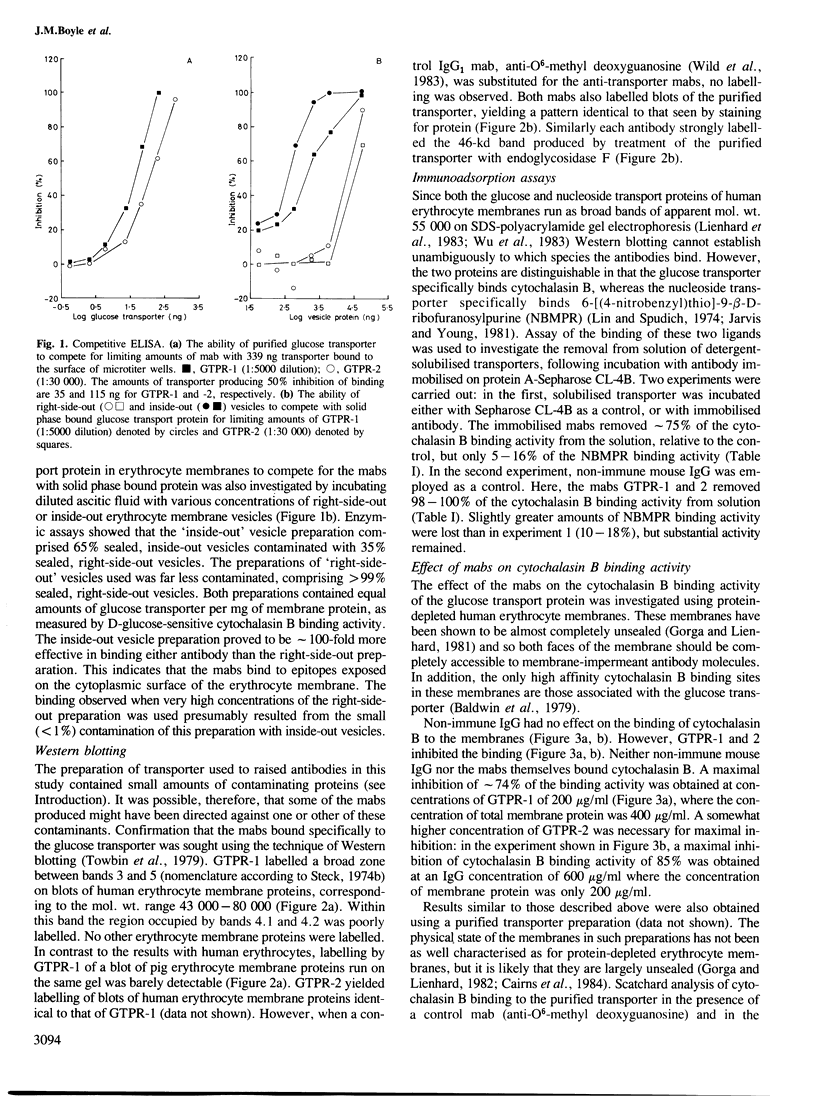
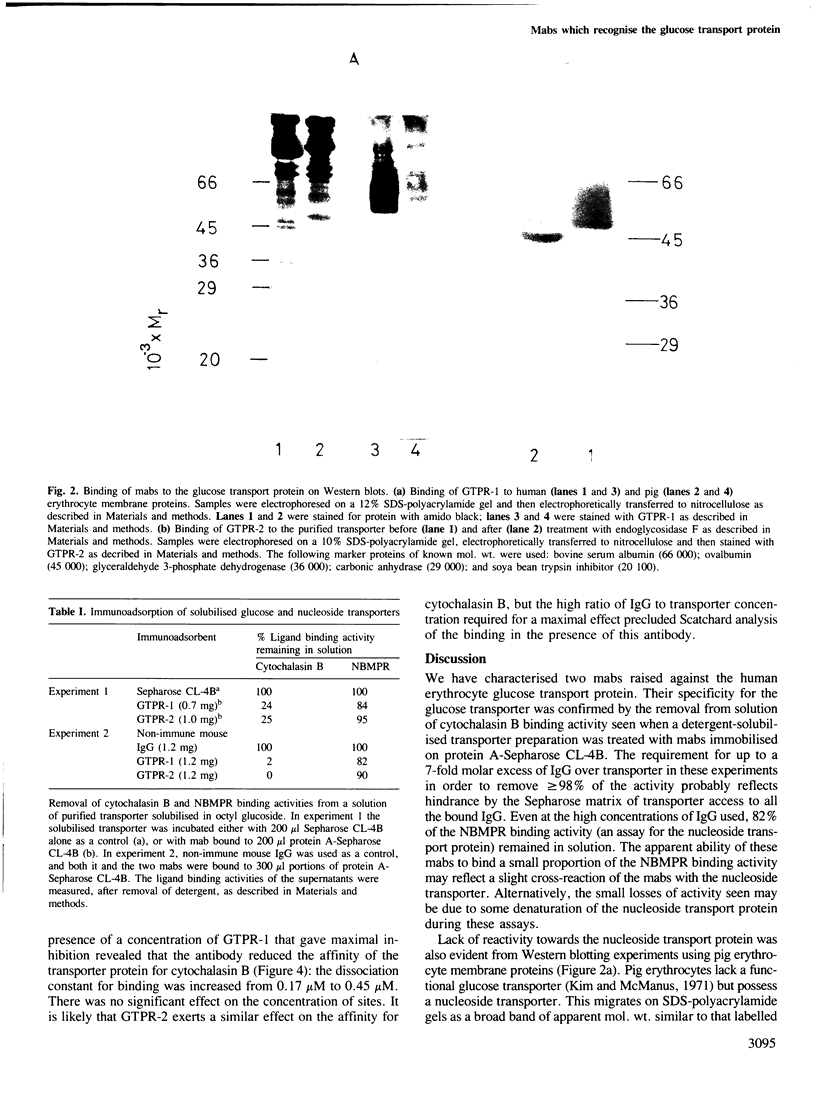
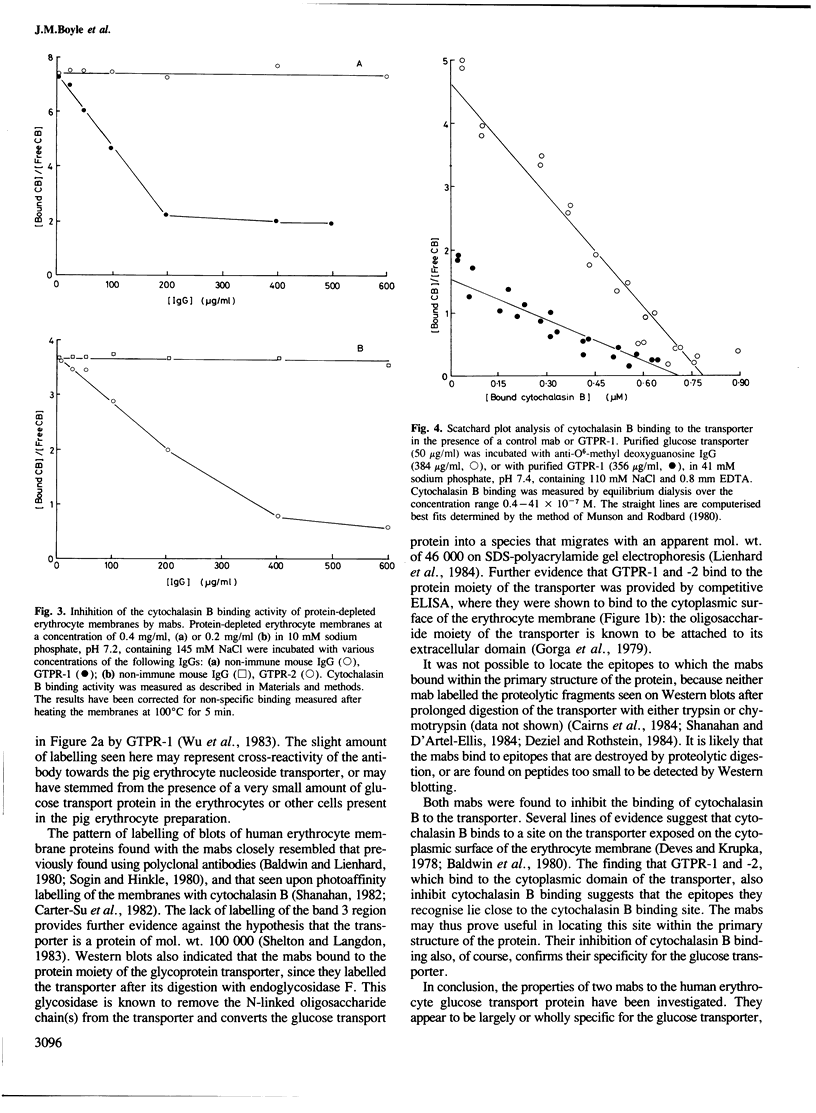
Abstract
Two monoclonal antibodies (mabs) of subclass IgG1 have been raised against the human erythrocyte glucose transport protein. The mabs bound to the purified glucose transporter in both its membrane-bound and detergent-solubilised forms. However, they exhibited little or no binding to the detergent-solubilised nucleoside transport protein, which is present as a minor contaminant in the glucose transport protein preparation. Both mabs inhibited the binding of cytochalasin B to the glucose transport protein, reducing the affinity of this binding by greater than 2-fold. Each mab labelled the transporter polypeptide on Western blots both before and after treatment of the protein with endoglycosidase F, indicating that the epitopes recognised were located on the protein moiety of the glycoprotein. However, the mabs did not bind to the large fragments produced by tryptic or chymotryptic digestion of the native protein, although both mabs were shown to bind to sites on the cytoplasmic surface of the erythrocyte membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M., Gorga J. C., Lienhard G. E. The monosaccharide transporter of the human erythrocyte. Transport activity upon reconstitution. J Biol Chem. 1981 Apr 25;256(8):3685–3689. [PubMed] [Google Scholar]
- Baldwin J. M., Lienhard G. E., Baldwin S. A. The monosaccharide transport system of the human erythrocyte. Orientation upon reconstitution. Biochim Biophys Acta. 1980 Jul;599(2):699–714. doi: 10.1016/0005-2736(80)90211-4. [DOI] [PubMed] [Google Scholar]
- Baldwin S. A., Baldwin J. M., Gorga F. R., Lienhard G. E. Purification of the cytochalasin B binding component of the human erythrocyte monosaccharide transport system. Biochim Biophys Acta. 1979 Mar 23;552(1):183–188. doi: 10.1016/0005-2736(79)90257-8. [DOI] [PubMed] [Google Scholar]
- Baldwin S. A., Baldwin J. M., Lienhard G. E. Monosaccharide transporter of the human erythrocyte. Characterization of an improved preparation. Biochemistry. 1982 Aug 3;21(16):3836–3842. doi: 10.1021/bi00259a018. [DOI] [PubMed] [Google Scholar]
- Baldwin S. A., Lienhard G. E. Immunological identification of the human erythrocyte monosaccharide transporter. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1401–1408. doi: 10.1016/0006-291x(80)90575-6. [DOI] [PubMed] [Google Scholar]
- Cairns M. T., Elliot D. A., Scudder P. R., Baldwin S. A. Proteolytic and chemical dissection of the human erythrocyte glucose transporter. Biochem J. 1984 Jul 1;221(1):179–188. doi: 10.1042/bj2210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Rothstein A. Proteolytic cleavages of cytochalasin B binding components of band 4.5 proteins of the human red blood cell membrane. Biochim Biophys Acta. 1984 Sep 19;776(1):10–20. doi: 10.1016/0005-2736(84)90245-1. [DOI] [PubMed] [Google Scholar]
- Elbrink J., Bihler I. Membrane transport: its relation to cellular metabolic rates. Science. 1975 Jun 20;188(4194):1177–1184. doi: 10.1126/science.1096301. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Baldwin S. A., Lienhard G. E. The monosaccharide transporter from human erythrocytes is heterogeneously glycosylated. Biochem Biophys Res Commun. 1979 Dec 14;91(3):955–961. doi: 10.1016/0006-291x(79)91972-7. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Changes in the intrinsic fluorescence of the human erythrocyte monosaccharide transporter upon ligand binding. Biochemistry. 1982 Apr 13;21(8):1905–1908. doi: 10.1021/bi00537a031. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry. 1981 Sep 1;20(18):5108–5113. doi: 10.1021/bi00521a003. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Extraction and partial purification of the nucleoside-transport system from human erythrocytes based on the assay of nitrobenzylthioinosine-binding activity. Biochem J. 1981 Jan 15;194(1):331–339. doi: 10.1042/bj1940331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kim H. D., McManus T. J. Studies on the energy metabolism of pig red cells. I. The limiting role of membrane permeability in glycolysis. Biochim Biophys Acta. 1971 Jan 26;230(1):1–11. doi: 10.1016/0304-4165(71)90048-1. [DOI] [PubMed] [Google Scholar]
- Klip A., Walker D., Ransome K. J., Schroer D. W., Lienhard G. E. Identification of the glucose transporter in rat skeletal muscle. Arch Biochem Biophys. 1983 Oct 1;226(1):198–205. doi: 10.1016/0003-9861(83)90285-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Crabb J. H., Ransome K. J. Endoglycosidase f cleaves the oligosaccharides from the glucose transporter of the human erythrocyte. Biochim Biophys Acta. 1984 Jan 25;769(2):404–410. doi: 10.1016/0005-2736(84)90324-9. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Kim H. H., Ransome K. J., Gorga J. C. Immunological identification of an insulin-responsive glucose transporter. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1150–1156. doi: 10.1016/0006-291x(82)91090-7. [DOI] [PubMed] [Google Scholar]
- Lin S., Spudich J. A. Biochemical studies on the mode of action of cytochalasin B. Cytochalasin B binding to red cell membrane in relation to glucose transport. J Biol Chem. 1974 Sep 25;249(18):5778–5783. [PubMed] [Google Scholar]
- Molday R. S., MacKenzie D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983 Feb 1;22(3):653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Saffhill R., Strickland P. T., Boyle J. M. Sensitive radioimmunoassays for O6-n-butyldeoxyguanosine, O2-n-butylthymidine and O4-n-butylthymidine. Carcinogenesis. 1982;3(5):547–552. doi: 10.1093/carcin/3.5.547. [DOI] [PubMed] [Google Scholar]
- Salter D. W., Baldwin S. A., Lienhard G. E., Weber M. J. Proteins antigenically related to the human erythrocyte glucose transporter in normal and Rous sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1540–1544. doi: 10.1073/pnas.79.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. F. Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J Biol Chem. 1982 Jul 10;257(13):7290–7293. [PubMed] [Google Scholar]
- Shanahan M. F., D'Artel-Ellis J. Orientation of the glucose transporter in the human erythrocyte membrane. Investigation by in situ proteolytic dissection. J Biol Chem. 1984 Nov 25;259(22):13878–13884. [PubMed] [Google Scholar]
- Shelton R. L., Jr, Langdon R. G. Reconstitution of glucose transport using human erythrocyte band 3. Biochim Biophys Acta. 1983 Aug 24;733(1):25–33. doi: 10.1016/0005-2736(83)90087-1. [DOI] [PubMed] [Google Scholar]
- Sogin D. C., Hinkle P. C. Immunological identification of the human erythrocyte glucose transporter. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5725–5729. doi: 10.1073/pnas.77.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland P. T., Boyle J. M. Characterisation of two monoclonal antibodies specific for dimerised and non-dimerised adjacent thymidines in single stranded DNA. Photochem Photobiol. 1981 Nov;34(5):595–601. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. Kinetic properties of the reconstituted glucose transporter from human erythrocytes. J Biol Chem. 1981 Sep 10;256(17):8907–8914. [PubMed] [Google Scholar]
- Wheeler T. J., Simpson I. A., Sogin D. C., Hinkle P. C., Cushman S. W. Detection of the rat adipose cell glucose transporter with antibody against the human red cell glucose transporter. Biochem Biophys Res Commun. 1982 Mar 15;105(1):89–95. doi: 10.1016/s0006-291x(82)80014-4. [DOI] [PubMed] [Google Scholar]
- Wild C. P., Smart G., Saffhill R., Boyle J. M. Radioimmunoassay of O6-methyldeoxyguanosine in DNA of cells alkylated in vitro and in vivo. Carcinogenesis. 1983 Dec;4(12):1605–1609. doi: 10.1093/carcin/4.12.1605. [DOI] [PubMed] [Google Scholar]
- Wu J. S., Kwong F. Y., Jarvis S. M., Young J. D. Identification of the erythrocyte nucleoside transporter as a band 4.5 polypeptide. Photoaffinity labeling studies using nitrobenzylthioinosine. J Biol Chem. 1983 Nov 25;258(22):13745–13751. [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M., Robins M. J., Paterson A. R. Photoaffinity labeling of the human erythrocyte nucleoside transporter by N6-(p-Azidobenzyl)adenosine and nitrobenzylthioinosine. Evidence that the transporter is a band 4.5 polypeptide. J Biol Chem. 1983 Feb 25;258(4):2202–2208. [PubMed] [Google Scholar]
- Zoccoli M. A., Baldwin S. A., Lienhard G. E. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem. 1978 Oct 10;253(19):6923–6930. [PubMed] [Google Scholar]