoxide dismutase of yeast mitochondria, a gene previously assumed to code for the Rieske iron-sulphur protein. Eur J Biochem. 1985 Feb 15;147(1):153–161. doi: 10.1111/j.1432-1033.1985.tb08731.x. [DOI] [PubMed] [Google Scholar]
- Müller M., Blobel G. In vitro translocation of bacterial proteins across the plasma membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7421–7425. doi: 10.1073/pnas.81.23.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Ionophores. Methods Enzymol. 1979;55:435–454. doi: 10.1016/0076-6879(79)55058-7. [DOI] [PubMed] [Google Scholar]
- Sadler I., Suda K., Schatz G., Kaudewitz F., Haid A. Sequencing of the nuclear gene for the yeast cytochrome c1 precursor reveals an unusually complex amino-terminal presequence. EMBO J. 1984 Sep;3(9):2137–2143. doi: 10.1002/j.1460-2075.1984.tb02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of ADP/ATP carrier into mitochondria. Precursor imported in vitro acquires functional properties of the mature protein. J Biol Chem. 1984 Mar 25;259(6):3487–3491. [PubMed] [Google Scholar]
- Schleyer M., Schmidt B., Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982 Jun 15;125(1):109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Schmidt B., Wachter E., Sebald W., Neupert W. Processing peptidase of Neurospora mitochondria. Two-step cleavage of imported ATPase subunit 9. Eur J Biochem. 1984 Nov 2;144(3):581–588. doi: 10.1111/j.1432-1033.1984.tb08505.x. [DOI] [PubMed] [Google Scholar]
- Sebald W., Neupert W., Weiss H. Preparation of Neurospora crassa mitochondria. Methods Enzymol. 1979;55:144–148. doi: 10.1016/0076-6879(79)55020-4. [DOI] [PubMed] [Google Scholar]
- Takiguchi M., Miura S., Mori M., Tatibana M., Nagata S., Kaziro Y. Molecular cloning and nucleotide sequence of cDNA for rat ornithine carbamoyltransferase precursor. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7412–7416. doi: 10.1073/pnas.81.23.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Wright R. M., Ko C., Cumsky M. G., Poyton R. O. Isolation and sequence of the structural gene for cytochrome c oxidase subunit VI from Saccharomyces cerevisiae. J Biol Chem. 1984 Dec 25;259(24):15401–15407. [PubMed] [Google Scholar]
- Zimmermann R., Neupert W. Transport of proteins into mitochondria. Posttranslational transfer of ADP/ATP carrier into mitochondria in vitro. Eur J Biochem. 1980 Aug;109(1):217–229. doi: 10.1111/j.1432-1033.1980.tb04787.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Wickner W. Energetics and intermediates of the assembly of Protein OmpA into the outer membrane of Escherichia coli. J Biol Chem. 1983 Mar 25;258(6):3920–3925. [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Proteinaceous receptors for the import of mitochondrial precursor proteins. J Biol Chem. 1984 Jun 25;259(12):7850–7856. [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Transfer of proteins into mitochondria. Precursor to the ADP/ATP carrier binds to receptor sites on isolated mitochondria. J Biol Chem. 1983 Apr 10;258(7):4071–4074. [PubMed] [Google Scholar]
- von Heijne G. Analysis of the distribution of charged residues in the N-terminal region of signal sequences: implications for protein export in prokaryotic and eukaryotic cells. EMBO J. 1984 Oct;3(10):2315–2318. doi: 10.1002/j.1460-2075.1984.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



