Abstract
Objective
To compare differences between clinician perceptions of therapeutic substitutes for antipsychotics prescribed to patients with dementia in long term care (LTC) and published evidence.
Methods
A mixed-methods approach that included a drug information search, online survey of 55 LTC clinicians and a comprehensive literature review was used. For 41 pharmacologic antipsychotic substitute candidates identified, LTC clinicians rated the likelihood they would substitute each for patients with dementia and identified non-pharmacologic antipsychotic substitutes. The quality of evidence supporting the most likely antipsychotic substitutes was assessed using a modified GRADE approach.
Results
Among 36 (65%) of LTC clinicians responding, the pharmacologic candidates deemed likely or somewhat likely to be substituted for an antipsychotic were: valproic acid, serotonin modulator antidepressants, short-acting benzodiazepines, serotonin reuptake inhibitor antidepressants, alpha-adrenoceptor antagonist, buspirone, acetaminophen, serotonin-norepinephrine reuptake inhibitor antidepressants, memantine, and a cholinesterase inhibitor. High quality evidence supporting these substitutions existed for only memantine and cholinesterase inhibitors, while high quality evidence cautioning against this substitution existed for valproic acid. Activities and music therapy were the most commonly cited non-pharmacologic substitutes but the supporting evidence for each is sparse.
Conclusion
Perceptions of LTC clinicians regarding substitutes for antipsychotics in LTC patients with dementia vary widely and are often discordant with published evidence.
Keywords: Dementia, antipsychotic, BPSD, long-term care
Introduction
Approximately one-half of long-term care (LTC) patients have dementia, of which nearly one-third to one-fifth receive an antipsychotic despite not having a diagnosis that would warrant such prescribing. (Chen et al., 2010; Centers for Medicare and Medicaid Services [CMS], 2015; Kuehn, 2013) Extensive evidence has demonstrated marginal clinical benefit of using antipsychotics in dementia management and serious adverse events including stroke, myocardial infarction, and death (Tan et al., 2015; Tolppanen et al., 2016; Wang et al., 2005). Since 2008, the Food and Drug Administration (FDA) has required a boxed warning on all antipsychotics, warning of these serious risks and noting these medications are not approved for the treatment of dementia. Antipsychotic use reportedly decreased by 11% among patients who are managed in an outpatient setting in response to such warnings (Desai, Heaton, & Kelton, 2012), however, changes in the prevalence of antipsychotic use in the LTC setting since 2005 are more mixed.
In 2012, the Centers for Medicare and Medicaid Services (CMS) launched a national initiative to reduce unnecessary antipsychotic drug use in LTC facilities and to improve dementia care (CMS, 2015). This effort included a well-publicized and multimodal campaign of social media videos, online training materials, and outreach to state-level LTC stakeholders. Many LTC facilities have since reduced antipsychotic use in their dementia population. However, the problematic behaviors often associated with dementia require alternative treatments and approaches.
Comprehensive information and guidance are lacking about safe and effective substitutes for antipsychotics (Briesacher, Tjia, Field, Peterson, & Gurwitz, 2013; Levinson, 2011). Additionally, clinician attitudes toward and knowledge about the use of these alternatives are unknown (Gould, Tilly, & Reed, 2009). Non-pharmacological interventions have shown some evidence in effectively treating behavioral and psychiatric symptoms of dementia (BPSD) (Gitlin, Kales, & Lyketsos, 2012). However, reported efficacy is inconsistent across different interventions and implementation may be challenging given staffing shortages and the training that is required for these interventions to be effective (The Henry J. Kaiser Family Foundation, The Kaiser Commission on Medicaid and the Uninsured, 2013).
The primary objective of this study was to identify the perceptions of LTC clinicians about potential substitute therapies for antipsychotics (both pharmacologic and non-pharmacologic) for patients with dementia in LTC and compare these perceptions with published evidence. This work is part of a larger investigation sponsored by the National Institute on Aging (R21AG049269) to track changes in prescribing patterns in the LTC setting following initiatives by CMS to reduce antipsychotic use in LTC.
Methods
We used a mixed-methods approach to identify potential pharmacologic and non-pharmacologic substitutes for antipsychotics in the steps outlined below. All aspects of this study were within the ethical guidelines of human subject research and approved by Northeastern University’s Institutional Review Board.
Identification of potential antipsychotic substitutes
Two experienced clinical pharmacists with training and expertise in geriatric care searched the 2015 American Hospital Formulary Service (AHFS) database to identify all pharmacologic classes with both central nervous system activity and potential to be perceived as a clinically plausible treatment for patients with dementia (McEvoy, Litvak, & Welsh, 2011). This process identified 10 AHFS medication groups encompassing 41 different medication/medication classes that were deemed to be potential pharmacologic substitute candidates (Table S1). This list was compared with other reviews on this topic (Kales, Gitlin, & Lyketsos, 2015; Sink, Holden, & Yaffe, 2005).
Survey instrument development
A 57-item survey was developed by two geriatric pharmacists John Devlin, Carla Bouwmeester (JD, CB) and a geriatrician Meenakshi Patel (MP) to evaluate LTC providers’ perceptions regarding potential substitutes for antipsychotics in patients with dementia who have BPSD (see Appendix A of the supplement). The survey asked respondents to use a 4-item Likert scale (4 = very likely; 1 = never) to rate the likelihood that each of the 41 pharmacologic candidates would substitute for antipsychotics in the treatment of dementia. In addition, three free-text boxes were offered as open-solicitation of non-pharmacologic substitutes. The survey also asked three questions about perceptions surrounding the impact of the CMS initiative to reduce antipsychotic prescribing in their facility as well as demographic and professional practice characteristics.
LTC provider panel development
The investigator team identified a purposive sample (Lavrakas, 2008) of authoritative front-line LTC clinicians from professional networks as a first-round seed panel. Using an adaptation of the snowball sampling method (Goodman, 1961), the seed panel members were then invited to nominate additional LTC clinicians in iterative rounds until sampling targets were met. Sampling targets included representatives from the four major US geographic census regions and the four professional groups most likely to influence antipsychotic prescribing in LTC: psychiatrists, geriatricians, advanced practice nurses, and clinical pharmacists. These targets were met after three rounds.
We opted to use a snowball sampling approach because the types of clinicians (i.e. LTC physicians, psychiatrists, pharmacists, and advanced practice RNs) who influence antipsychotic and substitute prescribing in the LTC setting are diverse. The fact that no single database exists to identify these clinicians makes them difficult to identify. If we had tried to survey a database of all consultant pharmacists or all LTC physicians our response rate may have been low and thus potential issues with response bias could have existed and we wanted to ensure that we had LTC clinicians and researchers who were motivated and interested in responding.
Survey administration
The survey was formatted into web-based survey software (Qualtrics, Provo, UT, USA). Participants were emailed a link to the survey and reminded weekly to respond. All responses were anonymous, and no compensation was offered for participation. Consent was assumed by participation. Survey collection ended after three weeks.
Data analysis
Survey data was downloaded into an Excel database (Microsoft, Seattle, WA, USA) and descriptive statistics generated for each item. Ratings of never/not likely were grouped together due to insufficient sample size. Candidates receiving a median likelihood rating ≥2 (somewhat likely or very likely) were deemed likely antipsychotic substitutes. Non-pharmacologic candidates were grouped into broad categories ranked according to response frequency. Those mentioned by two or more LTC experts were deemed substitutes. Subgroup analyses were conducted across the following domains: (1) providers’ prescribing abilities and (2) whether or not they currently provide any clinical services in a LTC facility. Mean differences were tested using the Mann–Whitney U-test. Microsoft Excel 2010 and SAS® V9.4 (Cary, NC, USA) were used for data tabulation and analysis.
Evidence summary
For each substitute identified in the provider survey, we conducted a structured review of the evidence supporting use in LTC for dementia or BPSD, particularly agitation. We searched the Cochrane Database of Systematic Reviews and PubMed using generic and brand names of potential pharmacologic substitutes, MeSH terms and a modified Cochrane Highly Sensitive Search Strategy developed by a medical librarian. We limited the search to randomized controlled trials (RCTs), meta-analyses, and systematic reviews published since 1990 (the first year that an atypical antipsychotic was marketed in the US (Shen, 1999)) focused on LTC dementia populations (Tables S2 and S3). We assessed the quality of evidence using an adaptation of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines (Balshem et al., 2011). Substitutes with evidence from Cochrane systematic reviews received a rating of 4, high-quality RCTs or meta-analyses received a 3, and those with evidence from RCTs with bias risk received a 2. Conclusions drawn from the evidence describe both benefit and safety of each substitute for treating patients with dementia in LTC (summarized in Tables 1 and 2 for more details see Tables S4, S5a, and S5b).
Table 1.
Evidence for use of medications/pharmacologic classes most likely to be substituted for an antipsychotic in long-term care residents with dementiaa.
| Drugs | Quality of evidenceb |
Summary of evidence |
Conclusions |
|---|---|---|---|
| Valproic acid | Strong | Not useful | Valproate preparations are not recommended for management of dementia. Valproate therapy is not effective in treating dementia-associated agitation and is associated with significant adverse effects. |
| Serotonin modulator antidepressant | Moderate | May be useful | Trazodone may reduce dementia-associated agitation and improve sleep in this population but evidence remains limited and safety concerns exist with its use in older adults. |
| Short-acting benzodiazepine | Moderate | May be useful | Short-acting benzodiazepines may improve time to sleep onset and reduce dementia-associated agitation but evidence is limited and benzodiazepines are associated with falls, delirium, and reduced sleep quality. |
| Serotonin reuptake inhibitor (SSRI) antidepressant | Moderate | Cautious use | Sertraline and citalopram are associated with modest reductions in dementia-associated agitation and psychosis. Serious safety concerns exist with their use in older adults. |
| Alpha-adrenoceptor antagonists (A1A) | Moderate | May be useful | Prazosin has comparable improvement in agitation and aggression to antipsychotics. Evidence of efficacy of other A1A drugs is lacking. |
| Buspirone | Weak | May be useful | Buspirone may reduce dementia-associated delusions, aggression, and anxiety but evidence is very limited and safety concerns, particularly in older adults, exist. |
| Acetaminophen | Moderate | May be useful | Acetaminophen may reduce dementia-associated agitation and discomfort in the context of an overall pain management protocol but evidence is limited. Few safety concerns exist. |
| Serotonin-norepinephrine reuptake inhibitor (SNRI) antidepressant | Moderate | May be useful | Dextromethorphan-quinidine may reduce dementia-associated agitation but the evidence remains limited and safety concerns exist with use in older adults. |
| Memantine | Strong | Useful | Memantine modestly reduces agitation in moderate to severe Alzheimer’s disease. Safety concerns are minor. |
| Cholinesterase inhibitor | Strong | Useful | Donepezil, galantamine, and rivastigmine improve cognition in mild to moderate dementia. Safety concerns are minor. |
Note:
Presented in order of most likely to less likely substitute based on respondent rating.
Strong = GRADE 4, Moderate = GRADE 3, Weak = GRADE 2.
Table 2.
Non-pharmacologic antipsychotic substitutes for long-term care residents with dementia: survey reporting frequency and evidence summarya.
| Non-pharmacologic categories |
Frequency of response |
Quality of evidencea |
Summary of evidence |
Conclusions |
|---|---|---|---|---|
| Activities | 11 | Moderate | May be useful | Activities (particularly those incorporating redirection) have shown some benefit in reducing agitation. |
| Music therapy | 9 | Moderate | May be useful | Music therapy (often accompanied by redirection) may reduce agitation in mild or moderate dementia but lacks enough high-quality evidence to definitively conclude benefit. |
| Exercise | 6 | Weak | May be useful | Incongruence and low quality of evidence make the current role of exercise unclear. |
| Redirection | 6 | None | May be useful | No evidence exists to support the use of redirection (independent of a more complex intervention) as a strategy to reduce agitation. |
| Unmet medical need | 6 | Weak | May be useful | Although assessing for unmet medical needs such as glasses or hearing aids is recommended in the literature, there are no rigorous studies that examine this specifically, in reducing agitation. |
| Sensory intervention | 5 | Moderate | Useful | Sensory interventions (e.g. Montessori or multisensory stimulation) have been shown to reduce agitation. |
| Restraints | 4 | None | May be useful | No evidence exists examining use of restraints to reduce agitation. |
| Social contact | 4 | None | May be useful | No evidence exists to support the use of social contact (independent of a more complex intervention) as a useful therapy for reducing agitation. |
| Changing the environment | 3 | Weak | May be useful | Few studies examine physical environmental changes. Inconclusive results prevent any determination of benefit. |
| Light therapy | 3 | Moderate | Not useful | Light therapy has not been shown effective for reducing agitation and in fact may worsen it. |
| Pet therapy | 3 | Weak | May be useful | Limited evidence shows potential benefit to support the use of live or simulated pet therapy to reduce agitation. |
Note:
Presented in order of frequency suggested by respondents.
Strong = GRADE 4, Moderate = GRADE 3, Weak = GRADE 2.
Results
Among the 55 LTC providers invited, 36 participated (64% response rate). Among responders, 13 were prescribers (i.e. psychiatrists, geriatricians, advanced nurse practitioners, or physician assistants) and 19 were non-prescribers. Non-prescribers were clinical pharmacists with advanced geriatric pharmacy training that routinely consult with and directly influence LTC prescribing. Twenty-three reported providing clinical services to a LTC facility. Full demographic parameters are presented in Table S6.
Substitution practices
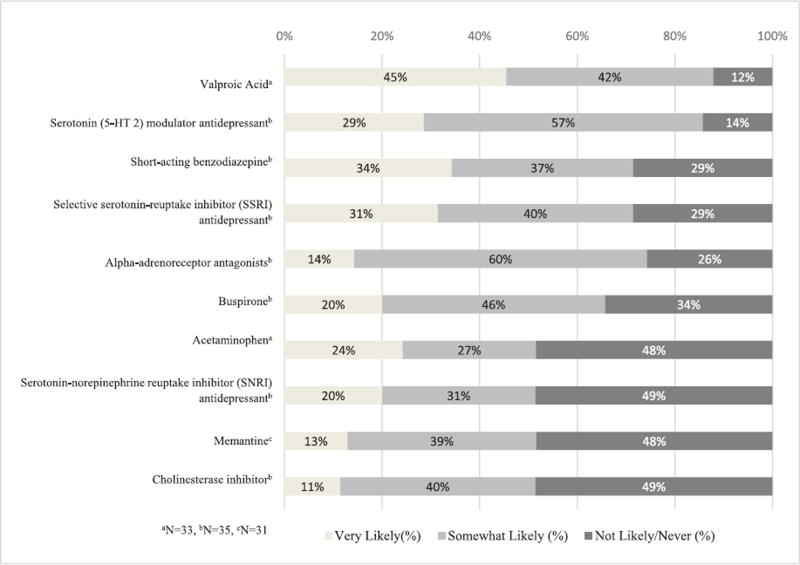
The 10 medications rated as most likely to be substituted for an antipsychotic prescribed to LTC patients with dementia, in rank order are: valproic acid1, serotonin modulator antidepressants, short-acting benzodiazepines, serotonin reuptake inhibitor (SSRI) antidepressants, alpha-adrenoceptor antagonist (A1A), buspirone, acetaminophen, serotonin-norepinephrine reuptake inhibitor (SNRI) antidepressants, memantine, and cholinesterase inhibitors (Figure 1 and Figure S1) (Figure 1 near here). Subgroup analyses revealed that antipsychotic substitution preferences were not influenced by prescribing status, LTC clinical involvement, or prescriber training (i.e. psychiatrists vs. geriatricians) (Tables S7 and S8).
Figure 1. Medications most likely to be substituted for antipsychotics in long-term care residents with dementia.
Note: This figure presents the distribution of respondent ratings for the pharmacologic candidates with median rating ≥2 (i.e. likely to be substitutes for an antipsychotic). Data is presented in order of highest likelihood rating to lower likelihood rating.
Clinicians suggested 16 non-pharmacologic interventions that could be used as a substitute for antipsychotics when treating dementia. Activities and music therapy were the most commonly cited substitutes, identified by one-third and one-quarter of respondents (see Table S9 for full list).
Evidence summary
Table 1 summarizes the evidence-base for the most highly rated pharmacologic substitutes for antipsychotics in LTC dementia patients (Table 1 near here). Most substitutes had little to no evidence supporting their use in this patient population. The exceptions were memantine and cholinesterase inhibitors, each of which has high-quality evidence supporting some benefit in treating dementia or BPSD in the LTC setting. Of note, the evidence for valproic acid (the top-rated pharmacologic substitute for an antipsychotic) was high quality but strongly cautioned against use in this population due to safety concerns. The evidence for the other substitutes ranged from moderate to weak, and was often insufficient to conclude whether a benefit exists.
Table 2 presents the evidence for non-pharmacologic substitutes (Table 2 near here). None had strong levels of evidence supporting benefits in this patient population, however moderate evidence was available supporting activities and sensory interventions. The other substitutes had insufficient or no evidence to conclude any benefit.
Perceptions of antipsychotic prescribing practices and substitution activities
Nearly all experts agreed that antipsychotics are overused in patients with dementia and the 2012 CMS initiative led to a decrease in antipsychotic prescribing in their LTC facility (88% and 78%, respectively). Most experts (63%) also agreed that they had seen increases in substitution of antipsychotics with CNS-active medications as a result of the CMS campaign (see Table S10 for details.)
Discussion
This investigation represents the first comparison between the perceptions of LTC clinicians and the graded evidence regarding the use of either pharmacologic or non-pharmacologic substitutes for antipsychotic therapy in LTC patients with dementia. The most common antipsychotic medication substitutes ranged across a number of different pharmacological classes including benzodiazepines, antidepressants, anticonvulsants, analgesics, and cholinesterase inhibitors. Reported non-pharmacologic substitutes varied widely and included different cognitive, physical, and environmental strategies as well as restraints. Across the many pharmacological and non-pharmacologic substitutes reported, no clear preference emerged favoring any one strategy.
Our summary of the current evidence surrounding therapeutic substitutes for antipsychotics reveals large gaps in the knowledge base, especially for LTC patients with dementia. The evidence to support a safety or clinical advantage over antipsychotics with the identified pharmacologic and nonpharmacologic substitutes is largely lacking or discordant. Valproic acid serves as a good example. It was aggressively promoted in the 1990s to LTC clinicians as an off-label treatment for behavioral problems in patients with dementia. (Department of Justice, Office of Public Affairs, 2012) However, more recent randomized clinical trials have failed to demonstrate benefit in dementia management and showed association with serious safety concerns (Lonergan & Luxenberg, 2009).
Our comparison of LTC clinician perceptions and the published evidence demonstrates a dichotomy between what providers say are likely substitutes for antipsychotics in this population and what the literature says are safe and effective treatments. We suspect that long-established prescribing habits of using older, inexpensive medications with sedative properties may explain these perceptions more than evidence- based decision-making. Comments from the survey helped to corroborate this apparent discord:
There are LTC facilities that I work with where the medical directors are insisting to use short-acting benzodiazepines such as lorazepam as a substitute for antipsychotic therapy, irregardless [sic] of the potential adverse effects associated with this class of agents.
Similarly, another respondent commented about substitution with buspirone:
“This is one we see a lot of” as “the preferred agent of a single provider at our facility. I don’t especially like this choice and haven’t observed this to be effective…”
While the exact reasons why the LTC experts surveyed appear to support the use of these agents with little to no evidence remains unclear, this may relate to off-label marketing, a lack of familiarity with published evidence, and an underestimation of the potential risks of these medications in older adults.
Our findings are in agreement with prior studies that examined the FDA black box warning and the Omnibus Budget Reconciliation Act of 1987 (OBRA-87) in community and nursing home-dwelling residents with dementia, respectively (Lantz & Nebenzahl, 1996; Martinez, Jones, & Rietbrock, 2013). In both cases, the use of both antidepressants and anxiolytics (e.g. benzodiazepines) increased as substitutions for antipsychotics. Unique to our study is the perception that valproic acid is a likely substitute for antipsychotics. This perception does not agree with any previous study of the effects of an antipsychotic reduction program and could be attributed to the off-label marketing of Depakote to treat behavioral disturbances of dementia by Abbott Laboratories between 1998 and 2006 (Department of Justice, Office of Public Affairs, 2012).
Our study had several potential limitations. While the use of snowball sampling has well-documented limitations, it remains the best method to explore the opinions of difficult-to-reach populations (Goodman, 1961; Lavrakas, 2008). A larger sample might have identified different distributions in diversity of clinicians who are involved in both practice and research in this setting as well as additional antipsychotic substitution trends. Our results cannot be extrapolated to other countries where substitution practices or clinician diversity may be different. Our results may not represent the opinions of LTC professionals (e.g. bedside nurses) not surveyed or LTC providers who did not participate. Our survey asked about perceptions of antipsychotic substitutes among dementia patients without providing a context for factors like actual patient care or LTC resources that might influence response. We are unable to make conclusions about treatment of specific symptoms of dementia such as BPSD. Further, we have summarized the evidence for only patients with dementia who reside in the LTC setting; evidence may exist for dementia patients in other practice settings that could inform antipsychotic substitution practices. The survey tool was designed to produce an exhaustive capture of perceived substitutes rather than assessment of appropriateness, and it was not validated. The non-pharmacologic substitutes were self-reported.
Nevertheless, we believe our comparison of LTC provider perceptions with the limited available evidence supporting benefit of potential pharmacologic and non-pharmacologic substitutes for antipsychotics in LTC patients with dementia highlights an important potential clinical practice gap. We believe our findings and the evidence summarized here provide primary targets for future research and CMS quality initiatives. There are interventions identified here with potential value in the care of LTC patients with dementia yet require more study to verify benefits and safety and adjust clinician perceptions.
Conclusions
In conclusion, our study offers a novel comparison of clinician perceptions to published evidence for pharmacologic and non-pharmacologic substitutes for antipsychotics prescribed to patients with dementia residing in LTC. Our hope is that the supporting evidence presented here can be used as preliminary information for furthering the academic and clinical dialogues around better decision-making in LTC of patients with dementia.
Acknowledgments
We acknowledge Carrie Price, MLS, of Welch Medical Library, for help adapting the literature search strategy. Also Carla Bouwmeester, PharmD and Meenakshi Patel, MD for assisting with survey development.
Funding
This work was supported by the National Institute on Aging [grant number R21AG049269].
Footnotes
Supplemental data for this article can be accessed at, http://dx.doi.org/10.1080/13607863.2016.1277974.
Valproic acid includes Depakote.
Disclosure statement
Brianne Olivieri-Mui, John Devlin, Aileen Ochoa, Danielle Schenck, and Becky Briesacher declare that they have no conflicts of interest.
References
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Briesacher BA, Tjia J, Field T, Peterson D, Gurwitz JH. Antipsychotic use among nursing home residents. JAMA. 2013;309(5):440–442. doi: 10.1001/jama.2012.211266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. Nursing home data compendium 2013. Rockville, MD: 2015. [Google Scholar]
- Chen Y, Briesacher BA, Field TS, Tjia J, Lau DT, Gurwitz JH. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Archives of Internal Medicine. 2010;170(1):89–95. doi: 10.1001/archinternmed.2009.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Justice, Office of Public Affairs. Abbott Labs to Pay $1.5 Billion to Resolve Criminal & Civil Investigations of Off-label Promotion of Depakote [Press Release] 2012 May 7; Retrieved from https://www.justice.gov/opa/pr/abbott-labs-pay-15-billion-resolve-criminal-civil-investigations-label-promotion-depakote.
- Desai VCA, Heaton PC, Kelton CML. Impact of the Food and Drug Administration’s antipsychotic black box warning on psychotropic drug prescribing in elderly patients with dementia in outpatient and office-based settings. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2012;8(5):453–457. doi: 10.1016/j.jalz.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LA. Snowball sampling. Annals of Mathematical Statistics. 1961;32(1):148–170. [Google Scholar]
- Gould E, Tilly J, Reed P, editors. Alzheimer’s association campaign for quality care: Dementia care practice recommendations for professionals working in a home setting phase 4. 2009 Retrieved from https://www.alz.org/national/documents/phase_4_home_care_recs.pdf.
- The Henry J. Kaiser Family Foundation, The Kaiser Commission on Medicaid and the Uninsured. Overview of nursing facility capacity, financing, and ownership in the United States in 2011 (Publication No. 8456) 2013 Retrieved from https://kaiserfamilyfoundation.files.wordpress.com/2013/06/8456-overview-of-nursing-facility-capacity.pdf.
- Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. Efforts stall to curb nursing home antipsychotic use. JAMA. 2013;310(11):1109–1110. doi: 10.1001/jama.2013.276603. [DOI] [PubMed] [Google Scholar]
- Lantz CA, Nebenzahl E. Behavior and interpretation of the kappa statistic: Resolution of the two paradoxes. Journal of Clinical Epidemiology. 1996;49(4):431–434. doi: 10.1016/0895-4356(95)00571-4. [DOI] [PubMed] [Google Scholar]
- Lavrakas PJ, editor. Encyclopedia of survey research methods. Thousand Oaks, CA: SAGE; 2008. [Google Scholar]
- Levinson DR. Medicare atypical antipsychotic drug claims for elderly nursing home residents. (OEI-07-08-00150) Washington, DC: Office of the Inspector General; 2011. Retrieved from http://oig.hhs.gov/oei/reports/oei-07-08-00150.pdf. [Google Scholar]
- Lonergan E, Luxenberg J. Valproate preparations for agitation in dementia. The Cochrane Database Systematic Reviews. 2009;3:CD003945. doi: 10.1002/14651858.CD003945.pub3. [DOI] [PubMed] [Google Scholar]
- Martinez C, Jones RW, Rietbrock S. Trends in the prevalence of antipsychotic drug use among patients with Alzheimer’s disease and other dementias including those treated with antidementia drugs in the community in the UK: A cohort study. BMJ Open. 2013;3(1):e002080. doi: 10.1136/bmjopen-2012-002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy G, Litvak K, Welsh O. American Hospital Formulary Service, Drug Information 2015. Bethesda, MD: American Society of Hospital Pharmacists; 2011. [Google Scholar]
- Shen WW. A history of antipsychotic drug development. Comprehensive Psychiatry. 1999;40(6):407–414. doi: 10.1016/s0010-440x(99)90082-2. [DOI] [PubMed] [Google Scholar]
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. JAMA. 2005;293(5):596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- Tan L, Tan L, Wang HF, Wang J, Tan CC, Tan MS, Yu JT. Efficacy and safety of atypical antipsychotic drug treatment for dementia: A systematic review and meta-analysis. Alzheimer’s Research and Therapy. 2015;7(1):20. doi: 10.1186/s13195-015-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tolppanen AM, Koponen M, Tanskanen A, Lavikainen P, Sund R, Tiihonen J, Taipale H. Antipsychotic use and risk of hospitalisation or death due to pneumonia in persons with and without Alzheimer’s disease. Chest. 2016;150(6):1233–1241. doi: 10.1016/j.chest.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. New England Journal of Medicine. 2005;353(22):2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]