Abstract
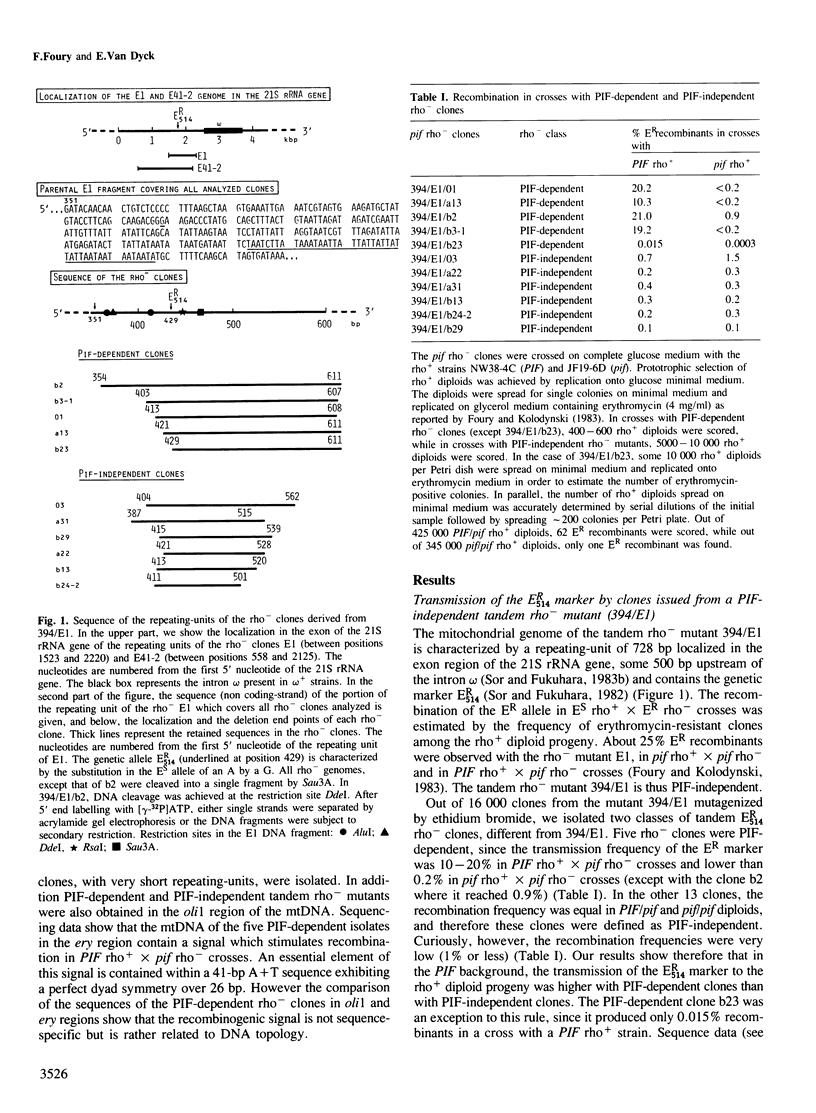
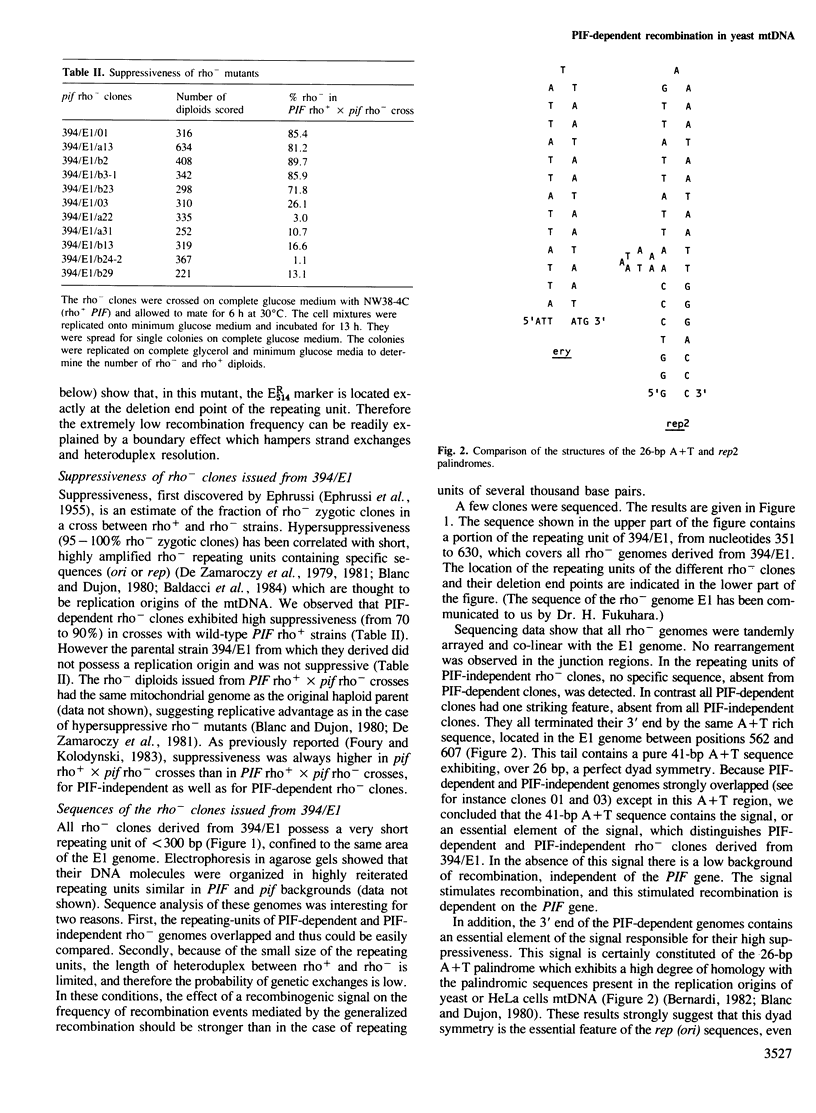
From their recombination properties, tandem rho- mutants of the mitochondrial genome of Saccharomyces cerevisiae were divided into two categories. In crosses between PIF-independent rho- and rho+ strains, the recombination frequency is low and similar in PIF/pif and pif/pif diploids. In crosses between PIF-dependent rho- and rho+ strains, the recombination frequency is stimulated 10-50 times in PIF/pif diploids and is drastically decreased in pif/pif diploids. These results suggest that a recombinogenic signal is present in the mitochondrial (mt) DNA of PIF-dependent rho- clones. This signal is not recognized in pif mutants. Sequence analysis of a series of small (<300 bp) overlapping tandem rho- genomes located in the ery region of the 21S rRNA gene led us to identify an essential element of this signal within a 41-bp A+T sequence exhibiting over 26 bp a perfect dyad symmetry. However the recombinogenic signal is not sequence-specific since the sequence described above does not characterize PIF-dependent rho- clones located in the oli1 region. Our results rather suggest that the recombinogenic signal is related to the topology of rho- DNA. Denaturated sites in the double helix or cruciform structures elicited by local negative supercoiling might be preferred sites of the initiation of recombination.
Keywords: mtDNA, recombination, yeast, pif mutants
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci G., Chérif-Zahar B., Bernardi G. The initiation of DNA replication in the mitochondrial genome of yeast. EMBO J. 1984 Sep;3(9):2115–2120. doi: 10.1002/j.1460-2075.1984.tb02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. J. Torsional stress and local denaturation in supercoiled DNA. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3870–3874. doi: 10.1073/pnas.76.8.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Dujon B. Replicator regions of the yeast mitochondrial DNA responsible for suppressiveness. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3942–3946. doi: 10.1073/pnas.77.7.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Dujon B., Bolotin-Fukuhara M., Coen D., Deutsch J., Netter P., Slonimski P. P., Weill L. Mitochondrial genetics. XI. Mutations at the mitochondrial locus omega affecting the recombination of mitochondrial genes in Saccharomyces cerevisiae. Mol Gen Genet. 1976 Jan 16;143(2):131–165. doi: 10.1007/BF00266918. [DOI] [PubMed] [Google Scholar]
- Dujon B., Slonimski P. P., Weill L. Mitochondrial genetics IX: A model for recombination and segregation of mitochondrial genomes in saccharomyces cerevisiae. Genetics. 1974 Sep;78(1):415–437. doi: 10.1093/genetics/78.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi B., de Margerie-Hottinguer H., Roman H. SUPPRESSIVENESS: A NEW FACTOR IN THE GENETIC DETERMINISM OF THE SYNTHESIS OF RESPIRATORY ENZYMES IN YEAST. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1065–1071. doi: 10.1073/pnas.41.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye G., Fukuhara H., Grandchamp C., Lazowska J., Michel F., Casey J., Getz G. S., Locker J., Rabinowitz M., Bolotin-Fukuhara M. Mitochondrial nucleic acids in the petite colonie mutants: deletions and repetition of genes. Biochimie. 1973;55(6):779–792. doi: 10.1016/s0300-9084(73)80030-6. [DOI] [PubMed] [Google Scholar]
- Foury F., Goffeau A. Genetic control of enhanced mutability of mitochondrial DNA and gamma-ray sensitivity in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6529–6533. doi: 10.1073/pnas.76.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foury F., Kolodynski J. pif mutation blocks recombination between mitochondrial rho+ and rho- genomes having tandemly arrayed repeat units in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5345–5349. doi: 10.1073/pnas.80.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard C., Strauss F., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Nature. 1980 Jan 10;283(5743):218–220. doi: 10.1038/283218a0. [DOI] [PubMed] [Google Scholar]
- Hsieh T. S., Wang J. C. Thermodynamic properties of superhelical DNAs. Biochemistry. 1975 Feb 11;14(3):527–535. doi: 10.1021/bi00674a011. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Dujon B. The intron of the mitochondrial 21S rRNA gene: distribution in different yeast species and sequence comparison between Kluyveromyces thermotolerans and Saccharomyces cerevisiae. Mol Gen Genet. 1983;192(3):487–499. doi: 10.1007/BF00392195. [DOI] [PubMed] [Google Scholar]
- Kitts P., Richet E., Nash H. A. Lambda integrative recombination: supercoiling, synapsis, and strand exchange. Cold Spring Harb Symp Quant Biol. 1984;49:735–744. doi: 10.1101/sqb.1984.049.01.083. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Kroeger P. E., Brougham M. J., Holloman W. K. Topological linkage of circular DNA molecules promoted by Ustilago rec 1 protein and topoisomerase. Cell. 1983 Oct;34(3):919–929. doi: 10.1016/0092-8674(83)90549-4. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Slonimski P. P. Electron microscopy of analysis of circular repetitive mitochondrial DNA molecules from genetically characterized rho- mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1976 Jul 5;146(1):61–78. doi: 10.1007/BF00267984. [DOI] [PubMed] [Google Scholar]
- Locker J., Lewin A., Rabinowitz M. The structure and organization of mitochondrial DNA from petite yeast. Plasmid. 1979 Apr;2(2):155–181. doi: 10.1016/0147-619x(79)90036-2. [DOI] [PubMed] [Google Scholar]
- Locker J., Rabinowitz M., Getz G. S. Tandem inverted repeats in mitochondrial DNA of petite mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1366–1370. doi: 10.1073/pnas.71.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michel F., Grandchamp C., Dujon B. Genetic and physical characterization of a segment of yeast mitochondrial DNA involved in the control of genetic recombination. Biochimie. 1979;61(9):985–1010. doi: 10.1016/s0300-9084(80)80254-9. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cruciform structures in supercoiled DNA. Nature. 1981 Feb 5;289(5797):466–470. doi: 10.1038/289466a0. [DOI] [PubMed] [Google Scholar]
- Perlman P. S. Genetic analysis of petite mutants of Saccharomyces cerevisiae: transmissional types. Genetics. 1976 Apr;82(4):645–663. doi: 10.1093/genetics/82.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Complete DNA sequence coding for the large ribosomal RNA of yeast mitochondria. Nucleic Acids Res. 1983 Jan 25;11(2):339–348. doi: 10.1093/nar/11.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Identification of two erythromycin resistance mutations in the mitochondrial gene coding for the large ribosomal RNA in yeast. Nucleic Acids Res. 1982 Nov 11;10(21):6571–6577. doi: 10.1093/nar/10.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Unequal excision of complementary strands is involved in the generation of palindromic repetitions of rho- mitochondrial DNA in yeast. Cell. 1983 Feb;32(2):391–396. doi: 10.1016/0092-8674(83)90458-0. [DOI] [PubMed] [Google Scholar]
- Strausberg R. L., Vincent R. D., Perlman P. S., Butow R. A. Asymmetric gene conversion at inserted segments on yeast mitochondrial DNA. Nature. 1978 Dec 7;276(5688):577–583. doi: 10.1038/276577a0. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Baldacci G., Bernardi G. Putative origins of replication in the mitochondrial genome of yeast. FEBS Lett. 1979 Dec 15;108(2):429–432. doi: 10.1016/0014-5793(79)80580-3. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Gene. 1983 Mar;21(3):193–202. doi: 10.1016/0378-1119(83)90002-1. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Marotta R., Faugeron-Fonty G., Goursot R., Mangin M., Baldacci G., Bernardi G. The origins of replication of the yeast mitochondrial genome and the phenomenon of suppressivity. Nature. 1981 Jul 2;292(5818):75–78. doi: 10.1038/292075a0. [DOI] [PubMed] [Google Scholar]


