Abstract
Non-transformed steroid receptors have an approximately 8S sedimentation coefficient that corresponds to an oligomeric structure of 250-300 kd which includes a non-hormone binding 90-kd protein. A monoclonal antibody BF4 raised against the purified, molybdate-stabilized, 8S progesterone receptor (8S-PR) from chick oviduct, recognizes 8S forms of all steroid hormone receptors. BF4 was found specific for a 90-kd protein present in great abundance in all chicken tissues, including that present in 8S-forms of steroid receptors. Here, using immunological and biochemical techniques, we demonstrate that this ubiquitous BF4-positive 90-kd protein is in fact the chicken 90 kd heat-shock protein (hsp 90): it increased in heat-shocked chick embryo fibroblasts, and displayed identical migration in two-dimensional gel electrophoresis and the same V8 peptide map as the already described hsp 90. We discuss the possibility that the interaction between hsp 90 and steroid hormone-binding subunits may play a role in keeping the receptor in an inactive form.
Full text
PDF
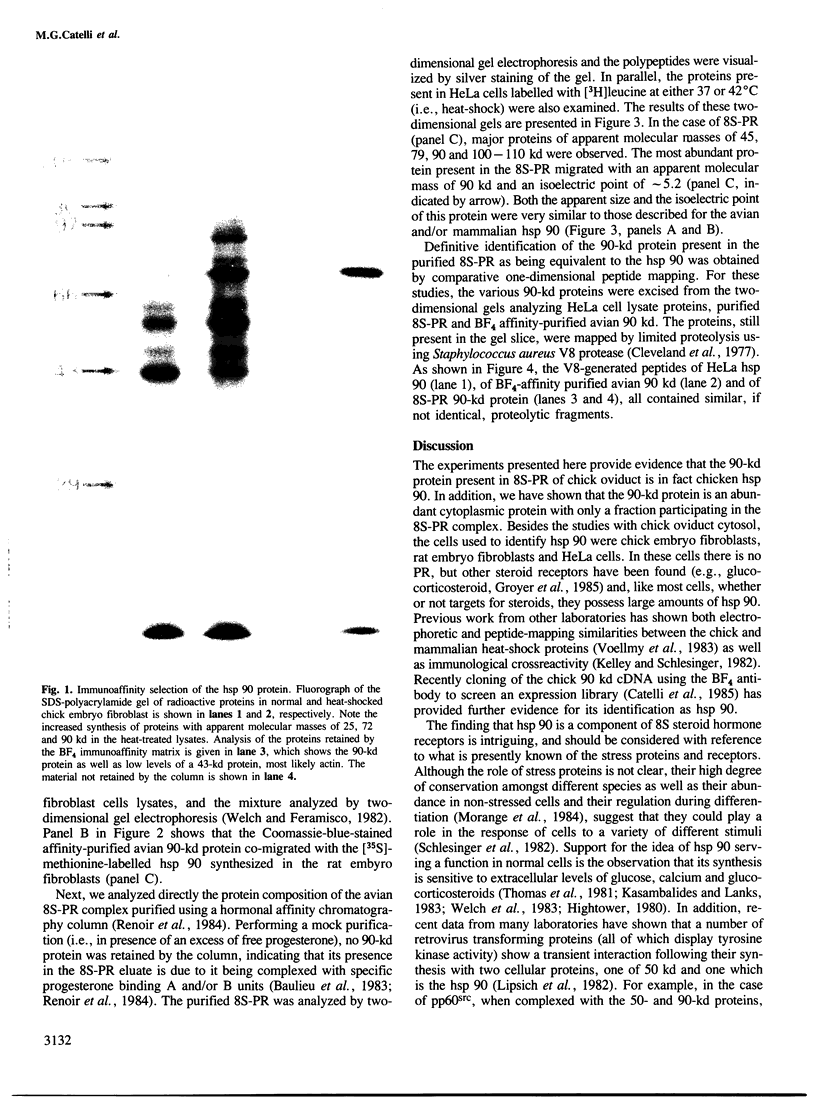
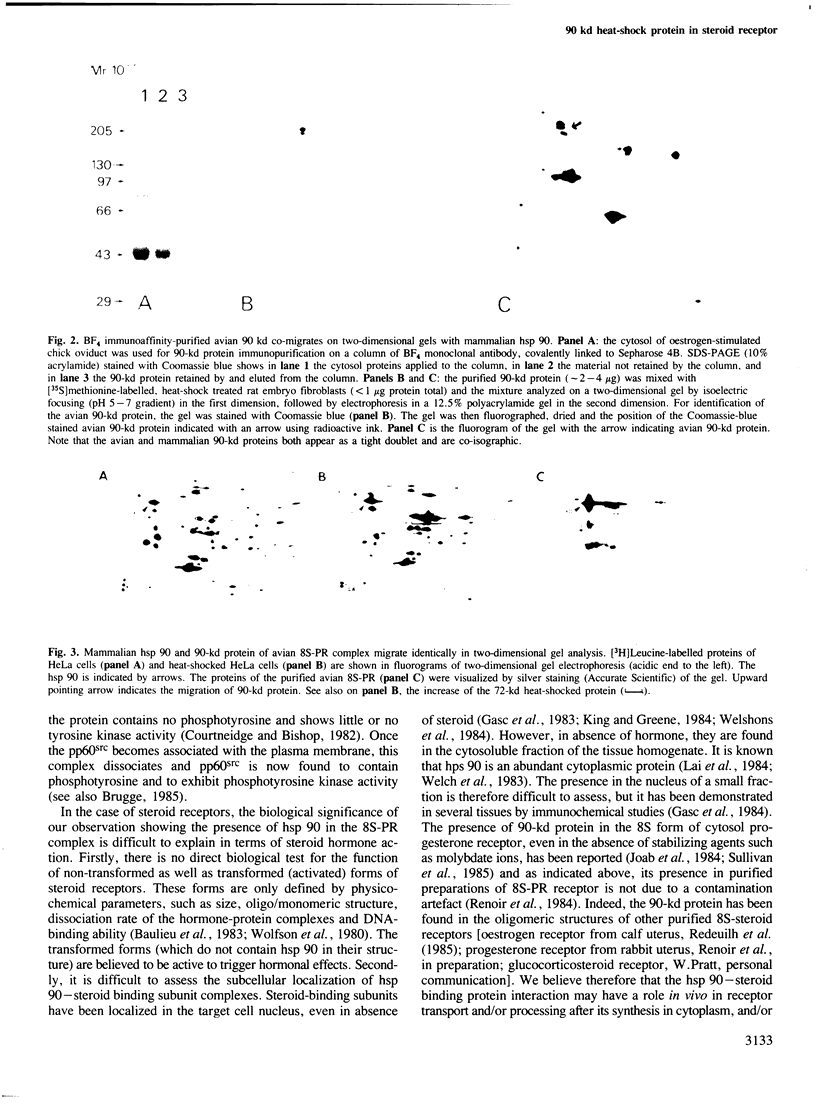


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E., Alberga A., Jung I., Lebeau M. C., Mercier-Bodard C., Milgrom E., Raynaud J. P., Raynaud-Jammet C., Rochefort H., Truong H. Metabolism and protein binding of sex steroids in target organs: an approach to the mechanism of hormone action. Recent Prog Horm Res. 1971;27:351–419. doi: 10.1016/b978-0-12-571127-2.50033-x. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J., Yonemoto W., Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983 Jan;3(1):9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Feramisco J. R., Helfman D. M. Cloning of the chick hsp 90 cDNA in expression vector. Nucleic Acids Res. 1985 Sep 11;13(17):6035–6047. doi: 10.1093/nar/13.17.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Courtneidge S. A., Bishop J. M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. J., Puri R. K., Toft D. O. Phosphorylation in vivo of chicken oviduct progesterone receptor. J Biol Chem. 1982 Dec 10;257(23):14226–14230. [PubMed] [Google Scholar]
- Garcia T., Tuohimaa P., Mester J., Buchou T., Renoir J. M., Baulieu E. E. Protein kinase activity of purified components of the chicken oviduct progesterone receptor. Biochem Biophys Res Commun. 1983 Jun 29;113(3):960–966. doi: 10.1016/0006-291x(83)91092-6. [DOI] [PubMed] [Google Scholar]
- Gasc J. M., Renoir J. M., Radanyi C., Joab I., Tuohimaa P., Baulieu E. E. Progesterone receptor in the chick oviduct: an immunohistochemical study with antibodies to distinct receptor components. J Cell Biol. 1984 Oct;99(4 Pt 1):1193–1201. doi: 10.1083/jcb.99.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Groyer A., Le Bouc Y., Joab I., Radanyi C., Renoir J. M., Robel P., Baulieu E. E. Chick oviduct glucocorticosteroid receptor. Specific binding of the synthetic steroid RU 486 and immunological studies with antibodies to chick oviduct progesterone receptor. Eur J Biochem. 1985 Jun 3;149(2):445–451. doi: 10.1111/j.1432-1033.1985.tb08945.x. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Joab I., Radanyi C., Renoir M., Buchou T., Catelli M. G., Binart N., Mester J., Baulieu E. E. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. 1984 Apr 26-May 2Nature. 308(5962):850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- Kasambalides E. J., Lanks K. W. Dexamethasone can modulate glucose-regulated and heat shock protein synthesis. J Cell Physiol. 1983 Jan;114(1):93–98. doi: 10.1002/jcp.1041140116. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Lai B. T., Chin N. W., Stanek A. E., Keh W., Lanks K. W. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984 Dec;4(12):2802–2810. doi: 10.1128/mcb.4.12.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Cutt J. R., Brugge J. S. Association of the transforming proteins of Rous, Fujinami, and Y73 avian sarcoma viruses with the same two cellular proteins. Mol Cell Biol. 1982 Jul;2(7):875–880. doi: 10.1128/mcb.2.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morange M., Diu A., Bensaude O., Babinet C. Altered expression of heat shock proteins in embryonal carcinoma and mouse early embryonic cells. Mol Cell Biol. 1984 Apr;4(4):730–735. doi: 10.1128/mcb.4.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanyi C., Joab I., Renoir J. M., Richard-Foy H., Baulieu E. E. Monoclonal antibody to chicken oviduct progesterone receptor. Proc Natl Acad Sci U S A. 1983 May;80(10):2854–2858. doi: 10.1073/pnas.80.10.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader W. T., Birnbaumer M. E., Hughes M. R., Weigel N. L., Grody W. W., O'Malley B. W. Studies on the structure and function of the chicken progesterone receptor. Recent Prog Horm Res. 1981;37:583–633. doi: 10.1016/b978-0-12-571137-1.50017-7. [DOI] [PubMed] [Google Scholar]
- Sherman M. R., Stevens J. Structure of mammalian steroid receptors: evolving concepts and methodological developments. Annu Rev Physiol. 1984;46:83–105. doi: 10.1146/annurev.ph.46.030184.000503. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Vroman B. T., Bauer V. J., Puri R. K., Riehl R. M., Pearson G. R., Toft D. O. Isolation of steroid receptor binding protein from chicken oviduct and production of monoclonal antibodies. Biochemistry. 1985 Jul 16;24(15):4214–4222. doi: 10.1021/bi00336a060. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Welch W. J., Mathews M. B., Feramisco J. R. Molecular and cellular effects of heat-shock and related treatments of mammalian tissue-culture cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):985–996. doi: 10.1101/sqb.1982.046.01.092. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Bromley P., Kocher H. P. Structural similarities between corresponding heat-shock proteins from different eucaryotic cells. J Biol Chem. 1983 Mar 25;258(6):3516–3522. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Perlman A. J., Tata J. R. Transient paralysis by heat shock of hormonal regulation of gene expression. EMBO J. 1984 Dec 1;3(12):2763–2770. doi: 10.1002/j.1460-2075.1984.tb02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson A., Mester J., Chang-Ren Y. "Non-activated" form of the progesterone receptor from chick oviduct: characterization. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1577–1584. doi: 10.1016/s0006-291x(80)80078-7. [DOI] [PubMed] [Google Scholar]






