Abstract
Drug addiction is a chronic disease that is shaped by alterations in neuronal function within the cortical-basal ganglia-thalamic circuit. However, our understanding of how this circuit regulates drug-seeking remains incomplete, and relapse rates remain high. The midline thalamic nuclei are an integral component of the cortical-basal ganglia-thalamic circuit and are poised to mediate addiction behaviors, including relapse. It is surprising that little research has examined the contribution of midline thalamic nuclei and their efferent projections in relapse. To address this, we expressed inhibitory, Gi/o-coupled DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) in a subset of the midline thalamic nuclei or in midline thalamic nuclei neurons projecting to either the nucleus accumbens or the amygdala. We examined the effect of transiently decreasing activity of these neuronal populations on cue-induced and cocaine-primed reinstatement of cocaine-seeking. Reducing activity of midline thalamic nuclei neurons attenuated both cue-induced and cocaine-primed reinstatement, but had no effect on cue-induced reinstatement of sucrose-seeking or locomotor activity. Interestingly, attenuating activity of efferent projections from the anterior portion of midline thalamic nuclei to the nucleus accumbens blocked cocaine-primed reinstatement but enhanced cue-induced reinstatement. Decreasing activity of efferent projections from either the posterior midline thalamic nuclei to the nucleus accumbens or the midline thalamic nuclei to amygdala had no effect. These results reveal a novel contribution of subsets of midline thalamic nuclei neurons in drug-seeking behaviors, and suggest that modulation of midline thalamic nuclei activity may be a promising therapeutic target for preventing relapse.
Keywords: DREADDs, cocaine, rat, neural circuits, addiction
Graphical Abstract
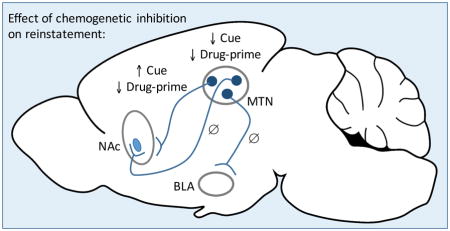
Chemogenetic inhibition of midline thalamic nuclei (MTN) decreases both cue-induced and cocaine-primed reinstatement of cocaine-seeking in rats. Inhibition of efferent projections from anterior MTN to the nucleus accumbens (NAc) has similar effects on cocaine-primed reinstatement but enhances cue-induced reinstatement. However, this same manipulation in efferent projections from posterior MTN to the NAc or in efferent projections to the basolateral nucleus of the amygdala (BLA) had no effect. These data suggest that modulation of MTN activity may be a promising therapeutic target for preventing relapse.
INTRODUCTION
Drug addiction is a chronic disease characterized by compulsive drug-taking and high rates of relapse during abstinence. Indeed, at least 40–60% of addicts seeking treatment will relapse at least once during the progression of their disease (McLellan et al., 2000). Although many molecular and cellular adaptations thought to underlie addiction have been identified within the cortico-basal ganglia-thalamic system, these findings have yet to translate into effective treatment options (Koob & Volkow, 2009; Sesack & Grace, 2009; Steketee & Kalivas, 2011).
A key node in the cortico-basal ganglia-thalamic circuit is the midline (e.g., paraventricular PVT; intermediodorsal IM; and mediodorsal MD) and intralaminar (e.g., centromedian CM) nuclei of the thalamus (MTN). These glutamatergic nuclei are uniquely situated to integrate information regarding the sensory and motivational state of an animal in order to guide behavioral responses to motivationally salient stimuli (Haight & Flagel 2014; James & Dayas 2013; Kelley et al. 2005; Van der Werf et al., 2002). Specifically, MTN afferents originate in subcortical regions involved in arousal and visceral sensation, such as the hypothalamus, dorsal raphe, locus coeruleus, periaqueductal gray, and reticular activating system (Van der Werf et al., 2002). In addition, MTN have reciprocal connections with limbic structures involved in the regulation of emotion, motivation, and addiction, such as prefrontal cortex (PFC) and basolateral amygdala (BLA) (Hsu & Price, 2009; Li & Kirouac, 2007; Li & Kirouac, 2011; Van der Werf et al., 2002; Berendse & Groenewegen, 1990; Yager et al., 2015). Lastly, MTN receive information regarding on-going motivated behavior via inputs from output structures of the basal ganglia system (O’Donnell et al., 1997). Thus, it is surprising that there are not more studies examining the role of MTN, and MTN inputs to downstream structures, in relapse. Nonetheless, work to date has found that neuronal activity in MTN increases following exposure to drug-associated cues and that lesions and/or inactivation of PVT reduces reinstatement of drug-seeking to cues, context or a drug prime (Dayas et al., 2008; Hamlin et al., 2009; James et al., 2010; James et al., 2011; Matzeu et al., 2015a; Matzeu et al., 2015b; Wedzony et al. 2003).
In addition, persistent, glutamate-dependent morphological, physiological, and electrochemical changes occur in the nucleus accumbens (NAc) following drug exposure, which leads to an enhancement in glutamatergic signaling that is particularly important for mediating relapse (Berke & Hyman, 2000; Everitt, 2014; Lüscher & Malenka, 2011; Steketee & Kalivas, 2011; Wolf, 2016). Although glutamatergic inputs from the cortex are well-known regulators of NAc neurons, given that MTN are a dense source of striatal glutamate, synapse directly onto medium spiny neurons, and regulate dopamine release from ventral tegmental area terminals within the NAc, the role of these afferents in relapse behaviors also deserves consideration (Berendse & Groenewegen, 1990; Frassoni et al., 1997; Lei et al., 2013; Parsons et al., 2006; Su & Bentivoglio, 1990; Wall et al., 2013).
To begin to address these issues, we used viral-mediated gene transfer techniques to express Gi/o-DREADDs (Designer Receptors Exclusively Activated by Designer Drugs; hM4Di) selectively in MTN, MTN projections to nucleus accumbens (MTN-NAc), or MTN projections to basolateral amygdala (MTN-BLA). DREADDs are activated by clozapine-N-oxide (CNO), which leads to a transient increase in Gi/o-signaling cascades in targeted neurons (Rogan and Roth, 2011; Roth, 2016; Zhu and Roth, 2014). In addition to a reduction in intracellular cAMP levels, activation of hM4Di also decreases neuronal activity in glutamatergic neurons via activation of G-protein-coupled inwardly rectifying potassium channels and a reduction in neurotransmitter release (Armbruster et al., 2007; Kerstetter et al., 2016; Roth, 2016; Zhu et al., 2016). Using these tools, we assessed how transiently inhibiting neuronal activity in subsets of midline thalamic nuclei neurons impacts cue-induced and cocaine-primed reinstatement of drug-seeking in rats.
MATERIALS AND METHODS
Animals
All experiments were approved by the Seattle Children’s Research IACUC and adhered to National Institutes of Health (NIH) guidelines. Male Sprague-Dawley rats (n=85, Envigo, Livermore, CA, USA) weighing 250–274g upon arrival were pair-housed and maintained on a 12 h light/dark cycle. Rats were pair-housed throughout the experiment, except for a group of rats (n=5) that were singled-housed following catheter surgeries. Rats were mildly food restricted (i.e., fed 16–20 g per day and exhibited steady weight gain over the course of a study) starting five days prior to the beginning of each experiment. Food restriction was necessary for sucrose self-administration and included on all experiments for consistency. All behavior tests occurred during the light cycle. Twenty-three rats were excluded from analysis because of catheter failures (n=9), inability to learn self-administration (n=3), resistance to extinction (n=4), and virus localization (n=7).
Drugs
CNO was obtained from the NIH as part of the Rapid Access to Investigative Drug Program funded by the National Institute of Neurological Disorders and Stroke (Bethesda, MD, USA). CNO was administered intraperitoneally (ip) in a volume of 1 mL/kg at doses of 5 mg/kg (in 6% DMSO/sterile water) or 10 mg/kg (in 12% DMSO/sterile water). These doses of CNO were based on prior studies which demonstrated that CNO has no off-target effects on cue-induced or cocaine-primed reinstatement (Kerstetter et al., 2016; Mahler et al., 2014). The concentration of DMSO used in the vehicle corresponded to the maximum dose of CNO used in an experiment. Injections of vehicle and CNO were performed in a counterbalanced manner for all experiments and were administered 30 min prior to behavior testing. Cocaine HCl (obtained from National Institute of Drug Addiction, Bethesda, MD, USA) was dissolved in sterile 0.9% saline. Gentamycin (1 mg/kg IV), sodium brevital (10 mg/mL IV), Baytril (20–25 mg/kg SC), meloxicam (0.2 mg/kg SC), beuthanasia, and isoflurane (2–4%, inhaled) were dissolved in 0.9% sterile saline (all obtained from Patterson Veterinary, Columbus, OH, USA).
Viral vectors
Non-Cre recombinase (Cre-) dependent (AAV5-hSYN-hM4Di-mCherry) and Cre-dependent (AAV5-hSYN-DIO-hM4Di-mCherry) hM4Di-DREADD viral vectors were packaged in adenoassociated virus (AAV) serotype 5 at the University of North Carolina viral vector core (Chapel Hill, NC, USA) with a titer of ~1 × 109 viral genomes/μL. Canine adenovirus expressing Cre (CAV2-Cre) had a titer of ~2.5 × 109 viral genomes/μl and was prepared as previously described (Kremer et al., 2000).
Surgical techniques
Rats were anesthetized with isoflurane during all surgical procedures, and rats received an injection of meloxicam prior to surgery for analgesia. Rats were monitored for at least 3 days following each surgical procedure. Using standard stereotaxic procedures, viruses were injected into MTN, NAc (targeting both core and shell) and/or BLA via 33-gauge needles attached to gas-tight syringes (Hamilton Company, Reno, NV, USA) were placed above the region of interest. The following stereotaxic coordinates relative to Bregma (in mm) were used for virus injections (presented as brain region, anterior/posterior, medial/lateral, dorsal/ventral, virus, injection volume): MTN, −2.5, ±0.5, −6, hM4Di, 0.5μL; NAc, +1.8, ±1.4, −7.5, CAV2-Cre, 0.5μL; BLA, −2.3, ±4.8, −8, CAV2-Cre, 0.3μL. All viruses were infused over 1 min, and needles were left for an additional 5 minutes to allow for diffusion. All rats received hM4Di injections into MTN to allow for a within-subject experimental design. Previous studies in our lab and others have demonstrated no off-target effects of CNO in a variety of behaviors and at a range of doses, including those used in these studies (Ferguson et al. 2010; Ferguson et al., 2013; Kerstetter et al., 2016; Mahler et al., 2014). Behavioral testing with CNO occurred at least 28 days following viral infusion to allow for adequate viral expression in cell bodies and terminal regions.
For cocaine self-administration experiments, chronic indwelling catheters were placed into the right jugular vein and attached to a back-mounted port, as previously described (Crombag et al., 2000). Stainless steel caps were attached to the back ports to prevent damage due to pair-housing. Catheters were flushed daily with gentamycin, and rats received prophylactic injections of Baytril for at least 5 days post-surgery.
Cocaine self-administration
Self-administration chambers
Behavioral training and testing was performed in standard operant self-administration chambers equipped with retractable levers and stimulus lights located on the front wall (Med Associates, Fairfax, VT, USA). The back wall contained a white house light. A syringe pump, located outside of the box, delivered cocaine via tubing attached to a swivel and the catheter backport. All tubing was attached to a suspended swivel, which allowed rats to move freely within the chambers.
Cocaine self-administration: FR training
At least 3 days following catheter implantation, rats were trained to lever press for cocaine on an FR1 schedule of reinforcement. Rats received at least 5 training sessions and continued training until they met criterion (10 active lever presses in less than 2h; training amounts ranged from 5–10 sessions). The beginning of the session was indicated by insertion of the two levers into the chamber. Completion of the FR on the active lever resulted in a single cocaine infusion (0.4 mg/kg/infusion in 50 μL over 2.8 s) and illumination of the stimulus light above the active lever (4 s). Responses on the inactive lever had no programmed consequences (i.e., no cocaine and no light). Additional presses on the active lever during the cue light presentation were recorded, but did not yield any additional cocaine (i.e., 4 s time-out). The location of the active lever was counterbalanced across rats. The session ended after 2 h or when rats received 20 cocaine infusions, whichever came first.
Intermittent access to cocaine self-administration
Following completion of FR training, rats underwent 14 sessions of intermittent access to cocaine (Zimmer et al., 2012). During each session, rats were given periods of 5 min access to cocaine followed by 25 min time-out periods where the levers were retracted and cocaine was not available. This pattern repeated for a total of 125 min (5 cocaine-available periods and 4 cocaine-unavailable periods). Completion of an FR1 during the cocaine-available periods resulted in a single cocaine infusion (0.4 mg/kg/infusion in 50 μl over 2.8 s) and illumination of the stimulus light above the active lever (4 s).
Extinction
Rats next underwent extinction training. During these 2 h sessions, responses on the active lever had no programmed consequences (i.e., no cocaine delivery or illumination of the cue light). Extinction training continued for at least 7 sessions and until rats pressed the active lever less than 25 times for two consecutive days. The duration of extinction training ranged from 7–16 sessions.
Cue-induced reinstatement of cocaine-seeking
After rats reached extinction criteria, they underwent multiple tests of cue-induced reinstatement. For each test, rats were connected to the infusion line and placed into the chamber. The 2 h session began with the extension of the levers into the chamber and illumination of the cue light previously paired with cocaine for 10s. After the initial presentation of the cue light, each active lever press yielded in illumination of the cue light for 4 s. Responses on each lever were recorded during the cue-light presentation. Rats underwent additional extinction training between the reinstatement sessions until they met criteria (2 consecutive days of less than 25 lever presses). An initial group of rats (n=5; Figure 1D) underwent 4 reinstatement tests in order to determine whether cue-induced reinstatement of cocaine-seeking decreased across sessions. In the experiment assessing the effect of decreasing MTN activity, rats underwent 3 reinstatement tests. Thirty min prior to the test sessions, rats received injections of vehicle (VEH) or CNO (5 or 10 mg/kg, ip). The order of drug pre-treatment was counterbalanced across rats. For all other DREADD experiments, rats underwent 2 reinstatements tests where they received vehicle or CNO (5 mg/kg, ip) counterbalanced across rats 30 min prior to the test session.
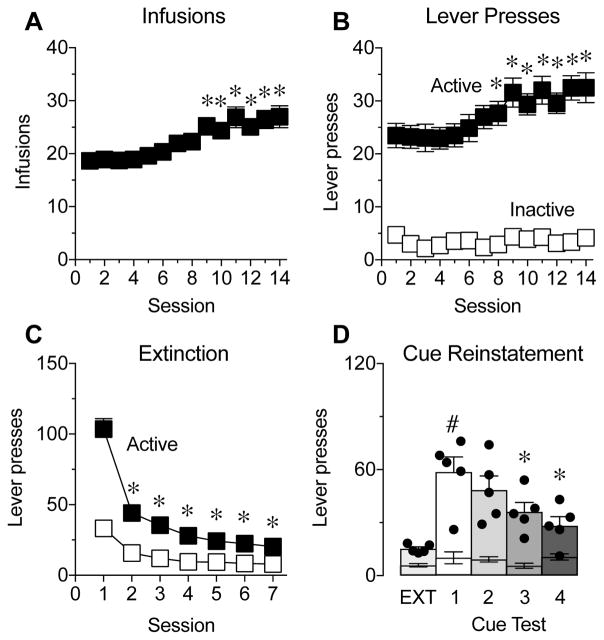
Figure 1. Intermittent access to cocaine self-administration leads to escalation of cocaine intake.
(A,B) Number of infusions earned (A) and active (black squares) and inactive (white squares) lever presses (B) during 14 sessions of intermittent access to cocaine self-administration (N=38; data is pooled from all of the DREADD cocaine self-administration experiments). *P<0.05 compared to session 1, Dunnett’s multiple comparisons test. (C) Number of active (black squares) and inactive (white squares) lever presses during the first 7 days of extinction (EXT). *P<0.05 compared to session 1, Dunnett’s multiple comparisons test. (D) Active (bars and dots) and inactive (lower error bars) lever presses during extinction (Data averaged across extinction sessions before and between the cue tests) and 4 cue-induced reinstatement tests (N=5). #P<0.05 compared to EXT, Sidak multiple comparisons test; *P<0.05 compared to cue test 1, Dunnett’s multiple comparisons test. Data represent mean ± SEM.
Cocaine-primed reinstatement of cocaine-seeking
After rats underwent their last cue-induced reinstatement test and met extinction criteria, they underwent two drug-primed reinstatement tests. We have previously found that cocaine-primed reinstatement of cocaine-seeking is maintained across two reinstatement sessions (Kerstetter et al., 2015). Thirty min prior to each test session, rats received injections of vehicle or CNO (5 mg/kg, ip). The order of drug pre-treatment was counterbalanced across rats. Rats then received a single injection of cocaine (10 mg/kg, ip) prior to placement into the operant chamber. The rats were connected to the infusion line and the 2 h session began with the extension of the levers into the chamber. Active and inactive lever presses were recorded, but had no programmed consequences (i.e., the cue light associated with the active lever did not illuminate). Rats underwent additional extinction training between the reinstatement sessions until they met criteria (2 consecutive days of less than 25 lever presses).
Sucrose self-administration, extinction and cue-induced reinstatement of sucrose-seeking
Sucrose self-administration was similar to FR training for cocaine self-administration. At 5 days following viral infusions, rats were trained, during their light cycle, to lever press for sucrose pellets (Bio-Serv, Flemington, NJ, USA) on an FR1 schedule. After 3–4 days of training, rats underwent 2 weeks of sucrose self-administration. The beginning of the session was indicated by insertion of the two levers into the chamber, which remained extended for the duration of the 1 h session. An intermittent access schedule was not used for this experiment in order to ensure sufficient acquisition of the operant response. There was no limit to the number of sucrose pellets rats could earn during each session. Responses on the active lever resulted in delivery of a single pellet and illumination of the stimulus light above the active lever for 4 sec. Responses on the inactive lever had no programmed consequences (i.e., no sucrose pellet and no light). Additional presses on the active lever during the cue light presentation were recorded, but did not yield additional sucrose pellets. The location of the active lever was counterbalanced across rats. Rats then underwent extinction training for at least 4 days and until rats pressed the active lever less than 25 times for 2 consecutive days. The duration of extinction training ranged from 4–8 sessions. Cue-induced reinstatement sessions were identical to those described for reinstatement of cocaine-seeking, except the sessions were 1h.
Locomotor Activity
Three days after completion of the sucrose experiment, those same rats received an injection of VEH or CNO (5 mg/kg, ip). Thirty minutes later, they were placed into locomotor activity boxes (San Diego Instruments, San Diego, CA, USA), and behavior was measured for 60 min. In a separate experiment, naïve rats (n=10) received an injection of VEH or CNO (5 mg/kg, ip). Thirty minutes later, these rats received an injection of cocaine (15 mg/kg, ip) and were placed into locomotor activity boxes for 60 min. The number of crossovers (defined as two consecutive beam breaks) was used as an index of locomotor activity.
Immuohistochemistry
Injection accuracy was confirmed by visualization of mCherry immunofluorescence in targeted brain regions. Rats were anesthetized with Beuthanasia-D and transcardially perfused with PBS (pH 7.4) followed by 4% paraformaldehyde. Brains were removed and post-fixed overnight, then sliced into 60 μm sections on a vibrating microtome. Immunohistochemical analysis of mCherry-tagged hM4Di was performed as described (Kerstetter et al., 2016). Briefly, floating sections were washed in 0.5% Triton-X/PBS for 10 min, blocked in 0.25% Triton-X/5% normal goat serum (NGS)/PBS for 2 h, and incubated in 0.25% Triton-X/2.5% NGS/PBS containing an antibody to mCherry (1:400, rabbit host; Takara Bio USA, Mountain View, CA, USA, product #632496) with gentle agitation at room temperature overnight. Next, sections were rinsed 4 times in PBS and incubated in anti-rabbit Alexa 568 (red)-conjugated secondary antibody (1:400; Thermo Fisher Scientific, product #A-11036) for 2 h. Sections were then washed 2 times in PBS, mounted on slides, and cover-slipped with Vectashield mounting medium with DAPI. Z-stack images (12 steps spaced evenly throughout the 60 μm slices at either 10× or 40× magnification) were taken of mounted brain sections, captured using a Zeiss LSM 710 Confocal microscope, and processed using ImageJ software (NIH). For experiments with non-specific DREADD expression throughout MTN (Figures 2–3), mCherry expression had to be detected in at least 25 cells in each of the MTN (ie. mCherry expression in all 4 regions analyzed: PVT, IMD, MD, and CM). Furthermore, for output-specific studies (figures 4–5), rats had to have mCherry expression in axons/terminals of the output regions (i.e., NAc or BLA), as well as expression of mCherry in at least 25 cell bodies across PVT, IMD, and CM. If expression did not meet these criteria for any experiment, rats were excluded from the analysis.
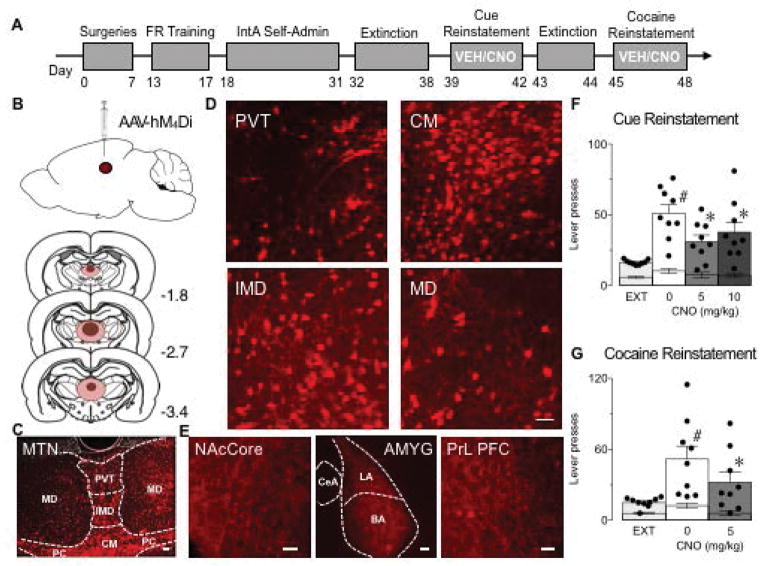
Figure 2. Reducing activity of MTN attenuates cue-induced and drug-primed reinstatement of cocaine-seeking.
(A) Illustration of the experimental design. Injections of vehicle (VEH) or clozapine-N-oxide (CNO) occurred at least 39 d post viral-infusion. (B) Top: Illustration of the viral vector targeting approach. An AAV vector expressing hM4Di (AAV-hM4Di) was injected into midline thalamic nuclei (MTN). Bottom: Illustration of viral spread in MTN. Dark red indicates robust expression in all rats and pink indicates areas of weaker expression and/or expression in a subset of the rats. The numbers next to each section indicate location in anterior-posterior plane relative to Bregma. (C,D) Representative sections of mCherry-tagged hM4Di immunofluorescence in MTN; paraventricular (PVT), centromedian (CM), interomediodorsal (IMD), mediodorsal (MD), and paracentral (PC) nuclei of the thalamus. (E) Representative sections of mCherry-tagged hM4Di immunofluorescence in MTN terminals and/or axons in nucleus accumbens core (NAcCore), lateral (LA) and basolateral (BLA) but not central (CeA) subregions of the amygdala (AMYG) and the prelimbic (PrL) subregion of the prefrontal cortex (PFC) 8–10 weeks following viral infusions. Scale bars, 50 μm. (F) Active (bars and dots) and inactive (lower error bars) lever presses during extinction (EXT) and cue-induced reinstatement of cocaine-seeking following VEH (0 mg/kg) or CNO treatment (5 or 10 mg/kg). #P<0.05 compared to EXT, Sidak multiple comparisons test; *P<0.05 compared to 0 mg/kg CNO, Dunnett’s multiple comparisons test. (G) Active (bars and dots) and inactive (lower error bars) lever presses during EXT and cocaine-primed reinstatement of cocaine-seeking following vehicle (0 mg/kg) or CNO treatment (5 mg/kg). #P<0.05 compared to EXT, Sidak multiple comparisons test; *P<0.05 compared to 0 mg/kg CNO, Sidak multiple comparisons test. N=9. Data represent mean ± SEM.
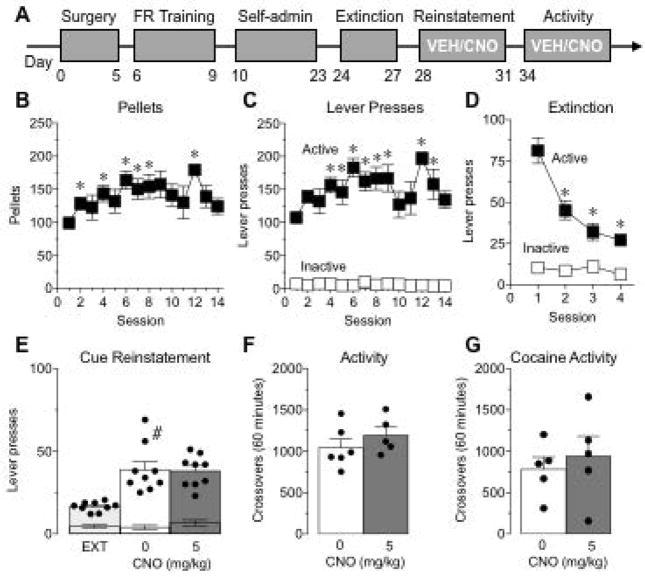
Figure 3. Reducing activity of MTN has no effect on cue-induced reinstatement of sucrose-seeking.
(A) Illustration of the experimental design. CNO testing occurred at least 28 d post viral-infusion. (B,C) Number of pellets earned (B) and active (black squares) and inactive (white squares) lever presses (C) during 14 sessions of sucrose self-administration (N=9). *P<0.05, compared to session 1, Dunnett’s multiple comparisons test. (D) Number of active (black squares) and inactive (white squares) lever presses during the first 4 days of extinction. *P<0.05, compared to session 1, Dunnett’s multiple comparisons test. (E) Active (bars and dots) and inactive (lower error bars) lever presses during extinction (EXT) and cue-induced reinstatement of sucrose-seeking following vehicle (0 mg/kg) or CNO (5 mg/kg) treatment. #P<0.05 compared to EXT, Sidak multiple comparisons test. (F,G) Total number of beam breaks during a baseline (F) or cocaine-induced (G; 15mg/kg) locomotor activity test session following vehicle or CNO (5 mg/kg) treatment. N=5–6/group. Data represent mean ± SEM.
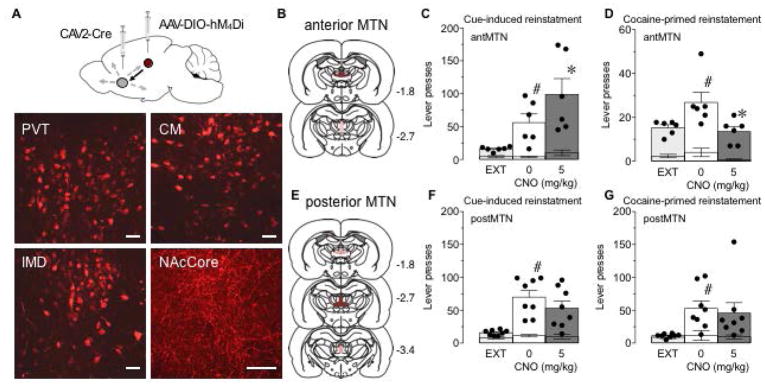
Figure 4. Reducing activity of anterior MTN projections to NAc enhances cue-induced reinstatement but blocks cocaine-primed reinstatement of cocaine-seeking.
(A) Top: Illustration of the intersectional viral vector approach. A retrogradely transported CAV2 expressing Cre-recombinase (CAV2-Cre) was injected into the NAc, and a Cre-dependent AAV expressing hM4Di (AAV-DIO-hM4Di) was injected into MTN. Bottom: Representative sections of mCherry-tagged hM4Di immunofluorescence in PVT, CM, IMD and NAc core. Scale bars, 50 μm. (B,E) Viral spread in anterior MTN group (B) and posterior MTN group (E). Dark red indicates robust expression in all rats and pink indicates areas of weaker expression and/or expression in a subset of the rats. The numbers next to each section indicate location in anterior-posterior plane relative to Bregma. (C,D) Active (bars and dots) and inactive (lower error bars) lever presses during extinction (EXT) and cue-induced (C) and cocaine-primed (D) reinstatement of cocaine-seeking following vehicle (0 mg/kg) or CNO treatment (5 mg/kg) in rats that had hM4Di expression in anterior MTN. #P<0.05 compared to EXT and *P<0.05 compared to 0 mg/kg CNO; Sidak’s multiple comparisons test. N=6. (F,G) Active (bars and dots) and inactive (lower error bars) lever presses during EXT and cue-induced (F) and cocaine-primed (G) reinstatement of cocaine-seeking following vehicle (0 mg/kg) or CNO treatment (5 mg/kg) in rats that had hM4Di expression in posterior MTN. #P<0.05 compared to EXT, Sidak multiple comparisons test. N=8. Data represent mean ± SEM.
Figure 5. Reducing activity of MTN projections to BLA has no effect on reinstatement of cocaine-seeking.
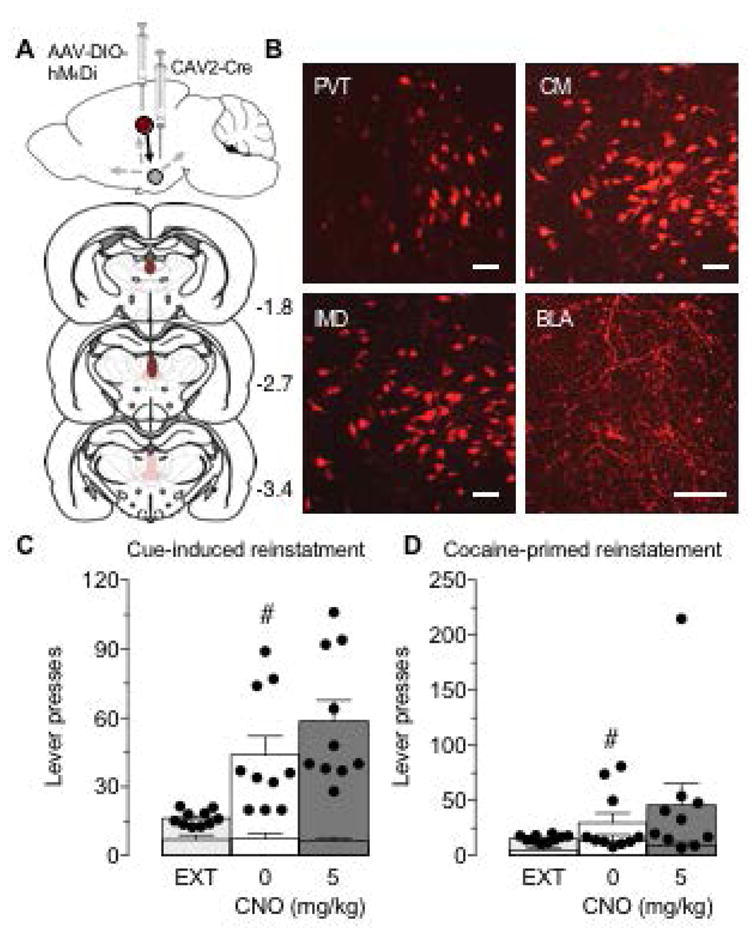
(A) Top: Illustration of the intersectional viral vector approach. A retrogradely transported CAV2 expressing Cre-recombinase (CAV2-Cre) was injected into the BLA, and a Cre-dependent AAV expressing hM4Di (AAV-DIO-hM4Di) was injected into MTN. Bottom: Viral spread in MTN. Dark red indicates robust expression in all rats and pink indicates areas of weaker expression and/or expression in a subset of the rats. The numbers next to each section indicate location in anterior-posterior plane relative to Bregma. (B) Representative sections of mCherry-tagged hM4Di immunofluorescence in PVT, CM, IMD and BLA. Scale bars, 50 μm. (C,D) Active (bars and dots) and inactive (lower error bars) lever presses during extinction (EXT) and cue-induced (C) and drug-primed (D) reinstatement of cocaine-seeking following vehicle (0 mg/kg) or CNO treatment (5 mg/kg). #P<0.05 compared to EXT, Sidak multiple comparisons test. N=10. Data represent mean ± SEM.
Data Analyses
All statistical analyses were determined a priori and performed with Prism 6 (Graphpad Software Inc, La Jolla, CA, USA). Earned infusions or pellets were analyzed with one-way repeated measures ANOVA. During self-administration and extinction, active and inactive lever presses during each session were analyzed with two-way repeated measures ANOVA (lever × session). Multiple two-way repeated measures ANOVA were determined a priori and carried out on cue-induced reinstatement of cocaine-seeking, drug-primed reinstatement, and cue-induced reinstatement of sucrose seeking data. First, to determine the effects of cue-exposure or a priming injection of cocaine on lever pressing, active and inactive lever presses during extinction and reinstatement (vehicle condition in all DREADD studies and session 1 for figure 1; phase) were tested with two-way repeated measures ANOVA (phase × lever). We found no significant differences in active or inactive lever presses during the extinction sessions preceding the vehicle compared to CNO injections in either the cue-induced or cocaine-primed reinstatement experiments (data not shown). Therefore, the active and inactive lever presses preceding vehicle and CNO injections were averaged. Second, to determine the effects of CNO (or three cue exposures) on lever pressing during reinstatement, active and inactive lever presses following vehicle and CNO (treatment) were assessed using two-way repeated measures ANOVA (treatment × lever). The effects of housing condition (pair- or single-housed) on lever pressing were tested with mixed two-way ANOVA (between-subjects housing condition × RM lever ANOVA). Locomotor activity was tested with an unpaired t-test. All ANOVA analyses were followed by Dunnett’s or Sidak’s multiple comparisons tests, as appropriate. For all comparisons, α ≤ 0.05. Data are graphed as mean ± SEM.
RESULTS
Intermittent access to cocaine self-administration leads to escalation of cocaine intake
All of the rats for the cocaine self-administration DREADD experiments underwent the same intermittent access to self-administration and extinction procedures prior to testing, and the data are pooled and shown in Figure 1A–C (n=38). A summary of the experimental design is shown in Figure 2A. Two weeks of intermittent access to cocaine leads to a significant increase in the total number of earned cocaine infusions (Fig. 1A; F(13,37)=11.25, P<0.0001) and total number of active lever presses (Fig. 1B; main effect of session F(13,481)=7.37, P<0.0001; main effect of lever F(1,37)=156.4, P<0.0001; lever × session interaction F(13,481)=6.45, P<0.0001), suggesting that this schedule is sufficient to produce rats that exhibit escalation of intake, which is a major hallmark of addiction-like behavior.
During extinction training, rats that underwent intermittent access to cocaine showed decreased responding across sessions (Fig. 1C; main effect of session F(6,222)=90.35, P<0.0001; main effect of lever F(1,37)=236.8, P<0.0001; lever × session interaction F(6,222)=74.53, P<0.0001). Following extinction, an initial group of rats (n=5) underwent 4 cue-induced reinstatement tests to assess the ability of a cue to reinstate cocaine-seeking across multiple sessions. As expected, rats exhibited robust reinstatement of active lever pressing during the first cue-induced reinstatement compared to extinction (Fig. 1D; main effect of phase F(1,4)=27.74, P=0.006; main effect of lever F(1,4)=37.87, P<0.004; phase × lever interaction F(1,4)=22.5, P=0.009). In addition, although active lever pressing decreased over the four cue-induced reinstatement sessions (Fig. 1D; main effect of session F(3,12)=3.77, P=0.04; main effect of lever F(1,4)=59.35, P=0.002; session × lever interaction F(3,12)=4.71, P=0.02), there were no significant differences in lever pressing across the first two reinstatement tests. These results support the use of a within-subjects cue-induced reinstatement design for the DREADD experiments. However, because of the trend toward a decrease in lever responses across sessions, vehicle and CNO injections were also counterbalanced across test sessions.
The majority of cocaine self-administration experiments are conducted in single-housed rats. Given that our animals were pair-housed, we compared self-administration behavior of pair-housed rats to single-housed rats. There were no significant differences between groups in the total number of earned cocaine infusions (Fig. S1A, no main effect of housing F(1,42)=1.49, P=0.23 and no session × housing interaction F(13,546)=0.17, P=1.00), total number of active lever presses (Fig. S1B; no main effect of housing F(1,42)=1.68, P=0.20 and no session × housing interaction F(13,546)=0.18, P=1.00) and total number of inactive lever presses (Fig. S1B; no main effect of housing F(1,42)=1.72, P=0.20 and no session × housing interaction F(13,546)=0.30, P=0.99).
In addition, there was no effect of housing condition on the total number of active lever presses (Fig. S1C; no main effect of housing F(1,42)=0.39, P=0.54 and no session × housing interaction F(6,252)=0.34, P=0.92) or inactive lever presses (Fig. S1C; no main effect of housing F(1,42)=1.86, P=0.18 and no session × housing interaction F(6,252)=1.8, P=0.1) during extinction. Finally, housing condition had no effect on active lever presses (Fig. S1D; no main effect of housing F(1,42)=0.28, P=0.60 and no session × housing interaction F(1,42)=0.2, P=0.66) or inactive lever presses (Fig. S1D; no main effect of housing F(1,42)=1.09, P=0.30 and no session × housing interaction F(1,42)=0.19, P=0.66) during cue-induced reinstatement. Therefore, pair-housing of rats represents an advantageous alternative to individual housing without hindering the ability to compare results to previous studies.
Reducing activity of MTN attenuates drug-seeking
To examine the effect of altering thalamic activity on cue-induced and cocaine-primed reinstatement of cocaine seeking, we injected an AAV expressing hM4Di into PVT (Fig. 2B). However, upon assessing viral spread with immunohistochemistry of the mCherry-tag on hM4Di, fluorescence was evident not only in the PVT, but also IMD, CM and MD (Fig. 2B–E); thus, we refer to our injections as targeted to MTN. In addition, we assessed mCherry fluorescence in MTN terminal regions throughout the cortico-basal ganglia-thalamic circuit. Consistent with anatomical tracing studies (Berendse and Groenewegen, 1990; Groenewegen et al., 1990; Li and Kirouac, 2011; Pinto et al., 2003; Su and Bentivoglio, 1990; Vertes and Hoover, 2008), high levels of mCherry fluorescence were observed throughout the rostral-caudal gradient of the NAc core, NAc shell, the BLA and lateral nucleus of the amygdala, and the cingulate, infralimbic and prelimbic regions of the prefrontal cortex (PFC) (Fig. 2E). Images shown for NAc and PFC in Fig. 2E are representative of the fluorescence observed in each sub-region of these areas, although expression is only shown for the NAc core and prelimbic region of PFC.
Following cocaine self-administration and extinction (data included in Fig. 1A–C), the role of MTN was assessed during cue-induced reinstatement of cocaine-seeking. Rats (n=9) received counterbalanced injections of VEH and CNO (5 and 10 mg/kg, ip) 30 min prior to three reinstatement tests. CNO-induced activation of hM4Di in PVT neurons has been shown to reduce excitatory post-synaptic currents in downstream NAc neurons, confirming that this method reduces thalamic activity (Zhu et al., 2016). As expected, rats pressed the active lever significantly more during the cue-induced reinstatement session following VEH compared to pressing during extinction (Fig. 2F; main effect of phase F(1,8)=37.62, P=0.0003; main effect of lever F(1,8)=94.54, P<0.0001; phase × lever interaction F(1,8)=33.48, P=0.0004). Reducing activity of MTN following either dose of CNO significantly attenuated lever pressing (Fig. 2F; main effect of treatment F(2,16)=4.11, P=0.04; main effect of lever F(1,8)=103.4, P<0.0001; no treatment × lever interaction F(2, 16)=2.70, P=0.097). This effect was due to a reduction in pressing on the active lever (Fig. 2F; 5 mg/kg CNO, P=0.003; 10 mg/kg CNO, P=0.04) and not the inactive lever (5 mg/kg CNO, P=0.83; 10 mg/kg CNO, P=0.80), suggesting the manipulation was effective at reducing cue-induced reinstatement of cocaine-seeking. Because we observed a similar reduction in active lever pressing following 5 and 10 mg/kg CNO, only the 5 mg/kg dose was used for subsequent experiments.
We then tested whether MTN also regulate drug-primed reinstatement of cocaine-seeking. Rats received counterbalanced injections of VEH or CNO (5 mg/kg, ip) 30 min prior to a cocaine injection (10 mg/kg, ip). When rats received VEH injections prior to the cocaine prime, they showed significantly higher levels of lever pressing on the active lever compared to extinction (Fig. 2G; main effect of phase F(1,8)=14.6, P=0.005; main effect of lever F(1,8)=20.84, P=0.002; phase × lever interaction F(1,8)=14.55, P=0.01;), which verifies that cocaine induces reinstatement following intermittent access to cocaine during self-administration and extinction in pair-housed rats. As with cue-induced reinstatement, activation of hM4Di in MTN significantly attenuated active lever pressing following a priming injection of cocaine (Fig. 2G; main effect of treatment F(1,8)=10.14, P=0.01; main effect of lever F(1,8)=18.8, P=0.003; no lever × treatment interaction F(1,8)=2.85, P=0.13) without altering inactive lever pressing (P>0.05), suggesting that reducing activity of MTN decreases cocaine-seeking to a drug prime.
It is possible that MTN regulate motivation for cues previously paired with any type of reward. To test this, a separate cohort of rats was trained to self-administer sucrose; a summary of the experimental design is show in Fig. 3A. Viral expression patterns were the same as in Fig. 2B–E. Rats (n=9) readily learned to self-administer sucrose pellets (Fig. 3B,C; pellets: F(13,8)=4.56, P<0.02; lever pressing: main effect of lever F(1,8)=145.8, P<0.0001; main effect of session F(13,104)=3.77, P<0.001; lever × session interaction F(13,104)=3.83, P<0.0001). Following completion of sucrose self-administration, rats underwent extinction training. Rats rapidly extinguished responding across sessions (Fig. 3D; main effect of session F(3,24)=31.34, P<0.0001; main effect of lever F(1,8)=81.46, P<0.0001; lever × session interaction F(3,24)=32.43, P<0.0001). Rats were then given injections of VEH or CNO (5 mg/kg, ip) in a counterbalanced order 30 min prior to tests for cue-induced reinstatement of sucrose-seeking. Following VEH, there was a significant increase in active lever pressing compared to pressing during extinction (Fig. 3E; main effect of phase F(1,8)=16.3, P=0.004; main effect of lever F(1,8)=73.77, P<0.0001; phase × lever interaction, F(1,8)=27.03, P=0.0008). However, decreasing activity of MTN had no effect on lever pressing (Fig. 3D; main effect of lever F(1,8)=82.02, P<0.0001, no main effect of treatment, F(1,8)=0.10, P=0.76, no lever × treatment interaction, F(1,8)=0.73, P=0.42), suggesting that MTN do not modulate cue-induced seeking to all palatable rewards.
Finally, in order to determine whether the decrease in active lever pressing following reinstatement of drug-seeking could be due to alterations in motor behavior, three days following reinstatement of sucrose-seeking, the rats were given either VEH (n=6) or CNO (5 mg/kg, ip; n=5) prior to placement in locomotor activity boxes. Decreasing activity of MTN had no effect on the number of crossovers made during a 1 h test session (Fig. 3F; t(9)=1.01, P=0.34). In addition, given a recent report that CNO can have locomotor effects in control animals that do not express DREADDs (MacLaren et al., 2016), the effect of CNO (5mg/kg, ip) was assessed on cocaine (15 mg/kg, ip)-induced locomotor activity in a naïve group of animals. CNO administration had no effect the number of crossovers induced by cocaine in a 1 h test session (Fig. 3G; t(8)=0.54, P=0.60). Taken together, these results suggest that MTN regulate drug-seeking responses to cocaine and associated cues, without altering general motivation or locomotor activity.
Reducing activity of anterior MTN-NAc enhances cue-induced reinstatement and blocks cocaine-primed reinstatement
Given that glutamatergic signaling within the NAc regulates both cue-induced and drug-primed reinstatement, and MTN are a dense source of NAc glutamate, we examined whether MTN-NAc projections were responsible for the attenuation in cocaine-seeking observed with the non-selective approach (Frassoni et al., 1997; Lei et al. 2013; McFarland et al., 2003; Park et al. 2002). The experimental design was the same as for the MTN manipulation (Fig. 2A) except rats were only tested for one dose of CNO on the cue-induced reinstatement test. Rats (n=14) received injections of a retrograde virus CAV2-Cre into NAc and a Cre-dependent AAV expressing hM4Di into MTN (Fig. 4A). This combinatorial technique resulted in robust expression of mCherry fluorescence in cell bodies of PVT, CM, and IMD as well as in axons/terminals in NAc (Fig. 4A). The pattern of fluorescence expression was more restricted than that seen with the non-selective approach (Fig. 2B–E), and we did not observe fluorescence in axons/terminals within the BLA, PFC, or dorsal striatum (data not shown). For behavioral analysis, rats were split into two groups based on whether mCherry fluorescence occurred in anterior (Fig. 4B) or posterior (Fig. 4E) MTN.
Following cocaine self-administration and extinction (data included in Fig. 1A–C), the effect of transiently decreasing activity of anterior MTN-NAc projections was examined in reinstatement of cocaine-seeking. Although cue-induced reinstatement was observed following VEH (Fig. 4C; main effect of phase F(1,5)=9.43, P=0.03; main effect of lever F(1,5)=26.08, P=0.004; phase × lever interaction F(1,5)=10.60, P=0.02), decreasing activity of anterior MTN-NAc significantly enhanced active lever pressing for cocaine-associated cues without affecting inactive lever pressing (Fig. 4C; main effect of lever F(1,5)=21.16, P=0.006; main effect of treatment not quite significant, F(1,5)=5.53, P=0.065; treatment × lever interaction F(1,5)=7.46, P=0.04). In addition, active lever pressing was significantly increased when VEH preceded a priming injection of cocaine (Fig. 4D; main effect of phase F(1,5)=8.98, P=0.03; main effect of lever F(1,5)=37.95, P=0.002; no phase × lever interaction, F(1,5)=3.62, P=0.12), but attenuating activity of anterior MTN-NAc significantly decreased active lever pressing without altering inactive lever pressing (Fig. 4D; main effect of treatment F(1,5)=12.56, P=0.02; main effect of lever F(1,5)=35.42, P=0.002; no treatment × lever interaction F(1,5)=2.88, P=0.15), suggesting that cocaine-primed reinstatement was blocked.
In contrast to the bidirectional effects of hM4Di activation in anterior MTN-NAc on relapse behaviors, dampening activity of posterior MTN-NAc (Fig. 4E) had no effect on either cue-induced (Fig. 4F; no main effect of treatment F(1,7)=0.90, P=0.37; main effect of lever, F(1,7)=79.27, P<0.0001; no treatment × lever interaction F(1,7)=1.55, P=0.25) or cocaine-primed reinstatement (Fig. 4G; no main effect of treatment F(1,7)=0.27, P=0.62; main effect of lever, F(1,7)=8.62, P=0.02; no treatment × lever interaction F(1,7)=0.15, P=0.71).
MTN-BLA do not regulate reinstatement of cocaine-seeking
Given that the amygdala has been shown to regulate both cue-induced and cocaine plus cue-primed reinstatement, and we observed bright labeling of mCherry fluorescence in both the BLA following the non-selective MTN manipulation (Fig. 2E), we next wanted to determine whether MTN-BLA efferents also regulate relapse behavior (Kantak et al., 2002; Bossert et al. 2013; Lee et al., 2013; Stefanik & Kalivas, 2013; Yager et al., 2015). Using the same combinatorial approach as with MTN-NAc (except the CAV2-Cre was injected into BLA rather than NAc), we expressed mCherry-tagged hM4Di selectively in MTN-BLA neurons (n=10) (Fig. 5A,B). We observed expression of mCherry fluorescence in PVT, CM, and IM, as well as in axons/terminals in the BLA (Fig. 5B). We did not detect expression of mCherry fluorescence in axons/terminals in central amygdala (data not shown, but pattern similar to Figure 2E). Following cocaine self-administration and extinction (data included in Fig. 1A–C), the effect of transiently decreasing activity of MTN-BLA projections was examined in reinstatement of cocaine-seeking. We found that reducing activity of these projections had no effect on either cue-induced (Fig. 5C; significant main effect of lever, F(1,9)=55.17, P<0.0001, no main effect of treatment, F(1,9)=1.49, P<0.25, no treatment × lever interaction, F(1,9)=2.19, P=0.17) or cocaine-primed (Fig. 5D; significant main effect of lever, F(1,9)=6.86, P=0.03, no main effect of treatment, F(1,9)=0.27, P=0.62, no treatment × lever interaction, F(1,9)=0.97, P=0.35) reinstatement of cocaine-seeking. Together, these data suggest that MTN regulate cocaine-seeking behaviors via their projections to NAc but not BLA.
DISCUSSION
We utilized an intermittent access to cocaine self-administration paradigm for these studies, which regulates the temporal dynamics of drug intake to produce a spiking pattern of brain cocaine concentration, rather than the stable levels of cocaine that occur during continuous access models (Allain et al. 2015; Kawa et al., 2016; Zimmer et al., 2012a; Zimmer et al., 2012b). Interestingly, we found that intermittent access leads to an escalation of cocaine intake after limited self-administration experience (<14 sessions). To our knowledge, this phenomenon has previously been reported in this timeframe only following extended access (ie. 6 h) to cocaine or in 1 h sessions if preceded by several shorter training sessions, and is consistent with a recent report of escalation after prolonged (36 sessions) intermittent access to cocaine (Ahmed and Koob, 1998; Beckmann et al., 2012; Edwards and Koob, 2013; Kawa et al., 2016). Escalation is thought to be an early indicator of increased vulnerability to relapse and is associated with enhanced reinstatement behavior (Deroche et al., 1999; Kippin et al., 2006; Mantsch et al., 2004; Vanderschuren and Everitt, 2004). Furthermore, intermittent access increases motivation for cocaine intake relative to continuous access paradigms (Zimmer et al., 2012a). Thus, intermittent access to cocaine self-administration not only mimics the temporal pattern of drug intake seen in addicts but offers a powerful preclinical model for inducing addiction-like behaviors in a relatively short period of time.
Studies have previously used lesions and pharmacological inactivation to examine the role of the PVT in relapse to drugs, so we first combined the intermittent access paradigm with chemogenetic and viral-mediated gene transfer techniques to see if similar results could be observed with DREADD-induced dampening of thalamic activity. Gi/o-coupled DREADDs were initially targeted toward the PVT, but viral expression was more diffuse than expected, extending into MD, IM, and CM nuclei. We found that transiently reducing activity of MTN during reinstatement attenuated both cue-induced and drug-primed cocaine-seeking. These results are consistent with studies that found that temporary inactivation of PVT decreased both cue-induced and cocaine-primed reinstatement of cocaine-seeking, and lesions of PVT reduced context-induced reinstatement of alcohol-seeking (Hamlin et al., 2009; James et al., 2010; Matzeu et al., 2015a). Furthermore, increases in markers of neuronal activation (i.e., cFos) in MD, IM, and PVT have been reported following exposure to visual, olfactory, or contextual cues paired with either cocaine or alcohol self-administration (Dayas et al., 2008; Hamlin et al., 2009; James et al., 2011; Marchant et al., 2010; Barson et al., 2014; Matzeu et al. 2015; Wedzony et al. 2003). Although studies have not examined the effect of altering activity of IM or CM in relapse behaviors, lesions of MD do not alter cocaine-primed or stress-induced reinstatement of drug-seeking (McFarland and Kalivas, 2001; James et al., 2011). Thus, we cannot rule out the contribution of IM and CM, in addition to the PVT, in the observed behavioral effects.
We next aimed to determine whether DREADD-mediated inhibition of MTN also attenuates seeking for natural rewards and found that this manipulation had no effect on cue-induced reinstatement of sucrose-seeking. Although these results are consistent with studies that have found no increase in neuronal activation in PVT following exposure to sucrose-paired stimuli nor an effect of PVT lesions on sucrose-seeking, other studies have shown that PVT, CM, and MD can regulate responses to cues associated with palatable food rewards (Wedzony et al., 2003; Kelley et al., 2005; Matzeu et al., 2015a; Schiltz et al., 2007; Igelstrom et al., 2010). The differences in results are likely due to methodological differences, including type of reward and operant paradigm, as well as the manipulations that were used.
While previous work, together with our study, supports a clear role for MTN in reinstatement of drug-seeking induced by cues, context and drugs, it does not resolve which set(s) of MTN efferents are critical for regulating relapse behaviors since all outputs were equally affected by the manipulations. Indeed, consistent with prior tracing studies, we examined fluorescence in regions throughout the cortico-basal ganglia circuit following hM4Di injections into MTN and found that the MTN project densely to NAc and BLA, as well as to PFC (Berendse and Groenewegen, 1990; Frassoni et al., 1997; Groenewegen et al., 1990; Li and Kirouac, 2007; Pinto et al., 2003; Su and Bentivoglio, 1990; Van der Werf et al., 2002; Vertes and Hoover, 2008). In addition, we have previously shown that activating hM4Di in PFC decreases neuronal activity in efferent projections to the NAc, and others have demonstrated hM4Di can attenuate neurotransmitter release of neurons expressing hM4Di and reduce activity of downstream neurons (Kerstetter et al., 2016; Stachniak et al., 2014; Zhu et al., 2016).
To begin to identify which target regions integrate information from the MTN during reinstatement, we used a combinatorial viral vector approach to express Gi/o-coupled DREADDs selectively in MTN-NAc. These neurons were targeted because glutamatergic signaling in NAc regulates relapse, and MTN neurons send a dense glutamatergic projection to NAc (Berendse and Groenewegen, 1990; Frassoni et al., 1997; Groenewegen et al., 1990; Li and Kirouac, 2007; McFarland et al., 2003; Park et al., 2002; Su and Bentivoglio, 1990; Yager et al. 2015). Notably, this approach led to a more restricted pattern of hM4Di expression compared to non-Cre-dependent targeting of the MTN, with fluorescence found primarily in PVT, CM, and IMD. In addition, the pattern of fluorescence varied across animals such that some had strong expression in anterior regions of MTN whereas others had strong expression in posterior regions. Given that the more anterior aspects of these nuclei project to NAc shell whereas the more central/posterior aspects project primarily to NAc core (Berendse and Groenewegen, 1990; Li and Kirouac, 2007) and that the NAc shell and core can differentially regulate reinstatement (Anderson et al., 2002; Fuchs et al., 2004; Ma et al., 2014; Schmidt et al., 2006; Schmidt et al., 2005a), we assessed the effects of dampening activity in anterior or posterior MTN-NAc independently on both cue- and drug primed-reinstatement.
Activation of Gi/o-coupled DREADDs in anterior MTN-NAc blocked reinstatement of cocaine-seeking following a priming injection of cocaine, which mirrored the results of the non-selective manipulation. Thus, activity in anterior MTN-NAc neurons is necessary to drive thalamic-mediated aspects of cocaine-primed reinstatement behavior. MTN projections to the NAc not only converge onto dendritic spines of GABAergic medium spiny projection neurons but also onto dopamine terminals (Lei et al., 2013; Pinto et al., 2003; Wall et al., 2013). Given that electrical stimulation of MTN leads to glutamate-dependent increases in dopamine release in the NAc (Parsons et al., 2006), it is likely that dampening activity of glutamatergic MTN-NAc reduced both glutamatergic and dopaminergic influences onto medium spiny neurons following the drug prime, both of which could lead to a decrease in the output of these neurons and the observed reduction of drug-primed reinstatement.
In contrast to the effect on drug-prime reinstatement, attenuating activity of anterior MTN-NAc projections enhanced cue-induced reinstatement of cocaine-seeking. Thus, these data suggest that anterior MTN-NAc are likely regulating cue-induced reinstatement via a different mechanism than for that of the drug-prime, and future studies are warranted to explore this. Nonetheless, because the anterior MTN-NAc manipulation produced a result that was opposite to that of the non-projection-specific MTN manipulation on cue-induced reinstatement, these findings suggest that the ability of MTN to regulate cue-driven relapse is driven by multiple downstream connections. Although the role of posterior MTN-NAc and MTN-BLA projections were also assessed in both cue-induced and drug-primed reinstatement, transiently decreasing activity in either of these MTN efferent projections had no effect.
The lack of effect in posterior MTN-NAc projections is consistent with a recent study that used a viral manipulation (which corresponded to the same posterior MTN region targeted in the present study to permanently block neurotransmission in PVT neurons projecting to NAc, and found no effect of this manipulation on incubation of cocaine craving, a cue-dependent form of cocaine-seeking (Neumann et al., 2016). Although it is surprising that decreasing anterior but not posterior MTN-NAc projections is sufficient to regulate drug-seeking, these results are consistent with the emerging view that even within a single structure, there are subtle differences in how sub-regions regulate relapse behaviors. For example, recent studies have demonstrated dissociable roles of prelimbic and infralimbic PFC projections to NAc core and shell, respectively, in regulation of drug-seeking (Ma et al., 2014). In addition, lesion studies have demonstrated dissociable roles of the anterior and posterior regions of BLA in reinstatement behaviors (Kantak et al., 2002).
While it is possible that the combinatorial viral vector approach resulted in an insufficient number of infected neurons to produce an effect in the posterior MTN-NAc and MTN-BLA experiments, this is unlikely as neuronal infection rates were comparable to those observed within the anterior MTN-NAc projection, and behavioral effects were observed following manipulation of that region. Thus, one likely target for MTN regulation of drug-seeking is the PFC, as the PFC showed moderate fluorescent expression following DREADD expression in MTN, and is activated by cues associated with drug use, regulates cue-induced and drug prime reinstatement, and projects to both the NAc core and shell (Ciccocioppo et al., 2001; Feil et al., 2010; Goldstein and Volkow, 2011; Kufahl et al., 2009; McFarland et al., 2003).
It should be noted that 5 mg/kg CNO in the absence of DREADD expression (the primary dose used in our experiments) has recently been found to alter acute responses to a single dose of amphetamine, suggesting that CNO can have off-target effects on behavior (MacLaren et al., 2016). However, our lab and others have demonstrated CNO has no effect on cue-induced or drug-primed reinstatement, cocaine self-administration, amphetamine sensitization, and operant food-responding in GFP-injected or no virus control rats (Ferguson et al., 2010; Ferguson et al., 2013; Kerstetter et al., 2016; Mahler et al., 2014). Within this manuscript, the lack of effect of CNO on inactive lever presses, sucrose-seeking, basal and cocaine-induced locomotor activity, and in the posterior MTN-NAc and MTN-BLA manipulations also supports the idea that 5 mg/kg CNO alone does not impact operant learning and cocaine-seeking. Thus, off-target effects of CNO are unlikely to account for the effects of hM4Di activation in MTN and MTN-NAc during reinstatement described here.
In summary, this research not only confirms that MTN regulate both cue-induced and cocaine-primed reinstatement of cocaine-seeking, but extends previous work by beginning to define the downstream projections of MTN that are important targets for this modulation. In particular, MTN neurons projecting to the NAc shell seem to be especially critical for driving reinstatement behaviors. It has recently been argued that MTN should be included in maps of the addiction circuit and our findings strongly support that notion (James and Dayas, 2013; Martin-Fardon and Boutrel, 2012). Given that relapse can be triggered by drug-associated stimuli and relapse rates remain high, this work has important clinical implications as it suggests that MTN are a promising, novel therapeutic target.
Supplementary Material
Acknowledgments
This work was supported by US NIH grant R01 DA036582 (SMF).
ABBREVIATIONS
- AAV
adenoassociated virus
- AMYG
amygdala
- BLA
basolateral amygdala
- CeA
central amygdala
- CM
centromedian nucleus of the thalamus
- CNO
clozapine-N-oxide
- DREADD
Designer Receptors Exclusively Activated by Designer Drugs
- EXT
extinction
- hM4Di
Gi/o-coupled, inhibitory DREADD
- IM
intermediodorsal nucleus of the thalamus
- LA
lateral amygdala
- MD
mediodorsal nucleus of the thalamus
- MTN
midline and intralaminar nuclei of the thalamus
- MTN-BLA
midline thalamic nuclei neurons projecting to basolateral amygdala
- MTN-NAc
midline thalamic nuclei neurons projecting to nucleus accumbens
- NAc
nucleus accumbens
- NAcCo
nucleus accumbens core
- PC
paracentral nucleus of the thalamus
- PFC
prefrontal cortex
- PrL
prelimbic cortex
- PVT
paraventricular nucleus of the thalamus
- VEH
vehicle
Footnotes
AUTHOR CONTRIBUTIONS
AMW, LMY, EAD, and CTL performed the behavioral and immunohistochemical experiments. JFN provided the CAV2-cre. AMW, LMY, and SMF designed the overall study. AMW and SMF wrote the manuscript. All authors contributed to data interpretation and manuscript editing.
FINANCIAL DISCLOSURES
All authors report no financial disclosures or conflicts of interest.
DATA ACCESSIBILITY STATEMENT
All data associated with this work is available upon request. Please send your request to the corresponding author (smfergus@uw.edu).
References
- Ahmed SH, Koob GH. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Allain F, Minogianis E, Roberts DCS, Samaha A. How fast and how often: the pharmacokinetics of drug use are decisive in addiction. Neuroscience and Biobehavioral Reviews. 2015;56:166–79. doi: 10.1016/j.neubiorev.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell Attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2002;168(1–2):132–38. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences. 2007;104(12):5163–68. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addiction Biology. 2014;20(3):469–81. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich J, Bardo MT. Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated Acquisition? Psychopharmacology. 2012;222(2):257–67. doi: 10.1007/s00213-012-2641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. The Journal of Comparative Neurology. 1990;299(2):187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25(3):515–32. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229(3):453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield LA, Hart G, Balleine BW. The role of the anterior, mediodorsal, and parafascicular thalamus in instrumental conditioning. Frontiers in Systems Neuroscience. 2013;7:51. doi: 10.3389/fnsys.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JR, Jansen HT, Sorg BA. Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug and Alcohol Dependence. 2014;134:387–90. doi: 10.1016/j.drugalcdep.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo RP, Sanna P, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proceedings of the National Academy of Sciences. 2001;98(4):1976–81. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. The European Journal of Neuroscience. 2003;18(5):1286–94. doi: 10.1046/j.1460-9568.2003.02833.x. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus Accumbens mediates relapse in cocaine addiction. The Journal of Neuroscience. 2000;20(15):RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93(4):1359–67. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behavioral Brain Research. 2000;116(1):1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biological Psychiatry. 2008;63(2):152–57. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Deroche V, Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. The European Journal of Neuroscience. 1999;11(8):2731–36. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behavioural Pharmacology. 2013;24(5–6):356–62. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories - indications for novel treatments of addiction. European Journal of Neuroscience. 2014;40(1):2163–82. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neuroscience and Biobehavioral Reviews. 2010;35(2):248–75. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Phillips PE, Roth BL, Wess J, Neumaier JF. Direct-pathway striatal neurons regulate the retention of decision-making strategies. The Journal of neuroscience. 2013;33(28):11668–76. doi: 10.1523/JNEUROSCI.4783-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nature Neuroscience. 2010;14(1):22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassoni C, Spreafico R, Bentivoglio M. Glutamate, aspartate and co-localization with calbindin in the medial thalamus an immunohistochemical study in the rat. Experimental Brain Research. 1997;115(1):95–104. doi: 10.1007/pl00005689. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176(3–4):459–65. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience. 2011;12(11):652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Progress in Brain Research. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Haight JL, Flagel SB. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Frontiers in Behavioral Neuroscience. 2014;8:79. doi: 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Fraser KM, Akil H, Flagel SB. Lesions of the paraventricular nucleus of the thalamus differentially affect sign- and goal-tracking conditioned responses. European Journal of Neuroscience. 2015;42(7):2478–88. doi: 10.1111/ejn.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. European Journal of Neuroscience. 2009;29(4):802–12. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Igelstrom KM, Herbison AE, Hyland BI. Enhanced c-Fos expression in superior colliculus, paraventricular thalamus and septum during learning of cue-reward association. Neuroscience. 2010;168(3):706–14. doi: 10.1016/j.neuroscience.2010.04.018. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199(C):235–42. doi: 10.1016/j.neuroscience.2011.09.047. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Days CV. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS ONE. 2010;5(9):e12980. doi: 10.1371/journal.pone.0012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Dayas CV. What about me…? The PVT: a role for the paraventricular thalamus (PVT) in drug-seeking behavior. Frontiers in Behavioral Neuroscience. 2013;7:18. doi: 10.3389/fnbeh.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Grueter BA. Cocaine experience enhances thalamo-accumbens N-methyl-D-aspartate receptor function. Biological Psychiatry. 2016;80(9):671–81. doi: 10.1016/j.biopsych.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. The Journal of Neuroscience. 2002;22(3):1126–36. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology. 2016;233(19–20):3587–3602. doi: 10.1007/s00213-016-4393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BE, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. The Journal of Comparative Neurology. 2005;493(1):72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Wunsch AM, Nakata KG, Donckels E, Neumaier JF, Ferguson SM. Corticostriatal afferents modulate responsiveness to psychostimulant drugs and drug-associated stimuli. Neuropsychopharmacology. 2016;41(4):1128–37. doi: 10.1038/npp.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187(1):60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. Journal of virology. 2000;74(1):505–12. doi: 10.1128/jvi.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63(10):823–35. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51(3):533–45. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- Lee BR, Yao-Ying M, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schluter OM, Dong Y. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nature Neuroscience. 2013;16(11):1644–51. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Deng Y, Liu B, Mu S, Guley NM, Wong T, Reiner A. Confocal laser scanning microscopy and ultrastructural study of VGLUT2 thalamic input to striatal projection neurons in rats. The Journal of Comparative Neurology. 2013;521(6):1354–77. doi: 10.1002/cne.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. The Journal of Comparative Neurology. 2007;506(2):263–87. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Structure and Function. 2011;217(2):257–73. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69(4):650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schluter OM, Huang YH, Dong Y. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–67. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD. Clozapine N-oxide administration produces behavioral effects in long-evans rats: implications for designing DREADD experiments. eNeuro. 2016;3(5):1–14. doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones GJ. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature Neuroscience. 2014;17(4):577–85. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175(1):1–11. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. The Journal of neuroscience. 2010;30(42):14102–15. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Boutrel B. Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Frontiers in Behavioral Neuroscience. 2012;6:75. doi: 10.3389/fnbeh.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Weiss F, Martin-Fardon R. Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking Behavior. Neuroscience Letters. 2015a;608(C):34–39. doi: 10.1016/j.neulet.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Cauvi G, Kerr TM, Weiss F, Martin-Fardon R. The paraventricular nucleus of the thalamus is differentially recruited by stimuli conditioned to the availability of cocaine versus palatable food. Addiction Biology. 2015b;22(1):70–77. doi: 10.1111/adb.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience. 2001;21(21):8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish C, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience. 2003;23(8):3531–37. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–95. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Frontiers in systems neuroscience. 2013;7(37):1–19. doi: 10.3389/fnsys.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PA, Wang Y, Yan Y, Wang Y, Ishikawa M, Cui R, Huang YH, Sesack SR, Schluter OM, Dong Y. Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacology. 2016;41(9):2399–2410. doi: 10.1038/npp.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. The Journal of neuroscience. 2008;28(17):4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. The Journal of neuroscience. 2002;22(7):2916–25. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. The Journal of Comparative Neurology. 2006;500(6):1050–63. doi: 10.1002/cne.21224. [DOI] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens Shell: ultrastructural characteristics and spatial relationships with dopamine afferents. The Journal of Comparative Neurology. 2003;459(2):142–55. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacological Reviews. 2011;63(2):291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. DREADDs for neuroscientists. Neuron. 2016;89(4):683–94. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz CA, Bremer QZ, Landry QF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biology. 2007;5(1):16–20. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. European Journal of Neuroscience. 2006;23(1):219–28. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. European journal of pharmacology. 2005;526(1–3):65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2009;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus midbrain pathway for feeding behavior. Neuron. 2014;82(4):797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Frontiers in Behavioral Neuroscience. 2013;7(213):1–6. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacological Reviews. 2011;63(2):348–65. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HS, Bentivoglio M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. Journal of Comparative Neurology. 1990;297(4):582–93. doi: 10.1002/cne.902970410. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus: anatomical and functional evidence for participation in processes of arousal and awareness. Brain research. 2002;39(2–3):107–40. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305(5686):1017–19. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. The Journal of Comparative Neurology. 2008;508(2):212–37. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79(2):347–60. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, Mackowiak M, Rogowski A, Kostowski W, Bienkowski P. Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedeberg’s Archives of Pharmacology. 2003;368(5):331–41. doi: 10.1007/s00210-003-0811-7. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Whitelaw RB, Robbins TW, Everitt BJ. Excitotoxic lesions of the mediodorsal thalamic nucleus attenuate intravenous cocaine self-administration. Psychopharmacology. 1998;140(2):225–32. doi: 10.1007/s002130050761. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nature Reviews Neuroscience. 2016;17(6):351–65. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301(C):529–41. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CD, Deutch AD. The effects of thalamic paraventricular nucleus lesions on cocaine-induced locomotor activity and sensitization. Pharmacology Biochemistry and Behavior. 1998;60(3):753–58. doi: 10.1016/s0091-3057(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Zhu H, Roth BL. Silencing synapses with DREADDs. Neuron. 2014;82(4):723. doi: 10.1016/j.neuron.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wienecke CFR, Nachtrab G, Chen X. A thalamic input to the Nucleus accumbens mediates opiate dependence. Nature. 2016;530(7589):219–22. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DCS. The motivation to self-administer Is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology. 2012a;37(8):1901–10. doi: 10.1038/npp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Dobrin CA, Roberts DCS. Examination of behavioral strategies regulating cocaine intake in rats. Psychopharmacology. 2012b;225(4):935–44. doi: 10.1007/s00213-012-2877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.