Abstract
Inhalation delivery of prostaglandin E (PGE2) in combination with selected siRNA(s) was proposed for the efficient treatment of idiopathic pulmonary fibrosis (IPF). Nanostructured lipid carriers (NLC) were used as a delivery system for PGE2 with and without siRNAs targeted to MMP3, CCL12, and HIF1Alpha mRNAs. The model of IPF was developed in SKH1 mice by intratracheal administration of bleomycin at a dose of 1.5 U/kg. Results showed that NLC-PGE2 in combination with three siRNAs delivered locally to the lungs by inhalation markedly reduced mouse body mass, substantially limited hydroxyproline content in the lungs and disturbances of the mRNAs and protein expression, restricted lung tissue damage and prevented animal mortality. Our data provide evidence that IPF can be effectively treated by inhalation of the NLC-PGE2 in combination with siRNAs delivered locally into the lungs. This effect could not be achieved by using NLC containing just PGE2 or siRNA(s) alone.
Keywords: Nanostructured lipid carriers, lung damage, PGE2, MRI, CT imaging, inhalation
Graphical Abstract
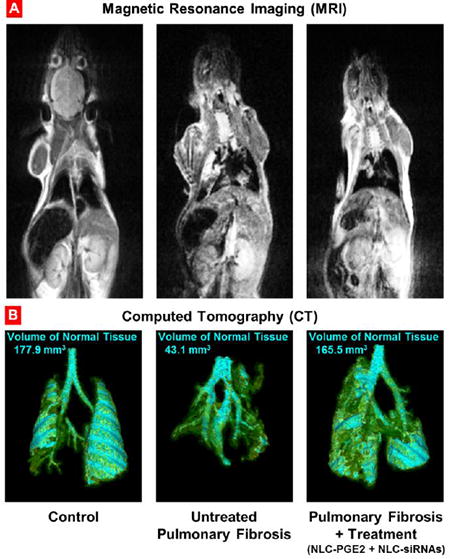
Development and treatment of pulmonary fibrosis. Representative magnetic resonance (A) and computed tomography (B) images of control (healthy) mouse, untreated and treated mice with pulmonary fibrosis. Mice were treated with NLC-PGE2 and mixture of three siRNAs (MMP3, CCL12, and HIF1A) delivered by NLC twice a week for three weeks starting one day later after the bleomycin administration. Images were taken at the end of treatment. Cyan-colored areas represent normal lung tissue. Yellow color indicates normal connective tissue while green color shows fibrotic tissue that is not normally associated with lung segmentations.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a severe lung disease wherein lung tissue becomes thickened, stiff, and scarred. According to Hutchinson et al, the disease affects up to 16.3 per 100 ,000 per year in the US1. At present, there are no FDA-approved therapies for IPF; however, a considerable amount of recent research and numerous clinical trials have been carried out in order to improve knowledge of this disease and to evaluate a variety of therapeutic agents2-4. IPF patients may ultimately require supportive oxygen therapy or pulmonary transplantation. Consequently, the development of a novel effective treatment for this disturbing disease is urgently needed. As was shown previously in our lab5, pulmonary fibrosis can be effectively treated by the inhalation delivery of a liposomal form of prostaglandin E2 (PGE2) locally into the lungs. PGE2, a cyclooxygenase-derived lipid mediator, has attracted considerable attention for its role in the development and progression of IPF and as a possible therapeutic agent for this disease6. The results of the previous investigations made the liposomal form of PGE2 an attractive drug for inhalation therapy of IPF. The main reason for the successful treatment was that local delivery of the drug into the lungs could enhance its therapeutic efficiency and potentially prevent serious systemic adverse “off-target effects” of PGE2 and protein suppression by siRNA in other organs. We found that at least 80% of particles were retained in the lungs after inhalation and did not enter into the systemic circulation. However, signs of inflammation, edema and hypoxia features were still observed in the lungs after the treatment. We also showed that liposomal PGE2 limited but not completely downregulated the synthesis of the proteins which caused lung damage during the IPF development.
The present study is aimed at developing a unique therapeutic approach based on the delivery to the lungs of nanostructured lipid carriers (NLC) designed for inhalation administration of PGE2 in combination with siRNA in order to limit lung damage caused by IPF and prevent further development of the disease by suppressing the synthesis of pro-fibrotic proteins. The following three targeted proteins were selected based on our previous study5: MMP3 (matrix metalloproteinase), CCL12 (chemokine), and HIF1A (hypoxia-inducible-factor). The major improvements and differences from the existing approaches and our previous investigations include: (1) combinatorial treatment with PGE2 and siRNAs targeted to major proteins responsible for lung damage induced by fibrosis and inflammation; (2) delivery of PGE2 and siRNAs locally to the lungs, limiting their adverse side-effects to healthy tissues and increased the efficiency of the treatment; (3) use of the modified nanostructured lipid carriers for pulmonary (inhalation) local delivery of siRNA in combination with PGE2 in order to improve the stability and solubility of active components and enhance drug and siRNA content in the lungs.
Methods
Materials
Precirol ATO 5 was generously provided by Gattefossé USA (Paramus, NJ). Soybean phosphatidylcholine (SPC), triethylamine (TEA), squalene, Tween-80, and mannitol were purchased from Sigma Aldrich (St. Louis, MO). DOTAP (1,2-dioleoyl-3-trimethylammonium-propane (chloride salt) was obtained from Avanti Polar Lipids (Alabaster, AL). PGE2 was obtained from Apichem Chemical Technology Co., Ltd. (Shanghai, China) and bleomycin was purchased from Sigma Aldrich (Ronkonkoma, NY). The selected siRNAs were custom synthesized according to our design by Ambion (Austin, TX). Hairless SKH1-Hr mice, 6–8 weeks-old, were purchased from Charles River Laboratories (Wilmington, MA).
Synthesis of nanostructured lipid carriers (NLCs) containing PGE2 and siRNA
Drug-loaded NLCs were prepared by a modified melted ultrasonic dispersion method as previously described7-9. Briefly, prior to NLC preparation, PGE2 was dissolved in 1 mL of DMSO and added to the hot lipid phase consisting of 100 mg Precirol ATO 5 (solid lipid), 100 mg squalene (liquid/lipid) and 5 mg SPC (lipophilic emulsifier). The aqueous phase was prepared by dissolving 250 mg Tween-80 (surfactant) and 25 mg DOTAP (cationic lipid) in 10 mL of deionized water. Both phases were maintained for 15 min at 80 °C in an oil bath under magnetic stirring. The hot lipid phase then was added slowly to the aqueous solution and dispersed using a high-speed homogenizer (PRO Scientific Inc. Oxford, CT) for 5 min at 12,000 rpm. The crude emulsion was additionally treated by a probe type ultrasonicator (Fisher Scientific Model 120 Sonic Dismembrator, Bridgewater, NJ) for 5 min at 3 W. The hot emulsion was cooled at 4 °C on an ice bath, maintaining the mechanical stirring for 60 min. After preparation, the NLC was purified by dialysis (MWC 10,000) and subjected to lyophilization. Mannitol (5%) was added into NLC suspension as cryoprotector. The obtained powder was stored at 4 °C until further use. The siRNA complexes were prepared at w/w (weight NLC/weight siRNA) ratio of 117:1 in water by adding stock solution of NLC into a prepared siRNA solution (MMP3 siRNA, CCL12 siRNA, and HIF1A siRNA 1:1:1). The final concentrations of siRNAs (MMP3 siRNA, CCL12 siRNA, and HIF1A siRNA 1:1:1) and PGE2 loaded into NLC were 128.5 μg/mL and 20 mM, respectively. The samples were vortexed, and the solutions were then incubated at room temperature for 30 min to ensure complex formation.
Particle size and zeta potential
The particle size distribution and zeta potential were measured by Malvern ZetaSizer NanoSeries (Malvern Instruments Enigma Business Park, UK) according to the manufacturer's instructions. All measurements were carried out at room temperature. Each parameter was measured three times for each batch, and average values and standard deviations were calculated.
Quantitative HPLC method for PGE2 concentration evaluation
The aliquots of NLC were disrupted in isopropanol in a ratio of 10:90 (NLC/isopropanol) and the concentration PGE2 was determined by high-performance liquid chromatography (HPLC) using a symmetry C18 column (150 mm × 4.6 mm (Water Corporation, Milford, MA) operated at room temperature. The mobile phase consisted of methanol/water/ammonium acetate of 10/90/2 mM, v/v/concentration; the flow rate was set to 1.0 ml/min, wavelength 196 nm. The chromatographic apparatus consisted of a Model 1525 pump (Waters Instruments, Milford, MA), a Model 717 Plus auto-injector (Waters Instruments, Milford, MA) and a Model 2487 variable wavelength UV detector (Waters Instruments, Milford, MA) connected to the Millennium software
Animal model of IPF
Experiments were performed on healthy 6–8 weeks old SKH1-hr hairless mice (20–25 g) obtained from Charles River Laboratories (Wilmington, MA). Veterinary care followed the guidelines described in the guide for the care and use of laboratory animals (AAALAC) as well as the requirements established by the animal protocol approved by the Rutgers Institutional Animal Care and Use Committee (IACUC). All mice were housed in micro-isolator cages under pathogen-free conditions at room temperature with humidity of 40 ± 15% and a 12 h light/dark cycle. Mice were anesthetized via intraperitoneal injection of 80 mg/kg ketamine and 10–12 mg/kg xylazine (Butler-Schine Animal Health Inc, Dublin, Ohio). Once anesthetized, the mouse was placed on the tilting rodent work stand (Hallowell EMC, Pittsfield, MA) in supine position and restrained in position by an incisor loop. The tongue was then extruded via rotation with a cotton tip applicator. The larynx was visualized using a modified 4 mm ear speculum attached to the operating head of an ophthalmoscope (Wellch Allyn, Skaneateles Falls, NY). The modified speculum, acting in an inverted fashion as a laryngoscope blade, provided dorsal displacement of the tongue and magnification of the laryngeal opening. Bleomycin was administered intratracheally at a dose of 1.5 U/kg. The development of fibrosis was confirmed by different types of imaging, measurement of hydroxyproline concentration in lung tissues, histopathology, body and lung mass and overexpression of genes involved in the development of IPF.
In vivo Magnetic Resonance Imaging (MRI) and Computed Tomography (CT)
Mice were anesthetized with isoflurane (4% for induction of anesthesia and 1–2% for maintenance) using XGI-8 Gas Anesthesia System (Xenogen, Alameda, CA) for all imaging procedures. MRI was performed using Fast Spin Echo (T2) and Gradient Echo (T1) sequences. Both scans utilized 16 slices at 1.5 mm thickness with a field of view of 100 mm. Scans were combined in the analysis software, reviewed and segmented.
CT was performed using high current (400 mA) and high voltage (45 kV) with a “High Resolution” file size. Lungs were digitally isolated from the image using a CT-ROI (-999 to 10) and then segmented into four components based on voxel intensity, which generally represents tissue radio-density. Volumes of each component were then determined.
Treatment
Mice were treated by inhalation twice weekly for three weeks, starting one day after the bleomycin administration, with NLC-PGE2, NLC-siRNA(s), and NLC-PGE2 + NLC-siRNAs targeted to MMP3, CCL12 and HIF1A mRNAs. A previously developed instillation unit consisting of a Collison nebulizer connected to four-port, nose-only exposure chambers was used for inhalation delivery of drug formulations as previously developed and validated in our lab5, 9, 10. NLCs were aerosolized at the flow rate of 2 L/min for 10 min. Animal mass was measured daily throughout the study. After the three week treatment period, all mice were anesthetized with isoflurane and euthanized. The lungs were excised and used for further analysis.
Gene expression
Mouse lungs were extracted; trachea and mainstem bronchi were separated, frozen and homogenized. Total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. First-strand cDNA was synthesized with Ready-To-Go You- Prime First-Strand Beads (Amersham Biosciences, Piscataway, NJ) with 1 μg of total cellular RNA (from 107 cells) and 100 ng of random hexadeoxynucleotide primer (Amersham Biosciences, Piscataway, NJ). After synthesis, the reaction mixture was immediately subjected to quantitative polymerase chain reaction (QPCR). A standard Mouse Fibrosis RT Profiler™ PCR Array panel from SABiosciences (Quiagen, Valencia, CA) was used. The assay was performed on lung samples from healthy mice (control), mice with lung fibrosis and mice with lung fibrosis treated with different drug/siRNA formulations. QPCR was performed using SYBER Green Master Mix as detection agent. A fold-change less than 1 (control) represented downregulation of the correspondent gene. The fold-change was calculated using the following formula: 2(−ΔCCT))5.
Selection of siRNA sequences
Three targeted genes/proteins involved in the development of lung damage induced by bleomycin, were previously selected5. These proteins are: MMP3 (matrix metalloproteinases), (NCBI Ref Seq: NM_010809.2), CCL12 chemokines (NCBI Ref Seq: NM_011331.2), and HIF1A hypoxia inducible factor alpha (NCBI Ref Seq: NM_010431.2). The best sequences of siRNA(s) targeted to MMP3, CCL12 and HIF1A mRNAs were selected in special studies.
We followed the general guidelines commonly acceptable for designing silencing siRNAs as proposed by Pei and Tuschl11 and refined by Raynolds et al 12. According to the approach, the length of the siRNA is limited to19-23 nucleotides, outside from the region of 50-100 bp from the start and the termination codon. The sequence should have a moderate GC content (30-52%), and should not contain internal repeats. It is preferable to have at least 3 A/Us at position 15-19, A in position 6 and avoid G/C at position 19.
Three independent predictive online platforms were utilized for designing of siRNAs that target mRNAs: AsiDesigner from Korea Research Institute of Bioscience and Biotechnology (KRIBB), siDirect 2.013 and siDesign Center from Dharmacon, Inc. These platforms utilize slightly different methodology and filter for siRNA selection and were able to produce from 50 to 100 potential siRNA candidates. Based on the aforementioned rules, we chose siRNA sequences that were consensual among all three predictive platforms. Three sequences for each of targeted mRNA were selected:
MMP3: 1) 5′-CGAUGAUGAACGAUGGACA-3′; 2) 5′-GUGGUACCCACCAAGUCUA-3′; 3) 5′-GAAAUCAGUUCUGGGCUAU-3′.
CCL12: 1) 5′-CUCAUAGCUACCACCAUCA-3′; 2) 5′-CCAGUCACGUGCUGUUAUA-3′; and 3) 5′-GGACCAUACUGGAUAAGGA-3′; HIF1A: 1) 5′-AGAUGACGGCGACAUGGUU-3′; 2) 5′-CUCCAAGUAUGAGCACAGU-3′; 3) 5′-CAGUUACGAUUGUGAAGUU-3′.
Then, the selected sequences were tested in vitro in order to identify those that more effectively suppress the expression of targeted proteins. To this end, A549 human alveolar basal epithelia lung adenocarcinoma cells were incubated with NLC-siRNA targeted to MMP3(1), MMP3(2), MMP3(3); CCL12(1), CCL12(2), CCL12(3), HIF1A(1), HIF1A(2), HIF1A(3). Gene expression was measured by the QPCR and presented as a fold-change as described above.
Hydroxyproline assay
Three weeks after bleomycin instillation, lungs were harvested, homogenized in distilled water and examined using a Bio-vision hydroxyproline assay kit (Biovision, Mount View, CA, USA). Homogenized lung tissues were hydrolyzed in 12 N HCl at 120 °C for 3 h in pressure-tight vials. After this, 10 μl of samples were allocated to a 96 well plate and dried under vacuum. Oxidation buffer with chloramine T was added to each sample at room temperature for 5 min, and the samples were then incubated in dimethylaminobenzaldehyde (DMAB) reagent for 90 min at 60 °C. Samples were cooled and absorbance at 560 nm was measured using an automated microplate plate reader. Six concentrations of hydroxyproline standard dilutions (from 0 to 1 μg/well) were used to plot a hydroxyproline standard curve.
Histopathology
Mice were euthanized and necropsied 3 weeks after treatment with bleomycin or bleomycin plus drug and siRNA. Lungs were rapidly removed and fixed in 10% neutral-buffered formalin for hours. They were then trimmed for full-lung sections and were dehydrated in ascending ethanols, cleared in xylene, and embedded in Paraplast-Plus. Five-micron sections were cut and stained with either hematoxylin-eosin for general morphology or Masson's trichrome for connective tissue, and examined with a Zeiss Axiophot microscope.
Statistical analysis
Data were analyzed using descriptive statistics, single-factor analysis of variance (ANOVA), and presented as mean values ± standard deviation (SD) from four-to-eight independent measurements. The comparison among groups was performed by the independent sample Student's t-test. The difference between variants was considered significant if P < 0.05.
Results
Selection of targeted genes
In order to examine the mechanisms of the development of fibrosis induced by intratracheal instillation of bleomycin we studied the profiles of the expression of 84 key genes involved in tissue transformation during wound repair and development of fibrosis. These data were obtained using the standard Mouse Fibrosis RT Profiler™ PCR Array panel (Qiagen, Valencia, CA). The results showed that after instillation of bleomycin, 24 studied genes were upregulated by more than 5 times while 7 out of 84 genes were downregulated more than 5-fold. Among the genes which were overexpressed we chose three that could serve as biomarkers reflecting the IPF development: MMP3 from the matrix metalloproteinases family, CCL12 from the cytokine family, and HIF1A from the hypoxia-inducible-factors family. The overexpression pattern of the corresponding proteins is usually present in lungs of mice with IPF induced by bleomycin5, 14, 15. The results previously obtained by our group indicated the abundant overexpression of MMP3, CCL12, HIF1A genes in mice during the development of IPF5. Previously we showed that PGE2 delivered into the lungs by inhalation directly resulted in the substantial reduction of lung damage induced by bleomycin5. We hypothesized that the delivery of PGE2 in combination with suppression of all of these three key genes (MMP3, CCL12, HIF1A) could further enhance therapeutic results.
Selection of siRNA sequences
Hence, targeting of these three genes (MMP3, CCL12, HIF1A) using corresponding siRNAs in order to inhibit the proteins synthesis potentially could limit the fibrotic lungs damage. In order to test this hypothesis, the most efficient sequences of corresponding siRNAs were selected. Three siRNA sequences from the each of the mentioned mRNAs (MMP3, CCL12, and HIF1A) were tested on the A549 human alveolar basal epithelial lung adenocarcinoma cells. The most efficient sequences from the each family were chosen for the future in vivo studies: MMP3 - sequence 2, CCL12 - sequence 1 and HIF1A -sequence 2 (Figure 1).
Figure 1. Selection of siRNA sequences.
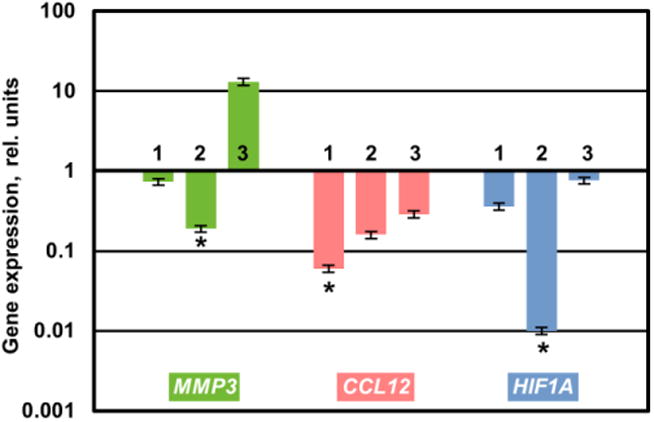
Three siRNA sequences for each gene (MMP3, CCL12, and HIF1A) were selected and tested for the suppression of the targeted mRNAs. A549 human aveolar basal epithelial adenocarcinoma lung cells were incubated with siRNAs delivered by NLC. Gene expression was measured by the QPCR and presented as fold change relative to the untreated cells. Means ± SD are shown. *The most efficient siRNA sequences chosen for the further in vivo study.
Validation of experimental model of lung fibrosis
In order to validate the previously developed model of pulmonary fibrosis5, IPF was induced in mice by intratrachel instillation of 1.5 U/kg bleomycin (the dose was previously selected5). MRI and CT imaging, changes in animal body and lung mass, hydroxyproline content in the lung tissues, histopathology and mouse survival data, were used as hallmarks of the development of IPF in experimental animals. MRI and CT images confirmed the development of IPF in animals after instillation of bleomycin (Figure 2). It was found that after instillation of bleomycin, the volume of normal tissues in the lungs decreased more than 4-fold. The measurements showed that the total body mass decreased in almost 1.8 fold while the lung mass increased statistically significantly (Figure 3 A, B). Instillation of bleomycin also increased the hydroxyproline content in the lungs in 2.7 times (Figure 3, B), which correlated with the development of fibrosis noted in lung sections. Finally, the development of IPF caused the death of experimental animals where all mice from the group treated with bleomycin developed pulmonary fibrosis and died within 13 days after instillation (Figure 4, Curve 2). Taken together, these data clearly confirmed that intratrachel instillation of 1.5 U/kg bleomycin induced heavy and progressive pulmonary fibrosis.
Figure 2. Development and treatment of pulmonary fibrosis.
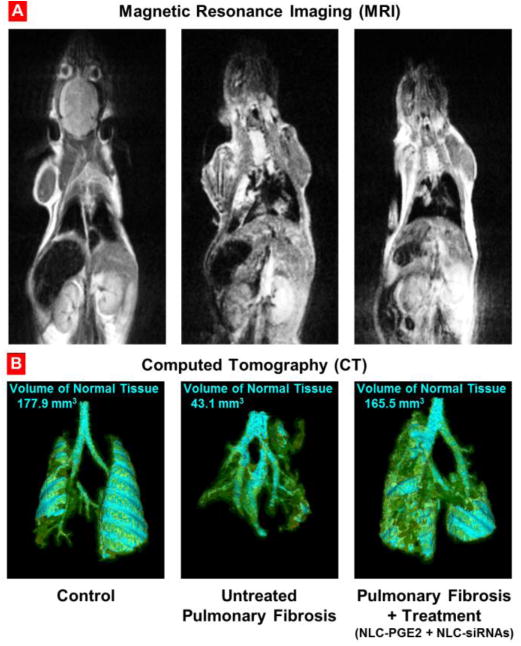
Representative magnetic resonance (A) and computed tomography (B) images of control (healthy) mouse, untreated and treated mice with pulmonary fibrosis. Pulmonary fibrosis was induced by intratracheal instillation of 1.5 U/kg of bleomycin. Mice were treated with NLC-PGE2 and mixture of three siRNAs (MMP3, CCL12, and HIF1A) delivered by NLC twice a week for three weeks starting one day later after the bleomycin administration. Images were taken at the end of treatment. Cyan-colored areas represent normal lung tissue. Yellow color indicates normal connective tissue while green color shows fibrotic tissue that is not normally associated with lung segmentations. Areas of lung that appear to be “missing” represent dense fibrotic tissue, hemorrhage and/or edema that cannot be captured by segmentation technique applied.
Figure 3. Inhalation treatment of pulmonary fibrosis.
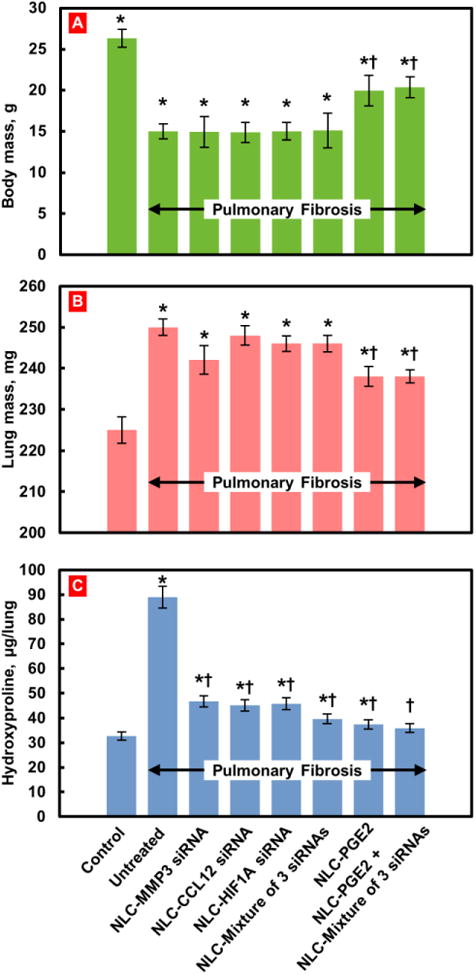
Changes in body (A) and lung (B) mass and hydroxyproline content in the lungs (C). Pulmonary fibrosis was induced by intratracheal instillation of 1.5 U/kg of bleomycin. Mice were treated with substances indicated twice a week for three weeks starting one day later after the bleomycin administration. At the end of treatment, lungs were harvested, weighted, homogenized and hydroxyproline content in the lungs was measured. Means ± SD are shown. *P < 0.05 when compared with control mice; †P < 0.05 when compared with untreated mice with pulmonary fibrosis.
Figure 4. Survival of mice (Kaplan-Meier survival plot) with pulmonary fibrosis.
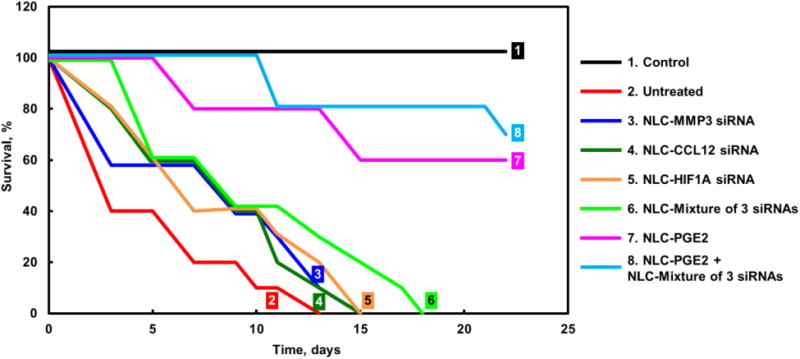
Pulmonary fibrosis was induced by intratracheal instillation of 1.5 U/kg of bleomycin. Mice were treated with substances indicated twice a week for three weeks starting one day later after the bleomycin administration.
Characterization of nanoparticles
Initially, the size of empty NLC was 250 ± 30 nm and increased to 400 ± 50 nm after complexation with siRNA. Zeta potential of empty NLC was +15 ± 4 mV while mixing with siRNA almost completely eliminated the surface charge of entire complexes.
Treatment of IPF using NLC containing siRNAs and/or PGE2
One-day after bleomycin instillation mice were randomly divided into the following eight groups: 1 -Healthy animals (control); 2 - Mice with fibrosis (bleomycin); 3 - Mice with fibrosis treated by inhalation with NCL-MMP3 siRNA; 4 - Mice with fibrosis treated by inhalation with NCL-CCL12 siRNA; 5 - Mice with fibrosis treated by inhalation with NCL- HIF1A siRNA; 6 - Mice with fibrosis treated by inhalation with NCL-MMP3, CCL12, HIF1A siRNAs; 7 - Mice with fibrosis treated by inhalation with NCL-PGE2; and 8 - Mice with fibrosis treated by inhalation with NCL-PGE2 and mixture of three siRNAs (MMP3, CCL12, HIF1A). The mice were treated twice weekly for three weeks starting one day after the bleomycin administration.
Treatment of mice with IPF with a single or a mixture of all three selected siRNAs had no change statistically significant effect on body and lung mass of experimental animals (Figure 3, A, B). In contrast, each of the siRNAs as well as their combination significantly decreased the hydroxyproline content in the lungs (Figure 3, C). The inhalation of each siRNA alone or in combination substantially decreased the expression of each targeted genes (Figure 5) and induced changes in the expression of profibrotic genes (Figure 6). As the result, the treatment with each siRNA and their combination decreased the mortality of mice with IPF. However, all mice still died within 23-28 days after bleomycin administration (Figure 4, Curves 3-6).
Figure 5. Expression of targeted genes in lung tissues of mice with pulmonary fibrosis.
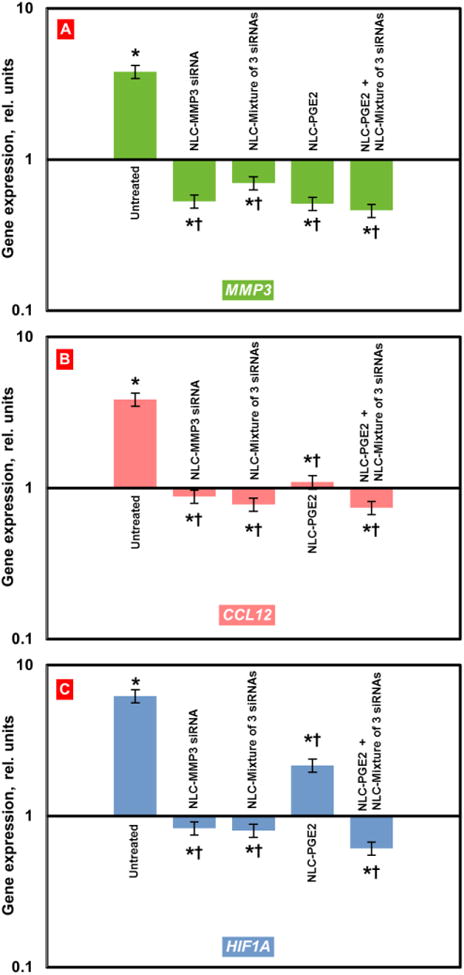
Pulmonary fibrosis was induced by intratracheal instillation of 1.5 U/kg of bleomycin. Mice were treated with substances indicated twice a week for three weeks starting one day later after the bleomycin administration. At the end of treatment, lungs were harvested and homogenized. The expression of MMP3 (A), CCL12 (B) and HIF1A (C) genes was measured by the QPCR and presented as fold change relative to the healthy animals. Means ± SD are shown. *P < 0.05 when compared with control mice; † P < 0.05 when compared with untreated mice with pulmonary fibrosis.
Figure 6. Expression of profibrotic genes in lung tissues of mice with pulmonary fibrosis.
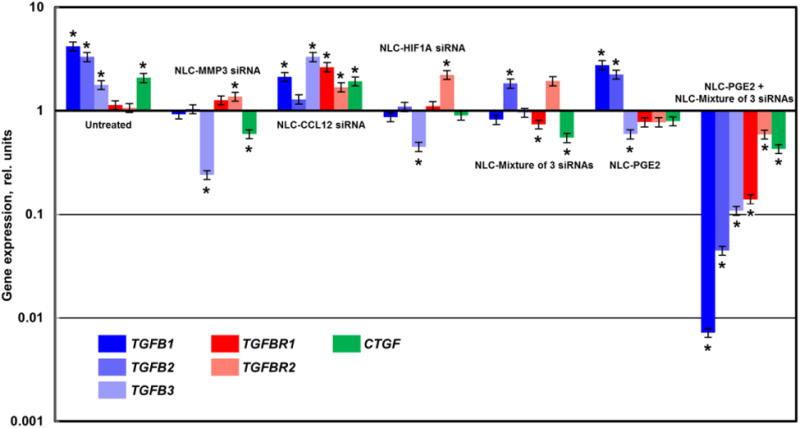
Pulmonary fibrosis was induced by intratracheal instillation of 1.5 U/kg of bleomycin. Mice were treated with substances indicated twice a week for three weeks starting one day later after the bleomycin administration. At the end of treatment, lungs were harvested and homogenized The expression of genes encoding transforming growth factor-β cytokines (TGFB1-3), transforming growth factor-β receptors (TGFBR1-2) and smooth muscle α actin (CTGF) was measured by the QPCR and presented as fold change relative to the healthy animals. Means ± SD are shown. *P < 0.05 when compared with control mice.
Treatment of mice with fibrosis by NLC containing PGE2 resulted in a moderate therapeutic effect: body mass was notably higher, whereas hydroxyproline concentration in lungs and lung mass were lower significantly when compared with untreated animals or animals treated with NLC containing just a single siRNA or the mixture of all three siRNAs (Figure 3). In terms of gene expression, NLC-PEG2 significantly suppressed all targeted genes (Figure 5) and some of the pro-fibrotic genes, specifically TGFB3, TGFBR1, 2, CTGF. As a result, about 60% of mice survived bleomycin injection after the treatment with NLC-PGE2.
However, the best therapeutic results were achieved when mice received combinatorial treatment that consisted of nanostructured lipid carriers containing PGE2 in combination with all three siRNAs (MMP3, CCL12, HIF1A). Positive effect of treatment with NLC-PGE2 + mixture of three siRNAs was confirmed by MEI and CT (Figure 2). Consequently, use of NLC loaded with PGE2 and mixture of three siRNAs led to the substantial reduction of lung inflammation and decrease in the formation of connective tissue. It was also found that the volume of fibrotic tissue in the lungs after the treatment was 3.8-fold lower when compared with bleomycin-treated mice with IPF and was almost the same as normal values in healthy animals. Mice body mass remained lower and lung mass higher when compared with those in healthy mice though hydroxyproline content in lungs was almost normalized after three weeks of inhalation treatment with NLC containing PGE2 and mixture of three siRNAs (Figure 3). It is interesting that the expression of all three targeted genes was substantially decreased in mice with IPF after the inhalation treatment with PGE2 in combination with three siRNAs delivered by NLC (Figure 5).
Expression of pro-fibrotic genes
Combinatorial treatment also suppressed expression all of the studied pro-fibrotic genes (Figure 6). In addition to the targeted genes, the expression of the connective tissue growth factor CTGF and genes associated with TGF-beta transmembrane receptors (TGFB1, TGFB2, TGFB3, TGFBR1, and TGFBR2) were monitored. As previously reported 16-18 and confirmed in this study, the level of the expression of these genes was elevated during the development of IPF (Figure 6), although, the level of elevation differed among the studied genes. It is known that the overexpression of the TGF-beta receptors in tissues occurs during the formation of the new fibrotic lesion but not in established lesions16. These data correlate with our results since the experimental mice were in the early stage of the fibrosis development. The CTGF gene is known to play a critical role in the pathogenesis of pulmonary fibrosis as well17. Moreover, recent studies have identified that TGF-β-regulated genes, or connective tissue growth factor (CTGF), mediate the effector function of TGF-β in the pathogenesis of pulmonary fibrosis17, 19. Figure 6 clearly demonstrates that inhalation delivery with nanoparticles containing PGE2 and mixture of three siRNAs (targeted to MMP3, CCL12, and HIF1A) sufficiently downregulated the expression of CTGF and TGFB1, TGFB2, TGFB3, TGFBR1, TGFBR2 genes. At the same time, treatment with NLC-PGE2 or NLC-siRNA alone or NLC-PGE2 in combination of all three siRNAs only partially suppressed synthesis of the studied genes. Furthermore, particles containing just CCL12 siRNA showed no appreciable effect on either connective tissue growth factor or TGF-beta transmembrane receptor expression levels in mouse lung.
Histopathology
Control Groups: Lungs of untreated mice (Controls) showed patent alveolar architecture with no evidence of inflammation, hemorrhage or other pathology (Figure 7a). Trichrome staining was observed around blood vessels and bronchi, but was not detected in the alveolar walls (Figure 7b). Group F: Lungs of bleomycin-treated mice displayed diffuse, severe alveolar injury, characterized by destruction and collapse of alveolar walls, infiltration of the alveolar spaces by a mixed population of inflammatory cells, including PMNs and macrophages, and reactive alveolar pneumocytes, and focal hemorrhage (Figure 7a). Trichrome staining revealed a fine, lacey deposition of connective tissue along the alveolar walls, indicative of early fibrosis (Figure 7b). Group F+T: Lungs of mice with bleomycin plus drug and siRNA showed significant protection from bleomycin-induced injury. The alveolar structure was essentially normal, with only focal hemorrhage and no inflammatory infiltrates (Figure 7a). Some focal emphysema-like alveolar dilation was observed at the margin of the lung parenchyma, possibly representing agonal hyperinflation. Bronchi and blood vessels were unremarkable. Faint trichome staining was noted around the bronchi and adventitia of large blood vessels, but not in the alveolar walls (Figure 7b).
Figure 7. Histopathology of lung.
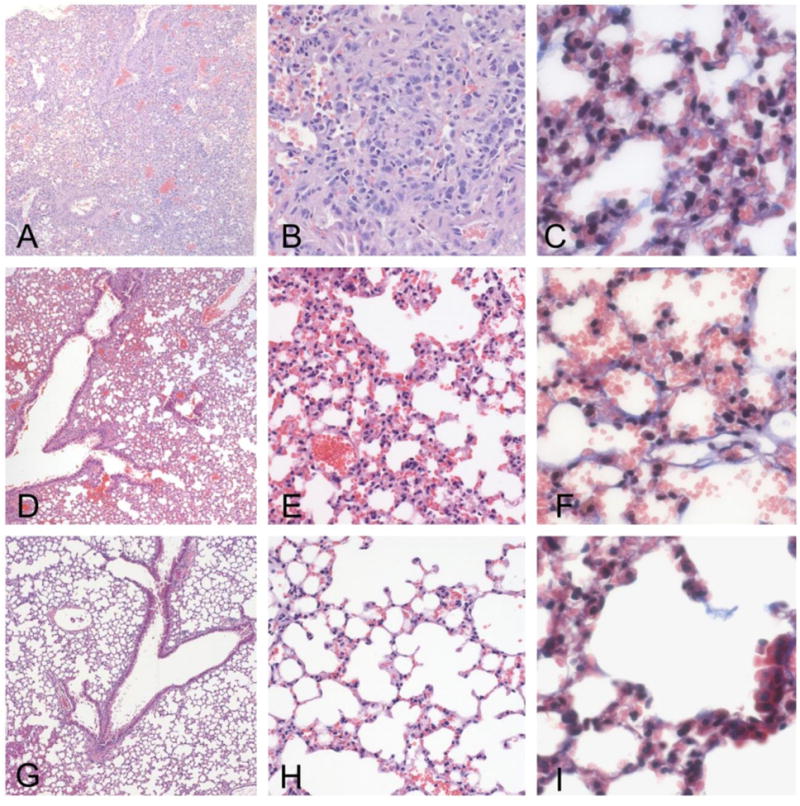
Lungs treated with bleomycin display severe injury, characterized by the obliteration of the alveolar space by proliferating stromal cells and a mixed inflammatory infiltrate, consisting primarily of macrophages. The infiltration was confluent and occupied sizable portions of the lung (a - X10, b - X20). Proliferation of fibrous tissue was observed along the alveolar walls by trichrome staining (c - 40X). Lungs of mice treated with bleomycin plus PGE2 and siRNA displayed a significant protective effect of treatment (7d - X10, e - X20). The alveolar structure was largely intact, with only small areas of infiltration and parenchymal consolidation. Only modest increase in connective tissue was observed by trichrome staining (d - X40). Lungs of control mice animals demonstrate a patent alveolar structure, with no inflammation, consolidation or fibrosis. (f - X10, g - X20, f - X40).
Discussion
Present study is aimed at developing a unique therapeutic approach based on nanostructured lipid carriers designed for inhalation delivery of PGE2 in combination with siRNA to the lung in order to limit lung damage caused by IPF and prevent further development of the disease. The proposed NLC represents a new generation of lipid nanoparticles, which were developed to exploit arising advantages from different drug carriers including liposomes5. NLC were developed by nanostructuring the lipid matrix in order to give more flexible control of drug release, increasing the loading efficiency for drugs like PGE2 which possesses poor water solubility, and preventing drug leakage. Among the different approaches to modeling pulmonary fibrosis, bleomycin remains the most frequently used agent and can be given directly into the airway by intratracheal administration20, 21. The major advantage of the intratracheal bleomycin model is the short time between the drug instillation and the manifestation of fibrosis. The results obtained in our earlier study showed high efficacy of liposomal PGE2 locally delivered to the lungs in limiting the signs of lung damage. However, signs of limited fibrosis, inflammation, edema and hypoxia features could still be observed in the lungs after the treatment5. Based on our preliminary results, we selected three proteins which were significantly overexpressed in the lung tissues with IPF and were not completely suppressed by liposomal PGE2. These proteins are: matrix metalloproteinase (MMP3), chemokine (CCL12) and hypoxia-inducible-factor alpha (HIF1A). siRNA sequences were selected for the suppression of targeted mRNAs. We hypothesized that the suppression of these proteins would further enhance the therapeutic efficacy of PGE2 in treatment of IPF and improve treatment outcome. To verify the hypothesis, three selected siRNAs that showed the most efficient protein suppression were chosen for the treatment of mice with IPF.
The results obtained in the present experimental study demonstrated for the first time that treatment of pulmonary fibrosis using NLC loaded with PGE2 and combination of three siRNAs targeted to MMP3, CCL12, and HIF1A delivered by inhalation resulted in increased survival of animals and limited the development of all clinical measurements of IPF following intratracheal instillation of bleomycin. Specifically, the current treatment prevented the loss of body mass, substantially limited hydroxyproline content in the lungs and disturbances in the mRNA expressions, restricted lung tissue damage, fibrosis and completely prevented animal mortality. These preventive effects of PGE2 in combination with protein suppressed result from ability of such combinatorial treatment to limit fibroblast proliferation, activation, migration, collagen secretion, and/or myofibroblast differentiation6, 22, 23,24. The experimental data support our hypothesis and underscore the therapeutic promise of this treatment's approach. It is highly likely that the normalization of the expression of genes encoding proteins responsible for inflammation and fibrosis after bleomycin instillation observed in the present study plays a central role in the prevention of lung injury and animal mortality. Suppression of HIF1A protein also plays a role in limiting fibrotic lung damage since it is generally believed that fibrosis is accompanied by hypoxia and activation of major hypoxic signaling pathways involved in the development and compensation of fibrotic damage25. Among the proteins significantly upregulated during the development of IPF were those encoding multiple matrix metalloproteinases (MMPs), collagens, and cytokines and growth factors. Elevated levels of MMP3 gene and protein expression have been described in IPF lung tissue, with MMP3 protein expression localized in alveolar and bronchiolar epithelial cells26. It is also known that CCL12 is involved in the pathogenesis of IPF, being a relevant ligand for the in vivo recruitment of fibroblasts and the pro-fibrotic effects on multiple cell types27, 28. However, the separate suppression of any single profibrotic RNA and protein synthesis could not significantly limit IPF development, whereas complex treatment which included PGE2 and siRNA combination effectively slowed down or even stop the disease progression.
It is well known that the transforming growth factor-beta (TGFB) family of receptors plays an important role in the initiation of the signal transduction events leading to mitogenic responses and initiation of fibrosis by inducing myofibroblast differentiation in lungs28. The connective tissue growth factor (CTGF) is overexpressed by fibroblasts. Additionally, CTGF and TGF-beta act together to promote sustained fibrosis17. Thus the constitutive overexpression of CTGF by fibroblasts present in fibrotic lesions would be expected to directly contribute to chronic progressive fibrosis. Notably, combinatorial local lung delivery by NLCs of PGE2 and mixture of three selected siRNAs (MMP3, CCL12, HIF1A) was able to downregulate the synthesis of five genes (TGFB1, TGFB2, TGFB3, TGFBR1, and TGFBR2) belonging to the transforming growth factors family and CTGF. This fact led to the suggestion that the mechanisms of the pulmonary fibrosis development are very complex and induce the up-regulation of particular genes triggering activation of different signal transduction pathways.
In summary, our data provide evidence that the unique therapeutic approach based on NLC designed for inhalation delivery of PGE2 in combination with mixture of selected siRNAs (MMP3, CCL12, HIF1A) results in the substantial limitation of lung damage caused by IPF and prevents further progression of the disease. The results of present investigations make this validated methodology an attractive approach for inhalation treatment of idiopathic pulmonary fibrosis.
Acknowledgments
This work was supported in part by grants from National Institutes of Health (R01 HL118312 and P30 ES005022). The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46:795–806. doi: 10.1183/09031936.00185114. [DOI] [PubMed] [Google Scholar]
- 2.Covvey JR, Mancl EE. Recent evidence for pharmacological treatment of idiopathic pulmonary fibrosis. Ann Pharmacother. 2014;48:1611–9. doi: 10.1177/1060028014551015. [DOI] [PubMed] [Google Scholar]
- 3.Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res. 2007;24:819–41. doi: 10.1007/s11095-006-9216-x. [DOI] [PubMed] [Google Scholar]
- 4.Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99:308–19. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- 5.Ivanova V, Garbuzenko OB, Reuhl KR, Reimer DC, Pozharov VP, Minko T. Inhalation treatment of pulmonary fibrosis by liposomal prostaglandin E2. Eur J Pharm Biopharm. 2013;84:335–44. doi: 10.1016/j.ejpb.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004;25:40–6. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Huang ZR, Hua SC, Yang YL, Fang JY. Development and evaluation of lipid nanoparticles for camptothecin delivery: a comparison of solid lipid nanoparticles, nanostructured lipid carriers, and lipid emulsion. Acta Pharmacol Sin. 2008;29:1094–102. doi: 10.1111/j.1745-7254.2008.00829.x. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Nie SF, Kong J, Li N, Ju CY, Pan WS. A controlled-release ocular delivery system for ibuprofen based on nanostructured lipid carriers. Int J Pharm. 2008;363:177–82. doi: 10.1016/j.ijpharm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171:349–57. doi: 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainelis G, Seshadri S, Garbuzenko OB, Han T, Wang Z, Minko T. Characterization and application of a nose-only exposure chamber for inhalation delivery of liposomal drugs and nucleic acids to mice. J Aerosol Med Pulm Drug Deliv. 2013;26:345–54. doi: 10.1089/jamp.2011-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei Y, Tuschl T. On the art of identifying effective and specific siRNAs. Nat Methods. 2006;3:670–6. doi: 10.1038/nmeth911. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–30. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 13.Naito Y, Yoshimura J, Morishita S, Ui-Tei K. siDirect 2.0: updated software for designing functional siRNA with reduced seed-dependent off-target effect. BMC Bioinformatics. 2009;10:392. doi: 10.1186/1471-2105-10-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–81. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePianto DJ, Chandriani S, Abbas AR, Jia G, N'Diaye EN, Caplazi P, et al. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax. 2015;70:48–56. doi: 10.1136/thoraxjnl-2013-204596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. Faseb j. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, et al. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60:2142–55. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 19.Leask A. Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis. Keio J Med. 2004;53:74–7. doi: 10.2302/kjm.53.74. [DOI] [PubMed] [Google Scholar]
- 20.Egger C, Cannet C, Gerard C, Jarman E, Jarai G, Feige A, et al. Administration of bleomycin via the oropharyngeal aspiration route leads to sustained lung fibrosis in mice and rats as quantified by UTE-MRI and histology. PLoS One. 2013;8:e63432. doi: 10.1371/journal.pone.0063432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tassali N, Bianchi A, Lux F, Raffard G, Sanchez S, Tillement O, et al. MR imaging, targeting and characterization of pulmonary fibrosis using intra-tracheal administration of gadolinium-based nanoparticles. Contrast Media Mol Imaging. 2016 doi: 10.1002/cmmi.1703. [DOI] [PubMed] [Google Scholar]
- 22.Bozyk PD, Moore BB. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:445–52. doi: 10.1165/rcmb.2011-0025RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol. 2008;39:482–9. doi: 10.1165/rcmb.2008-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh M, Stewart A, Tucker DE, Bonventre JV, Murphy RC, Leslie CC. Role of cytosolic phospholipase A(2) in prostaglandin E(2) production by lung fibroblasts. Am J Respir Cell Mol Biol. 2004;30:91–100. doi: 10.1165/rcmb.2003-0005OC. [DOI] [PubMed] [Google Scholar]
- 25.Qian F, He M, Duan W, Mao L, Li Q, Yu Z, et al. Cross regulation between hypoxia-inducible transcription factor-1alpha (HIF-1alpha) and transforming growth factor (TGF)-ss1 mediates nickel oxide nanoparticles (NiONPs)-induced pulmonary fibrosis. Am J Transl Res. 2015;7:2364–78. [PMC free article] [PubMed] [Google Scholar]
- 26.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53:585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekert JE, Murray LA, Das AM, Sheng H, Giles-Komar J, Rycyzyn MA. Chemokine (C-C motif) ligand 2 mediates direct and indirect fibrotic responses in human and murine cultured fibrocytes. Fibrogenesis Tissue Repair. 2011;4:23. doi: 10.1186/1755-1536-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol. 2008;40:2174–82. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]


