Abstract
Objective:
Provide up-to-date detection rates for common mental disorders (CMD) and examine patient service-use since the Preferred Doctor scheme was introduced to France in 2005, with patients encouraged to register with and consult a family practitioner (FP) of their choice.
Methods:
Study of 1133 consecutive patients consulting 38 FPs in the Montpellier region, replicating a study performed before the scheme. Patients in the waiting room completed the self-report Patient Health Questionnaire (PHQ) and Client Service-Receipt Inventory with questions on registration with a Preferred Doctor and doctor-shopping. CMD was defined as reaching PHQ criteria for depression, somatoform, panic or anxiety disorder. For each patient, FPs completed a questionnaire capturing psychiatric caseness.
Results:
81.2% of patients were seeing their Preferred Doctor on the survey-day. Of those with a CMD, 52.6% were detected by the FP. This increased with CMD severity and comorbidity. Detected cases were more likely to be consulting their Preferred Doctor (84.7% versus 79.4% for non-detected cases, p = 0.05) rather than another FP. They declared more visits to psychiatrists (17.2% versus 6.7%, p = 0.002). There was no association with consultation frequency or doctor-shopping, which both declined between the two studies.
Conclusion:
The CMD detection rate is relatively high, with no increase compared to our previous study, despite a decline in doctor-shopping. An explanation is the same high proportion of patients visiting their usual FP on the survey-day at both periods, suggesting a limited impact of the scheme on care continuity. FP action taken highlights the importance of improving detection for providing care to patients with CMDs.
Keywords: common mental disorders, detection, family practice, service use
Abstract
Objectif:
Actualiser les taux de détection des troubles psychiatriques courants (TPC) par les médecins de famille (MF) et étudier l'utilisation des services de soins par les patients après l'introduction en France, en 2005, du dispositif du Médecin Traitant les encourageant à s'enregistrer auprès d'un MF de leur choix, responsable de la coordination des soins.
Méthodes:
Etude transversale de 1133 patients consécutifs consultant 38 MF (approximativement 30 patients par MF) dans la région de Montpellier, répétant une étude menée en 2003. Les patients ont répondu à des auto-questionnaires en salle d'attente, dont le Patient Health Questionnaire (PHQ) et le Client Service Receipt Inventory incluant des questions sur le choix du Médecin Traitant et le nomadisme médical. Les TPC étaient définis comme tout trouble somatoforme, dépressif (majeur, autre), panique ou anxieux, selon les critères du PHQ. Pour chaque patient, le MF remplissait un questionnaire et cotait la présence et la sévérité des troubles psychiatriques.
Résultats:
Le jour de l'enquête, 81,2% des patients consultaient leur Médecin Traitant. Parmi les patients présentant un TPC, 52,6% ont été détectés par le MF. Le taux de détection augmentait avec la sévérité des troubles et la comorbidité. Les patients détectés étaient plus nombreux à consulter leur Médecin Traitant (84,7% versus 79,4% pour les non détectés, p = 0,05). Ils étaient plus nombreux à déclarer des consultations au cours des six derniers mois auprès de psychiatres (17,2% versus 6,7% pour les non détectés, p = 0,002). Aucune association n'a été mise en évidence avec la fréquence de consultation ou le nomadisme médical, qui ont tous deux diminué après l'introduction du dispositif.
Conclusion:
Le taux de détection des TPC est relativement élevé et semblable à celui observé dans notre étude précédente, malgré une diminution du nomadisme médical. Cela pourrait s'expliquer par une proportion semblable dans les deux études de patients consultant leur MF habituel le jour de l'enquête, suggérant un impact limité du dispositif du Médecin Traitant sur la continuité des soins. Les actions entreprises par les MF en fin de consultation soulignent l'importance d'améliorer la détection des patients présentant des TPC.
Common mental disorders (CMDs), most frequently defined as mood, anxiety, somatoform, and substance use disorders, remain a major public health problem, with 12-month prevalence rates ranging from 10% to 20% in high-income countries.1 Most CMDs are managed in family practice,2,3 where there is widespread evidence that approximately 50% of cases are not detected.4–8 Although detection, mainly studied with regard to depression, has not always been shown to improve outcome,5,9 it is a crucial and necessary first step in providing care whether through watchful waiting or immediate intervention.10
Detection of CMDs varies widely among family practitioners (FPs) within and between countries.4,8,11 Detection is influenced by many factors, related to the FP,12–14 the patient,5,14–16 and the health care system.17 For example, detection appears to be higher in countries with more individualised care models characterised by a client-centred approach where there is continuity with the same FP.18 Detection increases with frequency of visits and when the patient is better known to the FP.5,12 Paradoxically, detection has also been shown to be higher in doctor-shopping patients, but only among those consulting different FPs for reasons of dissatisfaction with previous care.19 Doctor-shopping is defined here as consulting a different FP to one’s usual FP; consulting a different FP is often unavoidable (e.g., FP away or patient on holiday) but in some cases is explained by the patient looking for a different approach.
Many detection studies4,8,11 have been performed in countries with hierarchical primary health care models characterised by restricted access to care by different forms of gatekeeping, large group practices, and limited patient choice and access to a personal FP.20,21 Little is known about France, where FPs work mainly alone on a fee-for-service basis.22,23 In 2003, we carried out one of the only studies performed among randomly selected FPs using validated research instruments and found a 50% detection rate for CMDs.6,19,24,25 At the time, patients could access any FP or specialist as often as they wanted, as illustrated by the high level of doctor-shopping (28.2% had seen at least 2 different FPs in the past 6 months, 18.4% for practical reasons and 9.8% for reasons of dissatisfaction).19
In 2005, a new scheme was introduced with the aim of structuring access to both primary and secondary care.26 Patients (aged 16 years and above) register with an FP (or specialist doctor, although this is very rare: <1% of patients)27 of their choice (the “Preferred Doctor”), who is responsible for coordinating care and referring the patient if necessary to secondary care (to all specialist doctors except gynaecologists, ophthalmologists, and psychiatrists for patients below the age of 25 years). To date, at least 95% of patients have registered with a Preferred Doctor,26 and direct referrals have substantially declined.28 Financial sanctions in the form of lower reimbursements (reduced from 70% to 30%) (www.ameli.fr) are applied to patients who consult: without having registered with a Preferred Doctor, with a different FP to their Preferred Doctor unless justified (FP unavailable, FP or patient away), or directly with a specialist doctor without being referred by the FP. These changes can be seen as a progressive move toward a more structured organisation of care in a system that still remains relatively open and patient-led. Indeed, patients can choose their doctor and can cancel and register with a new Preferred Doctor at will. However, the scheme encourages loyalty, which is based mainly on trust, quality of care, familiarity, and availability of the FP.29 The scheme is likely to increase continuity of care between primary and secondary care and relational continuity with a single trusted doctor.30,31 Patients are likely to be better known to their FP, and those with a CMD are more likely to be detected18 and accurately diagnosed.32 We thus hypothesize that FP detection of CMDs as well as continuity of care will have increased since the introduction of the Preferred Doctor scheme.
The aim of our study is to provide up-to-date prevalence and detection rates for CMDs, overall and by diagnostic category, within the new gatekeeping scheme. We also aim to describe the health and service-use characteristics of patients with CMDs, overall and according to whether they are detected by the survey day FP. Case management according to detection is also examined. Findings are interpreted in the light of those obtained in the 2003 study prior to the scheme.
Methods
FP Sample
The study was conducted from February to July 2013 in family practices in and around the city of Montpellier. We followed as closely as possible the same methodological approach as a previous study performed in 2003.6
In 2003, a random sample of 46 FPs drawn from the regional FP lists participated, with a response rate of 32.7% (see Norton et al6 for a detailed description of the recruitment method). For the 2013 study, all 46 FPs were contacted again; 34 were still practicing in the study area (6 had retired, 4 were not reachable, and 2 no longer worked in family practice). In all, 29 (85.3%) agreed to participate again. Those who refused stated a lack of time or interest; they were mainly males (4/5) practicing in the urban study zone (3/5). To adjust the age distribution of the sample to that of the FPs in the study area, 9 additional, younger FPs were recruited. We initially contacted the FPs who had taken over the practices of those who had retired or moved since 2003, and then we recruited 3 FPs from professional lists covering the study areas, approaching the youngest in age first. In all, this convenience sample included 38 FPs, of whom 29 had participated in the first study.
Patient Sample
In each FP practice, a research assistant invited all consecutive eligible patients entering the waiting room to participate. Patient inclusion criteria were: age (18 years and above) and the ability to read and write French. Patients and FPs signed a consent form prior to participation as required by the Montpellier University Hospital ethics committee. It was clearly explained that both patient and FP responses would remain confidential, with a common identification number linking patient and FP questionnaires.
Patients were recruited until approximately 30 per FP had participated. The response rate was 67%. The main reason for refusing was an objection to giving their name and address on the consent form.
None of the patients had participated in the 2003 study.
Instruments
Patient Data
Before the consultation, patients in the waiting room completed self-report questionnaires, including an adapted version of the Client Service Receipt Inventory (CSRI)33 on service use and the Patient Health Questionnaire (PHQ).34
The CSRI was adapted for the French care system, so information was collected on the number of FP consultations as well as the number of different FPs consulted over the past 6 months. Patients who had seen at least 2 different FPs (survey day visit included) were asked to give their reasons, classified as practical only versus dissatisfaction with previous care. Patients reported all hospitalisations, consultations with specialist doctors (type of specialist, number of visits), and other carers (nurses, physiotherapists, psychologists, etc.), along with any use of medication for psychological or sleeping problems. Questions were also added with respect to patients’ current status vis-a-vis the Preferred Doctor scheme.
The PHQ is a validated instrument that allows the identification of both threshold disorders (major depressive disorder, panic disorder, other anxiety disorder, bulimia nervosa) and subthreshold disorders (other depressive disorder, and the PHQ-specific categories: somatoform disorder, probable alcohol abuse/dependence, binge-eating disorders), by applying DSM-IV diagnostic criteria algorithms.34,35 For somatoform disorders, patients meeting caseness criteria (applying the original algorithm to the somatoform module rather than PHQ-15 criteria)35 and rated by the FP as moderately or severely physically ill were reclassified as noncases. This was the only way to ensure that those with a possible “adequate biological explanation” for the symptoms were not given the diagnosis. The PHQ had already been translated into French for the 2003 study.6 Patients were considered as having a CMD if they reached criteria on the PHQ for a somatoform disorder, major or other depressive disorder, panic disorder, or other anxiety disorder.
Patients also completed a form on their sociodemographic characteristics, and level of disability was measured using the Brief Disability Questionnaire.8
FP Data
During the consultation, the FPs completed a short questionnaire adapted from the World Health Organisation Physician’s Encounter Form.36 The FPs were asked to estimate the severity of physical and psychiatric illness, regardless of presenting symptoms, by ticking a box on a 5-point scale ranging from completely healthy to severely ill, with a clear indication that ratings of 3 and above (mild, moderate, or severe) indicated caseness and required a diagnosis (open script). The FPs then indicated what actions were undertaken, such as providing listening and support, from a predefined list.
Statistical Analysis
Frequencies and medians (minimum-maximum) were used to describe the FP and patient samples. FPs who had participated in the previous study (n = 29) were compared with those recruited for the current study only (n = 9) by using Wilcoxon’s rank sum test and the chi-square test. For patients, all comparative analyses were performed using marginal Generalised Estimating Equations (GENMOD SAS procedure, option repeated) to take into account the 2-stage sampling process and clustering of patients by practice. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
The FP sample is described in Table 1. As expected, the 9 “new” FPs were younger (p = 0.0003) and, except for 2, had all practiced for less than 10 years. They were more likely to be in part-time practice (66.7% versus 17.2% for those who participated in both studies, p = 0.009), reflecting the higher proportion of female FPs (77.8% versus 34.5%, p = 0.05).
Table 1.
Description of family practitioner (FP) sample.
| 2013 study (N = 38) | ||
|---|---|---|
| n | % | |
| Practice setting | ||
| Urban | 27 | 71.1 |
| Semi-rural | 11 | 28.9 |
| Sex | ||
| Male | 21 | 55.3 |
| Female | 17 | 44.7 |
| Age (median [minimum-maximum]) | 38 | 51.5 [31-65] |
| FP since | ||
| <10 years | 7 | 18.4 |
| ≥10 years | 31 | 81.6 |
| Group practice | ||
| No—solo | 14 | 36.8 |
| Yes—partner FP(s) | 24 | 63.2 |
| Type of practice | ||
| Full-time | 27 | 71.1 |
| Part-time | 11 | 28.9 |
| Mental health training (past 3 years) | ||
| No | 18 | 47.4 |
| Yes, evening only | 5 | 13.2 |
| Yes, daytime seminar | 15 | 39.5 |
Among the patients, 35% were male, and 43% were aged 50 or above. Half (51%) were married or living with someone, and 31% were single. On the survey day, 81.2% of patients were seeing their Preferred Doctor and 94.8% reported having a Preferred Doctor, of which 44.9% had already changed Preferred Doctor at least once since 2005. Among the reasons for changing, 15.4% reported dissatisfaction with a previous FP and 3.3% lack of availability of FP; other reasons were patient or FP had moved (59.7%), FP had retired (16.5%), and distance to FP practice (5.1%).
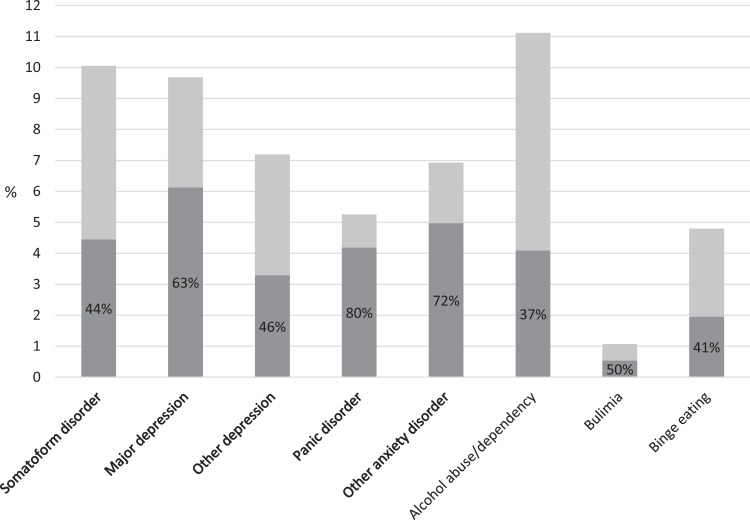
Of the patients, 25.5% met PHQ criteria for a CMD: 10% for a somatoform disorder; 7.2% and 9.6% for other and major depression, respectively; 5.2% for panic disorder; and 6.9% for other anxiety disorder. The FPs classified 30.6% of the patients as having a psychiatric disorder. The main diagnoses given were anxiety and/or panic disorder (43%), depression (21%), and anxiodepressive symptoms (17%). The FPs detected 52.6% of CMD cases (95% CI, 46.8 to 58.4), increasing with severity, from 44.2% for somatoform disorders to 79.7% for panic disorder (Figure 1). The detection rate for major and other depression combined was 60.3%; for a score of 10 or higher on the depression module (PHQ-9), the detection rate was 57.4%. The detection rate increased with the number of comorbid disorders: 46.6% for 1 disorder only, 53.7% for 2 disorders, and 82% for 3 or 4 disorders (collapsing major and minor depression and excluding alcohol and eating disorders) (p < 0.0001). Of the patients not reaching CMD criteria, 77.1% (95% CI, 74.2 to 79.9) were classified as noncases by the FP (specificity). Case detection was stratified according to whether the FPs had participated in the 2003 study: Among the 29 initial FPs, 47.9% (95% CI, 41.2 to 54.6) of cases were detected, for 66.4% (95% CI, 55.6 to 77.2) among the 9 “new” FPs (p = 0.007).
Figure 1.
Proportion of patients per Patient Health Questionnaire (PHQ) diagnostic category: overall (light + dark grey) and detected by the family practitioner (FP) (dark grey). Percentages correspond to FP detection rates per category. Bolded categories are included in the study definition of common mental disorder.
Patients with a CMD seeing their Preferred Doctor on the survey day were slightly more likely to be detected (p = 0.05) (Table 2). Similarly, they were less likely to be detected if seen by the FP for the first time (p = 0.02) (Table 3).
Table 2.
Patients’ sociodemographic characteristics and service use, according to CMD status and FP detection.a
| CMD = yes (n = 287) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CMD = no (n = 839) | CMD = yes (n = 287) | CMD not detected (n = 136) | CMD detected (n = 151) | |||||||
| n | % | n | % | p | n | % | n | % | p | |
| On the survey day | ||||||||||
| Sex: male | 320 | 38.1 | 75 | 26.1 | <0.0001 | 36 | 26.5 | 39 | 25.8 | 0.72 |
| Age | ||||||||||
| <30 y | 191 | 22.8 | 71 | 24.7 | 44 | 32.4 | 27 | 17.9 | ||
| 30-49 y | 265 | 31.6 | 112 | 39.0 | 50 | 36.8 | 62 | 41.1 | ||
| 50-64 y | 185 | 22.1 | 65 | 22.7 | 21 | 15.4 | 44 | 29.1 | ||
| >65 y | 198 | 23.6 | 39 | 13.6 | <0.0001 | 21 | 15.4 | 18 | 11.9 | 0.007 |
| Educational level | ||||||||||
| Low (<9 y) | 170 | 20.3 | 62 | 21.7 | 47 | 34.6 | 75 | 49.7 | ||
| Medium (9-12 y) | 220 | 26.2 | 80 | 28.0 | 64 | 47.1 | 61 | 40.4 | ||
| High (>12 y) | 449 | 53.5 | 144 | 50.4 | 0.54 | 25 | 18.4 | 15 | 9.9 | 0.82 |
| Marital status | ||||||||||
| Single | 244 | 29.1 | 104 | 36.2 | 57 | 41.9 | 47 | 31.1 | ||
| Marital life | 453 | 54.0 | 121 | 42.2 | 58 | 42.7 | 63 | 41.7 | ||
| Divorced/separated | 90 | 10.7 | 45 | 15.7 | 12 | 8.8 | 33 | 21.9 | ||
| Widowed | 52 | 6.2 | 17 | 5.9 | 0.003 | 9 | 6.6 | 8 | 5.3 | 0.03 |
| Living situation | ||||||||||
| Aloneb | 273 | 32.5 | 122 | 42.5 | 47 | 34.6 | 75 | 49.7 | ||
| With partnerb | 466 | 55.5 | 125 | 43.6 | 64 | 47.1 | 61 | 40.4 | ||
| Other | 100 | 11.9 | 40 | 13.9 | 0.002 | 25 | 18.4 | 15 | 9.9 | 0.004 |
| Occupation | ||||||||||
| Working | 402 | 48.0 | 123 | 42.9 | 59 | 43.4 | 64 | 42.4 | ||
| Unemployed | 79 | 9.4 | 43 | 15.0 | 20 | 14.7 | 23 | 15.2 | ||
| Retired | 223 | 26.6 | 49 | 17.1 | 22 | 16.2 | 27 | 17.9 | ||
| Other | 133 | 15.9 | 72 | 25.1 | <0.0001 | 35 | 25.7 | 37 | 24.5 | 0.96 |
| Disability level (score out of 11) | ||||||||||
| Low (0-1) | 330 | 39.5 | 38 | 13.2 | 23 | 16.9 | 15 | 9.9 | ||
| Moderate (2-5) | 298 | 35.7 | 85 | 29.6 | 42 | 30.9 | 43 | 28.5 | ||
| High (>5) | 208 | 24.9 | 164 | 57.1 | <0.0001 | 71 | 52.2 | 93 | 61.6 | 0.25 |
| Personal and social problemsc | ||||||||||
| No | 333 | 39.8 | 41 | 14.3 | 19 | 14.0 | 22 | 14.6 | ||
| A little | 382 | 45.7 | 99 | 34.5 | 52 | 38.2 | 47 | 31.1 | ||
| A lot | 121 | 14.5 | 147 | 51.2 | <0.0001 | 65 | 47.8 | 82 | 54.3 | 0.22 |
| Psychological reason for FP visitd | 75 | 8.9 | 78 | 27.2 | <0.0001 | 16 | 11.8 | 62 | 41.1 | <0.0001 |
| Seeing preferred doctor | ||||||||||
| Yes | 677 | 80.8 | 235 | 82.2 | 108 | 79.4 | 127 | 84.7 | ||
| No | 116 | 13.8 | 39 | 13.6 | 19 | 14.0 | 20 | 13.3 | ||
| Does not have one | 45 | 5.4 | 12 | 4.2 | 0.71 | 9 | 6.6 | 3 | 2.0 | 0.05 |
| In the past 6 months (CSRI) | ||||||||||
| Hospitalisation: yes | 113 | 13.5 | 57 | 19.9 | 0.006 | 23 | 17.0 | 34 | 22.5 | 0.07 |
| Visit to specialist doctor: yes | 426 | 50.9 | 188 | 65.7 | <0.0001 | 80 | 59.3 | 108 | 71.5 | 0.06 |
| Visit to psychiatrist: yes | 35 | 4.2 | 35 | 12.2 | <0.0001 | 9 | 6.7 | 26 | 17.2 | 0.002 |
| Visit to MHP: yes | 90 | 10.7 | 70 | 24.5 | <0.0001 | 23 | 17.0 | 47 | 31.1 | 0.006 |
| Doctor-shopping in FP | ||||||||||
| No | 705 | 84.3 | 235 | 81.9 | 108 | 79.4 | 127 | 84.1 | ||
| Yes, practical reason | 101 | 12.1 | 35 | 12.2 | 19 | 14.0 | 16 | 10.6 | ||
| Yes, dissatisfied reason | 30 | 3.6 | 17 | 5.9 | 0.12 | 9 | 6.6 | 8 | 5.3 | 0.48 |
| No. of visits to an FP ≥7 | 61 | 7.3 | 41 | 14.3 | 0.005 | 19 | 14.0 | 22 | 14.6 | 0.57 |
| Medicatione: yes | 181 | 21.6 | 140 | 48.8 | <0.0001 | 43 | 31.6 | 97 | 64.2 | <0.0001 |
aLess than 2% of missing values for all variables. CMD = common mental disorder; CSRI = Client Service Receipt Inventory; FP = family practitioner; MHP = mental health professional (psychiatrist, psychologist, or psychotherapist).
bWith or without children.
cBothered by.
dVisiting FP for a psychological reason or for a problem with a psychological cause.
eFor psychological reason or sleeping problems.
Table 3.
FP management of patients, according to CMD status and FP detection.a
| CMD = yes (n = 287) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CMD = no (n = 839) | CMD = yes (n = 287) | CMD not detected (n = 136) | CMD detected (n = 151) | |||||||
| n | % | n | % | p | n | % | n | % | p | |
| Patient seen for first time | 81 | 9.7 | 27 | 9.5 | 0.98 | 19 | 14.1 | 8 | 5.4 | 0.02 |
| FP action at the end of the visit | ||||||||||
| Prescription of anxiolytic or antidepressant medication | 51 | 6.1 | 46 | 16.1 | <0.0001 | 2 | 1.5 | 44 | 29.1 | <0.0001 |
| Prescription of any medication | 617 | 73.5 | 227 | 79.1 | 0.08 | 110 | 80.9 | 117 | 77.5 | 0.41 |
| Further investigation of somatic complaint | 128 | 15.3 | 41 | 14.3 | 0.53 | 20 | 14.8 | 21 | 13.9 | 0.99 |
| Referral to a specialist doctor | 109 | 13.0 | 57 | 19.9 | 0.002 | 20 | 14.8 | 37 | 24.5 | 0.18 |
| Referral to a psychiatrist | 3 | 0.4 | 7 | 2.4 | 0.008 | 0 | 0.0 | 7 | 4.6 | NV |
| Referral to another health care professional | 51 | 6.1 | 12 | 4.2 | 0.30 | 5 | 3.7 | 7 | 4.6 | 0.69 |
| Appointment made for follow-up visit | 78 | 9.3 | 34 | 11.9 | 0.13 | 15 | 11.1 | 19 | 12.7 | 0.27 |
| Discussion of problems, listening and support | 202 | 24.1 | 97 | 33.9 | 0.0006 | 31 | 23.0 | 66 | 43.7 | <0.0001 |
| Therapeutic education | 75 | 8.9 | 37 | 13.0 | 0.10 | 14 | 10.5 | 23 | 15.2 | 0.25 |
| Other (medical certificate) | 124 | 14.8 | 36 | 12.6 | 0.49 | 16 | 11.9 | 20 | 13.3 | 0.83 |
| Duration of visit | ||||||||||
| <10 min | 39 | 4.7 | 11 | 3.8 | 9 | 6.6 | 2 | 1.3 | ||
| 10 min | 245 | 29.4 | 59 | 20.6 | 31 | 22.8 | 28 | 18.5 | ||
| 10-20 min | 453 | 54.4 | 166 | 57.8 | 85 | 62.5 | 81 | 53.6 | ||
| 20-30 min | 63 | 7.6 | 38 | 13.2 | 6 | 4.4 | 32 | 21.2 | ||
| >30 min | 33 | 4.0 | 13 | 4.5 | 0.034 | 5 | 3.7 | 8 | 5.3 | 0.0002 |
aCMD = common mental disorder; FP = family practitioner; NV = test not valid.
Discussion
Our study confirms the high prevalence of CMDs seen in family practice.8,23,34,37 Detection rates, overall and for specific diagnoses, appear to be higher than those found elsewhere,4,34,38,39 when considering that our definition of CMDs includes subthreshold disorders. The detection rate is in keeping with our previous study,23 not confirming our hypothesis of an increase linked to the introduction of the Preferred Doctor scheme.40 While doctor-shopping has declined in the 10-year gap, the proportion of the patients seeing their usual or Preferred Doctor on the survey day has not changed. Patients with a CMD are more likely to be detected when they report higher secondary care service use. This is not the case for those who reported high attendance rates and doctor-shopping in family practice.
The detection rate is similar to that found in our previous study (51%; 95% CI, 45.5 to 56.6).40 When the sample is stratified according to participation in the previous study, the rate for the 29 initial FPs is very close to that obtained 10 years earlier. This could suggest stability over time in FP strategies and thresholds for detecting CMDs. The higher rate among the “new” FPs could be explained by differences in age, sex, and years of practice between the 2 subsamples, although there is little evidence in our study or elsewhere of a link between age and sex of FP and detection. It could also reflect greater emphasis on mental health during FP training.41 However, caution is required in interpreting our findings given the low number of cases per FP available for detection. We believe this accounts for the wide confidence intervals, rather than the wide variability in detection rates between FPs reported elsewhere.4,8,11
The lack of increase in detection rates between the 2 studies could be explained by the relatively high level of detection already found in 2003 (50%), especially considering that cross-sectional study designs tend to underestimate case detection.11,42 A cautious approach to detection is common; FPs preferring watchful waiting43 and discussion of symptoms to increase patient awareness and acceptation44 rather than immediate diagnosis and intervention. This is particularly true for mild conditions, as shown in our study where detection rates increase with the severity of disorders14,45 and comorbidity.46 Also, patients may chose not to disclose symptoms, and a normalising attribution style hinders and postpones detection.47 Last, studies often use categorical diagnostic classification systems, such as DSM, as their reference for detection. This approach is not always considered useful to FPs who prefer a dimensional approach based on symptom severity.48
In our 2013 study, we reported higher detection rates than in the PHQ validation study for mood (60.3% versus 51%) and anxiety disorder (66.7% versus 43%).34 Detection rates for major depression are also higher than in a recent large-scale study also using the PHQ (57.4% versus 51% for a PHQ-9 score ≥10).4 A meta-analysis on mild depression reported a detection rate of 33.8% compared with 46% in our study.38 For anxiety, our rates of 80% for panic disorder and 72% for other anxiety disorder can be compared to an overall rate obtained by meta-analysis of 44.5%.39
As reported elsewhere, our study shows CMDs to be linked to female gender, middle-age groups, marital status, occupation, disability, and personal and social problems.8,14,49,50 These characteristics are mirrored by higher FP detection rates for the 30- to 49-year age group,15,51 being divorced or separated, and living alone. Patients visiting for psychological reasons are more likely both to have a CMD and to be detected by the FP, which suggests that openly presenting psychological symptoms facilitates detection and increases its likelihood.47,52
Regarding service use, patients hospitalised or consulting a specialist doctor or psychiatrist in the past 6 months were more likely to meet CMD criteria.2 This is mirrored by a higher detection rate, despite being borderline significant for hospitalisation and number of specialist doctor visits.
Both number of FP visits25 and doctor-shopping19 have declined considerably since the 2003 study.40 In 2013, doctor-shopping was associated neither with having a CMD nor with FP detection; conversely, our findings from the 2003 study showed a higher proportion of CMD patients among doctor-shoppers whether for practical or dissatisfaction reasons. FP detection was also higher for doctor-shoppers but only for those reporting dissatisfaction with previous care.19 CMD patients consulted FPs more frequently, in keeping with the literature53,54 and our previous study.25 Frequent visits often reflect unmet needs55 or a higher level of patient needs.54 However, contrary to findings from elsewhere,12 this was not linked to detection in our study. This may be explained by a difference in the definition of frequent attendance, in terms of recall period, number of visits, and the lack of age and sex stratification of attendance.56
We found that seeing one’s Preferred Doctor on the survey day was associated with a slightly higher detection rate, in keeping with studies suggesting that FPs are better able to detect CMDs in patients with whom they are more familiar.12,16 Similarly, detection was lower for patients seen by the FP for the first time. Doctor-shopping had declined since the previous study, which could suggest a greater continuity of care. However, in each study, approximately the same proportion of patients declared seeing their usual or Preferred Doctor on the survey day,25 meaning that the scheme may only have formalised what already existed,57 with a limited impact on relational care continuity. This contradictory finding could be tentatively explained by the already high proportion of patients consulting their usual FP on the survey day prior to the scheme and possibly by different participation rates between patients seeing or not seeing their usual FP. There could also be a looser interpretation of what was considered as a “usual” FP in 2003 compared with the Preferred Doctor in 2013, which requires registration. Yet the lack of increase in continuity of care since the introduction of the scheme, as measured by the status (usual doctor or not) of the survey day FP, may explain our failure to find a significant increase in detection between the 2 surveys.
The most frequent action undertaken by the FP on the survey day was a prescription of medication. Patients reaching CMD criteria were more likely to be referred to a specialist doctor, specifically to a psychiatrist; there was no association with FP detection, which may be due to small numbers. CMD status and detection were both associated with longer consultation times, confirming that patients with psychiatric symptoms require more time.58 This is difficult to interpret in a cross-sectional study design as it is not possible to know whether detection increased due to longer visits or the opposite. In so far as longer consultations can be seen as beneficial to the patient,59 our findings highlight the importance of detection for providing adequate care.
The main limitation of our study is the lack of a gold standard for case-detection. Indeed, CMD caseness is based on self-reported symptoms, despite using the DSM-IV criteria-based PHQ designed specifically to overcome the difficulties of obtaining interview-based diagnoses in primary care. Also, the sample cannot be seen as representative of FPs practicing in the study area because of the initial low response rate (32.7%), although it is comparable to that reported in much family practice research.60 FPs particularly interested in mental health may have selectively agreed to participate, perhaps indicated by the retention rate for the 2003 FPs (85.3%). Cross-sectional designs for studying detection can be criticised because they tend to underestimate detection.11 Finally, comparisons between the 2003 and 2013 studies require caution given the other possible explanations for differences beyond the Preferred Doctor scheme.
The main strength of our study is the relatively large number of patients with a high participation rate, for whom data are available from both the patient and the FP. Unfortunately, this was not sufficient to study inter-FP detection rates given the limited number of CMD cases per FP. We used validated research tools, making our findings comparable to those of other studies. Also, the presence of a research assistant in the waiting room yielded high-quality data. We provide up-to-date findings on detection with the originality of comparable data from a previous study carried out using a similar approach in an overlapping sample of FPs.40 This is one of the only studies to provide comparable data obtained before and after the introduction of change in access and use of health care services.
The high but expected prevalence of CMDs in our study is coupled with a relatively high FP detection rate, similar to that in our previous study. Although doctor-shopping has declined since the introduction of the Preferred Doctor scheme, we saw no change over the 10-year period in the already high proportion of patients seeing their usual FP on the survey day. This suggests a limited impact of the scheme on relational continuity of care. It could explain the lack of increase in detection following the implementation of the scheme in 2005. Detected cases benefitted from longer consultation times with more support and advice and were more likely to receive prescriptions for psychotropic medication. This highlights the importance of improving detection as a crucial first step in tackling the growing public health and economic burden of CMDs.61 Further research is needed to better understand what interventions could help improve detection and patient management.
Acknowledgements
We would like to thank all the patients and FPs who participated in the study and the research assistants who helped collect and enter the data. The FPs were paid 150 € in 2003 and 300 € in 2013 (due to a higher number of patients to enroll and informed consent forms to sign) for their participation in the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by a Research Grant from the University Hospital of Montpellier in 2012. The hospital had no further role in the study design; in the collection, analysis, and interpretation of the data; or in the writing and submission of this article.
References
- 1. Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43:476–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kovess-Masfety V, Alonso J, Brugha TS, et al. Differences in lifetime use of services for mental health problems in six European countries. Psychiatr Serv. 2007;58:213–220. [DOI] [PubMed] [Google Scholar]
- 3. Lepine JP, Gastpar M, Mendlewicz J, et al. Depression in the community: the first pan-European study DEPRES (Depression Research in European Society). Int Clin Psychopharmacol. 1997;12:19–29. [PubMed] [Google Scholar]
- 4. Carey M, Jones K, Meadows G, et al. Accuracy of general practitioner unassisted detection of depression. Aust N Z J Psychiatry. 2014;48:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamphuis MH, Stegenga BT, Zuithoff NP, et al. Does recognition of depression in primary care affect outcome? The PREDICT-NL study. Fam Pract. 2012;29:16–23. [DOI] [PubMed] [Google Scholar]
- 6. Norton J, De Roquefeuil G, Boulenger JP, et al. Use of the PRIME-MD Patient Health Questionnaire for estimating the prevalence of psychiatric disorders in French primary care: comparison with family practitioner estimates and relationship to psychotropic medication use. Gen Hosp Psychiatry. 2007;29:285–293. [DOI] [PubMed] [Google Scholar]
- 7. Ormel J, Van Den Brink W, Koeter MW, et al. Recognition, management and outcome of psychological disorders in primary care: a naturalistic follow-up study. Psychol Med. 1990;20:909–923. [DOI] [PubMed] [Google Scholar]
- 8. Ustun TB, Sartorius N, eds. Mental Illness in General Health Care: An International Study. Chichester (UK: ): John Wiley & Sons; 1995. [Google Scholar]
- 9. Gilbody S, Sheldon T, Wessely S. Should we screen for depression? BMJ. 2006;332:1027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute for Clinical Excellence. Stepped care for people with common mental health disorders commissioning guide; 2011. Available from: https://www.nice.org.uk/guidance/cg123/resources/stepped-care-for-people-with-common-mental-health-disorders-commissioning-guide-433581949/chapter/1-Executive-Summary
- 11. Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet. 2009;374:609–619. [DOI] [PubMed] [Google Scholar]
- 12. Bushnell J. Frequency of consultations and general practitioner recognition of psychological symptoms. Br J Gen Pract. 2004;54:838–843. [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson C, Ostler K, Peveler RC, et al. Dimensional perspective on the recognition of depressive symptoms in primary care: The Hampshire Depression Project 3. Br J Psychiatry. 2001;179:317–323. [DOI] [PubMed] [Google Scholar]
- 14. Wittchen HU, Hofler M, Meister W. Prevalence and recognition of depressive syndromes in German primary care settings: poorly recognized and treated? Int Clin Psychopharmacol. 2001;16:121–135. [DOI] [PubMed] [Google Scholar]
- 15. Carey M, Yoong SL, Grady A, et al. Unassisted detection of depression by GPs: who is most likely to be misclassified? Fam Pract. 2015;32:282–287. [DOI] [PubMed] [Google Scholar]
- 16. Klinkman MS, Coyne JC, Gallo S, et al. False positives, false negatives, and the validity of the diagnosis of major depression in primary care. Arch Fam Med. 1998;7:451–461. [DOI] [PubMed] [Google Scholar]
- 17. Docherty JP. Barriers to the diagnosis of depression in primary care. J Clin Psychiatry. 1997;58(Suppl 1):5–10. [PubMed] [Google Scholar]
- 18. Ustun TB, Von Korff M. Primary mental health services: access and provision of care. In: Ustun TB, Sartorius N, eds. Mental Illness in General Health Care: An International Study. Chichester (UK): John Wiley & Sons; 1995. [Google Scholar]
- 19. Norton J, de Roquefeuil G, David M, et al. The mental health of doctor-shoppers: experience from a patient-led fee-for-service primary care setting. J Affect Disord. 2011;131:428–432. [DOI] [PubMed] [Google Scholar]
- 20. Bourgueil Y, Marek A, Mousques J. Three models of primary health care organisation in Europe, Canada, Australia and new Zealand. Quest Eco Santé: 2009;141. [Google Scholar]
- 21. Kringos D, Boerma W, Bourgueil Y, et al. The strength of primary care in Europe: an international comparative study. Br J Gen Pract. 2013;63:e742–e750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lecrubier Y, Boyer P, Lepine J, et al. Results from the Paris Centre. In: Ustun TB, Sartorius N, eds. Mental Illness in General Health Care: An International Study. Chichester (UK): John Wiley & Sons; 1995. [Google Scholar]
- 23. Norton J, de Roquefeuil G, David M, et al. [Prevalence of psychiatric disorders in French general practice using the patient health questionnaire: comparison with GP case-recognition and psychotropic medication prescription]. Encephale. 2009;35:560–569. [DOI] [PubMed] [Google Scholar]
- 24. Norton J, de Roquefeuil G, Benjamins A, et al. Psychiatric morbidity, disability and service use amongst primary care attenders in France. Eur Psychiatry. 2004;19:164–167. [DOI] [PubMed] [Google Scholar]
- 25. Norton J, David M, de Roquefeuil G, et al. Frequent attendance in family practice and common mental disorders in an open access health care system. J Psychosom Res. 2012;72:413–418. [DOI] [PubMed] [Google Scholar]
- 26. Dourgnon P, Guillaume S, Naiditch M, et al. Les assurés et le médecin traitant: premier bilan après la réforme. Quest Eco Santé. 2007;124. [Google Scholar]
- 27. CNAM. Médecin traitant, adopté par la majorité des Français, favorise la prévention: Point d’Information de la Caisse Nationale de l’Assurance Maladie, 2009. Available from: http://www.ameli.fr/fileadmin/user_upload/documents/Bilan_medecin_traitant_Vdef2.pdf
- 28. Le Fur P, Yilmaz E. Referral to specialist consultations in France in 2006 and changes since the 2004 Health Insurance reform. Quest Eco Santé. 2008;134. [Google Scholar]
- 29. Gerard L, Francois M, de Chefdebien M, et al. The patient, the doctor, and the patient’s loyalty: a qualitative study in French general practice. Br J Gen Pract. 2016;66:e810–e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haggerty JL, Roberge D, Freeman GK, et al. Experienced continuity of care when patients see multiple clinicians: a qualitative metasummary. Ann Fam Med. 2013;11:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waibel S, Henao D, Aller MB, et al. What do we know about patients’ perceptions of continuity of care? A meta-synthesis of qualitative studies. Int J Qual Health Care. 2012;24:39–48. [DOI] [PubMed] [Google Scholar]
- 32. Lester H, Tritter JQ, Sorohan H. Patients’ and health professionals’ views on primary care for people with serious mental illness: focus group study. BMJ. 2005;330:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beecham J, Knapp M. Costing psychiatric interventions. In: Thornicroft G, Brewin C, Wing J, eds. Measuring Mental Health Needs. London: Gaskell; 1992:163–183. [Google Scholar]
- 34. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. [DOI] [PubMed] [Google Scholar]
- 35. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. [DOI] [PubMed] [Google Scholar]
- 36. Von Korff M, Ustün TB. Methods of the WHO collaborative study on “Psychological Problems in General Health Care.” In: Ustun TB, Sartorius N, eds. Mental Illness in General Health Care: An International Study. Chichester (UK): John Wiley & Sons; 1995. [Google Scholar]
- 37. Ansseau M, Dierick M, Buntinkx F, et al. High prevalence of mental disorders in primary care. J Affect Disord. 2004;78:49–55. [DOI] [PubMed] [Google Scholar]
- 38. Mitchell AJ, Rao S, Vaze A. Can general practitioners identify people with distress and mild depression? A meta-analysis of clinical accuracy. J Affect Disord. 2011;130:26–36. [DOI] [PubMed] [Google Scholar]
- 39. Olariu E, Forero CG, Castro-Rodriguez JI, et al. Detection of anxiety disorders in primary care: a meta-analysis of assisted and unassisted diagnoses. Depress Anxiety. 2015;32:471–484. [DOI] [PubMed] [Google Scholar]
- 40. Norton J, David M, Gandubert C, et al. [Ability of French general practitioners to detect common mental disorders identified using the Patient Health Questionnaire: has this changed with the introduction of gatekeeping and registration with a preferred doctor?] L’Encéphale. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 41. Fovet T, Amad A, Geoffroy P, et al. Etat actuel de la formation des médecins généralistes à la psychiatrie et à la santé mentale en France. L’information Psychiatrique. 2014;90:319–322. [Google Scholar]
- 42. Kessler D, Bennewith O, Lewis G, et al. Detection of depression and anxiety in primary care: follow up study. BMJ. 2002;325:1016–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lampe L, Shadbolt N, Starcevic V, et al. Diagnostic processes in mental health: GPs and psychiatrists reading from the same book but on a different page. Australas Psychiatry. 2012;20:374–378. [DOI] [PubMed] [Google Scholar]
- 44. Oude Engberink A, Carbonnel F, David M, et al. Management of current psychiatric disorders: a French family physician experience. A qualitative study. Can J Psychiatry. 2016;61:413–421. [Google Scholar]
- 45. Aragones E, Pinol JL, Labad A, et al. Detection and management of depressive disorders in primary care in Spain. Int J Psychiatry Med. 2004;34:331–343. [DOI] [PubMed] [Google Scholar]
- 46. Piek E, Nolen WA, van der Meer K, et al. Determinants of (non-)recognition of depression by general practitioners: results of the Netherlands Study of Depression and Anxiety. J Affect Disord. 2012;138:397–404. [DOI] [PubMed] [Google Scholar]
- 47. Tylee A, Gandhi P. The importance of somatic symptoms in depression in primary care. Prim Care Companion J Clin Psychiatry. 2005;7:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pettersson A, Bjorkelund C, Petersson EL. To score or not to score: a qualitative study on GPs views on the use of instruments for depression. Fam Pract. 2014;31:215–221. [DOI] [PubMed] [Google Scholar]
- 49. Aragones E, Labad A, Pinol JL, et al. Somatized depression in primary care attenders. J Psychosom Res. 2005;58:145–151. [DOI] [PubMed] [Google Scholar]
- 50. Ansseau M, Fischler B, Dierick M, et al. Socioeconomic correlates of generalized anxiety disorder and major depression in primary care: the GADIS II study (Generalized Anxiety and Depression Impact Survey II). Depress Anxiety. 2008;25:506–513. [DOI] [PubMed] [Google Scholar]
- 51. Mitchell AJ, Rao S, Vaze A. Do primary care physicians have particular difficulty identifying late-life depression? A meta-analysis stratified by age. Psychother Psychosom. 2010;79:285–294. [DOI] [PubMed] [Google Scholar]
- 52. Kessler D, Lloyd K, Lewis G, et al. Cross sectional study of symptom attribution and recognition of depression and anxiety in primary care. BMJ. 1999;318:436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dowrick CF, Bellon JA, Gomez MJ. GP frequent attendance in Liverpool and Granada: the impact of depressive symptoms. Br J Gen Pract. 2000;50:361–365. [PMC free article] [PubMed] [Google Scholar]
- 54. Wyke S, Hunt K, Walker J, et al. Frequent attendance, socioeconomic status and burden of ill health: an investigation in the west of Scotland. Eur J Gen Pract. 2003;9:48–55. [DOI] [PubMed] [Google Scholar]
- 55. Smits FT, Brouwer HJ, Zwinderman AH, et al. Why do they keep coming back? Psychosocial etiology of persistence of frequent attendance in primary care: a prospective cohort study. J Psychosom Res. 2014;77:492–503. [DOI] [PubMed] [Google Scholar]
- 56. Vedsted P, Christensen MB. Frequent attenders in general practice care: a literature review with special reference to methodological considerations. Public Health. 2005;119:118–137. [DOI] [PubMed] [Google Scholar]
- 57. Auvray L, Doussain A, Le Fur P. Results from the 2002 report of the health and health care survey. Issues in Health Economics. 2003;78. [Google Scholar]
- 58. Kandel O, Ripault A, Jourdain M, et al. La durée de consultation intervient-elle dans la prescription de psychotropes? La Revue du Practicien. 2008;50:19–24. [PubMed] [Google Scholar]
- 59. Hutton C, Gunn J. Do longer consultations improve the management of psychological problems in general practice? A systematic literature review. BMC Health Serv Res. 2007;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Deehan A, Templeton L, Taylor C, et al. The effect of cash and other financial inducements on the response rate of general practitioners in a national postal study. Br J Gen Pract. 1997;47:87–90. [PMC free article] [PubMed] [Google Scholar]
- 61. Chisholm D, Sweeny K, Sheehan P, et al. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry. 2016;3:415–424. [DOI] [PubMed] [Google Scholar]