Abstract
Pain and stress share significant conceptual and physiological overlaps. Both phenomena challenge the body’s homeostasis and necessitate decision-making to help animals adapt to their environment. In addition, chronic stress and chronic pain share a common behavioral model of failure to extinguish negative memories. Yet, they also have discrepancies such that the final brain endophenotype of posttraumatic stress disorder, depression, and chronic pain appears to be different among the three conditions, and the role of the hypothalamic-pituitary-adrenal axis remains unclear in the physiology of pain. Persistence of either stress or pain is maladaptive and could lead to compromised well-being. In this brief review, we highlight the commonalities and differences between chronic stress and chronic pain, while focusing particularly on the central role of the limbic brain. We assess the current attempts in the field to conceptualize and understand chronic pain, within the context of knowledge gained from the stress literature. The limbic brain—including hippocampus, amygdala, and ventromedial prefrontal cortex—plays a critical role in learning. These brain areas integrate incoming nociceptive or stress signals with internal state, and generate learning signals necessary for decision-making. Therefore, the physiological and structural remodeling of this learning circuitry is observed in conditions such as chronic pain, depression, and posttraumatic stress disorder, and is also linked to the risk of onset of these conditions.
Keywords: pain, stress, limbic circuitry, hippocampus, amygdala, depression, posttraumatic stress disorder
Why Stress and Pain?
Stress-related psychiatric disorders, including depression and posttraumatic stress disorder (PTSD), are highly prevalent disabling illnesses with limited treatment options and poorly understood pathophysiology.1 Chronic pain is a widespread pathology afflicting 20%–30% of adults. Moreover, while treatment options are available, chronic pain continues to seriously affect the life quality of patients, and almost half of pain suffering individuals do not achieve adequate pain management.2 Better understanding of the overlapping and distinguishing features of chronic stress and pain could provide greater insight into the neurobiology of these processes, as well as contribute to rational drug development for these often comorbid conditions.3 In the current brief review, we describe the commonalities and differences of stress and pain, while primarily focusing on the maladaptive processes of chronic pain and chronic stress.
Pain and stress are two distinguished yet overlapping processes presenting multiple conceptual and physiological overlaps. Stress can be defined broadly as a process by which a challenging emotional or physiological event or series of events result in adaptive or maladaptive changes required to regain homeostasis and/or stability.4 Pain is the collection of emotional and sensory perceptions, as well as motor behaviors, resulting from the activation of the nociceptive pathways in response to harmful stimuli. The ability of the organism to adapt to stress or pain by regulating the internal milieu and maintaining stability is termed allostasis. Pain and stress are both adaptive in protecting the organism, for example, from physical injury or starvation. However, if either of the two processes becomes chronic, it can lead to long-term “maladaptive” changes in physiology and consequently behavior, resulting in suffering and compromised well-being.5 Taken together, these conceptualizations are clearly overlapping and offer an opportunity for theoretical and experimental exchanges between the two fields of study.
Researchers have adopted two, mutually non-exclusive, models linking pain and stress. The first model considers pain as one type of stress that adds strain on the organism. For example, chronic back pain (CBP) is conceptualized as a stress overload6 resulting in an increased risk for depression, alcohol abuse, or weight gain.5,7,8 In this model, chronic pain leads to “wear-and-tear”—also termed allostatic overload—in the body and brain “from chronic dysregulation (i.e., over-activity or inactivity) of physiological systems that are normally involved in adaptation to environmental challenge.”9 These wear-and-tear alterations result in compromised well-being, and/or social and occupational dysfunction. Persistent experience of pain (i.e., over-activity) can burden the brain and lead to deficits in decision-making.10–12 Conversely, fear of movement that would exacerbate pain could lead to a more sedentary lifestyle (i.e., inactivity) and weight gain. The second model depicts the cases in which wear-and-tear precipitates chronic pain. In this model, patients are faced with unpredictable stress that triggers pain—a migraine attack, for example—and leads to a vicious circle of “feed-forward” maladaptive physiological responses such as inflammation and brain damage and hence increased vulnerability to persistence of pain.13 The two models do not necessarily contradict each other, but rather borrow from the stress literature to provide either a causal conceptualization of the onset and persistence of chronic pain or of its long-term consequences. They also emphasize that stress and pain can be two nodes in a vicious circle of maladaptive responses to environmental challenges leading to compromised well-being.
In this review, we examine the important overlap between chronic pain and stress, while emphasizing differences between the two phenomena, which could have separate and even opposite neurobiological effects. We describe the commonalities and differences between chronic stress and chronic pain, with a special emphasis on the neurobiological underpinnings, where the brain limbic system14 stands as a central mediator of these two phenomena. We discuss in particular whether chronic pain can be considered under the larger process of stress or whether the two phenomena have different biological processes.
Socioeconomic Factors in Stress and Chronic Pain
There is evidence that disparity in many dimensions of socio-economic status (SES) such as income, education, and occupation, account for a significant variance of medical morbidities and mortalities.15,16 Studies have found a so-called “gradient” between occupational hierarchy and health disparities in adults. People in the bottom of the gradient have worse morbidities and mortalities.15 These SES disparities can in turn translate at the individual level to environmental stressors leading to a vulnerability to depression, substance use disorders, and obesity among others.4,17 Furthermore, children growing up in poor communities are at an increased risk of exposure to crime, economic hardship, and pollution18; this in turn can lead to adverse behavioral (e.g., emotional dysregulation)17,19 and neurodevelopmental outcomes (e.g., psychopathology and brain changes).20,21 While the brain is believed to be at the center of this process, the direct path linking SES factors to neurobiological brain adaptive and maladaptive responses remains largely unknown,9 with pain and stress as putative contributing factors.
The link between SES factors and exposure to stress is evident, given the broad definition of stress. However, the relationship between SES and chronic pain is less discernable. In the British Birth Cohort Study, a 45-year longitudinal study, increased risk for reporting pain as adults was found in individuals from a lower SES, and in those who experienced adverse life events as children. However, the increased risk was partly explained by other current life factors.22,23 In patients followed through the emergency room after a major physical trauma, a higher educational level was the only social factor associated with persistent back pain. Income and employment status before the injury were not associated with persistent back pain after the trauma.24 Educational level was also a protective factor against frequent knee pain in a cohort of Swedish patients examined for knee osteoarthritis.25 These findings support the presence of a link between social stressors, lower educational level, and onset of pain diatheses. Nevertheless, a recent literature review found no relationship between SES characteristics and the frequency of seeking a medical consultation for back pain.26 In addition, two longitudinal studies found no significant correlations between chronic pain, socio-demographic factors, adverse life events, and “dysfunction of the stress system.”27,28 These studies underscore the complexity of the relationship between social factors and chronic pain, while challenging the common wisdom of a direct link between social stressors such as low SES and the onset of chronic pain.
The Neurobiology of Stress and Pain
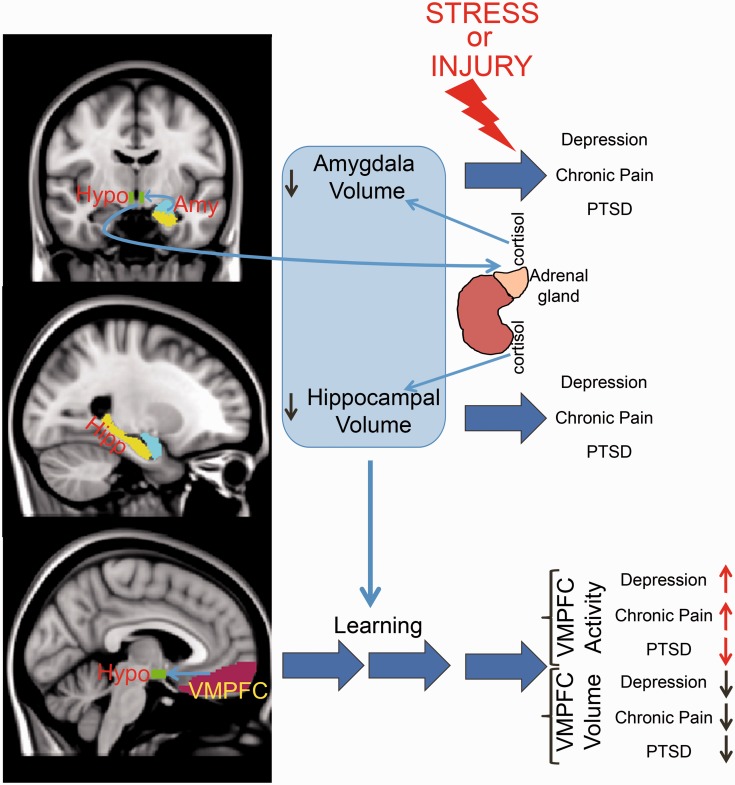
The brain plays a central role in stress and pain processes.4,29,30 As individuals interact with their environment, physical and psychological stressors can lead to adaptive or maladaptive neural and hormonal responses. Acute stress triggers the activation of the hypothalamic-pituitary-adrenal axis (HPA) leading to the release of adrenal glucocorticoids.29 These hormones have receptors concentrated in the limbic brain including the hypothalamus, amygdala, hippocampus, and prefrontal cortex (PFC).31,32 In the limbic system, glucocorticoids act as transcription factors and have therefore long-lasting effects on cellular function. Acute stress also activates the autonomic nervous system regulated by the brainstem,33 leading to increased blood pressure and diversion of blood from the gastrointestinal tract to the brain and muscles.29 In addition, perceived stress is integrated in the limbic brain with past experiences (i.e., memory), current physiological state (e.g., hunger/satiety), and decision-making. Subsequently, emotional states are updated accordingly (e.g., increased or decreased anxiety) with an ultimate effect on behavior (e.g., fight or flight). The limbic brain and HPA axis form an interconnected loop as projections from the hippocampus, amygdala, and PFC feed-back to the hypothalamus and regulate the stress responses and glucocorticoid release (Figure 1).34 Other brain areas have been also shown to be active during acute stress such as the insula and striatum.35
Figure 1.
Schematic depiction of the circuitry involved in chronic pain and chronic stress. Light-blue arrows indicate anatomical or physiological links. Dark blue arrows indicate time. Black and red arrows indicate magnitude. Abbreviations: Amy, Amygdala; Hipp, Hippocampus; Hypo, Hypothalamus; PTSD, post-traumatic stress disorder; VMPFC, ventro-medial prefrontal cortex.
Pain requires conscious perception of the nociceptive process. Nociceptive information is transmitted via peripheral A-δ and C-fibers to the brainstem and thalamus, where it is then relayed to multiple cortical and subcortical areas including primary and secondary somatosensory areas, anterior cingulate cortex, insula, amygdala, striatum, and medial PFC.36–39 Acute pain engenders both a sensory and an emotional experience and is an adaptive response protecting the body from tissue damage like a burning fire or the attack of a predator. Although acute pain can be easily conceptualized as a form of acute stress, the details of the neural and endocrine response to acute pain and acute stress can be different. For example, while it is known that both acute pain and stress activate the autonomic nervous system,29,40 evidence that acute pain activates the HPA axis and leads to peripheral adrenal cortisol secretion, one of the hallmark endocrine responses to stress, is unclear.41,42 Alternatively, at the brain level, functional magnetic resonance imaging studies of response to stress or pain demonstrate noticeable spatial overlap in the amygdala, hippocampus, striatum, insula, and anterior cingulate cortex.35,43
Learning and Neural Remodeling in Chronic Stress and Chronic Pain
Stress and pain engage the learning circuitry of the hippocampus, amygdala, and PFC (Figure 1), as animals interact adaptively with the challenges of their environment to maintain homeostasis.9,43 Animals have to learn about their environment to seek places where food can be found while avoiding the threat of an attack from a predator or the ingestion of poisonous substances. Fear (Pavlovian) conditioning and extinction are paradigms of learning in which both chronic pain and chronic stress can be conceptualized.44,45 In a Pavlovian model, a previously neutral stimulus acquires the ability to induce fear behavior in animals (e.g., conditioned stimulus) after being paired with an unconditioned painful stimulus, like a foot-shock.46 Extinction of the fear association, or the unlearning of the fear, occurs when the conditioned stimulus is presented multiple times without the unconditioned stimulus.47 PTSD and chronic pain can be considered conditions where the brain fails to extinguish the negative memory (i.e., memory of trauma or pain).44,45 Consistently, both PTSD and chronic pain patients show deficiency in extinction learning.48,49 In addition, similar to findings in traumatic stress preclinical literature,50 an animal model of chronic neuropathic pain shows impaired context-related fear extinction.51 The hippocampus, amygdala, and PFC each play a critical role in fear learning and extinction.45,52,53 The neurochemical properties of the learning circuitry and its adaptive response to chronic stress or pain are believed to be crucial in determining remission or persistence of pain and stress response beyond what is required for an evolutionary advantageous adaptive response.45,54–56 Below, we expand on details of the role played by each of these regions in chronic stress and chronic pain demonstrating the compelling conceptual overlap between the fields yet highlighting important empirical differences.
The hippocampus is active during acute stress,35 but rarely seen active during acute pain in humans.57 An intact hippocampus is important during acquisition of fear conditioning and association of context with stimuli that necessitates decision-making such as finding food or avoiding pain.58–62 In addition, the hippocampus contributes to contextual fear extinction.63,64 It contains glucocorticoid receptors,65 projects to the hypothalamus,66 and is thought to down-regulate the response to stress.9,67–69 Neurogenesis persists in the adult mammalian hippocampus70 and contributes to learning and memory.71 In humans, chronic pain and stress-related psychiatric disorders have been associated with shrinkage of the hippocampal volume.51,56,72,73 Vachon-Presseau et al. demonstrated that hippocampal volume is inversely correlated to elevated basal cortisol levels in CBP patients but not in matched healthy control arguing for a “stress model of chronic pain” centered on the hippocampus. Interestingly, smaller hippocampal volume predicts the risk of persistence of back pain after three years of a new episode of sub-acute back pain (SBP; pain duration 6–16 weeks),56 and is present in individuals at risk for PTSD and depression.74,75
Both chronic pain and stress were associated with suppressed hippocampal neurogenesis,51,72 a process that could be mediated by elevated glucocorticoids during stress.72 However, the relationship between neurogenesis and acute pain or stress is more complex. A recent study in rodents found that adult hippocampal neurogenesis is necessary for the emergence of pain behavior after nerve injury.76 Nevertheless, neurogenesis was suppressed once the pain became chronic,51 implying that the interaction between peripheral injury and central hippocampal learning mechanisms is critical for the onset of pain behavior. These results are in resemblance to findings by Kirby et al.77 showing that acute immobilization for 3 h, but not foot shock for 30 min, increased hippocampal neurogenesis. The effect of immobilization stress on neurogenesis could be reproduced with corticosterone injections, which leads to a delayed onset (at 2 weeks) of enhanced fear extinction.77 These studies highlight the fact that acute pain (i.e., foot shock) cannot be fully conceptualized as an acute stressor and that learning after nerve injury might have different long-term behavioral effects from stress. It also underscores the beneficial effects of acute stress, which increased neurogenesis and enhanced extinction. Consistently, Mutso et al.51 demonstrated impaired contextual fear extinction after peripheral nerve injury. In contrast, Kirby et al.77 demonstrated enhanced fear extinction two weeks after an acute stressor presentation.
The amygdala is another major node of the limbic brain (Figure 1) that is highly interconnected with the hippocampus.78 It plays a major role in emotional learning53 and in the response to stress and pain. The amygdala is active during response to threats such as angry faces79,80 and in response to acute pain.81,82 It is critical in the expression of fear46 and shows hyperactivity in chronic stress-related conditions such as PTSD, and in chronic pain disorders such as CBP or migraine.83–85 Animal data show that the amygdala plays a dual role in the perception of nociceptive input depending on the context of the painful stimulation. Lesion of the central nucleus of the amygdala (CeA) abolishes or decreases aversive stimulus-induced hypoalgesia (i.e., pain reduction).86 Corticosterone implant in the CeA enhances anxiety-like behavior and visceral hypersensitivity to balloon distention of the colon or acetic acid infusion in the colon.87 In addition, CeA neurons show increased sensitization in a rodent model of arthritis, independent of peripheral nociceptive input.88 In animal models, chronic stress and chronic pain are both associated with dendritic growth in the amygdala89–91 suggesting enhanced synaptic activity, possibly in response to increased glucocorticoid levels.92 At the macroscopic level, humans suffering from depression, PTSD, or chronic pain were found to have smaller amygdala,56,93–96 although not without inconsistency (e.g., Kuo et al.97). Interestingly, depressed patients on medications have increased amygdala volume.93 In addition, a cohort of 10 patients with hip osteoarthritis showed an increase in amygdala volume after total hip replacement and remission of pain98 suggesting that volume shrinkage is a consequence of chronic pain and depression, and could therefore recover if both conditions are adequately treated. This data, along with the decreased hippocampal volume in chronic pain, is consistent with the concept of allostatic-load from chronic pain as volumetric shrinkage can be considered the wear-and-tear manifested in the brain secondary to the chronic exposure to nociception lending support to the view that chronic pain can be considered a form of chronic stress.6 Nevertheless, other data showed that amygdala volume stays unchanged and predicts the persistence of back pain three years after a sub-acute episode of back pain,56 suggesting that a smaller amygdala volume could be a risk factor for chronic pain and not the consequence of exposure to chronic pain.
Both the hippocampus and amygdala are highly interconnected with the ventro-medial PFC (vmPFC)99,100 (Figure 1), which is a critical area in fear extinction52,54,101,102 and in assigning value to rewarding and aversive stimuli.103,104 vmPFC volume shrinks in chronic pain, PTSD, and depression.105,106 Activity in the vmPFC, on the other hand, increases after repeated acute stress in healthy subjects,35 and is increased in patients suffering from chronic pain and depression84,85,107–109 but is decreased in patients suffering from PTSD.45,110 In addition, vmPFC activity is positively correlated with pain intensity in CBP patients,84,85,107 but negatively correlated with severity of symptoms in PTSD. Therefore, the physiology of chronic pain and chronic stress might be diverging in the vmPFC. Behaviorally, altered vmPFC activity could explain impaired extinction in PTSD and impaired emotional decision-making in chronic pain10,11,111,112 and depression.113
Contentious Points in Borrowing From Stress to Explain Chronic Pain
Despite the significant neuroanatomical and physiological overlap reported above between chronic pain and chronic stress, upholding the stress model of chronic pain faces some challenges. First, as we outlined above, the data on the contribution of psycho-social factors and markers of biological stress to the onset or the persistence of chronic pain is conflicting.22,26–28 Second, the data on the “dysregulation of the HPA axis and cortisol” level in chronic pain does not fit any clear consistent pattern. As such, studies of chronic pain conditions have reported increases6,114–119 and decreases41,120–124 in cortisol level, while many studies reported no changes.125–128 Furthermore, different reports present conflicting data within the same condition such as CBP,6,41 fibromyalgia,125,129 or migraine.117,119,128 Third, the definition of stress is very broad; for example, showing violent pictures and acute aversive stimuli-like acute pain can be both stressful but involve different physiology. Furthermore, release of cortisol and activation of the hippocampus are often observed following stress,35 but rarely seen after acute pain.57 Similarly, although chronic conditions that are thought to arise after repeated stress or trauma such as PTSD and depression share markers of vulnerabilities with chronic pain within the limbic brain like a smaller hippocampus and a smaller amygdala,56,75,93–95 the brain endophenotypes appear to be different. For example, vmPFC global brain connectivity is decreased in depression,130 yet increased in PTSD131,132 and chronic pain.133 This observation does not preclude a role of stress physiology in the onset and persistence of chronic pain, but rather calls for more specific definitions of the biological markers of stress.
Conclusion
Taken together, the data discussed above provide a rationale for the attempts to use the stress model in chronic pain, yet emphasize the difficulties in classifying the concept of chronic pain under the general framework of chronic stress. We believe that unifying both processes under one theoretical framework would be enhanced by understanding how different chronic painful or stressful conditions induce continuous emotional learning centered particularly around the properties and remodeling of amygdala and hippocampus.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Murrough J, W. Abdallah, C. G., Mathew, S. Targeting glutamate signaling in depression: progress and prospects. Nat Rev Drug Discov. In press. [DOI] [PubMed]
- 2.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006; 10: 287–333. [DOI] [PubMed] [Google Scholar]
- 3.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med 2003; 163: 2433–2445. [DOI] [PubMed] [Google Scholar]
- 4.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry 2013; 73: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knaster P, Karlsson H, Estlander AM, Kalso E. Psychiatric disorders as assessed with SCID in chronic pain patients: the anxiety disorders precede the onset of pain. Gen Hosp Psychiatry 2012; 34: 46–52. [DOI] [PubMed] [Google Scholar]
- 6.Vachon-Presseau E, Roy M, Martel MO, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain 2013; 136: 815–827. [DOI] [PubMed] [Google Scholar]
- 7.Neumann L, Lerner E, Glazer Y, Bolotin A, Shefer A, Buskila D. A cross-sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin Rheumatol 2008; 27: 1543–1547. [DOI] [PubMed] [Google Scholar]
- 8.Wilson AC, Samuelson B, Palermo TM. Obesity in children and adolescents with chronic pain: associations with pain and activity limitations. Clin J Pain 2010; 26: 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 2010; 1186: 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain 2004; 108: 129–136. [DOI] [PubMed] [Google Scholar]
- 11.Verdejo-Garcia A, Lopez-Torrecillas F, Calandre EP, Delgado-Rodriguez A, Bechara A. Executive function and decision-making in women with fibromyalgia. Arch Clin Neuropsychol 2009; 24: 113–122. [DOI] [PubMed] [Google Scholar]
- 12.Walteros C, Sanchez-Navarro JP, Munoz MA, Martinez-Selva JM, Chialvo D, Montoya P. Altered associative learning and emotional decision making in fibromyalgia. J Psychosom Res 2011; 70: 294–301. [DOI] [PubMed] [Google Scholar]
- 13.Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron 2012; 73: 219–234. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam MM. Principles of behavioral and cognitive neurology. 2nd ed. Oxford, England: Oxford University Press; 2000:xviii, 540.
- 15.Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci 2010; 1186: 5–23. [DOI] [PubMed] [Google Scholar]
- 16.Volkers AC, Westert GP, Schellevis FG. Health disparities by occupation, modified by education: a cross-sectional population study. BMC Public Health 2007; 7: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: moving from markers to mechanisms of risk. Psychol Bull 2003; 129: 447–466. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci 2010; 1186: 37–55. [DOI] [PubMed] [Google Scholar]
- 19.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry 2006; 60: 296–301. [DOI] [PubMed] [Google Scholar]
- 20.Eluvathingal TJ, Chugani HT, Behen ME, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 2006; 117: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 21.Kim P, Evans GW, Angstadt M, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci U S A 2013; 110: 18442–18447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: results from the 1958 British Birth Cohort Study. Pain 2009; 143: 92–96. [DOI] [PubMed] [Google Scholar]
- 23.Macfarlane GJ, Norrie G, Atherton K, Power C, Jones GT. The influence of socioeconomic status on the reporting of regional and widespread musculoskeletal pain: results from the 1958 British Birth Cohort Study. Ann Rheum Dis 2009; 68: 1591–1595. [DOI] [PubMed] [Google Scholar]
- 24.Harris IA, Young JM, Rae H, Jalaludin BB, Solomon MJ. Factors associated with back pain after physical injury: a survey of consecutive major trauma patients. Spine 2007; 32: 1561–1565. [DOI] [PubMed] [Google Scholar]
- 25.Kiadaliri AA, Gerhardsson de Verdier M, Turkiewicz A, Lohmander LS, Englund M. Socioeconomic inequalities in knee pain, knee osteoarthritis, and health-related quality of life: a population-based cohort study in southern Sweden. Scand J Rheumatol 2017; 46(2): 143–151. [DOI] [PubMed] [Google Scholar]
- 26.Adamson J, Hunt K, Nazareth I. The influence of socio-demographic characteristics on consultation for back pain—a review of the literature. Fam Pract 2011; 28: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott AM, Smith BH, Hannaford PC, Smith WC, Chambers WA. The course of chronic pain in the community: results of a 4-year follow-up study. Pain 2002; 99: 299–307. [DOI] [PubMed] [Google Scholar]
- 28.Generaal E, Vogelzangs N, Macfarlane GJ, et al. Biological stress systems, adverse life events and the improvement of chronic multi-site musculoskeletal pain across a 6-year follow-up. J Pain 2017; 18(2): 155–165. [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904. [DOI] [PubMed] [Google Scholar]
- 30.Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015; 87: 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res 1996; 26: 235–269. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci 2000; 20: 4657–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 2002; 25: 433–469. [DOI] [PubMed] [Google Scholar]
- 34.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009; 10: 434–445. [DOI] [PubMed] [Google Scholar]
- 35.Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci U S A 2016; 113: 8837–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol 1996; 365: 640–658. [DOI] [PubMed] [Google Scholar]
- 37.Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience 2010; 168: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klop EM, Mouton LJ, Hulsebosch R, Boers J, Holstege G. In cat four times as many lamina I neurons project to the parabrachial nuclei and twice as many to the periaqueductal gray as to the thalamus. Neuroscience 2005; 134: 189–197. [DOI] [PubMed] [Google Scholar]
- 39.McMahon SB. Wall and Melzack's textbook of pain. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2013:xxix, 1153.
- 40.Saper CB. Pain as a visceral sensation. Prog Brain Res 2000; 122: 237–243. [DOI] [PubMed] [Google Scholar]
- 41.Muhtz C, Rodriguez-Raecke R, Hinkelmann K, et al. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med 2013; 14: 498–503. [DOI] [PubMed] [Google Scholar]
- 42.Goodin BR, Quinn NB, King CD, et al. Salivary cortisol and soluble tumor necrosis factor-alpha receptor II responses to multiple experimental modalities of acute pain. Psychophysiology 2012; 49: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 2007; 55: 377–391. [DOI] [PubMed] [Google Scholar]
- 44.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol 2008; 18: 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 2005; 48: 175–187. [DOI] [PubMed] [Google Scholar]
- 47.Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry 2006; 60(4): 329–336. [DOI] [PubMed] [Google Scholar]
- 48.Flor H, Knost B, Birbaumer N. The role of operant conditioning in chronic pain: an experimental investigation. Pain 2002; 95: 111–118. [DOI] [PubMed] [Google Scholar]
- 49.Wicking M, Steiger F, Nees F, et al. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiol Learn Mem 2016; 136: 116–126. [DOI] [PubMed] [Google Scholar]
- 50.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 2013; 14: 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mutso AA, Radzicki D, Baliki MN, et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci 2012; 32: 5747–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 2002; 420: 70–74. [DOI] [PubMed] [Google Scholar]
- 53.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184. [DOI] [PubMed] [Google Scholar]
- 54.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A 2005; 102: 10706–10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vachon-Presseau E, Tetreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016; 139: 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanz S, Seifert F, Maihofner C. Brain activity associated with pain, hyperalgesia and allodynia: an ALE meta-analysis. J Neural Transm (Vienna) 2011; 118: 1139–1154. [DOI] [PubMed] [Google Scholar]
- 58.Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci 1986; 100: 729–744. [DOI] [PubMed] [Google Scholar]
- 59.Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol 2011; 21: 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci U S A 1996; 93: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kahn MC, Bingman VP. Avian hippocampal role in space and content memory. Eur J Neurosci 2009; 30: 1900–1908. [DOI] [PubMed] [Google Scholar]
- 62.Sarel A, Finkelstein A, Las L, Ulanovsky N. Vectorial representation of spatial goals in the hippocampus of bats. Science 2017; 355: 176–180. [DOI] [PubMed] [Google Scholar]
- 63.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992; 106: 274–285. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa R, Fukushima H, Frankland PW, Kida S. Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. Elife 2016; 5: e17464 DOI: 10.7554/eLife.17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998; 19: 269–301. [DOI] [PubMed] [Google Scholar]
- 66.Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol 2006; 497: 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 1991; 12: 118–134. [DOI] [PubMed] [Google Scholar]
- 68.Herman JP, Schafer MK, Young EA, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci 1989; 9: 3072–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci 2011; 31: 9683–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gage FH. Mammalian neural stem cells. Science 2000; 287: 1433–1438. [DOI] [PubMed] [Google Scholar]
- 71.Denny CA, Kheirbek MA, Alba EL, et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 2014; 83: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus 2006; 16: 239–249. [DOI] [PubMed] [Google Scholar]
- 73.McCrae CS, O'Shea AM, Boissoneault J, et al. Fibromyalgia patients have reduced hippocampal volume compared with healthy controls. J Pain Res 2015; 8: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatr 2010; 67: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002; 5: 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Apkarian AV, Mutso AA, Centeno MV, et al. Role of adult hippocampal neurogenesis in persistent pain. Pain 2016; 157: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirby ED, Muroy SE, Sun WG, et al. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife 2013; 2: e00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 1998; 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 2001; 1: 70–83. [DOI] [PubMed] [Google Scholar]
- 81.Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron 2001; 32: 927–946. [DOI] [PubMed] [Google Scholar]
- 82.Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol 2009; 101: 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 2014; 35: 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006; 26: 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013; 136: 2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Helmstetter FJ. The amygdala is essential for the expression of conditional hypoalgesia. Behav Neurosci 1992; 106: 518–528. [DOI] [PubMed] [Google Scholar]
- 87.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 2004; 10: 221–234. [DOI] [PubMed] [Google Scholar]
- 88.Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol 2003; 89: 716–727. [DOI] [PubMed] [Google Scholar]
- 89.Tajerian M, Leu D, Zou Y, et al. Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology 2014; 121: 852–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A 2005; 102: 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol 2002; 87: 103–112. [DOI] [PubMed] [Google Scholar]
- 93.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatr 2008; 13: 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morey RA, Gold AL, LaBar KS, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatr 2012; 69: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burgmer M, Gaubitz M, Konrad C, et al. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med 2009; 71: 566–573. [DOI] [PubMed] [Google Scholar]
- 96.Mao CP, Yang HJ. Smaller amygdala volumes in patients with chronic low back pain compared with healthy control individuals. J Pain 2015; 16: 1366–1376. [DOI] [PubMed] [Google Scholar]
- 97.Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatr 2012; 69: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 2009; 29: 13746–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 1991; 313: 574–586. [DOI] [PubMed] [Google Scholar]
- 100.Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol 1995; 352: 567–593. [DOI] [PubMed] [Google Scholar]
- 101.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 2000; 20: 6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol 2006; 73: 61–71. [DOI] [PubMed] [Google Scholar]
- 103.Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol 2012; 22: 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuhn S, Gallinat J. The neural correlates of subjective pleasantness. Neuroimage 2012; 61: 289–294. [DOI] [PubMed] [Google Scholar]
- 105.Cauda F, Palermo S, Costa T, et al. Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. NeuroImage Clin 2014; 4: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 2009; 30: 3719–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci 2011; 31: 13981–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 2008; 28: 1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45: 651–660. [DOI] [PubMed] [Google Scholar]
- 110.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord 2012; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geha P, Dearaujo I, Green B, Small DM. Decreased food pleasure and disrupted satiety signals in chronic low back pain. Pain 2014; 155: 712–722. [DOI] [PubMed] [Google Scholar]
- 112.Berger SE, Baria AT, Baliki MN, et al. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res Notes 2014; 7: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Must A, Horvath S, Nemeth VL, Janka Z. The Iowa gambling task in depression—what have we learned about sub-optimal decision-making strategies? Front Psychol 2013; 4: 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vachon-Presseau E, Martel MO, Roy M, et al. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci 2013; 33: 6826–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sveinsdottir V, Eriksen HR, Ursin H, Hansen AM, Harris A. Cortisol, health, and coping in patients with nonspecific low back pain. Appl Psychophysiol Biofeedback 2016; 41: 9–16. [DOI] [PubMed] [Google Scholar]
- 116.Schell E, Theorell T, Hasson D, Arnetz B, Saraste H. Stress biomarkers' associations to pain in the neck, shoulder and back in healthy media workers: 12-month prospective follow-up. Eur Spine J 2008; 17: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leistad RB, Stovner LJ, White LR, Nilsen KB, Westgaard RH, Sand T. Noradrenaline and cortisol changes in response to low-grade cognitive stress differ in migraine and tension-type headache. J Headache Pain 2007; 8: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rainero I, Ferrero M, Rubino E, et al. Endocrine function is altered in chronic migraine patients with medication-overuse. Headache 2006; 46: 597–603. [DOI] [PubMed] [Google Scholar]
- 119.Patacchioli FR, Monnazzi P, Simeoni S, et al. Salivary cortisol, dehydroepiandrosterone-sulphate (DHEA-S) and testosterone in women with chronic migraine. J Headache Pain 2006; 7: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Buryanov A, Kostrub A, Kotiuk V. Endocrine disorders in women with complex regional pain syndrome type I. Eur J Pain 2017; 21(2): 302–308. [DOI] [PubMed] [Google Scholar]
- 121.Generaal E, Vogelzangs N, Macfarlane GJ, et al. Reduced hypothalamic-pituitary-adrenal axis activity in chronic multi-site musculoskeletal pain: partly masked by depressive and anxiety disorders. BMC Musculoskel Disord 2014; 15: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shahidi B, Sannes T, Laudenslager M, Maluf KS. Cardiovascular responses to an acute psychological stressor are associated with the cortisol awakening response in individuals with chronic neck pain. Physiol Behav 2015; 150: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lundh D, Hedelin H, Jonsson K, Gifford M, Larsson D. Assessing chronic pelvic pain syndrome patients: blood plasma factors and cortisol saliva. Scand J Urol 2013; 47: 521–528. [DOI] [PubMed] [Google Scholar]
- 124.Riva R, Mork PJ, Westgaard RH, Ro M, Lundberg U. Fibromyalgia syndrome is associated with hypocortisolism. Int J Behav Med 2010; 17: 223–233. [DOI] [PubMed] [Google Scholar]
- 125.Solberg Nes L, Carlson CR, Crofford LJ, de Leeuw R, Segerstrom SC. Self-regulatory deficits in fibromyalgia and temporomandibular disorders. Pain 2010; 151: 37–44. [DOI] [PubMed] [Google Scholar]
- 126.Wingenfeld K, Hellhammer DH, Schmidt I, Wagner D, Meinlschmidt G, Heim C. HPA axis reactivity in chronic pelvic pain: association with depression. J Psychosom Obstet Gynaecol 2009; 30: 282–286. [DOI] [PubMed] [Google Scholar]
- 127.Meeus M, Van Oosterwijck J, Ickmans K, et al. Interrelationships between pain processing, cortisol and cognitive performance in chronic whiplash-associated disorders. Clin Rheumatol 2015; 34: 545–553. [DOI] [PubMed] [Google Scholar]
- 128.Oncel C, Oflazoglu B, Forta H, Yucel N, Eren N. Plasma cortisol levels in migraineurs between attacks. Agri 2007; 19: 46–48. [PubMed] [Google Scholar]
- 129.Fischer S, Doerr JM, Strahler J, Mewes R, Thieme K, Nater UM. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome—the role of cortisol and alpha-amylase. Psychoneuroendocrinology 2016; 63: 68–77. [DOI] [PubMed] [Google Scholar]
- 130.Murrough JW, Abdallah CG, Anticevic A, et al. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum Brain Mapp 2016; 37: 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kennis M, van Rooij SJ, van den Heuvel MP, Kahn RS, Geuze E. Functional network topology associated with posttraumatic stress disorder in veterans. NeuroImage Clin 2016; 10: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdallah CG WK, Averill CL, Akiki T, Schweinsburg BC, Roy A, Martini B, Southwick S, Krystal JK, Scott JC. Anterior hippocampal dysconnectivity in posttraumatic stress Disorder: a dimensional and multimodal approach. Transl Psychiatr 2017; 7(2): e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mansour A, Baria AT, Tetreault P, et al. Global disruption of degree rank order: a hallmark of chronic pain. Sci Rep 2016; 6: 34853. [DOI] [PMC free article] [PubMed] [Google Scholar]