ABSTRACT
Topoisomerases solve critical topological problems in DNA metabolism and have long been regarded as the “magicians” of the DNA world. Here we present views from 2 of our recent studies indicating that Type IA topoisomerases from all domains of life often possess dual topoisomerase activities for both DNA and RNA. In animals, one of the 2 Type IA topoisomerases, Top3β, contains an RNA-binding domain, possesses RNA topoisomerase activity, binds mRNAs, interacts with mRNA-binding proteins, and associates with active mRNA translation machinery. The RNA-binding domain is required for Top3β to bind mRNAs and promote normal neurodevelopment. Top3β forms a highly conserved complex with Tudor-domain-containing 3 (TDRD3), a protein known to interact with translation factors, histones, RNA polymerase II, single stranded DNA and RNA. Top3β requires TDRD3 for its association with the mRNA translation machinery. We suggest that Type IA topoisomerases can be “magicians” for not only DNA, but also RNA; and they may solve topological problems for both nucleic acids in all domains of life. In animals, Top3β-TDRD3 is a dual-activity topoisomerase complex that can act on DNA to stimulate transcription, and on mRNA to promote translation.
KEYWORDS: FMRP, polyribosomes, topoisomerase, TDRD3, Top3β, Top3α
Introduction
The first DNA topoisomerase was discovered in E. coli by Dr. James Wang more than 4 decades ago (topoisomerase 1, abbreviated below as EcoTop1).1 Since then, DNA topoisomerases of different types been found in species from all domains of life.2-5 Numerous studies have shown that these enzymes can catalyze DNA strand passage reactions; and are essential to resolve topological stress produced during various DNA metabolic reactions, including replication, transcription, recombination, repair, and chromosome segregation. Their unique attribute of cutting and re-ligating DNA strands without leaving a trace makes them true “magicians of the DNA world”.2 Their defects can lead to reduced cell proliferation, shortened life-span,6 disease,7 and lethality.3,8
Topoisomerases have been classified in 2 major families based on how they cleave the DNA.2,5 Type I topoisomerases transiently cut one strand of the duplex, whereas Type II enzymes simultaneously cut both. These families are further divided into Type IA, IB, IIA and IIB. Type IA enzymes follow the stand-passage mechanism where they cut one strand, pass the unbroken strand through the broken ends of the other strand, and re-ligate the broken ends, resulting in altered linking numbers in DNA duplex. Type IB enzymes differ from Type IA in that they allow swiveling of broken ends around the intact stand to relieve the topoiological stress before re-ligating the broken ends. Specifically, Type IA topoisomerases can act on both double and single stranded (ds and ss) DNA to generate products with altered topology.9 Their reactions include: relaxation of supercoiled dsDNA;1 catenation or decatenation of dsDNA rings that contain at least one pre-existing ssDNA scission in one of the 2 rings;10-12 interconversion between ssDNA rings without knots and those with knots;13,14 intertwining of 2 ssDNA rings of complementary sequences into a closed duplex ring;15 and catenation or decatenation ssDNA rings.16-19 This ability to act on both double and single strand substrates makes Type IA enzymes ideal topoisomerases for RNA because typical RNA molecules (mRNA, tRNA, rRNAs) consist of both double and single-stranded regions.
The first hint that topoisomerases may act on RNA came from the study by DiGate and Marrians in 1992, in which they reported that E.coli topoisomerase 3 (EcoTop3) can catalyze cleavage of RNA.20 EcoTop3 is one of the 2 Type IA topoisomerases in this species (the other is EcoTop1). An elegant study by Seeman and colleagues in 1996 demonstrated that EcoTop3 can indeed catalyze an RNA strand passage reaction that converts a synthetic RNA circle without a knot to a trefoil knot, indicating that EcoTop3 does have RNA topoisomerase activity.21 However, unlike DNA topoisomerases that have been intensively investigated ever since their discovery, the RNA topoisomerase activity has attracted little attention for many years–there are no follow-up studies to link this activity to RNA metabolism, and no RNA topoisomerase activity has been reported in other species. A common thought has been that typical RNA molecules are single-stranded linear and not topologically constrained, so that they may not need topoisomerases.
The silence in the RNA topoisomerase field may finally come to an end in 2013 when our group and the group lead by Dr. Palotie have independently reported that a human Type IA topoisomerase, Top3β, can catalyze RNA strand-passage17 and cleavage reactions.7 The observed RNA topoisomerase activity is specific, because it is absent in the other Type IA topoisomerase in human, Top3α.17 Our group further showed that this difference in RNA topoisomerase activity is largely due to presence of an RNA binding domain, RGG-box, in Top3β but not in Top3α, as deletion of this domain diminished the RNA topoisomerase activity of Top3β.17 Importantly, the 2 studies provided several lines of evidence linking Top3β to mRNA translation. For example, both groups showed that Top3β forms a complex with Tudor domain-containing 3 (TDRD3),7,17 which interacts with a known mRNA-binding protein (RBP), FMRP (Fragile-X mental retardation protein)22 (Fig. 1, see the Animal Section). This is in contrast to Top3α which forms a structurally similar complex with RMI1, RMI2, and a well-characterized DNA helicase, BLM.23-25 Thus, these data suggest that human cells contain 2 homologous Type IA topoisomerase complexes–Top3α-RMI1-RMI2-BLM complex that functions in DNA repair, and the Top3β-TDRD3 complex that interacts with FMRP to participate in RNA metabolic reactions (Fig. 1).17 In support of the notion that the Top3β complex can act on RNA, Top3β has been found to co-localize in RNA stress granules with FMRP and TDRD3 in the cytoplasm,7,17 interact with the mRNA exon-junction complex,7 bind many mRNA targets of FMRP in vivo,17 associate with the active translation machinery (polyribosomes),7,17 and promote expression of a protein at synapse.17 Moreover, Top3β gene deletion has been linked to schizophrenia and intellectual disability,7 which is a mental disorder similar to the Fragile X syndrome caused by silencing of FMRP. Furthermore, Top3β-knockout mice and flies are defective in synapse formation,17 which mimics FMRP-knockout animals.26,27 Together, these data suggest that Top3β may work with FMRP and TDRD3 to regulate expression of mRNAs important for neurodevelopment and mental health.
Figure 1.
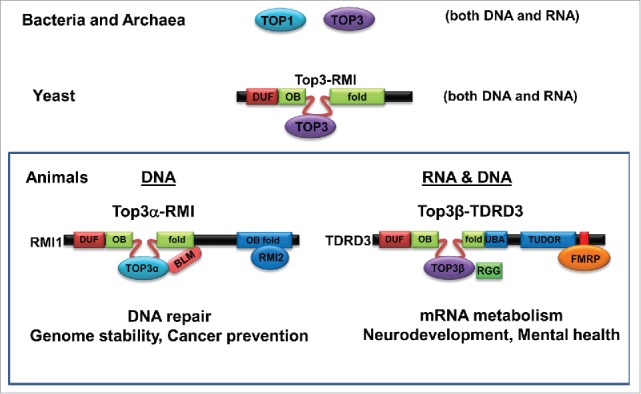
Type IA topoisomerases have evolved from single proteins with dual activities in microorganisms to multi-protein complexes with restricted activities in animals. Schematic representation of evolution of Type IA topoisomerases in DNA and RNA metabolism. In E. coli, both Type IA enzymes (Top1 and Top3) have dual activities for DNA and RNA. In yeast, the only Type IA enzyme is part of a complex (Top3-Rmi1) that also has dual activities for DNA and RNA. In animals, only one of the 2 Type IA paralogs, Top3β, has dual activity, whereas Top3α has activity for only DNA. Interestingly, Top3β, but not Top3α, contains an RNA-binding domain (RGG box) that is required for its RNA topoisomerase activity. Moreover, the 2 Top3 paralogs comprise 2 distinct complexes, with the Top3β complex containing a RNA binding protein (FMRP), whereas the Top3α complex containing a DNA helicase (BLM). These data argue that Type IA topoisomerases have evolved into 2 functional distinct complexes in animals, one for RNA and DNA (Top3β-TDRD3-FMRP), and one for DNA only (Top3α-Rmi1-Rmi2-BLM). This figure is adapted from Fig. 7A of a previous publication.28
In this review, we summarize several major points from our 2 recent studies28,29 which address several fundamental questions regarding RNA topoisomerases. These include: (i) their broad prevalence in all domains of life; (ii) their structural requirement that is common or different from that of DNA topoisomerases; (iii) their conserved association with mRNA translation machinery in animals; (iv) how they are targeted to mRNAs in animals; and (v) the evidence supporting their roles in neural development and diseases. We also discuss the possible origin and evolution of RNA topoisomerase activity and the Type IA topoisomerase complexes.
RNA topoisomerase activity is prevalent in Type IA topoisomerases from all domains of life
The first basic question addressed by our studies is how prevalent RNA topoisomerases are. Prior to our studies, RNA topoisomerase activity has been detected in only 2 Type IA topoisomerases, EcoTop321 and human Top3β.17 Because Type IA enzymes are the most ubiquitous topoisomerases present in nearly all species,4,30 these data raised a possibility that the RNA topoisomerase activity may be prevalent in broad species similarly as is the DNA topoisomerase activity. In a recent study from our group,28 we screened multiple published Type IA DNA topoisomerases from all 3 domains of life (bacteria, archaea, and eukarya) for RNA topoisomerase activity, using the same trefoil knot conversion assay described in the previous studies17,21 (Fig. 2A). To our satisfaction, we detected RNA topoisomerases activity in multiple Type IA enzymes from all 3 domains (Fig. 2B). These include EcoTop1, the prototype of Type 1A family and the first topoisomerase discovered;1 TmaTop1 from the thermophilic bacteria Thermotoga maritima; 2 enzymes from archaea Nanoarchaeum equitans (NeqTop1) and Sulpholobus solfataricus (SsoTop1); and Top3 of yeast saccharomyces crevisiae (a eukarya). Consistent with our findings, Hsieh and his colleagues have developed a different RNA topoisomerase assay, which is based on intertwining of 2 single stranded complementary RNA circles into a duplex RNA ring; and independently detected RNA topoisomerase activity in EcoTop1, NeqTop1, and Drosophila Top3β31 (See Fig. 2B for summary). More recently, Weizmann and colleagues have constructed new ssRNA knots as topological probes, and detected RNA topoisomerase activity in EcoTop1.32 These combined data suggest that the RNA topoisomerase activity is prevalent in Type IA topoisomerases from all domains of life. Interestingly, the enzymes with dual activities are mostly observed in unicellular organisms (bacteria, archaea and yeast) (Figs. 1 and 2B). This is in contrast to human, as only one of the 2 Type IA enzymes (Top3β) has dual activities, whereas the other one (Top3α) has only DNA activity.17 Specialization of the topoisomerase activity for each nucleic acid may be beneficial to eukarytes that have nuclear and cytoplasmic compartments, because it can prevent the DNA topoisomerases from binding to mRNAs and being mis-localized in the cytoplasm where mRNA translation occurs (see Discussion below on targeting of topoisomerase to mRNAs).
Figure 2.
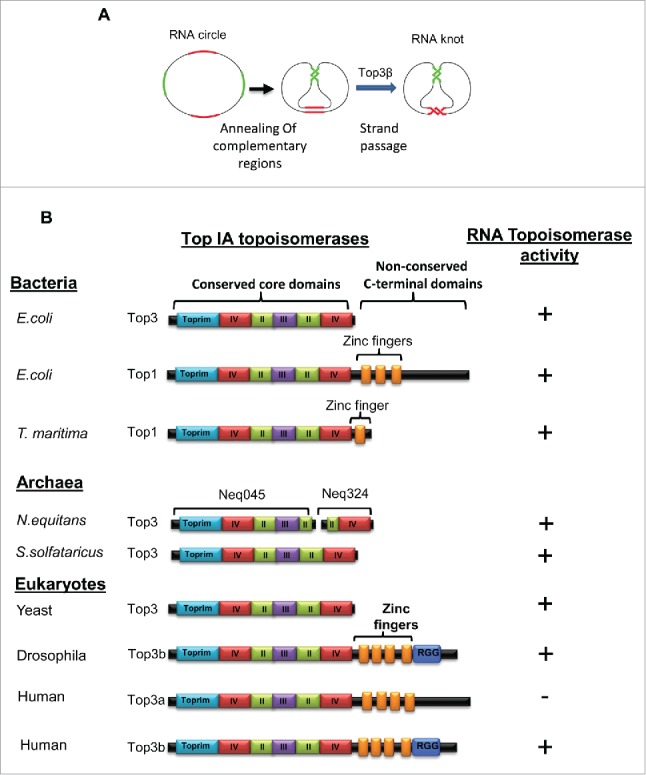
RNA topoisomerase activity is prevalent in Type IA topoisomerases from all 3 domains of life. (A) Schematic representation of the RNA topoisomerase assay. A synthetic circular RNA substrate contains 2 pairs of complementary regions (red and green) separated by single-stranded spacers (black). Through strand passage reactions, this substrate is converted to a trefoil knot in which the 2 pairs of complementary regions can form normal double helices. (B) Schematic representation of the domain structures of Type IA topoisomerases from different species (left) and their RNA topoisomerase activity.17,21,28,31 The conserved core domains and the non-conserved CTDs, including Zn-fingers (orange boxes), are indicated. Their RNA topoisomerase activity was shown on the right. Fig. 2A is adapted from Fig. 4 of the previous paper.17 Fig. 2B is adapted from 4 figures of another paper.28
Do all Type IA topoisomerases have RNA topoisomerase activity? Our screen revealed that some but not all Type IA enzymes possess RNA topoisomerase activity even in microorganisms. For example, we failed to observe RNA topoisomerase activity in Top1 from Mycobacterium tuberculosis and Mycobacterium smegmatis, as well as in 2 reverse gyrases from archaea.
RNA and DNA topoisomerase activities have common and unique structural requirement
The second question addressed by our studies is that as both RNA and DNA topoisomerase activities reside in the same Type IA enzymes, do these activities have common or different structural requirement. All Type IA enzymes utilize a strictly conserved Tyr residue to cleave DNA and form a transient covalent DNA-protein complex; and they absolutely require this Tyr residue for their DNA topoisomerase activity. We found that the same Tyr residue is also necessary for RNA topoisomerase activity for every recombinant Type IA enzyme which we tested.28 Thus, Type IA topoisomerases likely utilize the same catalytic residue to catalyze both DNA and RNA during topoisomerase reactions.
All Type IA enzymes have a group of highly conserved core domains, as well as a non-conserved C-terminal domain (CTDs) that is variable in lengths and numbers of Zinc-fingers (Fig. 2B). Inspection of the Type IA enzymes that carry RNA topoisomerase activity revealed that their CTDs are variable in lengths and may lack the Zinc-fingers (Fig. 2B), which imply that the conserved core domains are likely to be sufficient for the RNA topoisomerase activity. Consistent with this notion, we examined various mutants of EcoTop1, and found that its core domains are both necessary and sufficient to catalyze the RNA topoisomerase reaction, whereas its CTD is completely dispensable.28 This feature is similar to that of EcoTop1 in catalyzing DNA decatenation reaction,33 but different from the same enzyme in catalyzing relaxation of supercoiled DNA,34 as the core domains alone can catalyze the former reaction, whereas both core domains and CTD are needed for the latter reaction. Thus, bacterial Type IA enzymes have both common and different structural requirement for their dual activities. For simple DNA or RNA strand-passage reactions, such as conversion of a circle to a knot and decatenation, the core domains themselves are sufficient to perform the tasks. However, for a complex reaction on DNA duplex, such as supercoiled DNA relaxation, the CTD domain is required.
It needs to be pointed out that in human, the Type IA core domains alone are insufficient for RNA topoisomerase reactions. This is supported by findings that Top3α, which has intact core domains, lacks the RNA topoisomerase activity17 (Fig. 2B). It is also evidenced by data that the RNA topoisomerase activity of Top3β is strongly reduced when its C-terminal RGG box is deleted;17 and the activity is completely lost when its entire CTD domain is removed.28 The data suggest that during evolution, the Type IA core domains of animals have lost the ability to catalyze the strand-passage reactions by themselves. Instead, they have developed strong dependency on their unique CTDs for these reactions. One potential advantage of this dependency is that it enables regulation of topoisomerase activity by CTDs. For example, the CTD of Top3β can target the protein to mRNAs, whereas the CTD of Top3α cannot (see below).
RNA topoisomerases associate with polyribosomes in animals but not yeast or bacteria
The third question addressed by our studies is whether the association between the RNA topoisomerases and polyribosomes is conserved. We and others have previously shown that Top3β, along its interacting partners TDRD3 and FMRP, associate with polyribosomes in human cells,7,17 suggesting that Top3β may mediate mRNA translation together with its partners in eukaryotes. Because RNA topoisomerase activity is prevalent in Type IA topoisomerases from all 3 domains, we investigated whether the polyribosome association is a feature conserved in these enzymes across different domains. No polyribosome association was observed for 2 bacterial topoisomerases (EcoTop1 and EcoTop3), as well as yeast Top3.28 However, the polyribosome association was observed for Top3β from chicken DT40 cells and Drosophila S2 cells, both of which are animal species.28 The data provide evidence that the Type IA topoisomerases may participate in mRNA translation in animals.
The RNA topoisomerase Top3β depends on TDRD3 to associate with polyribosomes
The polyribosome association of human Top3β is dependent on its partner, TDRD3, as deletion of TDRD3 or mutations of its protein interaction domains disrupt the association.7 We found that this TDRD3-dependence is conserved in chicken and flies, as depleting TDRD3 from these cells strongly reduced the amount Top3β that associate with polyribosomes.28 Thus, TDRD3 may play a conserved role in targeting Top3β to the mRNA translation machinery in animals. Current evidence suggests that TDRD3 in animals can interact with multiple RNA binding proteins through its different protein-binding modules, such as its Tudor domain, FMRP-binding motif, and EBM (exon-junction-complex-binding) motif.7,17,k22,35 The Tudor domain can specifically recognize arginine-methylated RNA-binding proteins,35,36 including FMRP.17 Mutations in either Tudor or EBM motif of TDRD3 reduces the association between Top3β and polyribosomes, indicating that TDRD3-mediated protein-protein interactions recruit Top3β to the translation machinery.
Hsieh and colleagues have recently shown that TDRD3 has binding activities for both single-stranded RNA and DNA.31 Moreover, it can stimulate the topoisomerase activity of Top3β for both nucleic acids.31 Thus, TDRD3 is a perfect partner for the dual activity enzyme, Top3β, because it can help Top3β to bind and react with either ssDNA or ssRNA. We propose that TDRD3 plays 2 similar roles for Top3β on DNA or RNA: one, it helps to target Top3β to its site of action by protein-protein or protein-nucleic acid interactions; and 2, it stimulates the subsequent topoisomerase reaction on either RNA or DNA. In the case of RNA, TDRD3 can recruit Top3β to polyribosomes, RNA stress granules, and other translation factors. For reactions on DNA, TDRD3 has been shown to bind arginine-methylated histone tails through its Tudor domain;37 and this binding has been shown to recruit Top3β to chromatin to relax negatively supercoilded DNA and reduce R-loops formed during transcription.38 Moreover, TDRD3 has been reported to bind methylated carboxyl terminal domain of RNA polymerase II.39 Together, these data argue that Top3β-TDRD3 complex acts as a dual-activity complex that can promote biologic processes on either DNA or RNA.
Top3β is the major topoisomerase for mRNAs
The fourth question addressed by our studies is which topoisomerase works on mRNAs. Human cells contain 5 topoisomerases that are present in nucleus and/or cytoplasm, so that each of them could potentially bind mRNAs and regulate their topology. Three of the 5 topoisomerases (Top1, Top2α and Top3β) have previously been shown to bind directly to mRNAs in human cells using a method of UV-crosslinking coupled with mass spectrometry.40,41 These data raised a possibility that these 3 topoisomerases may bind mRNAs and regulate their topology. We used a variation of the above method–UV-crosslinking coupled with immunoblotting–to investigate which of the 5 topoisomerases directly binds mRNAs in human HEK293 cells.29 We found that Top3β is the only topoisomerase that is detectable in the mRNA-bound RNA-binding proteins (RBPs), whereas the other topoisomerases (Top1, Top2α, Top2β, and Top3α) are undetectable. In addition, we detected the presence of both interacting partners of Top3β–TDRD3 and FMRP. Together, these data suggest that Top3β is the major topoisomerase working on mRNAs in human cells.
Top3β is targeted to mRNAs by its unique RGG RNA-binding domain
An obvious question is why Top3β is the most abundant mRNA-binding topoisomerase. We found that only Top3β, but not any other topoisomerases, contains a recognizable RNA-binding domain, an RGG-box. This implies that Top3β is likely to be targeted to mRNAs by this domain. Consistent with this prediction, deletion of the RGG-box strongly reduced the amount of Top3β that associates with the mRNAs.29 This result is reminiscent of earlier data that deletion of RGG-box diminishes the RNA topoisomerase activity of Top3β.17 Together, these data reveal a crucial role of the RGG-box in targeting Top3β to RNAs and catalyzing topoisomerase reactions.
If the RGG-box is so important for Top3β to bind mRNAs, one would predict that it is highly conserved during evolution. Indeed, we found that the RGG-box is conserved in Top3β from not only animals, but also plants and fungi28 (Fig. 3A). Interestingly, the RGG-box is also present in Top3α of plants and several fungi species28 (Fig. 3A), which predicts that Top3α from these species may also be able to act on RNA. Future work is needed to explore this possibility.
Figure 3.
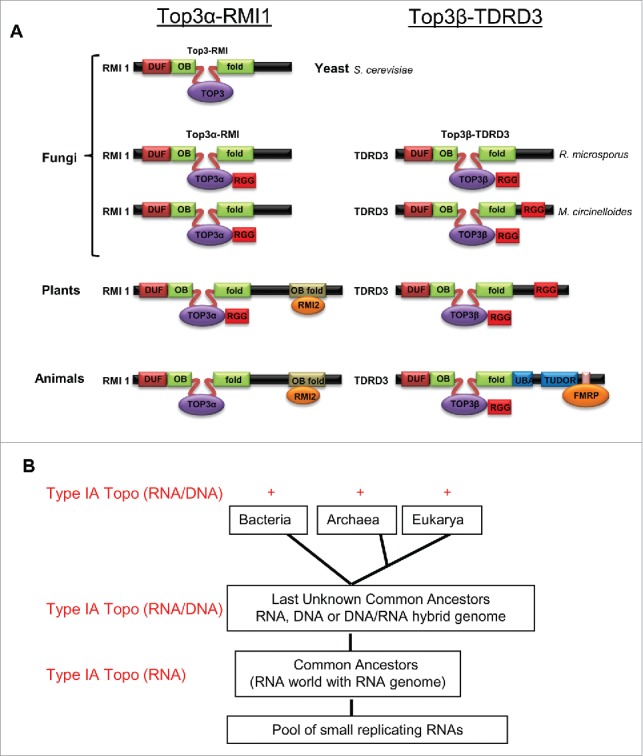
Type IA topoisomerases in eukaryotes have evolved into 2 distinct homologous complexes in animals and plants; the RNA topoisomerase activity of Type IA topoisomerases may have arisen earlier during evolution; (A) Schematic presentations show evolution of Top3 into 2 homologous complexes in eukaryotes, Top3α-RMI1 and Top3β-TDRD3, which have distinct functions in DNA and RNA metabolism, respectively. Yeast has a single copy of Top3 and RMI, which forms a complex required for maintenance of genomic stability. In Fungi like Mucor circinelloides and Rhizopus microspores, we predict that the Top3 enzyme has evolved into 2 paralogous complexes: Top3α-RMI1 and Top3β-TDRD3. Both complexes are also conserved in plants and animals. Top3α-RMI1 retains its function in DNA metabolism and maintenance of genomic stability, while Top3β-TDRD3 works in both DNA and RNA metabolism. The subunits of the 2 Top3 complexes, RMI1 and TDRD3, also share homology at their N-terminus, and emerged in above mentioned species of fungi. Their unique C-terminal domains provide protein-protein binding surfaces which target their respective topoisomerases to specific regulators, such as RMI2 or FMRP. Surprisingly, the C-terminal domains of TDRD3 in plants has more resemblance to those of fungi than to those of animals: they lack the UBA, TUDOR, and FMRP-interaction domains, but have the RGG-box which is also present in TDRD3 of several fungi species. Fungi and plants do not have orthologs of FMRP, which binds to the C-terminus of animal TDRD3. Accession numbers of Top3α, Top3β, RMI, TDRD3 homologs of M. circinelloides (Mc) and R. microsporus (Rm) are mentioned here. McTop3α - EPB81200, McRMI - EPB83693, RmTop3α - CEI89638, RmRMI - CEI87462, Mc Top3β - EPB85471, McTDRD3 - EPB83703, RmTop3β CEG78805 and RmTDRD3 GAN02671. (B) A model of origin and evolution of Type IA topoisomerases and their activity for RNA and DNA. It has been postulated that life starts with a pool of self-replicating RNAs; and there exists an RNA world with RNA genome before the current DNA world. We propose that Type IA enzymes may originate in the RNA world to solve RNA topological problems. When the RNA world evolved and was eventually replaced by the DNA world, these enzymes retained their RNA topoisomerase activity while developing a new activity for DNA. This may explain the prevalence of the RNA topoisomerase activity in Type IA enzymes from all 3 domains. Fig. 3A and 3B are reproduced from Fig. 7 and Fig. S7 of a previous publication.28
Top3β requires its RNA-binding and catalytic activity to promote neurodevelopment
An important issue addressed by our studies is whether Top3β requires its catalytic and RNA binding activity to function in vivo. Our previous studies have shown that Top3β is required for normal synapse formation using mouse and Drosophila as model systems.17 However, the study used gene-knockout animals that completely lack the Top3β protein, so that they did not provide answers to this question. In our new study, we rescued the Drosophila Topβb mutant by ectopically expressing wildtype and 2 mutants of Top3β that are either substituted of its catalytic Tyr residue to Phe, or deleted of its RGG-box. The first mutant completely lacks the topoisomerase activity, but has near normal mRNA-binding activity; whereas the second mutant has defective mRNA-binding activity, and reduced topoisomerase activity. We found that whereas the wildtype Top3β largely rescued the defective synapse formation of the Top3β mutant flies, neither mutants rescued the defect.29 Our data thus suggest that both RNA-binding and topoisomerase activities are needed by Top3β to promote neurodevelopment.
Top3β function is linked to mental disorders
Top3β genomic deletion has been linked to one of the most common mental disorders, Schizophrenia.7 We and others have previously found that the interaction between Top3β-TDRD3 complex and FMRP is disrupted by a Fragile X patient-derived point mutation.17,22 Because Fragile X syndrome is the most common known cause of autism spectrum disorders (ASD), these data argue that Top3β mutation could be a risk factor for schizophrenia and ASD. Consistent with this notion, Top3β genomic deletion has recently been observed in a patient with autism, cognitive impairment, facial dysmorphism and behavior concerns.42
In addition to the gene deletions, 2 de novo single nucleotide variants (SNVs) of Top3β, C666R and R472Q, have been reported in autism and schizophrenia patients, respectively.43,44 We noticed that these variants mutated highly conserved residues in the CTD and core domains of Top3β, respectively, suggesting that they may disrupt Top3β functions. In our latest study,29 we found that both mutants are defective in association with FMRP,29 which is similar to earlier data that a Fragile X patient-derived mutation disrupts the association between FMRP and Top3β-TDRD3.17,22 In addition, we found that the C666R variant is also defective in mRNA binding, in RNA topoisomerase activity, and in supporting normal synapse formation in Drosophila.29 Together, these data suggest that SNVs that compromise Top3β activities may contribute to abnormal neurodevelopment and mental dysfunction. Notably, whereas the C666R variant has not been detected in the general population, the R472Q variant has been detected with a frequency of 0.0004. It will be interesting to analyze whether individuals carrying R472Q allele have increased risk of developing mental disorders.
RNA topoisomerase activity may have arisen earlier during evolution
Type IA typoisomerases have been proposed to be present in the Last Unknown Common Ancestors (LUCAs) for the 3 domains of life, because of their near universal presence in all species30,45 (Fig. 3B). One evolutionary theory postulates that life originates from a pool of self-replicating RNAs, and there exists an RNA world with RNA genome before the current DNA world.46 There are also hypotheses that LUCAs may consist of organisms with genomes of RNA, or RNA-DNA hybrid, or DNA with an RNA replication intermediate.30,45,47 The discovery that RNA topoisomerase activity is prevalent in Type IA topoisomerases from 3 domains of life leads us to propose that these enzymes may have arisen earlier during evolution to solve RNA topological problems in the RNA world.28 The enzymes may have been evolved later to carry DNA topoisomerase activity in LUCA when DNA-based genome replaces the RNA genome. This proposal may help rationalize the findings that Type IA enzymes from all 3 domains have largely retained their dual activities for both nucleic acids.
Type IA topoisomerases have evolved into 2 distinct homologous complexes in animals and plants
We have previously observed that the 2 Type IA topoisomerases, Top3α and Top3β, are present in distinct complexes in human.17,23 Their direct interacting partners within each complex, RMI1 for Top3α and TDRD3 for Top3β, are also homologous to each other: they share 2 common domains at their N-terminus, DUF1767 domain and a discontinuous OB-fold with an intervening loop17,23,24 (Fig. 3A). Structural and functional analyses have revealed that the OB-fold and invervening loop of RMI1 and TDRD3 directly interact with Top3α or Top3β, respectively.17,48-50 Unlike the conserved N-terminus, the C-terminal domains of RMI1 and TDRD3 in animals are divergent: that of RMI1 consists of an OB-fold, which interacts with RMI223,25,50,51, as well as a DNA translocase, FANCM;52 whereas that of TDRD3 consists of multiple protein-interacting modules, UBA (ubiquitin association domain), Tudor, FMRP-interacting motif, and Exon-Junction-complex-binding motif.7,17,22,35 The last 3 domains of TDRD3 can interact with RNA-binding proteins and translation factors,7,17,22,35 consistent with the notion that TDRD3 targets Top3β to mRNA translation machinery.
Our BLAST searches of NCBI database revealed that RMI1 and TDRD3 are only present in eukaryotes, suggesting that mechanisms of Type IA enzymes in eukaryotes might be different from those in other domains of life. In addition, yeast has a single Top3 and a shorterned version of RMI1 that contains only the conserved N-terminal domains (Fig. 3A). The absence of the C-terminal protein-intection domains in yeast RMI1 may account for the lack of association between yeast Top3 and polyribosomes.28
Our BLAST searches also found that Top3α, Top3β, and their 2 partners, RMI1 and TDRD3, are present in plants and several fungi species. This suggests that Type IA topoisomerases from these species may form Top3α-RMI1 and Top3β-TDRD3 complexes that are similar to their counterparts in animals. However, TDRD3 from plants and fungi have 2 structural features different from their counterparts in animals (Fig. 3A): one, they contain an RGG-box that is absent in TDRD3 of animals; and 2, they lack the Tudor domain, FMRP-binding motif, and EBM motif present in the animal TDRD3. First, these data imply that RMI1 and TDRD3 are likely derived from a common ancestor that consists of only the DUF1767 domain and the OB-fold, which may resemble the RMI1 protein in yeast. They may have acquired their distinctive C-terminal domains later in evolution through gene fusion. These C-terminal domains may provide specific interface for DNA binding proteins, RNA binding proteins, or RNA, thus targeting their respective topoisomerases to appropriate action sites. Second, the data also argue that TDRD3 in fungi and plants may target Top3β to mRNA translation machinery differently compared with that of animals—they might directly recruit Top3β to mRNAs through their putative RGG boxes. Overall, our data suggest that Type IA topoisomerases are evolved from single enzymes in microorganisms to 2 multisubunit complexes with more specialized activities and interface in animals and plants.
Concluding Remarks and Future Work
A major discovery of our studies is that many Type IA DNA topoisomerases across all 3 domains of life have RNA topoisomerase activity. Because these enzymes possess dual activities, we propose that they should now be considered as “magicians” for not only DNA, but also RNA. In particular, our studies have provided new evidence for participation of one animal Type IA enzyme–Top3β–in mRNA metabolism. The evidence includes: one, Top3β is the most abundant mRNA-binding topoisomerase in human cells; 2, it is specifically targeted to mRNAs by its RNA-binding domain; 3, its RNA binding domain as well as the catalytic activity are required for neural development; 4, it has conserved association with the mRNA translation machinery in animals; and 5, this association requires its partner, TDRD3, which interacts many RBPs. It should be noted that Top3β also possesses DNA topoisomerase activity,53 and has been suggested to relax supercoiled DNA generated during transcription to reduce R-loop formation.38 Thus, Top3β could act as a dual-activity enzyme solving topological problems for DNA during transcription, and for mRNA during translation.
Is the RNA-binding and topoisomerase activity important for Top3β function? We have demonstrated that mutations disrupting the RNA-binding and catalytic activity of Top3β impair the capability of the protein to promote neurodevelopment.29 However, the same mutations can also disrupt the DNA topoisomerase activity of enzyme.17 At present, there is no separation-of-function mutant that inactivates one activity but leaves the other activity intact. Thus, it remains possible that it is the DNA topoisomerase activity or both activities of Top3β that are needed for normal neurodevelopment. Future work is needed to distinguish which activity is critical for Top3β function in vivo.
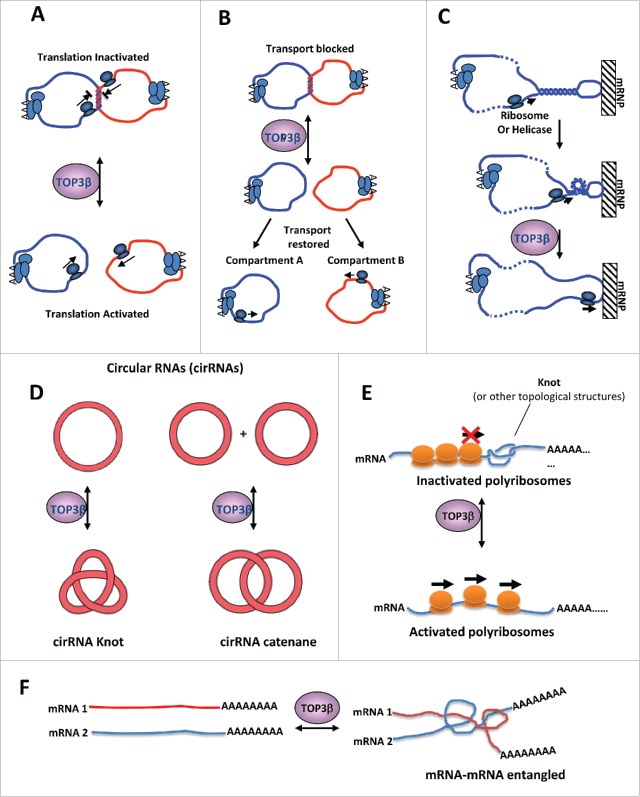
One unanswered question is what types of topological problems may arise during RNA metabolism? We have previously proposed several hypothetical situations where RNA topological problems may occur, which are then resolved by Top3β.17 For example, a linear mRNA may become topologically constrained by forming circular structures via protein-mediated interactions between the Poly-A binding proteins at the 3′ end of mRNA and the translation initation factors at the 5′ end.54 Top3β may catalyze catenation or decatenation of 2 mRNA circles, thus enhancing or inhibiting translation (Fig. 4A) or transport of mRNAs (Fig. 4B). In addition, helical torsions may arise when a translating ribosome or a helicase unwinds a duplex region in an mRNA hairpin (Fig. 4C). If the hairpin is bound to an immobile protein or cellular matrix, the helical torsion will not be relieved by rotation, and may impede progression of ribosomes or helicases during translation or transport. Top3β may relax the helical torsion through its strand passage activity. Moreover, a large family of circular RNAs have been identified from many species,55 and may play roles in neurodegenerative diseases.56,57 Top3β may interconvert these RNAs between circles and knots or catenane structures (Fig. 4D), thus affecting the their function. Furthermore, It is possible that linear RNAs may generate topological problems–they may form intramolecular or intermolecular knots, which may block mRNA translation (Fig. 4E). It is also possible that a linear RNA can get entangled with another linear RNA or DNA (Fig. 4F). The strand-passage activity of an RNA topoisomerase may solve these topological problems, thus facilitating various RNA-based reactions. It should be indicated that topoisomerases may function other than solving topological problems. For example, eukaryotic Top1 mutants that lack DNA topoisomerase activity can stimulate transcription initiation similarly as the wildtype protein.58,59 Thus, it remains to be determined whether Top3β and other Type IA enzymes function by solving RNA topological problems, or by althernative mechanisms, such as RNA binding or protein-protein interactions.
Figure 4.
Models for why RNA metabolism may create topological problems that need an RNA topoisomerase to solve. (A)(B) Models to illustrate that Top3β may catalyze catenation or decatenation of 2 mRNA circles, thus enhancing or inhibiting translation (B) or transport of mRNAs (C). It has been shown that mRNA circles can form when 2 ends of mRNA interacting with a common protein. (C) A model illustrates that helical torsions may arise when a translating ribosome or a helicase unwinds a duplex region in an mRNA hairpin. If the hairpin is bound to an immobile mRNP or cellular matrix, the helical torsion will not be relieved by rotation. Such torsion may impede progression of ribosomes or RNA helicases during translation or transport. Top3β can relax the helical torsion through its strand passage activity. (D) Top3β may interconvert circular RNAs to knots or catenane structures, which may affect the function of these RNAs. (E) A linear mRNA may form an intramocular knot, which may block progression of translating ribosomes. Top3β may catalyze knotting or unknotting of the linear mRNA, and thus affect translation. (F) Two linear mRNAs get entangled with each other, which may interfere with their functions. Top3β may catalyze entangling or segregation of these mRNAs, thus affecting their functions. This figure was adapted from Fig. S7 of a previous publication.17
The other important questions are which mRNAs are bound and regulated by Top3β? Does Top3β recognize some specific sequences or topological structures? Top3β-knockout mice have shortened life-span.6 Identification of Top3β-regulated mRNAs may shed new light on how the topoisomerases functions in promoting neurodevelopment, preventing mental dysfunction, and maintaining normal life-span.
Can other families of topoisomerases work on RNA? Several Type IB enzymes can catalyze cleavage of ribonucleotides incorporated into DNA.60,61 In addition, human Type II enzymes (Top2α and Top2β) can cleave DNA containing a single-ribonucleotide substitution.62 Moreover, human Type IB and Type II enzymes, Top1 and Top2α, respectively, have been shown to bind mRNAs in cells.40,41 Thus, these enzymes may be potential dual-activity topoisomerases. New assays will be needed to determine if these enzymes can act on RNA.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work is supported in part by the Intramural Research Program of the National Institute on Aging (Z01 AG000657–08), National Institutes of Health; the National Basic Research Program of China (2013CB911002) and National Natural Science Foundation of China (31661143040).
References
- 1.Wang JC. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol 1971; 55:523-33; PMID:4927945; https://doi.org/ 10.1016/0022-2836(71)90334-2 [DOI] [PubMed] [Google Scholar]
- 2.Wang JC. Cellular roles of DNA topoisomerases: A molecular perspective. Nat Rev Mol Cell Biol 2002; 3:430-40; PMID:12042765; https://doi.org/ 10.1038/nrm831 [DOI] [PubMed] [Google Scholar]
- 3.Pommier Y, Sun Y, Huang SN, Nitiss JL. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat Rev Mol Cell Biol 2016; 17(11):703-21; PMID:27649880; https://doi.org/ 10.1038/nrm.2016.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forterre P, Gadelle D. Phylogenomics of DNA topoisomerases: Their origin and putative roles in the emergence of modern organisms. Nucleic Acids Res 2009; 37:679-92; PMID:19208647; https://doi.org/ 10.1093/nar/gkp032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JC. DNA topoisomerases. Annu Rev Biochem 1996; 65:635-92; PMID:8811192; https://doi.org/ 10.1146/annurev.bi.65.070196.003223 [DOI] [PubMed] [Google Scholar]
- 6.Kwan KY, Wang JC. Mice lacking DNA topoisomerase IIIbeta develop to maturity but show a reduced mean lifespan. Proc Natl Acad Sci U S A 2001; 98:5717-21; PMID:11331780; https://doi.org/ 10.1073/pnas.101132498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoll G, Pietilainen OP, Linder B, Suvisaari J, Brosi C, Hennah W, Leppä V, Torniainen M, Ripatti S, Ala-Mello S, et al.. Deletion of TOP3beta, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat Neurosci 2013; 16:1228-37; PMID:23912948; https://doi.org/ 10.1038/nn.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Wang JC. Mammalian DNA topoisomerase IIIalpha is essential in early embryogenesis. Proc Natl Acad Sci U S A 1998; 95:1010-3; PMID:9448276; https://doi.org/ 10.1073/pnas.95.3.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JC. DNA topoisomerases. Annu Rev Biochem 1985; 54:665-97; PMID:2992360; https://doi.org/ 10.1146/annurev.biochem.54.1.665 [DOI] [PubMed] [Google Scholar]
- 10.Tse Y, Wang JC. E. coli and M. luteus DNA topoisomerase I can catalyze catenation of decatenation of double-stranded DNA rings. Cell 1980; 22:269-76; PMID:6253080; https://doi.org/ 10.1016/0092-8674(80)90174-9 [DOI] [PubMed] [Google Scholar]
- 11.DiGate RJ, Marians KJ. Identification of a potent decatenating enzyme from Escherichia coli. J Biol Chem 1988; 263:13366-73. PMID:2843517 [PubMed] [Google Scholar]
- 12.Brown PO, Cozzarelli NR. Catenation and knotting of duplex DNA by type 1 topoisomerases: A mechanistic parallel with type 2 topoisomerases. Proc Natl Acad Sci U S A 1981; 78:843-7; PMID:6262776; https://doi.org/ 10.1073/pnas.78.2.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu LF, Depew RE, Wang JC. Knotted single-stranded DNA rings: A novel topological isomer of circular single-stranded DNA formed by treatment with Escherichia coli omega protein. J Mol Biol 1976; 106:439-52; PMID:789893; https://doi.org/ 10.1016/0022-2836(76)90095-4 [DOI] [PubMed] [Google Scholar]
- 14.Du SM, Wang H, Tse-Dinh YC, Seeman NC. Topological transformations of synthetic DNA knots. Biochemistry 1995; 34:673-82; PMID:7819263; https://doi.org/ 10.1021/bi00002a035 [DOI] [PubMed] [Google Scholar]
- 15.Kirkegaard K, Wang JC. Escherichia coli DNA topoisomerase I catalyzed linking of single-stranded rings of complementary base sequences. Nucleic Acids Res 1978; 5:3811-20; PMID:214763; https://doi.org/ 10.1093/nar/5.10.3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Bachrati CZ, Ou J, Hickson ID, Brown GW. Human topoisomerase IIIalpha is a single-stranded DNA decatenase that is stimulated by BLM and RMI1. J Biol Chem 2010; 285:21426-36; PMID:20445207; https://doi.org/ 10.1074/jbc.M110.123216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu D, Shen W, Guo R, Xue Y, Peng W, Sima J, Yang J, Sharov A, Srikantan S, Yang J, et al.. Top3beta is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat Neurosci 2013; 16:1238-47; PMID:23912945; https://doi.org/ 10.1038/nn.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham RP, Wu AM, Shibata T, DasGupta C, Radding CM. Homologous pairing and topological linkage of DNA molecules by combined action of E. coli RecA protein and topoisomerase I. Cell 1981; 24:213-23; PMID:6263487 [DOI] [PubMed] [Google Scholar]
- 19.Takaku M, Takahashi D, Machida S, Ueno H, Hosoya N, Ikawa S, Miyagawa K, Shibata T, Kurumizaka H. Single-stranded DNA catenation mediated by human EVL and a type I topoisomerase. Nucleic Acids Res 2010; 38:7579-86; PMID:20639531; https://doi.org/ 10.1093/nar/gkq630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiGate RJ, Marians KJ. Escherichia coli topoisomerase III-catalyzed cleavage of RNA. J Biol Chem 1992; 267:20532-5. PMID:1383203 [PubMed] [Google Scholar]
- 21.Wang H, Di Gate RJ, Seeman NC. An RNA topoisomerase. Proc Natl Acad Sci U S A 1996; 93:9477-82; PMID:8790355; https://doi.org/ 10.1073/pnas.93.18.9477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linder B, Plottner O, Kroiss M, Hartmann E, Laggerbauer B, Meister G, Keidel E, Fischer U. Tdrd3 is a novel stress granule-associated protein interacting with the Fragile-X syndrome protein FMRP. Hum Mol Genet 2008; 17:3236-46; PMID:18664458; https://doi.org/ 10.1093/hmg/ddn219 [DOI] [PubMed] [Google Scholar]
- 23.Xu D, Guo R, Sobeck A, Bachrati CZ, Yang J, Enomoto T, Brown GW, Hoatlin ME, Hickson ID, Wang W. RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability. Genes Dev 2008; 22:2843-55; PMID:18923082; https://doi.org/ 10.1101/gad.1708608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, Li L, Wang W. BLAP75, an essential component of Bloom's syndrome protein complexes that maintain genome integrity. Embo J 2005; 24:1465-76; PMID:15775963; https://doi.org/ 10.1038/sj.emboj.7600622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh TR, Ali AM, Busygina V, Raynard S, Fan Q, Du CH, Andreassen PR, Sung P, Meetei AR. BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase-double Holliday junction dissolvasome. Genes Dev 2008; 22:2856-68; PMID:18923083; https://doi.org/ 10.1101/gad.1725108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 2001; 107:591-603; PMID:11733059; https://doi.org/ 10.1016/S0092-8674(01)00589-X [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci 2007; 27:3120-30; PMID:17376973; https://doi.org/ 10.1523/JNEUROSCI.0054-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad M, Xue Y, Lee SK, Martindale JL, Shen W, Li W, Zou S, Ciaramella M, Debat H, Nadal M, et al.. RNA topoisomerase is prevalent in all domains of life and associates with polyribosomes in animals. Nucleic Acids Res 2016; 44:6335-49; PMID:27257063; https://doi.org/ 10.1093/nar/gkw508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad M, Shen W, Li W, Xue Y, Zou S, Xu D, Wang W. Topoisomerase 3beta is the major topoisomerase for mRNAs and linked to neurodevelopment and mental dysfunction. Nucleic Acids Res 2016; PMID:27257063; https://doi.org/ 10.1093/nar/gkw1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forterre P, Gribaldo S, Gadelle D, Serre MC. Origin and evolution of DNA topoisomerases. Biochimie 2007; 89:427-46; PMID:17293019; https://doi.org/ 10.1016/j.biochi.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 31.Siaw GE, Liu IF, Lin PY, Been MD, Hsieh TS. DNA and RNA topoisomerase activities of Top3beta are promoted by mediator protein Tudor domain-containing protein 3. Proc Natl Acad Sci U S A 2016; 113(38):E5544-51; PMID:27582462; https://doi.org/ 10.1073/pnas.1605517113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D, Shao Y, Chen G, Tse-Dinh YC, Piccirilli JA, Weizmann Y. Synthesizing topological structures containing RNA. Nature Communications 2017; 8:14936; PMID:28361879; https://doi.org/ 10.1038/ncomms14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahumada A, Tse-Dinh YC. The role of the Zn(II) binding domain in the mechanism of E. coli DNA topoisomerase I. BMC Biochemistry 2002; 3:13; PMID:12052259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zumstein L, Wang JC. Probing the structural domains and function in vivo of Escherichia coli DNA topoisomerase I by mutagenesis. J Mol Biol 1986; 191:333-40; PMID:3029380; https://doi.org/ 10.1016/0022-2836(86)90130-0 [DOI] [PubMed] [Google Scholar]
- 35.Goulet I, Boisvenue S, Mokas S, Mazroui R, Cote J. TDRD3, a novel Tudor domain-containing protein, localizes to cytoplasmic stress granules. Hum Mol Genet 2008; 17:3055-74; PMID:18632687; https://doi.org/ 10.1093/hmg/ddn203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cote J, Richard S. Tudor domains bind symmetrical dimethylated arginines. J Biol Chem 2005; 280:28476-83; PMID:15955813; https://doi.org/ 10.1074/jbc.M414328200 [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Lu Y, Espejo A, Wu J, Xu W, Liang S, Bedford MT. TDRD3 is an effector molecule for arginine-methylated histone marks. Mol Cell 2010; 40:1016-23; PMID:21172665; https://doi.org/ 10.1016/j.molcel.2010.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, McBride KM, Hensley S, Lu Y, Chedin F, Bedford MT. Arginine methylation facilitates the recruitment of TOP3B to chromatin to prevent R loop accumulation. Mol Cell 2014; 53:484-97; PMID:24507716; https://doi.org/ 10.1016/j.molcel.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sims RJ 3rd, Rojas LA, Beck D, Bonasio R, Schuller R, Drury WJ 3rd, Eick D, Reinberg D, et al.. The C-terminal domain of RNA polymerase II is modified by site-specific methylation. Science 2011; 332:99-103; PMID:21454787; https://doi.org/22658674 10.1126/science.1202663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, et al.. Insights into RNA Biology from an Atlas of Mammalian mRNA-Binding Proteins. Cell 2012; 149:1393-406; PMID:22658674; https://doi.org/ 10.1016/j.cell.2012.04.031 [DOI] [PubMed] [Google Scholar]
- 41.Baltz AG, Munschauer M, Schwanhausser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, et al.. The mRNA-Bound proteome and its Global occupancy profile on protein-coding transcripts. Mol Cell 2012; 46:674-90; PMID:22681889; https://doi.org/ 10.1016/j.molcel.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 42.Kaufman CS, Genovese A, Butler MG. Deletion of TOP3B is associated with cognitive impairment and facial dysmorphism. Cytogenet Genome Res 2016; 150:106-11; PMID:27880953; https://doi.org/ 10.1159/000452815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, et al.. De novo gene disruptions in children on the autistic spectrum. Neuron 2012; 74:285-99; PMID:22542183; https://doi.org/ 10.1016/j.neuron.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, Levy S, Gogos JA, Karayiorgou M. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet 2012; 44:1365-9; PMID:23042115; https://doi.org/ 10.1038/ng.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leipe DD, Aravind L, Koonin EV. Did DNA replication evolve twice independently? Nucleic Acids Res 1999; 27:3389-401; PMID:10446225; https://doi.org/ 10.1093/nar/27.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole AM, Logan DT. Modern mRNA proofreading and repair: Clues that the last universal common ancestor possessed an RNA genome? Mol Biol Evol 2005; 22:1444-55; PMID:15774424; https://doi.org/ 10.1093/molbev/msi132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forterre P. Three RNA cells for ribosomal lineages and three DNA viruses to replicate their genomes: A hypothesis for the origin of cellular domain. Proc Natl Acad Sci U S A 2006; 103:3669-74; PMID:16505372; https://doi.org/ 10.1073/pnas.0510333103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goto-Ito S, Yamagata A, Takahashi TS, Sato Y, Fukai S. Structural basis of the interaction between Topoisomerase IIIbeta and the TDRD3 auxiliary factor. Sci Rep 2017; 7:42123; PMID:28176834; https://doi.org/ 10.1038/srep42123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bocquet N, Bizard AH, Abdulrahman W, Larsen NB, Faty M, Cavadini S, Bunker RD, Kowalczykowski SC, Cejka P, Hickson ID. Structural and mechanistic insight into Holliday-junction dissolution by topoisomerase IIIalpha and RMI1. Nat Struct Mol Biol 2014; 21:261-8; PMID:24509834; https://doi.org/ 10.1038/nsmb.2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang F, Yang Y, Singh TR, Busygina V, Guo R, Wan K, Wang W, Sung P, Meetei AR, Lei M. Crystal structures of RMI1 and RMI2, two OB-fold regulatory subunits of the BLM complex. Structure 2010; 18:1159-70; PMID:20826342; https://doi.org/ 10.1016/j.str.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoadley KA, Xu D, Xue Y, Satyshur KA, Wang W, Keck JL. Structure and cellular roles of the RMI core complex from the bloom syndrome dissolvasome. Structure 2010; 18:1149-58; PMID:20826341; https://doi.org/ 10.1016/j.str.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoadley KA, Xue Y, Ling C, Takata M, Wang W, Keck JL. Defining the molecular interface that connects the Fanconi anemia protein FANCM to the Bloom syndrome dissolvasome. Proc Natl Acad Sci U S A 2012; 109:4437-42; PMID:22392978; https://doi.org/ 10.1073/pnas.1117279109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson TM, Chen AD, Hsieh T. Cloning and characterization of Drosophila topoisomerase IIIbeta. Relaxation of hypernegatively supercoiled DNA. J Biol Chem 2000; 275:1533-40; PMID:106368419702200 [DOI] [PubMed] [Google Scholar]
- 54.Wells SE, Hillner PE, Vale RD, Sachs AB. Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell 1998; 2:135-40; PMID:9702200; https://doi.org/ 10.1016/S1097-2765(00)80122-7 [DOI] [PubMed] [Google Scholar]
- 55.Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol 2016; 17:205-11; PMID:26908011; https://doi.org/https://doi.org/ 10.1038/nrm.2015.32 [DOI] [PubMed] [Google Scholar]
- 56.Kumar L, Shamsuzzama Haque R, Baghel T, Nazir A. Circular RNAs: The emerging class of non-coding RNAs and their potential role in human neurodegenerative diseases. Mol Neurobiol 2016; 1-11; PMID:27796758; https://doi.org/ 10.1007/s12035-016-0213-8 [DOI] [PubMed] [Google Scholar]
- 57.You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al.. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci 2015; 18:603-10; PMID:25714049; https://doi.org/ 10.1038/nn.3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shykind BM, Kim J, Stewart L, Champoux JJ, Sharp PA. Topoisomerase I enhances TFIID-TFIIA complex assembly during activation of transcription. Genes Dev 1997; 11:397-407; PMID:9030691; https://doi.org/ 10.1101/gad.11.3.397 [DOI] [PubMed] [Google Scholar]
- 59.Merino A, Madden KR, Lane WS, Champoux JJ, Reinberg D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature 1993; 365:227-32; PMID:8396729; https://doi.org/ 10.1038/365227a0 [DOI] [PubMed] [Google Scholar]
- 60.Sekiguchi J, Shuman S. Site-specific ribonuclease activity of eukaryotic DNA topoisomerase I. Mol Cell 1997; 1:89-97; PMID:9659906; https://doi.org/ 10.1016/S1097-2765(00)80010-6 [DOI] [PubMed] [Google Scholar]
- 61.Kim N, Huang SN, Williams JS, Li YC, Clark AB, Cho JE, Kunkel TA, Pommier Y, Jinks-Robertson S. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 2011; 332:1561-4; PMID:21700875; https://doi.org/ 10.1126/science.1205016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Knudsen BR, Bjergbaek L, Westergaard O, Andersen AH. Stimulated activity of human topoisomerases IIalpha and IIbeta on RNA-containing substrates. J Biol Chem 1999; 274:22839-46; PMID:10428869; https://doi.org/ 10.1074/jbc.274.32.22839 [DOI] [PubMed] [Google Scholar]