Abstract
Context:
The 12-lipoxygenase (12-LO) pathway produces proinflammatory metabolites, and its activation is implicated in islet inflammation associated with type 1 and type 2 diabetes (T2D).
Objectives:
We aimed to test the efficacy of ML355, a highly selective, small molecule inhibitor of 12-LO, for the preservation of islet function.
Design:
Human islets from nondiabetic donors were incubated with a mixture of tumor necrosis factor α , interluekin-1β, and interferon-γ to model islet inflammation. Cytokine-treated islets and human islets from T2D donors were incubated in the presence and absence of ML355.
Setting:
In vitro study.
Participants:
Human islets from organ donors aged >20 years of both sexes and any race were used. T2D status was defined from either medical history or most recent hemoglobin A1c value >6.5%.
Intervention:
Glucose stimulation.
Main outcome measures:
Static and dynamic insulin secretion and oxygen consumption rate (OCR).
Results:
ML355 prevented the reduction of insulin secretion and OCR in cytokine-treated human islets and improved both parameters in human islets from T2D donors.
Conclusions:
ML355 was efficacious in improving human islet function after cytokine treatment and in T2D islets in vitro. The study suggests that the blockade of the 12-LO pathway may serve as a target for both form of diabetes and provides the basis for further study of this small molecule inhibitor in vivo.
A small molecule inhibitor of 12-lipoxygenase improved insulin secretion and oxygen consumption in human islets treated with proinflammatory cytokines or those from type 2 diabetic donors.
Islet inflammation is a key mechanism leading to functional β cell loss in both type 1 and type 2 diabetes (T1D, T2D). Proinflammatory mediators including cytokines and chemokines produced by immune cells, islet intrinsic molecules such as islet amyloid, and excess fatty acids from the circulation and released by lipases all contribute to islet inflammation (1–5). These molecules activate cascades, including the inflammasome, toll-like receptors, and endoplasmic reticulum (ER) stress, which can lead to loss of functional β cells (2, 5–7).
Proinflammatory lipid mediators generated from fatty acids, including arachidonic acid, are another important source of inflammation in islets under stress. In particular, the 12-lipoxygenase (12-LO) pathway (encoded by the ALOX12 gene) is the major LO enzyme in human islets and produces predominantly 12-S-hydroxyeicosatetraenoic acid (12-S-HETE) (7, 8). In the mouse islet, the functional pathways primarily producing 12-S-HETE include 12/15 LO encoded by the mouse Alox12 and mouse Alox15 genes (7–10). Increased expression of ALOX12 and related lipids has been demonstrated in human and rodent islets exposed to cytokines (7, 11). In addition, although ALOX12 expression is very low in healthy control islets, expression increases significantly in prediabetic and T1D and T2D islet samples (12, 13). 12-LO lipid products exert preinflammatory effects by activating downstream cytokines such as interleukin (IL)-12 or second messengers such as c-Jun N-terminal and p38 mitogen-activated protein kinase (p38-MAPK) (11, 14). Furthermore, ALOX12 activation can lead to mitochondrial and ER stress (15, 16).
The contribution of the 12-LO pathway in diabetes in mouse models has been tested using targeted knockout mice (17–21). However, the in vivo functional role in mice or direct testing of the role of ALOX12 to protect human islets requires a highly selective ALOX12 inhibitor (21, 22). The lack of a highly specific ALOX12 inhibitor has long been a limitation in the field; however, recent efforts in collaboration with the National Institutes of Health/National Center for Advancing Translational Sciences have led to the discovery of new chemical matter, including the structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino) benzenesulfonamides, with the lead compound ML355 [reported as compound 35 in (22, 23)] having submicromolar potency for 12-LO inhibition and greater than 50-fold selectivity relative to other LO enzymes (22, 23). ML355 also has favorable in vitro absorption, distribution, metabolism, and elimination (ADME) and an in vivo pharmacokinetic profile; thus, it has potential for clinical application (22, 23).
In the current study, we used ML355 to pharmacologically improve the dynamic function and metabolism of human islets exposed to proinflammatory cytokines (PICs) and islets isolated from T2D donors.
Materials and Methods
Culture of human islets
Human islets from nondiabetic or T2D donors were received from the Integrated Islet Distribution Program or Prodo Laboratories (Aliso Viejo, CA) with approval from the institutional review board at Eastern Virginia Medical School. Characteristics of islets and donors are summarized in Table 1. T2D status was determined by suppliers of islets. Islets were incubated in CMRL-1066 medium supplemented with 10% fetal bovine serum and 1% Pen-Strept overnight at 37°C in 5% CO2 for recovery from shipment. On the next day, islets were transferred to CMRL-1066 supplemented with 5% fetal bovine serum and 1% Pen-Strept. A portion of the islets were randomly selected and incubated with a mixture of human PICs, 0.57 mmol/L tumor necrosis factor α, 5.9 mmol/L interferon-γ, and 0.29 mmol/L IL-1β (all from BD Biosciences, San Jose, CA) in the presence or absence of 10 μmol/L ALOX12 inhibitor (ML355) for the indicated period. A group of islets was also incubated in the presence of 10 μmol/L p38-MAPK inhibitor (SB203580; Selleckchem, Houston, TX). ML355 and SB203580 were added 30 minutes before the addition of PIC. The synthesis and initial characterization of ML355 have been published (22, 23).
Table 1.
Characteristics of Human Islet Donors
| Sex | Age (y) | BMI (kg/m2) | Race | Cause of Death, A1c Value if Reported | Study Done | Source |
|---|---|---|---|---|---|---|
| Nondiabetic donors | ||||||
| F | 39 | 23.5 | Wh | Stroke | Figs. 1 and 3(b) | IIDP |
| M | 29 | 23.4 | Wh | Head trauma, A1c 5.1% | Figs. 1 and 3(b) | Prodo |
| M | 53 | 21.8 | Wh | Head trauma, A1c 5.5% | Fig. 1 | IIDP |
| F | 48 | 35 | Hisp | Stroke, A1c 5.7% | Fig. 1 | Prodo |
| M | 27 | 31.8 | Bl | Head trauma | Fig. 2 donor 1, Fig. 3(b) | Prodo |
| F | 51 | 24.4 | ND | Head trauma | Fig. 2 donor 2 | Prodo |
| F | 56 | 21.4 | Wh | Stroke | Fig. 2 donor 3, Fig. 3(b) | Prodo |
| M | 23 | 34.2 | Wh | Gunshot wound | Fig. 2 donor 4 | Prodo |
| F | 68 | 21.3 | Wh | Stroke | Fig. 2 donor 5 | Prodo |
| M | 50 | 31.7 | Bl | Head trauma | Fig. 2 donor 6 | IIDP |
| M | 52 | 33.3 | Wh | Head trauma, A1c 4.6% | Fig. 3(b) | IIDP |
| F | 45 | 26.6 | Wh | Stroke, A1c 4.5% | Fig. 3(b) and 3(d) | IIDP |
| F | 56 | 33.5 | Bl | Stroke after head trauma, A1c 5.2% | Fig. 3(b) and 3(d) | Prodo |
| F | 53 | 32 | Wh | Stroke, A1c 5.7% | Fig. 3(c) | Prodo |
| F | 59 | 28.2 | Hisp | Stroke A1c, 6.2% | Fig. 3(c) | IIDP |
| F | 43 | 22.4 | Wh | Anoxia, A1c 5.2% | Fig. 3(c) | Prodo |
| F | 33 | 34.2 | Bl | Stroke, A1c 5.5% | Fig. 3(c) | IIDP |
| F | 35 | 23.6 | Wh | Anoxia; A1c 4.6% | Fig. 3(d) | IIDP |
| T2D donors | ||||||
| M | 51 | 35.8 | Hisp | Head trauma, T2D × 15 y on oral agents, A1c 5.3% | Fig. 4 donor 1 | IIDP |
| F | 52 | 25.5 | Hisp | Stroke, T2D × 10 y, A1c 5.3% | Fig. 4 donor 2, Fig. 4(f) | IIDP |
| F | 37 | 39.3 | ND | Anoxia, T2D × 10 y on oral agents | Fig. 4 donor 3, Fig. 4(f) | Prodo |
| M | 51 | 24.6 | Hisp | Stroke, T2D diagnosed upon admission; A1c 6.9% | Fig. 4 donor 4, Fig. 4(f) | Prodo |
| M | 48 | 43.7 | Wh | Anoxia, T2D × 2 y on insulin and metformin, A1C 6.6% | Fig. 4 donor 5, Fig. 4(f) | IIDP |
| F | 58 | 36.3 | Wh | Stroke, T2D >10 y with ESRD on insulin, A1c 4.9% | Fig. 4 donor 6, Fig. 4(f) | IIDP |
| F | 41 | 43.1 | Hisp | Stroke, T2D of short duration, A1c 6.5% | Fig. 4(f) | Prodo |
Abbreviations: A1c, hemoglobin A1c; Bl, black or African American; BMI, body mass index; ESRD, end-stage renal disease; F, female; Hisp, Hispanic; IIDP, Integrated Islet Distribution Program; M, male; ND; not documented; Prodo, Prodo Laboratories; Wh, white.
Glucose-stimulated insulin secretion
Use of Krebs-Ringer buffer (KRB) for glucose-stimulated insulin secretion (GSIS) was previously published (24). For batch assays, islets treated with PIC in the presence or absence of ML355 for 24 hours were transferred to KRB without glucose and were incubated for 1 hour at 37°C in 5% CO2 in the absence of PIC or ML355. Thereafter, 50 islet equivalents (IEQs) per well were transferred to six-well plates filled with 1 mL of KRB containing 1 mmol/L or 18 mmol/L of glucose and were incubated for 1 hour at 37°C in 5% CO2. Each condition was performed in triplicate. After incubation, supernatant was collected for determination of insulin secretion by human insulin enzyme-linked immunosorbent assay (Mercodia, Winston-Salem, NC). Insulin from islet pellets was extracted with acidified ethanol as described to determine total insulin content (12). The insulin stimulation index was defined as the ratio of insulin secretion in 18 mmol/L of glucose over 1 mmol/L of glucose. Islet perifusion was performed as published using 500 IEQ of human islets after 24-hour incubation with or without ML355 in the presence or absence of PIC (12). In brief, islets were perifused with KRB containing 3 mmol/L of glucose followed by 23 mmol/L of glucose for 20 minutes and then 3 mmol/L of glucose. The samples were collected at 1 mL/min for human insulin measurement by enzyme-linked immunosorbent assay. The stimulation index was calculated as (average insulin secretion at 23 mmol/L of glucose)/(average insulin secretion at 3 mmol/L of glucose). The first-phase response was determined as (the average insulin secretion during peak 3 minutes of insulin response/the average secretion during 3 mmol/L of glucose). The second-phase response was determined as (the average insulin secretion between times 10 and 15 minutes of high glucose/the average secretion during 3 mmol/L of glucose). First/second is the ratio of the first and second phase responses as previously published (12).
Oxygen consumption rate
Islets treated with PIC in the presence or absence of ML355 for 24 hours were transferred to a XFe24 islet capture microplate at 200 IEQ per well, and an oxygen consumption rate (OCR) was obtained using XFe24 Seahorse extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA) according to the established protocol (25), with the following modifications. Measurement was started with Seahorse XF base medium without glucose. After three cycles of measurements, the following four reagents were sequentially added through ports: 20 mmol/L glucose, 5 μmol/L oligomycin, 5 μmol/L carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP), and 5 μmol/L rotenone, all at final concentration. Glucose response was defined as the difference between the highest OCR during the glucose phase and the OCR prior to glucose addition. Adenosine triphosphate (ATP) production was defined as the difference between the highest OCR during the glucose phase and the OCR at the end of the oligomycin phase. Maximal respiration was defined as the difference between the highest OCR during the FCCP phase and the OCR at the end of the assay. Islet culture and OCR were performed by separate individuals to avoid the introduction of bias.
Western blot
Islets were solubilized in CelLytic M (Sigma-Aldrich, St. Louis, MO) containing protease inhibitor to extract protein. Protein extracts were separated on Mini-PROTEAN® TGX™ Precast Gels (Bio-Rad, Hercules, CA) and transferred to Immobilon-FL PVDF Transfer Membranes (EMD Millipore, Billerica, MA). The blots were probed with the primary antibodies and then with the IRDye® 800CW and IRDye® 680RD secondary antibodies (LI-COR, Lincoln, NE) to detect protein bands with the Li-Cor Odyssey Imaging System (LI-COR). The rabbit polyclonal anti-phospho-P38 pThr180/pTyr182 antibody (44-684G; Thermo Fisher, Waltham, MA) was used at 1:500. The mouse monoclonal anti-p38α/β (A-12) antibody (sc-7972; Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:500.
All antibodies were previously validated.
Statistics
Data are presented as mean ± standard error of the mean and were analyzed using one-way analysis of variance with Dunnett’s posttest in comparison with control, Dunn’s multiple comparison test, Student t test, or Wilcoxon matched-pairs signed rank test (Prism software, Irvine, CA) as indicated in each figure. P < 0.05 was considered significant.
Results
ML355 improved GSIS and increased insulin content of human islets treated with PIC
PIC exposure of human and mouse β cells upregulated the 12-LO pathway, and the inhibition of the 12-LO pathway was implicated in protecting the viability and function of human and mouse β cells after PIC exposure (24). Therefore, we tested the efficacy of ML-335, a new, highly selective inhibitor of ALOX12 with a favorable ADME profile (22, 23), in protecting human islets against the impairment of GSIS by PIC.
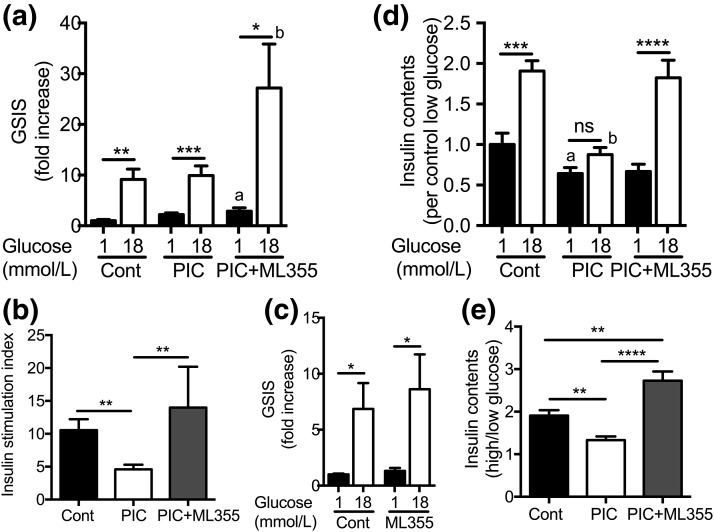
PIC treatment at the dose and duration used predominantly increased basal insulin secretion at 1 mmol/L of glucose and led to the reduction of further increases in insulin secretion at 18 mmol/L of glucose [Fig. 1(a)]. This is similar to data previously reported for human islets treated with PIC (26). As a result, the stimulation index was significantly reduced in PIC-treated human islets [Fig. 1(b)]. Coincubation of ML355 with PIC did not affect the rise in insulin secretion with low glucose but markedly increased insulin secretion at 18 mmol/L of glucose [Fig. 1(a)]. Therefore, the stimulation index was significantly improved in ML355 plus PIC–treated islets vs PIC alone [Fig. 1(b)]. ML355 did not alter GSIS in human islets untreated with PIC [Fig. 1(c)]. During the 1-hour batch assay, 18 mmol/L of glucose increased the insulin content of control human islets twofold, whereas the increase was blunted in PIC-treated human islets [Fig. 1(d) and 1(e)]. ML355 treatment allowed human islets treated with PIC to increase insulin content in response to high glucose [Fig. 1(d) and 1(e)].
Figure 1.
Batch incubation of human islets treated with PIC with or without 12-LO inhibitor. (a) GSIS was compared between human islets that were untreated (Cont), pretreated with PIC, or pretreated with PIC plus ML355 for 24 hours. GSIS was performed in the absence of PIC or ML355. Insulin secretion was expressed by taking the average insulin secretion of Cont at 1 mmol/L glucose as 1. (b) The insulin stimulation index was defined as described in the Methods. (c) GSIS was compared between untreated human islets (Cont) and islets treated with ML355 for 24 hours in the absence of PIC. Data are expressed as described in (a). (d) Insulin content in islet pellets was collected after GSIS. (e) Ratio of insulin content in the islet pellets. Each condition from a single donor was performed in triplicate, and data from three to four donors were combined (n = 8 to 12). *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001 by Student t tests. a: P < 0.05 one-way analysis of variance with Dunnett’s posttest in comparison with Cont 1, mmol/L glucose. b: P < 0.05 one-way analysis of variance with Dunnett’s posttest in comparison with Cont, 18 mmol/L glucose. ns, not statistically different.
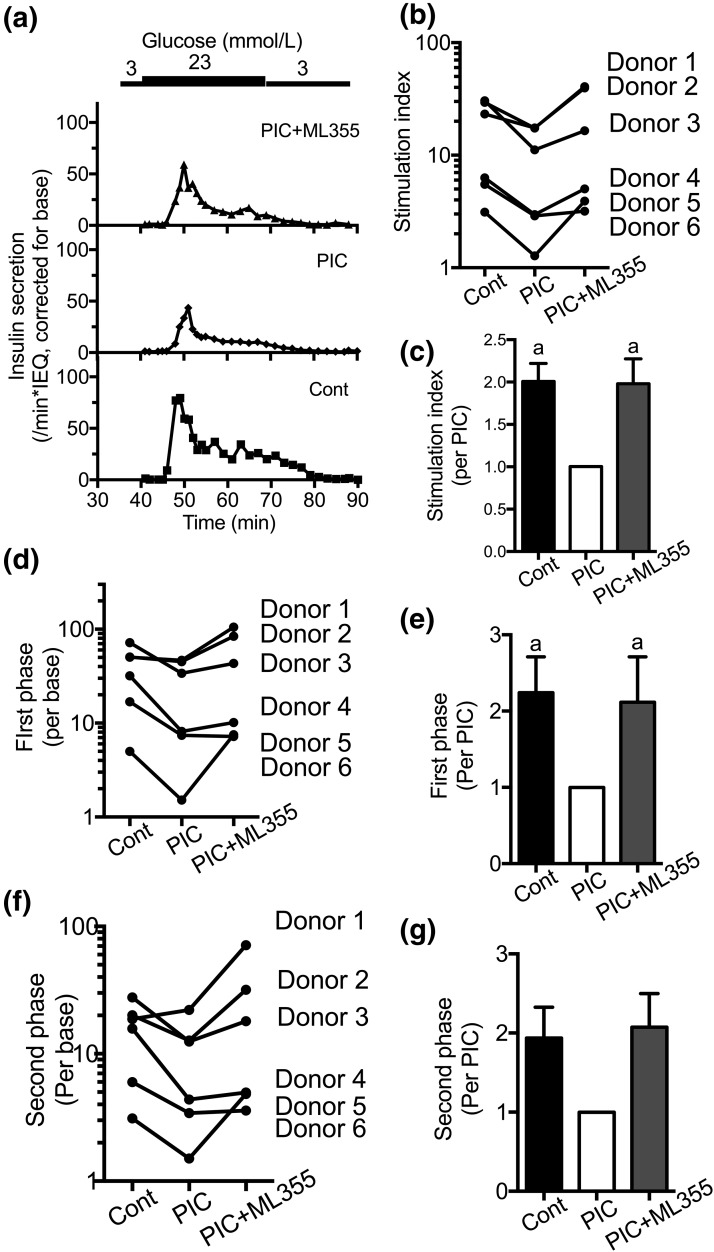
We also performed perifusion to determine the effects of PIC and ML355 on the first and second phases of insulin secretion. Control human islets demonstrated the robust first-phase insulin secretion typical of human islets [Fig. 2(a)] (12). PIC treatment blunted GSIS as seen by the reduction in the stimulation index and the first-phase response [Fig. 2(a–e)]. The second-phase response was also reduced for all samples except for one donor [Fig. 2(f) and 2(g)]. Coincubation of human islets with ML355 plus PIC preserved the simulation index and the first-phase response [Fig. 2(a–e)] and showed a trend for preservation for the second-phase response [Fig. 2(f) and 2(g)].
Figure 2.
Perifusion of human islets treated with PICs with or without 12-LO inhibitor. (a) A representative figure (donor 3) comparing perifusion of human islets that were untreated (Cont), treated with PIC, or treated with PIC in the presence of ML355 for 24 hours before perifusion. Data were expressed by taking insulin secretion at 3 mmol/L glucose (base) as 1. (b–g) Stimulation index, the first-phase response, and the second-phase response (as defined in the Methods) were obtained for six donors, as shown in (b), (d), and (f). Each parameter was also compared by taking the response of PIC as 1 in (c), (e), and (g) (n = 6). a: P < 0.05 vs PIC by Friedman test with Dunn’s multiple comparison test as posttest.
The inhibition of 12-LO improved oxygen consumption in human islets treated with PIC
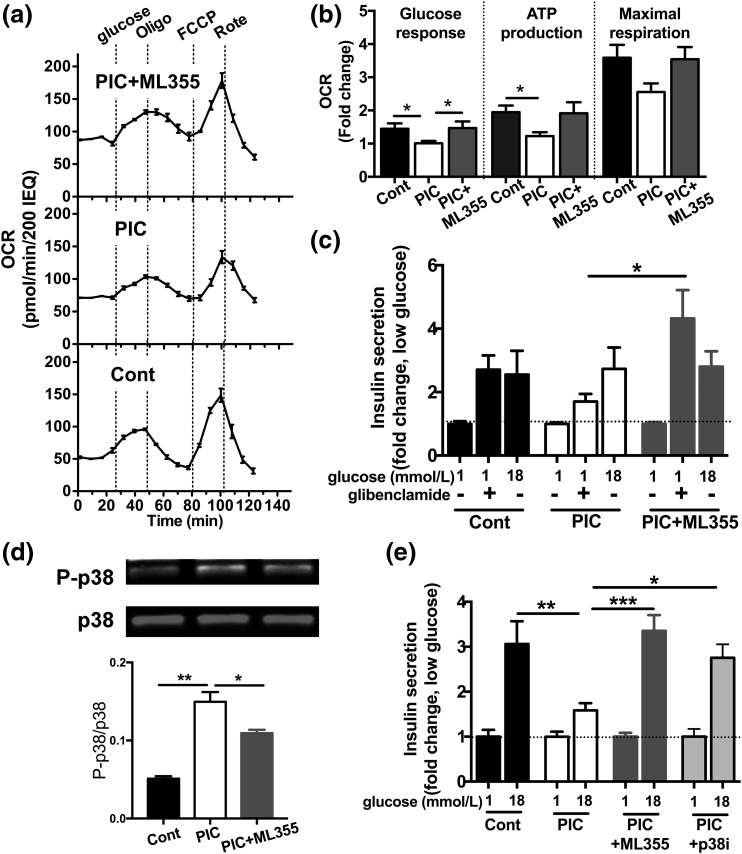
ATP production through oxidative phosphorylation and closure of the ATP-dependent potassium (KATP) channel is the key step in GSIS and couples glucose oxidation with insulin secretion (27). We determined the effects of PIC and ML355 treatment on human islets using OCR measured by the Seahorse metabolic analyzer as an index of ATP production and mitochondrial health (25). As shown in Fig. 3(a), human islets were sequentially treated with 20 mmol/L of glucose, oligomycin, FCCP, and rotenone. Compared with control islets, PIC blunted the glucose-stimulated increase in OCR and basal ATP production. Maximum respiration also showed the trend of reduction with PIC [Fig. 3(a) and 3(b)]. ML355 prevented the reduction of OCR by PIC, indicating the preservation of mitochondrial functions.
Figure 3.
Oxygen consumption and the response to glibenclamide in human islets treated with PIC with or without 12-LO inhibitor. (a) A representative figure (nondiabetic donor 3 of Fig. 2) comparing the OCR in human islets that were untreated (Cont), treated with PIC, or treated with PIC in the presence of ML355 for 24 hours (n = 3). (b) The OCR (as defined in the Methods) was compared among the Cont, PIC, and PIC plus ML355 groups, taking the average of the OCR in PIC-treated islets as 1. Data are mean ± standard error of the mean (SEM), combining three donors each in triplicate (n = 9). (c) Insulin secretion measured by batch assay at indicated concentrations of glucose with or without 10 μmol/L of glibenclamide in the Cont, PIC-treated, and PIC plus ML355−treated human islets. Insulin secretion was expressed by taking the average insulin secretion at 1 mmol/L glucose as 1. Data are mean ± SEM, combining four donors each in triplicate (n = 12). (d) Western blot compared total p38 (p38) and phospho-p38 (P-p38) in Cont, PIC-treated, and PIC plus ML355−treated human islets. The representative blot and the density of P-p38 corrected for p38 are shown (n = 3). (e) GSIS measured by batch assay at the indicated concentration of glucose in Cont, PIC-treated, PIC plus ML355−treated, and PIC plus SB203580 (p38i)−treated human islets. Insulin secretion was expressed by taking the average insulin secretion at 1 mmol/L glucose as 1. Data are mean ± SEM, combining two donors each in duplicate or triplicate (n = 4 to 6). *P < 0.05; **P < 0.01; ***P < 0.005 by Student t test.
We then tested whether ML355 improves insulin secretion at the step after the closure of the KATP channel by measuring insulin secretion in response to glibenclamide [Fig. 3(c)]. ML355 treatment significantly increased insulin secretion stimulated by glibenclamide in human islets treated with PIC [Fig. 3(c)]. Thus, ML355 improved the secretory response of human islets treated with PIC at the steps leading to KATP channel closure [Fig. 3(a) and 3(b)] and post−KATP channel [Fig. 3(c)].
In agreement with a previous study performed with a much less selective inhibitor of ALOX12 (28), improvement in islet functions by ML355 was associated with a reduction in phospho-p38 MAPK. p38-MAPK is considered one of the important pathways responsible for β cell dysfunction by PIC and is upregulated by PIC. Because ML355 was tested against all major human kinases, including p38-MAPK, and did not directly inhibit it, the effect of ML355 on p38-MAPK is secondary to reduction of 12-LO products. In support of the contribution of p38-MAPK in β cell dysfunction after PIC exposure, the p38-MAPK inhibitor SB203580 also prevented the reduction of GSIS by PIC [Fig. 3(e)]. The insulin stimulation indexes of the ML355-treated group and the SB203580-treated group were 3.36 ± 0.35 and 2.75 ± 0.30, respectively, implying that the inhibition of p38-MAPK activation contributes to preservation of GSIS by ML355 [Fig. 3(e)].
Incubation (24 hours) with 12-LO inhibitor improved glucose responses of human islets from T2D donors
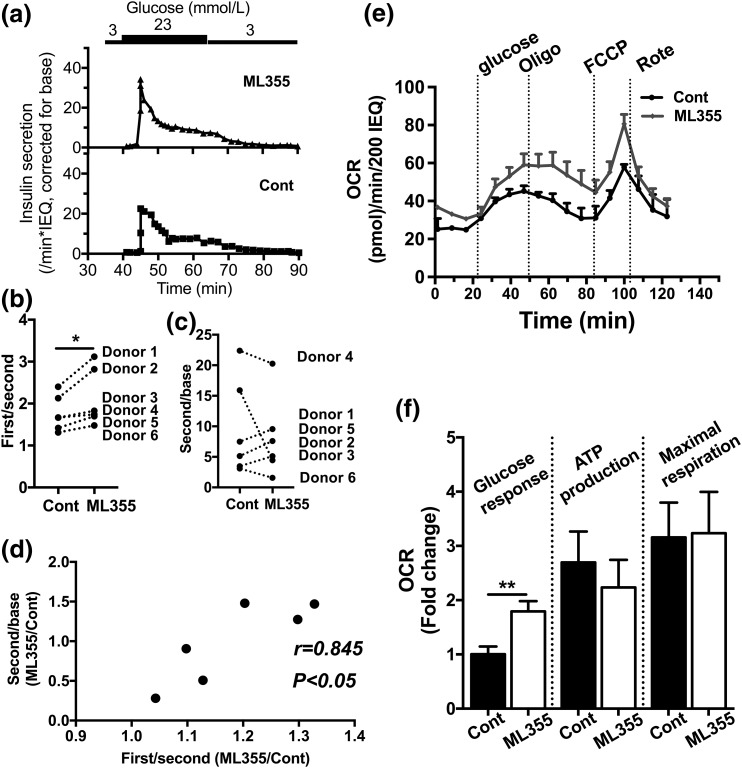
We previously reported an increase in ALOX12 expression in human islets from T2D donors (12, 13). Thus, we tested whether the inhibition of ALOX12 improved β cell function in human islets from T2D donors. In this prior study, we also reported that blunting of the first/second–phase response in perifusion was a useful index of first-phase insulin secretion in T2D human islets [Fig. 4(a)] (12). All six donors tested showed improvement in first-phase/second-phase insulin secretion after preincubation with ML355 [Fig. 4(a) and (b)], whereas the improvement in the second phase of insulin secretion was not persistent [Fig. 4(c)]. However, there was a positive correlation between improvement in first-phase secretion vs that of second-phase insulin secretion, as shown in Fig. 4(d). This result indicates that those donors with robust improvement in first-phase insulin secretion tended to improve the second phase of insulin secretion as well. Next, we compared the OCR with the Seahorse metabolic analyzer after ML355 pretreatment [Fig. 4(e)] and observed that ML355 increased the OCR in response to glucose in human T2D islets [Fig. 4(f)].
Figure 4.
Perifusion and OCR of human T2D islets treated with or without 12-LO inhibitor. (a) A representative figure (T2D donor 2) comparing perifusion of untreated human T2D islets (Cont) with islets treated with ML355 for 24 hours before perifusion. Data were expressed by taking insulin secretion at 3 mmol/L glucose (base) as 1. (b, c) The first-phase/second-phase response and the second phase response (as defined in the Methods) were obtained for six T2D donors. (d) Fold change between ML355 and Cont treatment of each donor from (b) plotted in the x-axis and each donor from (c) plotted in the y-axis. Pearson correlation coefficient is shown. (e) The representative figure [donor 3 of Fig. 4(b)] comparing the OCR (n = 3). (f) The OCRs (as defined in the Methods) were compared between the Cont and ML355 groups by taking the average of OCRs during the glucose challenge in Cont as 1. Data are mean ± SEM, combining six donors each in triplicate and quadruplicate (n = 21). *P < 0.05; **P < 0.01 by Wilcoxon matched-pairs signed rank test.
Discussion
There is substantial evidence that the 12-LO pathway serves as a therapeutic target for preservation of β cells in T1D and T2D. The expression of ALOX12 is increased in islets of T1D and T2D, ALOX12 activation produces 12-S-HETE that impairs the viability and function of β cells, and gene deletion prevents hyperglycemia in various mouse models of T1D and T2D (3, 7, 8, 16). Here, we demonstrated that ML355, a highly selective, new-generation, small molecule ALOX12 inhibitor, prevented the impairment of GSIS by PIC in nondiabetic human islets. Previously, we reported that an early generation of less-selective ALOX12 inhibitors, compounds 5 and 9, preserved GSIS in PIC-treated human islets (24). The current study further confirmed that the activation of the 12-LO pathway plays a key role in the impairment of GSIS during islet inflammation. Because ML355 holds high promise for in vivo use with superior ADME, its efficacy for islet protection under diabetogenic stress supports its future clinical utility in β cell preservation in diabetes. Importantly, we demonstrated that the ALOX12 inhibitor ML355 improved GSIS in human islets from T2D donors.
The mechanism by which ML355 improved the GSIS of PIC-treated islets appears to involve several targets. Although PIC treatment reduced the insulin content of human islets incubated in high glucose, human islets coincubated with ML355 showed insulin content similar to that of human islets not treated with PIC. It was previously reported that short-term incubation of human islets with glucose increased proinsulin production (29). Considering that insulin gene expression was not different between the groups (data not shown) and that the change in insulin content between low and high glucose was observed within 1 hour, ML355 likely preserves the efficiency of insulin production at the ER. Of note, activation of the 12-LO pathway was previously shown to cause ER stress (30). ML355 also prevented the reduction of the OCR by PIC in human islets, indicating that ML355 preserves mitochondrial health and supports ATP production. At the same time, insulin secretion in response to glibenclamide was improved with the addition of ML355 in PIC-treated human islets, indicating that the integrity of the secretory pathway after KATP channel closure is better preserved by inhibition of ALOX12. Collectively, ML355 prevented the impairment of functional parameters in PIC-exposed human islets through multiple pathways. This is not surprising given that islet inflammation associated with diabetes activates a wide array of stress pathways, including ER stress, mitochondrial dysfunction, and oxidative stress (3).
p38-MAPK is a stress response kinase known to be activated by multiple stressors, including PIC and hyperglycemia in β cells (31, 32). The activation of p38-MAPK in β cells treated with PIC is thought to contribute to β cell dysfunction, partly through the production of inducible nitric oxide synthase (33). We showed that ML355 was effective in blocking the activation of p38-MAPK by PIC in human islets and that ML355 was efficacious in preserving GSIS as a p38-MAPK inhibitor. It is important to determine whether 12-LO inhibitors such as ML355 effectively block p38-MAPK activation in response to other stimuli implicated in β cell dysfunction in diabetes, such as glucolipotoxicity.
Because the first phase of GSIS is critically important for the maintenance of glucose homeostasis, we performed perifusion to comprehensively assess the effect of 12-LO inhibition on two phases of GSIS in PIC-treated human islets and human islets from T2D donors. As shown in Fig. 2, the first phase was significantly reduced by PIC in human islets but was better maintained by preincubation with ML355, whereas the second phase showed a similar trend but failed to reach statistical significance. Overall, ML355 seems to be effective in preserving not only the total amount of insulin secreted (stimulation index) but also the dynamics of GSIS (first phase) in PIC-treated human islets.
Regarding human islets from T2D donors, we previously noted that blunting of the first phase is a key feature of T2D donor islets compared with nondiabetic human islets (12). In vitro treatment of ML355 improved the first-phase response of human islets from T2D donors, whereas the effect of ML355 on the second-phase response varied. However, islets from T2D donors with large improvement in the first-phase of GSIS also showed a trend toward increased second phase as well. Considering that ALOX12 expression of T2D islets varies (12) and that T2D human islets contain donors at variable stages of the disease, future studies to identify the parameters associated with robust responses to ML355 will be helpful. This information will be important when considering future clinical trials using 12-LO inhibition for the improvement of β cell function in T2D.
When reported, hemoglobin A1c level was not overtly elevated in our T2D donors, indicating well-controlled diabetes, mild disease, or secondary effects from chronic illness (Table 1). Although perifusion and/or the OCR showed impaired response to glucose, as expected in our cohort of T2D islets, future studies to test the efficacy of ML355 in islets from human T2D donors with overtly elevated hemoglobin A1c levels will be important. Nevertheless, it is intriguing that short-term in vitro incubation with ML355 produced significant improvement in GSIS and OCR in T2D islets.
Because the current study of human islets was limited to a short-term in vitro setting, the long-term effects of ML355 on human islets need to be established in a model such as human islets transplanted in immunodeficient mice or humanized mice expressing a human form of ALOX12.
In summary, a new, highly selective, small molecule inhibitor of the 12-LO pathway improved β cell function in human islets under inflammatory conditions and in islets from T2D donors. Our results further support the key contribution of the 12-LO pathway in the development of β cell demise in T1D and T2D. Furthermore, the 12-LO inhibitor may serve as a therapeutic option to preserve β cells under diabetogenic stress along with other anti-inflammatory therapeutics such as IL-1β and nuclear factor κB−targeted molecules (34).
Acknowledgments
A portion of the human islets were provided to J.L.N. through the Integrated Islet Distribution Program. We thank Dr. Ted Holman (University of California, Santa Cruz) for discussion and critical reading of the manuscript.
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation (from Sanofi/JDRF collaboration grant to J.L.N.) and National Institutes of Health Grants R01-HL112605 (to J.L.N.), R01-DK090490 (to Y.I.), and R01-DK105588 (to J.L.N. and R.G.M.).
Acknowledgments
Author contributions: J.L.N. conceptually designed the study. K.M., A.X., and Y.I. designed the experiments. Y.I. and J.L.N. supervised the experiments. K.M., A.X., S.H.P., L.G., L.J., T.B., J.R.W., and D.A.T.-F. tested ML355 in human islet experiments. D.K.L. and D.J.M. produced ML355. K.M., A.X., S.H.P., L.J., and Y.I. performed data analyses. R.G.M., Y.I., and J.L.N. interpreted the data, and Y.I. and J.L.N. wrote the manuscript. The rest of authors provided intellectual input for the drafting of the manuscript. All authors approved the final version of the manuscript.
Disclosure Summary: The authors declare that no duality of interest is associated with this manuscript.
Footnotes
- 12-LO
- 12-lipoxygenase
- 12-S-HETE
- 12-S-hydroxyeicosatetraenoic acid
- ADME
- absorption, distribution, metabolism, and elimination
- ATP
- adenosine triphosphate
- ER
- endoplasmic reticulum
- FCCP
- carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- GSIS
- glucose-stimulated insulin secretion
- IEQ
- islet equivalent
- IL
- interleukin
- KATP
- ATP-dependent potassium
- KRB
- Krebs-Ringer buffer
- OCR
- oxygen consumption rate
- p38-MAPK
- p38 mitogen-activated protein kinase
- PIC
- proinflammatory cytokine
- T1D
- type 1 diabetes
- T2D
- type 2 diabetes.
References
- 1.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52(8):1686–1688. [DOI] [PubMed] [Google Scholar]
- 2.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15(4):518–533. [DOI] [PubMed] [Google Scholar]
- 3.Imai Y, Dobrian AD, Morris MA, Nadler JL. Islet inflammation: a unifying target for diabetes treatment? Trends Endocrinol Metab. 2013;24(7):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1β production and β-cell dysfunction. Diabetes. 2014;63(5):1698–1711. [DOI] [PubMed] [Google Scholar]
- 5.Bone RN, Gai Y, Magrioti V, Kokotou MG, Ali T, Lei X, Tse HM, Kokotos G, Ramanadham S. Inhibition of Ca2+-independent phospholipase A2β (iPLA2β) ameliorates islet infiltration and incidence of diabetes in NOD mice. Diabetes. 2015;64(2):541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westwell-Roper C, Nackiewicz D, Dan M, Ehses JA. Toll-like receptors and NLRP3 as central regulators of pancreatic islet inflammation in type 2 diabetes. Immunol Cell Biol. 2014;92(4):314–323. [DOI] [PubMed] [Google Scholar]
- 7.Imai Y, Dobrian AD, Morris MA, Taylor-Fishwick DA, Nadler JL. Lipids and immunoinflammatory pathways of beta cell destruction. Diabetologia. 2016;59(4):673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50(1):115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai Y, Dobrian AD, Weaver JR, Butcher MJ, Cole BK, Galkina EV, Morris MA, Taylor-Fishwick DA, Nadler JL. Interaction between cytokines and inflammatory cells in islet dysfunction, insulin resistance and vascular disease. Diabetes Obes Metab. 2013;15(Suppl 3):117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta. 2015;1851(4):308–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48(3):486–495. [DOI] [PubMed] [Google Scholar]
- 12.Butcher MJ, Hallinger D, Garcia E, Machida Y, Chakrabarti S, Nadler J, Galkina EV, Imai Y. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57(3):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grzesik WJ, Nadler JL, Machida Y, Nadler JL, Imai Y, Morris MA. Expression pattern of 12-lipoxygenase in human islets with type 1 diabetes and type 2 diabetes. J Clin Endocrinol Metab. 2015;100(3):E387–E395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton MK, Rubinstein T, Puré E. Cellular and molecular mechanisms of the selective regulation of IL-12 production by 12/15-lipoxygenase. J Immunol. 2006;176(1):265–274. [DOI] [PubMed] [Google Scholar]
- 15.Nazarewicz RR, Zenebe WJ, Parihar A, Parihar MS, Vaccaro M, Rink C, Sen CK, Ghafourifar P. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys. 2007;468(1):114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tersey SA, Maier B, Nishiki Y, Maganti AV, Nadler JL, Mirmira RG. 12-Lipoxygenase promotes obesity-induced oxidative stress in pancreatic islets. Mol Cell Biol. 2014;34(19):3735–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103(10):1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, Kropf E, Rival C, Ma K, Carter JD, Tersey SA, Nunemaker CS, Nadler JL. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57(1):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol Endocrinol Metab. 2008;295(5):E1065–E1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole BK, Morris MA, Grzesik WJ, Leone KA, Nadler JL. Adipose tissue-specific deletion of 12/15-lipoxygenase protects mice from the consequences of a high-fat diet. Mediators Inflamm. 2012;2012:851798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green-Mitchell SM, Tersey SA, Cole BK, Ma K, Kuhn NS, Cunningham TD, Maybee NA, Chakrabarti SK, McDuffie M, Taylor-Fishwick DA, Mirmira RG, Nadler JL, Morris MA. Deletion of 12/15-lipoxygenase alters macrophage and islet function in NOD-Alox15(null) mice, leading to protection against type 1 diabetes development. PLoS One. 2013;8(2):e56763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luci D, Jameson JB II, Yasgar A, Diaz G, Joshi N, Kantz A, Markham K, Perry S, Kuhn N, Yeung J, Schultz L, Holinstat M, Nadler J, Taylor-Fishwick DA, Jadhav A, Simeonov A, Holman TR, Maloney DJ. Discovery of ML355, a Potent and Selective Inhibitor of Human 12-Lipoxygenase. Bethesda, MD: Probe Reports from the NIH Molecular Libraries Program; 2010. [PubMed] [Google Scholar]
- 23.Luci DK, Jameson JB II, Yasgar A, Diaz G, Joshi N, Kantz A, Markham K, Perry S, Kuhn N, Yeung J, Kerns EH, Schultz L, Holinstat M, Nadler JL, Taylor-Fishwick DA, Jadhav A, Simeonov A, Holman TR, Maloney DJ. Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. J Med Chem. 2014;57(2):495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor-Fishwick DA, Weaver J, Glenn L, Kuhn N, Rai G, Jadhav A, Simeonov A, Dudda A, Schmoll D, Holman TR, Maloney DJ, Nadler JL. Selective inhibition of 12-lipoxygenase protects islets and beta cells from inflammatory cytokine-mediated beta cell dysfunction. Diabetologia. 2015;58(3):549–557. [DOI] [PubMed] [Google Scholar]
- 25.Wikstrom JD, Sereda SB, Stiles L, Elorza A, Allister EM, Neilson A, Ferrick DA, Wheeler MB, Shirihai OS. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets [published correction appears in PLoS One. 2013;8(12)]. PLoS One. 2012;7(5):e33023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eizirik DL, Sandler S, Welsh N, Cetkovic-Cvrlje M, Nieman A, Geller DA, Pipeleers DG, Bendtzen K, Hellerström C. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994;93(5):1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162–185. [DOI] [PubMed] [Google Scholar]
- 28.Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase products reduce insulin secretion and beta-cell viability in human islets. J Clin Endocrinol Metab. 2010;95(2):887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eizirik DL, Korbutt GS, Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest. 1992;90(4):1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole BK, Kuhn NS, Green-Mitchell SM, Leone KA, Raab RM, Nadler JL, Chakrabarti SK. 12/15-Lipoxygenase signaling in the endoplasmic reticulum stress response. Am J Physiol Endocrinol Metab. 2012;302(6):E654–E665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15(8):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidarala V, Veluthakal R, Syeda K, Vlaar C, Newsholme P, Kowluru A. Phagocyte-like NADPH oxidase (Nox2) promotes activation of p38MAPK in pancreatic β-cells under glucotoxic conditions: evidence for a requisite role of Ras-related C3 botulinum toxin substrate 1 (Rac1). Biochem Pharmacol. 2015;95(4):301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishiki Y, Adewola A, Hatanaka M, Templin AT, Maier B, Mirmira RG. Translational control of inducible nitric oxide synthase by p38 MAPK in islet β-cells. Mol Endocrinol. 2013;27(2):336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donath MY. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia. 2016;59(4):679–682. [DOI] [PubMed] [Google Scholar]