Abstract
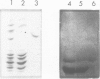
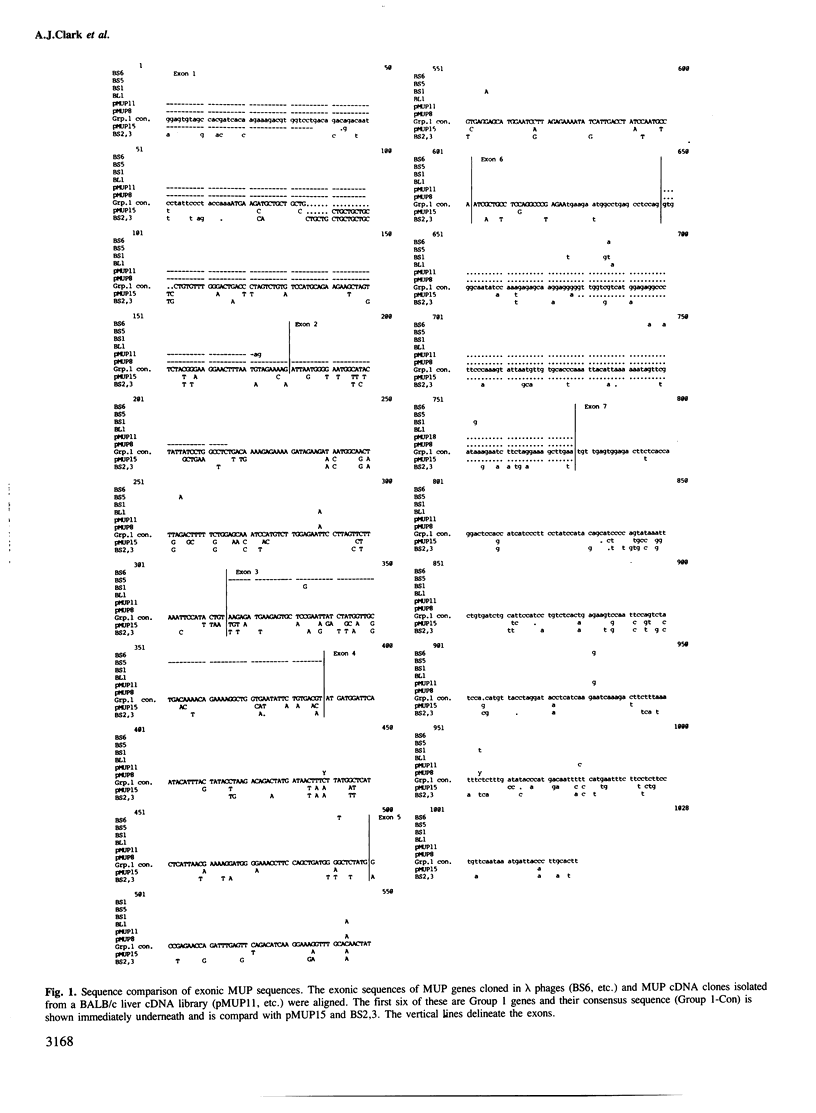
Here we compare the exonic sequences of four Group 1 mouse major urinary protein (MUP) genes and four Group 1 cDNA sequences. These define seven different nucleotide sequences which differ from each other by 0.35% of bases on average, and which would code for seven different MUP proteins that could probably be resolved physically into at least five classes. The sequences differ at 13 nucleotide positions and at six codons, and although they are closely related their descent cannot be described by a simple series of duplications. We also describe the sequence of another liver cDNA (pMUP15) which has diverged from the Group 1 consensus sequence in 14.6% of bases. The divergence is much greater over exons 1-3 than over exons 4-6, suggesting that an ancestral gene conversion event has occurred. pMUP15 also differs from the Group 1 genes in having a longer signal peptide sequence and a different splice configuration between exons 6 and 7. Unlike the Group 1 sequences, pMUP15 contains a potential N-linked glycosylation site. Other published work has shown that a shorter cDNA clone which is identical over their common sequence to pMUP15 codes for MUP proteins that are unusually large in size and acidic in pI. We show here that mouse urine does indeed contain a glycosylated MUP protein with those properties, presumably the product of the gene that corresponds to pMUP15.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. O., Clark A. J., Clissold P. M., Hainey S., Francke U. Two main groups of mouse major urinary protein genes, both largely located on chromosome 4. EMBO J. 1982;1(5):615–620. doi: 10.1002/j.1460-2075.1982.tb01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Selman G. G., Hickman J., Black L., Saunders R. D., Clark A. J. The 45-kb unit of major urinary protein gene organization is a gigantic imperfect palindrome. Mol Cell Biol. 1985 Jul;5(7):1591–1600. doi: 10.1128/mcb.5.7.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Clissold P. M., Al Shawi R., Beattie P., Bishop J. Structure of mouse major urinary protein genes: different splicing configurations in the 3'-non-coding region. EMBO J. 1984 May;3(5):1045–1052. doi: 10.1002/j.1460-2075.1984.tb01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Clissold P. M., Bishop J. O. Variation between mouse major urinary protein genes isolated from a single inbred line. Gene. 1982 Jun;18(3):221–230. doi: 10.1016/0378-1119(82)90159-7. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Ghazal P., Bingham R. W., Barrett D., Bishop J. O. Sequence structures of a mouse major urinary protein gene and pseudogene compared. EMBO J. 1985 Dec 1;4(12):3159–3165. doi: 10.1002/j.1460-2075.1985.tb04059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Hickman J., Bishop J. A 45-kb DNA domain with two divergently orientated genes is the unit of organisation of the murine major urinary protein genes. EMBO J. 1984 Sep;3(9):2055–2064. doi: 10.1002/j.1460-2075.1984.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clissold P. M., Bishop J. O. Molecular cloning of cDNA sequences transcribed from mouse liver endoplasmic reticulum poly(A)mRNA. Gene. 1981 Nov;15(2-3):225–235. doi: 10.1016/0378-1119(81)90132-3. [DOI] [PubMed] [Google Scholar]
- Clissold P. M., Bishop J. O. Variation in mouse major urinary protein (MUP) genes and the MUP gene products within and between inbred lines. Gene. 1982 Jun;18(3):211–220. doi: 10.1016/0378-1119(82)90158-5. [DOI] [PubMed] [Google Scholar]
- Clissold P. M., Hainey S., Bishop J. O. Messenger RNAs coding for mouse major urinary proteins are differentially induced by testosterone. Biochem Genet. 1984 Apr;22(3-4):379–387. doi: 10.1007/BF00484236. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Orkin S. H., Hamer D. H. Abnormal RNA splicing causes one form of alpha thalassemia. Cell. 1982 Jul;29(3):895–902. doi: 10.1016/0092-8674(82)90451-2. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Ghosh P. K., Benz E. J., Jr, Reddy V. B., Lebowitz P., Forget B. G., Weissman S. M. Abnormally spliced messenger RNA in erythroid cells from patients with beta+ thalassemia and monkey cells expressing a cloned beta+-thalassemic gene. Cell. 1982 Mar;28(3):585–593. doi: 10.1016/0092-8674(82)90213-6. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., Clark A. J., Bishop J. O. Evolutionary amplification of a pseudogene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4182–4185. doi: 10.1073/pnas.82.12.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A., Toole J. J. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell. 1979 Jun;17(2):449–457. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- Hong G. F. A systemic DNA sequencing strategy. J Mol Biol. 1982 Jul 5;158(3):539–549. doi: 10.1016/0022-2836(82)90213-3. [DOI] [PubMed] [Google Scholar]
- Knopf J. L., Gallagher J. F., Held W. A. Differential, multihormonal regulation of the mouse major urinary protein gene family in the liver. Mol Cell Biol. 1983 Dec;3(12):2232–2240. doi: 10.1128/mcb.3.12.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., Woodworth-Gutai M., Gross K. W., Held W. A. Subfamilies of the mouse major urinary protein (MUP) multi-gene family: sequence analysis of cDNA clones and differential regulation in the liver. Nucleic Acids Res. 1984 Aug 10;12(15):6073–6090. doi: 10.1093/nar/12.15.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Maniatis T. The structure of a human alpha-globin pseudogene and its relationship to alpha-globin gene duplication. Cell. 1980 Sep;21(2):537–544. doi: 10.1016/0092-8674(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Shaw P. H., Held W. A., Hastie N. D. The gene family for major urinary proteins: expression in several secretory tissues of the mouse. Cell. 1983 Mar;32(3):755–761. doi: 10.1016/0092-8674(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Szoka P. R., Paigen K. Regulation of mouse major urinary protein production by the Mup-A gene. Genetics. 1978 Nov;90(3):597–612. doi: 10.1093/genetics/90.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]