Abstract
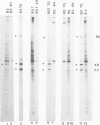
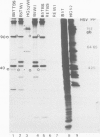
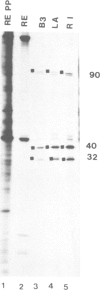
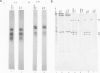
The cell proteins expressed in rat embryo cells transformed by herpes simplex virus (HSV) have been analysed by immunoprecipitation assays to determine those polypeptides which can be identified by immunoprecipitation with the sera of tumour-bearing animals and also with antisera to herpes simplex infected cells. Cell polypeptides commonly recognised by both these sera have been further characterised using a monoclonal antibody directed against a cellular polypeptide which accumulates on HSV-2 lytic infection. This monoclonal antibody recognises in HSV-transformed cells polypeptides of mol. wts. 90 000, 40 000 and 32 000. Further studies show that the accumulation of these polypeptides in HSV-transformed cells is not HSV specific but is a common feature of transformation or of cells which have been immortalised. We suggest that cellular polypeptides accumulating as a result of HSV infection may be of importance in the initiation of transformation by HSV, i.e., at the level of immortalisation of cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E., Kaufman R. H., Melnick J. L., Levy A. H., Rawls W. E. Seroepidemiologic studies of herpesvirus type 2 and carcinoma of the cervix. IV. Dysplasia and carcinoma in situ. Am J Epidemiol. 1973 Aug;98(2):77–87. doi: 10.1093/oxfordjournals.aje.a121541. [DOI] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. C., Murray B. K. Conversion of herpetic lesions to malignancy by ultraviolet exposure and promoter application. J Gen Virol. 1981 Aug;55(Pt 2):305–313. doi: 10.1099/0022-1317-55-2-305. [DOI] [PubMed] [Google Scholar]
- Bültjens T. E., Macnab J. C. Characterization of rat embryo cells transformed by ts mutants and sheared DNA of herpes simplex virus types 1 and 2 and a derived tumor cell line. Cancer Res. 1981 Jun;41(6):2540–2547. [PubMed] [Google Scholar]
- Cameron I. R., Park M., Dutia B. M., Orr A., Macnab J. C. Herpes simplex virus sequences involved in the initiation of oncogenic morphological transformation of rat cells are not required for maintenance of the transformed state. J Gen Virol. 1985 Mar;66(Pt 3):517–527. doi: 10.1099/0022-1317-66-3-517. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Docherty J. J., Subak-Sharpe J. H., Preston C. M. Identification of a virus-specific polypeptide associated with a transforming fragment (BglII-N) of herpes simplex virus type 2 DNA. J Virol. 1981 Oct;40(1):126–132. doi: 10.1128/jvi.40.1.126-132.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Dunlop M. Trans activation of plasmid-borne promoters by adenovirus and several herpes group viruses. Nucleic Acids Res. 1984 Aug 10;12(15):5969–5978. doi: 10.1093/nar/12.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. Transformation of rodent cells by a cloned DNA fragment of herpes simplex virus type 2. J Virol. 1981 May;38(2):749–760. doi: 10.1128/jvi.38.2.749-760.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnürch H. G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983 Jan;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Tumorigenic transformation induced by a specific fragment of DNA from herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2279–2283. doi: 10.1073/pnas.77.4.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaThangue N. B., Shriver K., Dawson C., Chan W. L. Herpes simplex virus infection causes the accumulation of a heat-shock protein. EMBO J. 1984 Feb;3(2):267–277. doi: 10.1002/j.1460-2075.1984.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- MacNab J. C. Tumour production by HSV-2 transformed lines in rats and the varying response to immunosuppression. J Gen Virol. 1979 Apr;43(1):39–56. doi: 10.1099/0022-1317-43-1-39. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Transformation of rat embryo cells by temperature-sensitive mutants of herpes simplex virus. J Gen Virol. 1974 Jul;24(1):143–153. doi: 10.1099/0022-1317-24-1-143. [DOI] [PubMed] [Google Scholar]
- Macnab J. C., Visser L., Jamieson A. T., Hay J. Specific viral antigens in rat cells transformed by herpes simplex virus type 2 and in rat tumors induced by inoculation of transformed cells. Cancer Res. 1980 Jun;40(6):2074–2079. [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J., Clements J. B. DNA sequence homology between two co-linear loci on the HSV genome which have different transforming abilities. EMBO J. 1983;2(11):1953–1961. doi: 10.1002/j.1460-2075.1983.tb01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E. Cytology and histopathology of cervical herpes simplex infection. Cancer. 1966 Jul;19(7):1026–1031. doi: 10.1002/1097-0142(196607)19:7<1026::aid-cncr2820190718>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W., Connell J. R. Induction of immortality is an early event in malignant transformation of mammalian cells by carcinogens. Nature. 1982 Oct 14;299(5884):633–635. doi: 10.1038/299633a0. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- Notarianni E. L., Preston C. M. Activation of cellular stress protein genes by herpes simplex virus temperature-sensitive mutants which overproduce immediate early polypeptides. Virology. 1982 Nov;123(1):113–122. doi: 10.1016/0042-6822(82)90299-9. [DOI] [PubMed] [Google Scholar]
- Paraskeva C., Brown K. W., Dunn A. R., Gallimore P. H. Adenovirus type 12-transformed rat embryo brain and rat liver epithelial cell lines: adenovirus type 12 genome content and viral protein expression. J Virol. 1982 Nov;44(2):759–764. doi: 10.1128/jvi.44.2.759-764.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Kitchener H. C., Macnab J. C. Detection of herpes simplex virus type-2 DNA restriction fragments in human cervical carcinoma tissue. EMBO J. 1983;2(7):1029–1034. doi: 10.1002/j.1460-2075.1983.tb01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Lonsdale D. M., Timbury M. C., Subak-Sharpe J. H., Macnab J. C. Genetic retrieval of viral genome sequences from herpes simplex virus transformed cells. Nature. 1980 Jun 5;285(5764):412–415. doi: 10.1038/285412a0. [DOI] [PubMed] [Google Scholar]
- Park M., Macnab J. C. Induction of a latent herpes simplex virus from a rat tumour initiated by herpes simplex virus-transformed cells. J Gen Virol. 1983 Mar;64(Pt 3):755–758. doi: 10.1099/0022-1317-64-3-755. [DOI] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. State of the viral DNA in rat cells transformed by polyoma virus. I. Virus rescue and the presence of nonintergrated viral DNA molecules. J Virol. 1976 May;18(2):436–444. doi: 10.1128/jvi.18.2.436-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Reyes G. R., LaFemina R., Hayward S. D., Hayward G. S. Morphological transformation by DNA fragments of human herpesviruses: evidence for two distinct transforming regions in herpes simplex virus types 1 and 2 and lack of correlation with biochemical transfer of the thymidine kinase gene. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):629–641. doi: 10.1101/sqb.1980.044.01.066. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Klagsbrun M. Serum-free growth of normal and transformed fibroblasts in milk: differential requirements for fibronectin. J Cell Biol. 1981 Feb;88(2):294–300. doi: 10.1083/jcb.88.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonka V., Kanka J., Hirsch I., Závadová H., Krcmár M., Suchánková A., Rezácová D., Broucek J., Press M., Domorázková E. Prospective study on the relationship between cervical neoplasia and herpes simplex type-2 virus. II. Herpes simplex type-2 antibody presence in sera taken at enrollment. Int J Cancer. 1984 Jan 15;33(1):61–66. doi: 10.1002/ijc.2910330111. [DOI] [PubMed] [Google Scholar]
- Walker A. I., Hunt T., Jackson R. J., Anderson C. W. Double-stranded DNA induces the phosphorylation of several proteins including the 90 000 mol. wt. heat-shock protein in animal cell extracts. EMBO J. 1985 Jan;4(1):139–145. doi: 10.1002/j.1460-2075.1985.tb02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentz W. B., Heggie A. D., Anthony D. D., Reagan J. W. Effect of prior immunization on induction of cervial cancer in mice by herpes simplex virus type 2. Science. 1983 Dec 9;222(4628):1128–1129. doi: 10.1126/science.6316503. [DOI] [PubMed] [Google Scholar]
- Wentz W. B., Reagan J. W., Heggie A. D., Fu Y. S., Anthony D. D. Induction of uterine cancer with inactivated herpes simplex virus, types 1 and 2. Cancer. 1981 Oct 15;48(8):1783–1790. doi: 10.1002/1097-0142(19811015)48:8<1783::aid-cncr2820480815>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Macnab J. C., Subak-Sharpe J. H. The structure and biological properties of herpes simplex virus DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):657–666. doi: 10.1101/sqb.1974.039.01.079. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events? Lancet. 1982 Dec 18;2(8312):1370–1372. doi: 10.1016/s0140-6736(82)91273-9. [DOI] [PubMed] [Google Scholar]