Abstract
Background
Dried leaf Artemisia annua (DLA) has shown efficacy against Plasmodium sp. in rodent studies and in small clinical trials. Rodent malaria also showed resiliency against the evolution of artemisinin drug resistance.
Purpose
This is a case report of a last resort treatment of patients with severe malaria who were responding neither to artemisinin combination therapy (ACT) nor i.v. artesunate.
Study Design
Of many patients treated with ACTs and i.v. artesunate during the 6 mon study period, 18 did not respond and were subsequently treated with DLA Artemisia annua.
Methods
Patients were given a dose of 0.5 g DLA per os, twice daily for 5d. Total adult delivered dose of artemisinin was 55 mg. Dose was reduced for body weight under 30 kg. Clinical symptoms, e.g. fever, coma etc., and parasite levels in thick blood smears were tracked. Patients were declared cured and released from hospital when parasites were microscopically undetectable and clinical symptoms fully subsided.
Results
All patients were previously treated with Coartem® provided through Santé Rurale (SANRU) and following the regimen prescribed by WHO. Of 18 ACT-resistant severe malaria cases compassionately treated with DLA, all fully recovered. Of the 18, this report details two pediatric cases.
Conclusions
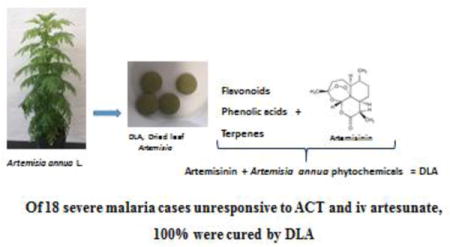
Successful treatment of all 18 ACT-resistant cases suggests that DLA should be rapidly incorporated into the antimalarial regimen for Africa and possibly wherever else ACT resistance has emerged.
Graphical abstract
Background
The entire Democratic Republic of Congo (DRC) is at risk of malaria. In 2013 >25,000 deaths occurred; many were children under 5 [WHO, 2015]. In 2005 the DRC adopted free artemisinin combination therapy (ACT) for all ages in the public sector. ACTs provided by SANRU (Santé Rurale) via the Global Fund remain the front line treatment. Recently in the Goma area of the DRC, out of thousands of malaria cases successfully treated with ACTs, a few cases that did not respond began emerging, so a compassionate effort to save their lives ensued.
Artemisia annua L. qualifies as a generally recognized as safe (GRAS) plant [Duke 2001] from which artemisinin is extracted and purified to produce ACT. The plant has been safely consumed in various forms, e.g. as a tea infusion [Hsu, 2006] and leaf powder [Onimus et al. 2013], for millennia [Hsu 2006; Weathers et al. 2014a]. Furthermore, an increasing body of evidence has shown in both rodents and humans that per os consumption of the dried leaves is efficacious [Elfawal et al. 2012, 2015; ICIPE, 2005; Onimus et al. 2013; Weathers et al. 2014b], provides more bioavailable artemisinin [Weathers et al. 2011, 2014b; Desrosiers and Weathers 2016], may reduce emergence of artemisinin drug resistance [Elfawal et al. 2015], and is highly cost effective [Weathers et al. 2014a].
ACT-resistant cases of malaria were encountered in North Kivu Province, DRC, and those patients subsided into severe malaria. As defined by WHO [WHO 2014] the symptoms of severe malaria may include loss of consciousness, respiratory distress, convulsions, prostration, shock, pulmonary edema, abnormal bleeding, severe gastric distress, and jaundice. There were 18 patients who met the criteria for severe malaria, but who failed to respond to either ACT or i.v. artesunate. Compressed leaf tablets of Artemisia annua (dried leaf Artemisia, DLA) were used to treat those 18 patients, all of whom then recovered fully. Here we describe two of those 18 cases in detail.
Methods
Plant material: preparation and analysis
Leaves of Ethiopian-grown A. annua L. [Anamed A-3; https://www.anamed-edition.com/en/artemisia-annua-anamed.html ] were harvested from field-grown plants in their vegetative stage, dried and then powdered. In March 2015, the powdered dried leaves were compressed without excipient [Weathers and Towler 2014] into 500 mg tablets by Ancient Formulas Inc., a cGMP facility in Wichita, KS, USA for Plesion International, Inc., a nonprofit, charitable NGO located in New Holland, PA, USA. Plesion is affiliated with the HEAL Africa Hospital in Goma, DRC. Samples of DLA tablets were re-powdered, extracted, and analyzed for artemisinin and other phytochemicals according to methods using GC-MS and spectroscopy [Towler and Weathers 2015]. The GC-MS method for monoterpenes was modified as: 40°C held for 5 min, increased to 250°C at 4°C/min, increased to 300°C at 25°C/min, held for 5 min. Thin layer chromatography (TLC) fingerprints used 20 × 20 cm 60 F254 Si-Gel plastic plates, 10.5 μm particle size, 200 μm thickness. Artemisinin and terpenoids were stained with p-anisaldehyde reagent containing (v/v) 97:2:1 glacial acetic acid, sulfuric acid, p-anisaldehyde, and heated at 105°C for 10 min. Flavonoids were stained with 2% (w/v) aluminum chloride in methanol.
Patient selection and treatment
About 18% of malaria patients presented with severe malaria and were treated with ACT (Coartem®), in the Rwanguba Health Zone March–August, 2016. Typically for uncomplicated malaria, patients are treated for 3d with Coartem®, consisting of artemether-lumefantrine (AL; 20 mg artemisinin, 120 mg lumefantrine) with dosage defined by Novartis [2015]. The ACTs were purchased from the EU by SANRU and provided as validated drugs. Severe malaria is treated with intravenous (i.v.) artesunate [WHO 2012]. AL and i.v. artesunate were administered per WHO guidelines. Some patients (18 in all: 6 males, 12 females, ages 14 mon to 60 years) responded neither to AL nor i.v. artesunate and thus were treated with DLA ~24 h post i.v. artesunate, which had followed AL ACT treatment. Adults (>30 kg) were given one 500 mg DLA tablet (5.5 mg artemisinin/tablet) of DLA twice daily for 5d; this was 55 mg artemisinin in toto per adult. Children were apportioned tablets by weight: for those 5 to <15 kg, a quarter-tablet was given; for 15–30 kg, a half-tablet was administered. Total artemisinin per child was thus 13.75 mg and 27.5 mg for the quarter and half-tablet regimens, respectively. For those in coma or too young to swallow tablets, the dose was crushed, mixed with clean water, and delivered by nasogastric (NG) tube. Malaria infection was confirmed using a WHO approved Rapid Diagnostic Test (RDT) [WHO 2017]. Parasites in thick blood smears from all patients were tracked for 5d. Patients were released from hospital only after all clinical symptoms disappeared and parasites were undetectable in thick blood smears. For the two detailed cases, written, signed consent was obtained ex post facto. The Institutional Review Board for the Committee of Research at HEAL Africa granted permission for a retrospective evaluation of patient data regarding these case reports of patients treated with Artemisia annua tablets in the Rwanguba hospital.
Results and Discussion
After 3d of AL, all 18 patients still had blood parasites and fever. All subsequently progressed into severe malaria with symptoms beyond fever and headache: 7 had anemia; 4 had vomiting and diarrhea; 6 had convulsions, some with coma and fever >40°C; 1 had vomiting, diarrhea, and was comatose. After unsuccessful AL treatment, all 18 patients were subsequently treated with i.v. artesunate per WHO guidelines for severe malaria. When i.v. artesunate also failed, DLA was used. All 18 patients who were treated with DLA tablets recovered fully with undetectable trophozoites (tr) in thick blood smears (tr/field). Similar to observations by ICIPE [2005], DLA tablets were well tolerated with no observed side effects. Two pediatric cases are described in further detail.
An underweight (17 kg) 14 yr old female was treated at a rural dispensary with ACT and then at home for 3d. Fever persisted, so she was transferred to hospital with convulsions. She lost consciousness twice en route. At hospital she was given i.v. artesunate (2.4 mg/kg) for 5d, but fever persisted and her blood still showed 0–2 tr/field. Day 5 she was given DLA (half-tablet twice a day for 3d) per os; on day 8 her fever was gone, her blood was tr negative, and she was released.
A 5 yr old male was treated with ACT at home with no success, then transferred to hospital with fever, anemia, respiratory infection, and convulsions leading to coma. He was treated for 5d with ampicillin (200 mg/kg/day) and gentamicin (4 mg/kg/day). Artesunate i.v. was also begun: 2.4 mg/kg twice on day 1, then once daily to complete 5d. Concurrent nonmalarial infections were halted, so the other antibiotics were stopped. The patient still had malaria (0–7 tr/field) and now was comatose, so A. annua treatment began. He received a half tablet DLA crushed in water delivered by NG tube, twice daily for 3d. Day 8 after admission to hospital, his parasite count dropped to 0–2 tr/field and he was out of coma. DLA treatment was continued for 2 more days (5d total), after which parasites were undetectable. The boy subsequently was able to resume normal activities, so he was discharged the next day.
This study is consistent with earlier rodent malaria studies that showed DLA was successful in eliminating artemisinin resistant parasites [Elfawal et al. 2015]. Although patient follow-up was not done 28 d after treatment to determine recrudescence, the results of these case studies suggested that consumption of dried leaf A. annua tablets thwarted ACT-resistant malaria.
DLA tablets contained (per g DW) (Table 1) 10.97, 2.54, 0.89, 1.30, 3.91, and 18.32 mg, respectively, of artemisinin, deoxyartemisinin, arteannuin B, artemisinic acid, dihydroartemisinic acid, and total flavonoids (as quercetin equivalents). Although artemisinin and flavonoids have reported antimalarial activity in the nanomolar and micromolar range, respectively, Suberu et al. [2013] observed that arteannuin B, artemisinic and dihydroartemisinic acids also are weak antimalarials. Each also demonstrated synergism that is seemingly dependent on their concentration relative to artemisinin [Suberu et al. 2013].
Table 1.
Phytochemical content of DLA tablets used in this study.
| Phytochemical | Concentration (mg/g DW) |
|---|---|
| Artemisinic compounds | |
| Artemisinin | 10.97 |
| Artemisinic acid | 1.30 |
| Dihydroartemisinic acid | 3.91 |
| Arteannuin B | 0.89 |
| Deoxyartemisinin | 2.54 |
| Phenolic acids | |
| Chlorogenic acid | 10.94 |
| Rosmarinic acid | nd |
| Coumarins | |
| Scopoletin | 2.47 |
| Flavonoids | |
| Total flavonoids | 18.32 |
| Artemetin | nd |
| Casticin | nd |
| Chrysoplenetin | 0.50 |
| Chrysoplenol D | 0.35 |
| Eupatorin | nd |
| Isovitexin | nd |
| Kaempferol | nd |
| Luteolin | nd |
| Myrcetin | nd |
| Quercetin | nd |
| Nonartemisinic Terpenes | |
| 1,8 cineole (eucalyptol) | nd |
| β-pinene | nd |
| β-neoclovene | nd |
| Borneol | nd |
| Camphor | 0.27 |
| Caryophyllene | nd |
| Caryophyllene oxide | nd |
| Phytol | 0.56 |
| Spathulenol | nd |
| Thujone | nd |
nd, not detected
Flavonoids are another important group of phytochemicals in A. annua that also have antimalarial activity, but weaker than artemisinin. Indeed, A. annua can produce as many as 40 different flavonoids [Ferreira et al. 2010]. To our knowledge only five flavonoids frequently found in A. annua cultivars have been studied for synergism with artemisinin. Of these, chrysoplenetin, chrysoplenol-D, and eupatorin, showed a significant improvement in their IC50 in the presence of artemisinin [Liu et al. 1992]. Of the total flavonoids measured, only chrysoplenetin and chrysoplenol-D were specifically identified via TLC and GC-MS in the DLA tablets (Figure 1; Table 1).
Figure 1.
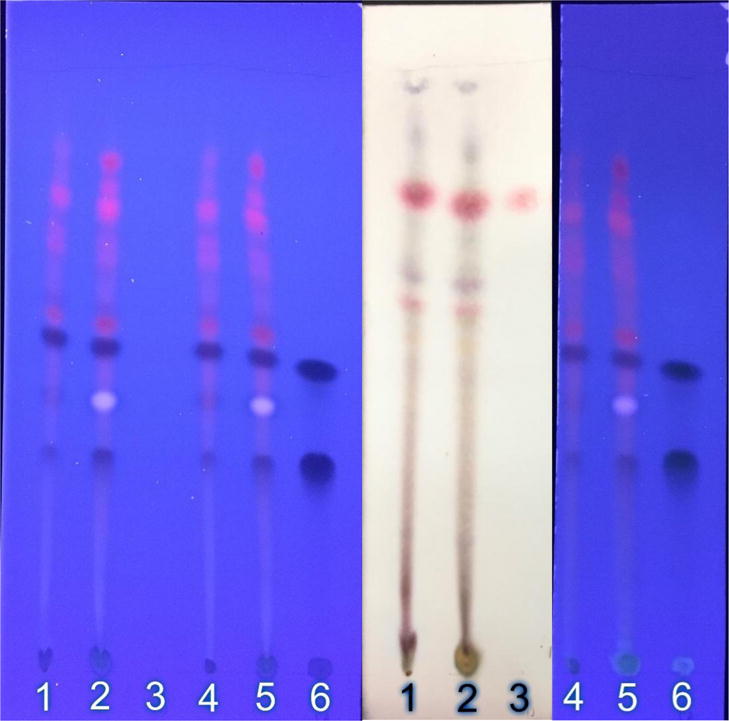
TLC fingerprint of methanol (MeOH) and methylene chloride (MeCl2) extracts of the Anamed A-3 Ethiopian grown Artemisia annua dried leaf tablets used in the study. Lane identification: 1: MeCl2 extract of DLA tablet powder (2 mg); 2: MeOH extract (2 mg); 3: artemisinin standard (5 µg); 4: MeCl2 extract; 5: MeOH extract; 6: (top to bottom) chrysoplenetin, chrysoplenol-D, chlorogenic acid standards (10 µg). Left photo, unstained TLC under UV illumination; middle photo, p-anisaldehyde stain under visible light; right photo, aluminum chloride stain under UV illumination.
While not an exhaustive list, other phytochemicals measured in DLA tablet extracts are listed in Table 1 including the coumarin, scopoletin, the phenolic acid, chlorogenic acid, and other terpenoids, e.g. the monoterpene camphor and diterpene alcohol phytol. Of these, only chlorogenic acid and phytol have demonstrated antimalarial activity [Suberu et al. 2013; Grace et al. 2012].
When leaves are dried prior to DLA tablet formation, many volatile monoterpenes are eliminated or severely reduced except for camphor [Weathers and Towler 2014]. TLC fingerprints of DLA extracted with two solvents are shown in Figure 1 and demonstrate via differential staining the plethora of terpenoids and flavonoids present in the DLA used to treat these patients. Many of these extracted compounds are as yet unidentified in this cultivar. Many of the phytochemicals found in A. annua also have not been thoroughly tested for synergism with artemisinin.
Although there is the possibility of a synergistic effect between DLA and residual artesunate from the i.v. treatments, the pharmacokinetics of artesunate makes that unlikely as by the time the DLA was given, about 24 h post i.v. artesunate, the drug had already been long eliminated from the body. The T1/2 of i.v. artesunate in human patients with severe or moderately severe malaria is 2.3 or 4.3, min, respectively, and depending on how the drug was delivered, plasma levels for both were undetectable after 6 h, all well before any DLA treatment began [Davis et al. 2001]. Artesunate, however, is considered a prodrug for dihydroartemisinin (DHA), the metabolic product of artesunate. DHA persists longer in the serum. The T1/2 of DHA produced after i.v. artesunate delivery in human patients with severe or moderately severe malaria is ~40 or 64 min, respectively [Davis et al. 2001], and again depending on length of drug delivery, extrapolation of DHA plasma levels in patients with severe malaria showed that it was undetectable ≤ 9 h later [Davis et al. 2001]. Considering that DLA was administered about 24 h after last i.v. artesunate, synergism between LA and residual DHA also seems unlikely. Taken together, the results of this study suggest that DLA alone successfully treated artemisinin-resistant malaria in these patients.
Conclusions
To our knowledge this is the first report of dried-leaf Artemisia annua controlling ACT-resistant malaria in humans. These 18 cases occurred over six months (Figure 2). They represented ~0.09 % of total ACT-treated patients in the same time and location, and demonstrated that oral consumption of dried leaf tablets of A. annua has possible utility in rescuing patients from ACT and i.v. artesunate failures. More comprehensive clinical trials on patients with ACT-resistant malaria are warranted and should include dosing studies with DLA containing different ratios of, e.g. artemisinin and flavonoids, and also patient follow-up through 28d to track recrudescence.
Figure 2.
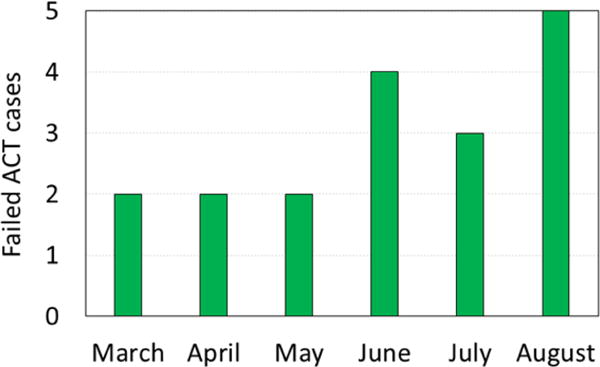
ACT and i.v. artesunate failure cases out of thousands of successfully ACT-treated malaria cases over 6 months in 2016 in North Kivu province, Democratic Republic of Congo.
Acknowledgments
Thanks to the nonprofit charitable NGO, Plesion International, Inc., for providing Artemisia annua tablets for patients. We are grateful for Award Number NIH-R15AT008277-01 from the National Center for Complementary and Integrative Health that enabled PJW and MJT to provide a phytochemical analysis of the plant material used in treating these 18 patients. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary and Integrative Health or the National Institutes of Health.
List of abbreviations
- ACT
artemisinin combination therapy
- AL
artemether-lumefantrine
- DLA
dried leaf Artemisia annua
- i.v
intravenous
- tr
trophozoites
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Author contributions.
NBD, physician attending patients receiving ACT or plant material, analyzed data; LMK, physician attending patients, ethical committee adviser, edited paper; PGB, pharmacist, supplied plant material tablets, edited paper; RLW, pharmacist, obtained plant material, had tablets cGMP manufactured, edited paper; MJT, analyzed plant material, edited paper; PJW, analyzed plant material, analyzed data, wrote article.
Contributor Information
Nsengiyumva Bati Daddy, Medical Director, Rwanguba Hospital, Rwanguba, N. Kivu, Democratic Republic of the Congo.
Luc Malemo Kalisya, Director HEAL Africa Hospital, Goma, Democratic Republic of the Congo (DRC).
Pascal Gisenya Bagire, Pharmacy Representative, Plesion International Inc., Edmonton, AB, Canada.
Robert L. Watt, Executive Director Pharmaceuticals, Plesion International Inc., Coatesville, PA, 19320, USA.
Melissa J. Towler, Research Scientist, Biology and Biotechnology, Worcester Polytechnic Institute, Worcester MA 01609, USA.
Pamela J. Weathers, Professor of Biology and Biotechnology and Professor of Biomedical Engineering, Worcester Polytechnic Institute, Worcester MA 01609, USA.
References
- Davis TME, Phuang HL, Ilett KE, Nguyen CH, Batty KT, Phuong VDB, Powell SM, Thien HV, Binh TQ. Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria. Antimicrobial Agents and Chemotherapy. 2001;45:181–186. doi: 10.1128/AAC.45.1.181-186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers MR, Weathers PJ. Effect of leaf digestion and artemisinin solubility for use in oral consumption of dried Artemisia annua leaves to treat malaria. Journal of Ethnopharmacology. 2016;190:313–318. doi: 10.1016/j.jep.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke JA. Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press; Boca Raton, FL: 2001. p. 70. [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM. Dried whole plant Artemisia annua as an antimalarial therapy. PLOS ONE. 2012;7(12):e52746. doi: 10.1371/journal.pone.0052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Weathers PJ, Rich SM. Dried whole plant Artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artemisinin. PNAS USA. 2015;112:821–826. doi: 10.1073/pnas.1413127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JF, Luthria DL, Sasaki T, Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:3135–3170. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MH, Lategan C, Graziose R, Smith PJ, Raskin I, Lila MA. Antiplasmodial activity of the ethnobotanical plant Cassia fistula. Natural Product Communications. 2012;7:1263–1266. [PubMed] [Google Scholar]
- Hsu E. The history of qing hao in the Chinese materia medica. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100:505–8. doi: 10.1016/j.trstmh.2005.09.020. [DOI] [PubMed] [Google Scholar]
- ICIPE. Whole-leaf Artemisia annua-based antimalarial drug: report on proof-of-concepts studies. 2005 unpublished copy available at: http://bit.ly/1vCHQkH Accessed Jan. 27, 2017.
- Liu KC, Yang SL, Roberts MF, Elford BC, Phillipson JD. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Reports. 1992;11:637–640. doi: 10.1007/BF00236389. [DOI] [PubMed] [Google Scholar]
- Novartis. Novartis Malaria Treatment. :2015. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/coartem.pdf Accessed Jan. 27, 2017.
- Onimus M, Carteron S, Lutgen P. The surprising efficiency of Artemisia annua powder capsules. Medicinal and Aromatic Plants. 2013;2:3. doi: 10.4172/2167-0412.1000125. [DOI] [Google Scholar]
- Suberu JO, Gorka AP, Jacobs L, Roepe PD, Sullivan N, Barker GC, Lapkin AA. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract–possible synergistic and resistance mechanisms. PLoS One. 2013;8:e80790. doi: 10.1371/journal.pone.0080790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler MJ, Weathers PJ. Variations in key artemisinic and other metabolites throughout plant development in a clonal cultivar of Artemisia annua for possible therapeutic use. Industrial Crops and Products. 2015;67:185–191. doi: 10.1016/j.indcrop.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Towler MJ, Hassanali A, Lutgen P, Engeu PO. Dried-leaf Artemisia annua, pACT: a practical malaria therapeutic for developing countries? World Journal of Pharmacology. 2014a;3:39–55. doi: 10.5497/wjp.v3.i4.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Elfawal MA, Towler MJ, Acquaah-Mensah G, Rich SM. Pharmacokinetics of artemisinin delivered by oral consumption of Artemisia annua dried leaves (pACT) in healthy vs. Plasmodium chabaudi-infected mice. Journal of Ethnopharmacology. 2014b;153:732–736. doi: 10.1016/j.jep.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DW. Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochemistry Reviews. 2011;10:173–183. doi: 10.1007/s11101-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Towler MJ. Changes in key constituents of clonally propagated Artemisia annua L. during preparation of compressed leaf tablets for possible therapeutic use. Industrial Crops and Products. 2014;62:173–178. doi: 10.1016/j.indcrop.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Management of severe malaria. 2012 http://apps.who.int/iris/bitstream/10665/79317/1/9789241548526_eng.pdf Accessed Jan. 27, 2017.
- WHO. Severe Malaria. Tropical Medicine and International Health. 2014;19(Suppl 1):7–131. doi: 10.1111/tmi.12313_2. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2015. 2015 http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ Accessed Jan. 27, 2017.
- WHO. 2017 http://www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/en/ Accessed Jan. 30, 2017.


