Abstract
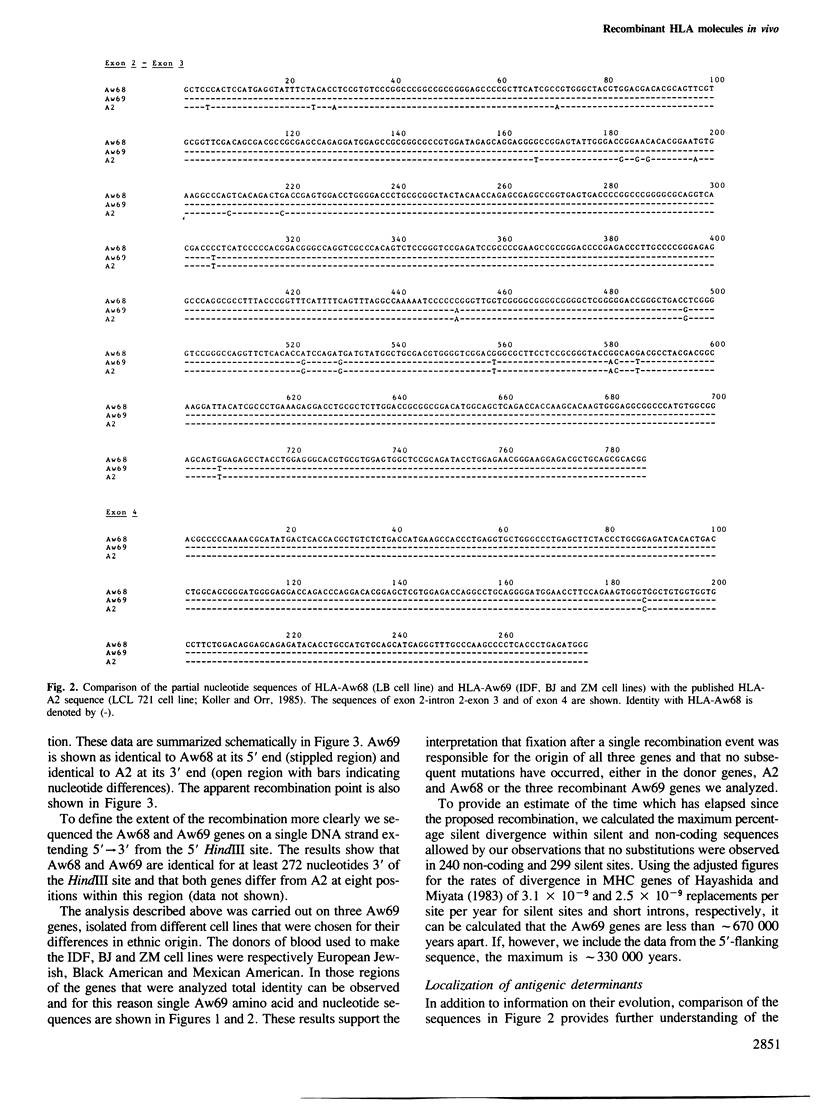
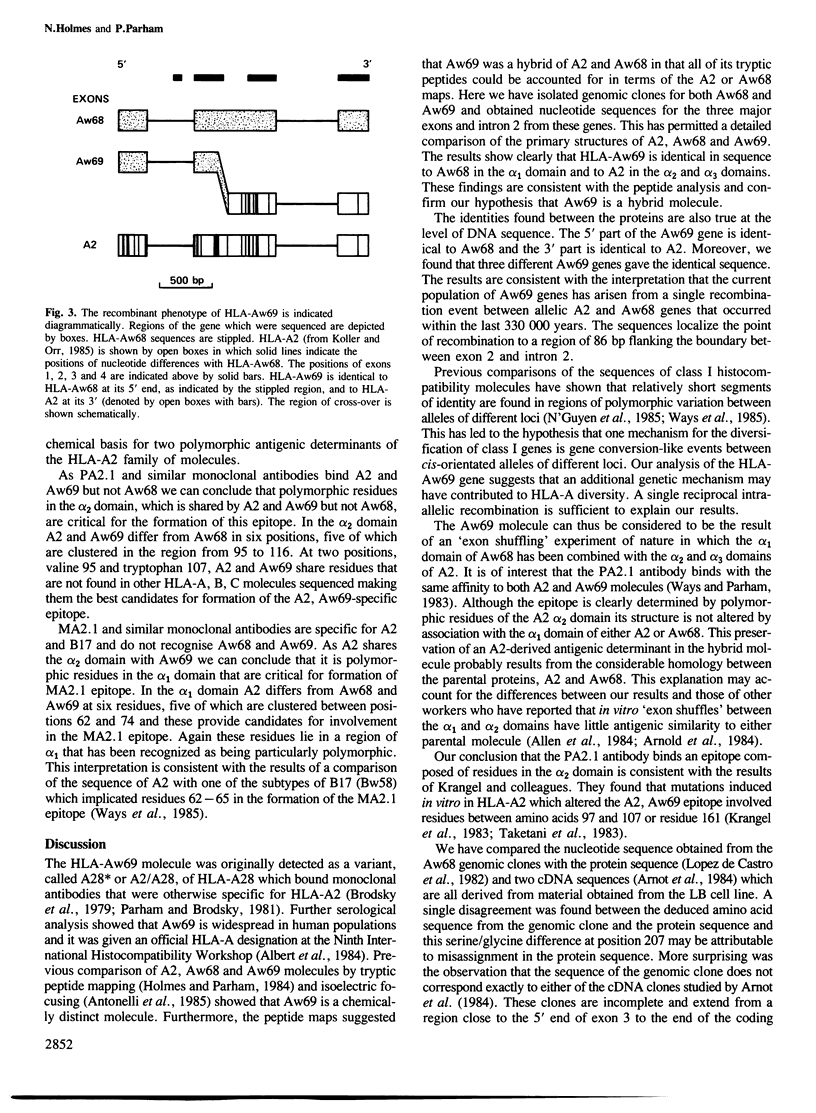
The human class I histocompatibility molecule HLA-Aw69 has serological and structural properties which suggested it was a hybrid of the allelic products HLA-A2 and HLA-Aw68. We have now isolated three genes for HLA-Aw69 and one gene for HLA-Aw68. The sequences of exons encoding the entire extracellular portion of the molecule and of intron 2 have been determined. Their comparison with the published sequence of HLA-A2 proves that HLA-Aw69 is a hybrid molecule with complete identity to HLA-Aw68 in the alpha 1 domain and with HLA-A2 in the alpha 2 and alpha 3 domains. This comparison also localised regions involved in the epitopes recognised by monoclonal antibodies. The three HLA-Aw69 genes obtained from unrelated individuals of diverse ethnic backgrounds are identical. All results are consistent with HLA-Aw69 having arisen by a single reciprocal recombination event between the HLA-Aw68 and HLA-A2 genes somewhere in a region of 86 bp about the 3' donor splice site of exon 2. Estimates of the silent mutation rate in HLA genes suggest this event occurred not more than 330 000 years ago. Intra-allelic reciprocal recombination thus represents a further mechanism in addition to gene conversion for the generation of novel class I histocompatibility alleles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Burgert H. G., Hamann U., Hämmerling G., Kees U., Kvist S. Cytolytic T cells recognize the two amino-terminal domains of H-2 K antigens in tandem in influenza A infected cells. Cell. 1984 Aug;38(1):79–87. doi: 10.1016/0092-8674(84)90528-2. [DOI] [PubMed] [Google Scholar]
- Arnot D., Lillie J. W., Auffray C., Kappes D., Strominger J. L. Inter-locus and intra-allelic polymorphisms of HLA class I antigen gene mRNA. Immunogenetics. 1984;20(3):237–252. doi: 10.1007/BF00364206. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Kostyu D. D., Strominger J. L., Krangel M. S. Delineation of immunologically and biochemically distinct HLA-A2 antigens. J Immunol. 1982 Aug;129(2):730–734. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Collins F., Kole R., Stoeckert C. J., Jr, Jagadeeswaran P., Duncan C. H., Weissman S. M., Jagadeeswaran P., Pan J., Forget B. G. Sequences of human repetitive DNA, non-alpha-globin genes, and major histocompatibility locus genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1079–1086. [PubMed] [Google Scholar]
- Gotch F. M., Kelly C., Ellis S. A., Wallace L., Rickinson A. B., van der Poel J., Crumpton M. J., McMichael A. J. Characterization of the HLA-A2.2 subtype: T cell evidence for further heterogeneity. Immunogenetics. 1985;21(1):11–23. doi: 10.1007/BF00372237. [DOI] [PubMed] [Google Scholar]
- Hayashida H., Miyata T. Unusual evolutionary conservation and frequent DNA segment exchange in class I genes of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 May;80(9):2671–2675. doi: 10.1073/pnas.80.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes N., Parham P. Molecular characterization of HLA-A28*, a novel HLA product, and its relationship to HLA-A28 and HLA-A2. Immunogenetics. 1984;20(2):103–115. doi: 10.1007/BF00364482. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Orr H. T. Cloning and complete sequence of an HLA-A2 gene: analysis of two HLA-A alleles at the nucleotide level. J Immunol. 1985 Apr;134(4):2727–2733. [PubMed] [Google Scholar]
- Koller B. H., Sidwell B., DeMars R., Orr H. T. Isolation of HLA locus-specific DNA probes from the 3'-untranslated region. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5175–5178. doi: 10.1073/pnas.81.16.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S., Taketani S., Pious D., Strominger J. L. HLA-A2 mutants immunoselected in vitro. Definition of residues contributing to an HLA-A2-specific serological determinant. J Exp Med. 1983 Jan 1;157(1):324–336. doi: 10.1084/jem.157.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Malissen B., Jordan B. R. Exon/intron organization and complete nucleotide sequence of an HLA gene. Proc Natl Acad Sci U S A. 1982 Feb;79(3):893–897. doi: 10.1073/pnas.79.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Parham P., Rust N., Brodsky F. A monoclonal antibody that recognizes an antigenic determinant shared by HLA A2 and B17. Hum Immunol. 1980 Sep;1(2):121–129. doi: 10.1016/0198-8859(80)90099-3. [DOI] [PubMed] [Google Scholar]
- N'Guyen C., Sodoyer R., Trucy J., Strachan T., Jordan B. R. The HLA-AW24 gene: sequence, surroundings and comparison with the HLA-A2 and HLA-A3 genes. Immunogenetics. 1985;21(5):479–489. doi: 10.1007/BF00430931. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Parham P., Androlewicz M. J., Brodsky F. M., Holmes N. J., Ways J. P. Monoclonal antibodies: purification, fragmentation and application to structural and functional studies of class I MHC antigens. J Immunol Methods. 1982 Sep 17;53(2):133–173. doi: 10.1016/0022-1759(82)90137-5. [DOI] [PubMed] [Google Scholar]
- Parham P., Brodsky F. M. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981 Dec;3(4):277–299. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Russo C., Ng A. K., Pellegrino M. A., Ferrone S. The monoclonal antibody CR11-351 discriminates HLA-A2 variants identified by T cells. Immunogenetics. 1983;18(1):23–35. doi: 10.1007/BF00401353. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan T., Sodoyer R., Damotte M., Jordan B. R. Complete nucleotide sequence of a functional class I HLA gene, HLA-A3: implications for the evolution of HLA genes. EMBO J. 1984 Apr;3(4):887–894. doi: 10.1002/j.1460-2075.1984.tb01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S., Krangel M. S., Pious D., Strominger J. L. Structural analysis of HLA-A2 antigen from immunoselected mutant 8.6.1: further definition of an HLA-A2-specific serological determinant. J Immunol. 1983 Dec;131(6):2935–2938. [PubMed] [Google Scholar]
- Ways J. P., Parham P. The binding of monoclonal antibodies to cell-surface molecules. A quantitative analysis with immunoglobulin G against two alloantigenic determinants of the human transplantation antigen HLA-A2. Biochem J. 1983 Nov 15;216(2):423–432. doi: 10.1042/bj2160423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- van der Poel J. J., Pool J., Goulmy E., van Rood J. J. Differential recognition of the serologically defined HLA-A2 antigen by allogeneic cytotoxic T cells. II. Definition of three HLA-A2 subtypes by CTLs. Immunogenetics. 1983;17(6):599–608. doi: 10.1007/BF00366128. [DOI] [PubMed] [Google Scholar]


