Abstract
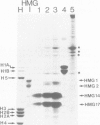
Chicken erythrocyte nuclei were incubated with DNA intercalating agents in order to isolate from chromatin specific DNA-binding proteins whose binding specificity may be determined by DNA secondary and/or tertiary structure. The intercalating agents ethidium bromide (EtBr) and propidium iodide induce the specific release of high mobility group proteins HMG 14 and HMG 17 under low ionic strength conditions. Chloroquine (CQ) intercalation also results in the selective liberation of HMG 14 and HMG 17, but, in addition, selectively releases other nuclear proteins (including histone H1A) in a pH- and ionic strength-dependent fashion. The use of this new 'elutive intercalation' technique for the isolation and purification of 'sequence-specific' and 'helix-specific' DNA-binding proteins is suggested.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frederick C. A., Grable J., Melia M., Samudzi C., Jen-Jacobson L., Wang B. C., Greene P., Boyer H. W., Rosenberg J. M. Kinked DNA in crystalline complex with EcoRI endonuclease. Nature. 1984 May 24;309(5966):327–331. doi: 10.1038/309327a0. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy W B., Wong N. C., Dixon G. H. Selective association of the trout-specific H6 protein with chromatin regions susceptible to DNase I and DNase II: possible location of HMG-T in the spacer region between core nucleosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2810–2814. doi: 10.1073/pnas.74.7.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B., Kuehl L., Dixon G. H. The release of high mobility group protein H6 and protamine gene sequences upon selective DNase I degradation of trout testis chromatin. Nucleic Acids Res. 1980 Jul 11;8(13):2859–2869. doi: 10.1093/nar/8.13.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottspeich F. Identification of the phenylthiohydantoin derivatives of amino acids by high pressure liquid chromatography, using a ternary, isocratic solvent system. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1829–1834. doi: 10.1515/bchm2.1980.361.2.1829. [DOI] [PubMed] [Google Scholar]
- Nickol J. M., Felsenfeld G. DNA conformation at the 5' end of the chicken adult beta-globin gene. Cell. 1983 Dec;35(2 Pt 1):467–477. doi: 10.1016/0092-8674(83)90180-0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Paton A. E., Wilkinson-Singley E., Olins D. E. Nonhistone nuclear high mobility group proteins 14 and 17 stabilize nucleosome core particles. J Biol Chem. 1983 Nov 10;258(21):13221–13229. [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Sandeen G., Wood W. I., Felsenfeld G. The interaction of high mobility proteins HMG14 and 17 with nucleosomes. Nucleic Acids Res. 1980 Sep 11;8(17):3757–3778. doi: 10.1093/nar/8.17.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter H., Bode J. The binding sites for large and small high-mobility-group (HMG) proteins. Studies on HMG-nucleosome interactions in vitro. Eur J Biochem. 1982 Oct;127(2):429–436. doi: 10.1111/j.1432-1033.1982.tb06890.x. [DOI] [PubMed] [Google Scholar]
- Spiker S., Mardian J. K., Isenberg I. Chomosomal HMG proteins occur in three eukaryotic kingdoms. Biochem Biophys Res Commun. 1978 May 15;82(1):129–135. doi: 10.1016/0006-291x(78)90586-7. [DOI] [PubMed] [Google Scholar]
- Strätling W. H., Seidel I. Relaxation of chromatin structure by ethidium bromide binding: determined by viscometry and histone dissociation studies. Biochemistry. 1976 Nov 2;15(22):4803–4809. doi: 10.1021/bi00667a009. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Johns E. W. The isolation, characterization and partial sequences of the chicken erythrocyte non-histone chromosomal proteins HMG14 and HMG17. Comparison with the homologous calf thymus proteins. Biochem J. 1980 Feb 1;185(2):383–386. doi: 10.1042/bj1850383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. M., Stearn C., Johns E. W. The primary structure of non-histone chromosomal protein HMG17 from chicken erythrocyte nuclei. FEBS Lett. 1980 Apr 7;112(2):207–210. doi: 10.1016/0014-5793(80)80181-5. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]
- Watson D. C., Wong N. C., Dixon G. H. The complete amino-acid sequence of a trout-testis non-histone protein, H6, localized in a subset of nucleosomes and its similarity to calf-thymus non-histone proteins HMG-14 and HMG-17. Eur J Biochem. 1979 Mar 15;95(1):193–202. doi: 10.1111/j.1432-1033.1979.tb12953.x. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B. The three-dimensional structure of DNA. Annu Rev Biochem. 1982;51:395–427. doi: 10.1146/annurev.bi.51.070182.002143. [DOI] [PubMed] [Google Scholar]