Abstract
With the increasing prevalence of end stage renal disease there is a growing need for hemodialysis. Arteriovenous fistulae (AVF) are the preferred type of vascular access for hemodialysis but maturation and failure continue to present significant barriers to successful fistula use. AVF maturation integrates outward remodeling with vessel wall thickening in response to drastic hemodynamic changes, in the setting of uremia, systemic inflammation, oxidative stress and preexistent vascular pathology. AVF can fail due to both failure to mature adequately to support hemodialysis, as well as development of neointimal hyperplasia (NIH) that narrows the AVF lumen, typically near the fistula anastomosis. Failure due to NIH involves vascular cell activation and migration and extracellular matrix remodeling with complex interactions of growth factors, adhesion molecules, inflammatory mediators, and chemokines, all of which result in maladaptive remodeling.
Different strategies have been proposed to prevent and treat AVF failure, based on current understanding of the modes and pathology of access failure; these approaches range from appropriate patient selection and use of alternative surgical strategies for fistula creation, to the use of novel interventional techniques or drugs to treat failing fistulae. Effective treatments to prevent or treat AVF failure requires a multidisciplinary approach involving nephrologists, vascular surgeons and interventional radiologists, allowing careful patient selection and the use of tailored systemic or localized interventions to improve patient-specific outcomes. This review provides contemporary information on the underlying mechanisms of AVF maturation and failure and discusses the broad spectrum of options that can be tailored for specific therapy.
Keywords: Arteriovenous fistula, maturation, maturation failure, angioplasty
Introduction
1. The prevalence of ESRD is increasing
Chronic kidney disease (CKD) is increasing in incidence worldwide, and with an estimated prevalence of 8%–16%, contributes significantly to the global burden of disease [1, 2]. The global prevalence of diabetes in adults is 9.1% (415 million people) according to a report published in 2015 by the International Diabetes Federation, rising beyond 10.4% (642 million people) by 2040 [3], largely due to the global increase in type 2 diabetes and obesity, especially in China, India and some developing countries in Africa [4–6]. This increase in the number of people developing diabetes has had a major impact on the development of diabetic kidney disease (DKD). DKD and an aging population have become the two challenges in managing end-stage renal disease (ESRD) worldwide. DKD is the leading cause of ESRD, accounting for approximately 50% of cases in the developed world. Although overall incidence rates for ESRD attributable to DKD have recently stabilized in the USA, these rates continue to rise in high-risk groups such as middle-aged African Americans, Native Americans, and Hispanics. The elderly population constitutes the fastest growing sector of the ESRD population and have unique needs by virtue of their high prevalence of comorbid conditions, slower progression of renal disease, and reduced survival; in the Medicare population alone, DKD-related expenditure among the elderly was nearly $25 billion in 2011 [7, 8]. With the increasing prevalence of ESRD, there is a growing need for renal replacement therapies (RRT).
2. AVF is the preferred form of RRT but is far from optimal
RRTs are the lifeline for ESRD patients. Modes of RRT include peritoneal dialysis (6.4%), renal transplant (29.3%), and hemodialysis (HD) (64.2%) [9, 10]. In 2010, 64.7% of patients in the United States with ESRD were treated with HD with either arteriovenous fistula (AVF), arteriovenous grafts (AVG), or tunneled and non-tunneled central venous catheters [11].
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines and the Fistula First Breakthrough Initiative prefer AVF as the optimal access for HD [12], as they have superior patency rates, fewer complications and lower health care costs [11, 13–15]. Additionally, a recent systematic review and meta-analysis on outcomes of vascular access for hemodialysis remains in support of autogenous access as the best approach when feasible: AVF were associated with the best patency and lowest infection and mortality outcomes, followed by AVG and catheters [16, 17]. AVF have also been recommended in the pediatric population [18].
However, AVF are not immediately available for use as an access for HD as they must mature, e.g. dilate and thicken. Unfortunately, AVF have a high rate of primary maturation failure with up to 60% not suitable for HD by 5 months after creation [19–22]. Furthermore, a recent systematic review and meta-analysis reported that the primary patency rates of AVF were 60% at 1 year and 51% at 2 years, with secondary patency rates of 71% at 1 year and 64% at 2 years, clearly suboptimal for a permanent treatment [23]. Although there are conflicting results regarding sex influence on AVF failure, most studies demonstrated that women have prolonged maturation time and decreased patency rate [17, 24, 25]; early thrombosis was also associated more frequently with women [26]. Controversially, AVF may not be favored for HD access in older patients [27–29]. Olsha et al demonstrated that 88% of their patients who were older than 80 years had vasculature suitable for autogenous access construction, with patency and complications similar to those of their younger counterparts, with adequate preoperative planning and postoperative maintenance [29]. However, elderly patients with ESRD frequently have a high prevalence of comorbidities, short life expectancy, and poor reported quality of life that is associated with lack of AVF maturation and diminished primary and cumulative AVF patency [28]; in these patients AVG placement might be more beneficial [27, 28, 30].
3. Lack of well-established clinical criteria to define AVF maturation or failure
AVF maturation is considered clinically successful if 6 weeks after surgery the fistula supports a flow of 600 mL/min, is located at a maximum of 6 mm from the skin surface and has a diameter of >6 mm [12], but this definition is difficult for clinical use. The North American Vascular Access Consortium definition may be more useful: a fistula is mature if it can be successfully used for dialysis with two-needle cannulation for two-thirds or more of all dialysis runs for 1 month and if it delivers the prescribed dialysis within the prescribed time frame [31].
Although there are some clinical criteria to define successful AVF maturation, the clinical definition of AVF failure is less clear, with frequent confusion between various types and stages of failure. Based on previous criteria and recent multicenter research [12, 26, 31], we have defined the 3 types of AVF failure as: early thrombosis, failure to mature, and late failure (Table 1).
Table 1.
Types of AVF failure.
| AVF failure type | Time | Definition | Pathophysiology |
|---|---|---|---|
| Early thrombosis | < 3 weeks | thrombosis of the access | Hypercoaguability |
| Failure to mature | < 6 months | Patent access but not suitable for cannulation or high efficiency hemodialysis | Inability to remodel outwardly |
| Late failure | > 3 months | a mature AVF used for at least 3 months that subsequently develops a problem requiring intervention | Neointimal hyperplasia |
Many variables contribute to successful AVF maturation or AVF failure: patient age, sex, presence of diabetes, obesity, vessel characteristics, surgical technique and surgeon experience, preoperative planning and mapping [32–34]. Successful access surgeons frequently adhere to the dictum that a successful AVF should be performed in the right patient at the right time in the right circumstances based upon comprehensive understanding of the mechanisms contributing to AVF maturation and AVF failure. The goal of this review paper is to give a basic understanding of the adaptive changes of AVF maturation as a framework to understand the mechanisms of AVF failure as well as subsequent treatments.
Mechanisms contributing to AVF maturation and failure
Mechanisms of AVF maturation
1. AVF maturation integrates outward remodeling and wall thickening
After AVF creation, the vein is exposed to a high flow, high shear stress, high pressure, and oxygen-rich arterial environment, leading to “maturation” of both the arterial inflow limb as well as venous outflow limb. Adaptation of the vein to the increased flow and shear stress of the arterial environment requires dilation by outward remodeling of the venous wall, (Poiseuille’s law), whereas increases in pressure and tensile stress result in wall thickening, (Laplace’s law). During this adaptive remodeling, hemodynamic changes are translated into endothelial and adventitial signaling, inducing structural changes in cells and the extracellular matrix (ECM); inflammation, growth factors and cell adhesion molecules in all three layers of the venous wall are involved with the process inducing remodeling (Figure 1).
Figure 1.
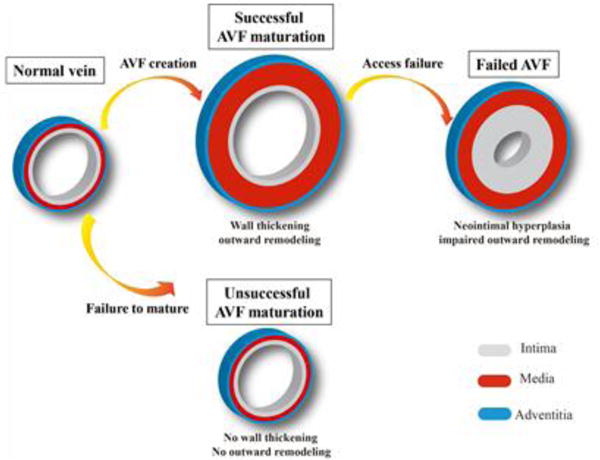
Schematic representation of the venous wall structural changes that occur after AVF creation. AVF successful maturation integrates wall thickening and outward remodeling. A failed AVF can be due to early failure to mature, with failure to develop outward remodeling or wall thickening, or may be due to later development of neointimal hyperplasia and impaired outward remodeling in a previously functional conduit.
1.1 Hemodynamic flow
Blood flow in the cephalic vein is normally approximately 28 ± 14 ml/min [35]. Successful radial-cephalic AVF have flow rates averaging between 600–1000 ml/min with higher peak flows in the larger diameter brachial-cephalic AVF. Dixon et al. reported that 90% of forearm AVF have flows between 500–2000 ml/min, whereas 90% of upper arm AVF have flows between 500–3000 ml/min [36]. Shear stress in the cephalic limb of a brachial-cephalic fistula increases from preoperative venous magnitudes of 5–10 dyne/cm2 to 24.5 dyne/cm2 after one week, which then normalizes to 10.4 dyne/cm2 over 3 months [37]. With these high magnitudes of flow in the AVF, the character of the flow may be disturbed, e.g. non-laminar and disordered, possibly even turbulent [38–41].
1.2 Outward remodeling and wall thickening
AVF maturation is the product of both vessel wall outward remodeling and thickening and is thought to be an adaptive process to the increased pressure, shear stress, and oxygen tension from the arterial inflow that is no longer attenuated by resistive forces of the arterioles and the capillary bed. Different from vein grafts, AVF adapt mainly via outward dilation and wall thickening with less intimal thickening [42]. Schwartz et al. confirmed this behavior using a rabbit model, showing that AVF are exposed to higher flow than vein grafts (AVF: 82 ± 17 ml/min; vein grafts: 16 ± 4 ml/min) as well as increased shear stress (AVF: 71 ± 50 dyne/cm2; VG: 0.96 ± 0.38 dyne/cm2). AVF showed increased dilation (AVF: 194%; VG: no change), whereas vein grafts were exposed to higher pressure (VG: 62 ± 3 mmHg; AVF: 6 ± 2 mmHg) and had increased myointimal area (VG: 4.72 ± 0.83 mm2; AVF: 1.9 ± 0.55 mm2)[43].
Outward remodeling is thought to be mediated by the venous endothelium and adventitia that sense hemodynamic forces and integrates these forces to allow successful adaption without loss of luminal area and vessel patency [21, 24, 44, 45]. Venous diameter expansion is a critical element of outward remodeling and predicts clinical success for both AVF and vein grafts [46–48]. Several studies examining venous dilation in AVF reported mean diameter increases from 2.3–3.2 to 5.8–6.6 mm by 3 months after fistula creation. These values reflect a 45%–86% increase within the first month and an increase of up to 179% after 3 months, corresponding to an average cross-sectional area of approximately 10–12 mm2, to normalize the shear stress [49, 50].
Wall thickening is the adaptation of the vessel wall to increased pressure. This process involves expansion of all the vessel layers via both ECM deposition and cell proliferation and migration [44, 45, 51]. Several types of cells are involved in wall thickening, including smooth muscle cells, adventitial fibroblasts, and bone marrow derived progenitor cells [45, 52–54]. The adventitial myofibroblast is critical during venous adaptation to arterial flow and helps maintain venous wall integrity and hemostasis after surgical creation of the AVF [45]. Myofibroblast precursors residing in the venous adventitia sense the abrupt mechanical forces produced by arterial flow to rapidly adjust their genomic expression program to help increase vascular resistance. This adaptive response includes the formation of bundles of contractile microfilaments and extensive cell-to-matrix attachment sites as well as the secretion of MMPs, collagen, and ECM proteins that strengthen the fistula wall [45, 55]. Targeting the adventitia to treat NIH is a newer strategy to prevent AVF venous stenosis [56].
1.3 Endothelial Signaling
Molecular signaling within the maturing AVF is of vital importance to understand and control the normal adaptive response of fistula maturation and the abnormal maladaptive response of fistula failure. The release of chemotactic and inflammatory mediators from the endothelium during surgical manipulation and hemodynamic variation are important during the initial phase of adaptation. Directly after AVF creation, high magnitudes of arterial flow result both in passive vascular distention and nitric oxide (NO) synthesis by endothelial cells with subsequent vascular smooth muscle cell (VSMC) relaxation, resulting in acute vasodilation [57–59]. NO is produced by endothelial nitric oxide synthase (eNOS), and is a potent vasodilator and signaling molecule with anti-inflammatory and anti-platelet properties [51, 59, 60]. eNOS may contribute to adaptive vein wall remodeling both through its anti-inflammatory and anti-thrombotic properties as well as through its anti-proliferative properties. Both eNOS and inducible nitric oxide synthase (iNOS) are upregulated in the AVF and may mediate adaptation; inhibition of eNOS results in increased MCP-1 and IL-8, leading to NIH [54, 61]. Endothelin-1 (ET-1) is an inflammatory mediator of vasoconstriction and endothelial proliferation. ET-1 expression is upregulated in the venous wall and within areas of NIH in AVF as well as in the plasma of patients with chronic renal failure and hemodialysis; ET-1 may mediate wall thickening in response to localized hemodynamic forces [62–64].
1.4 Matrix remodeling
Venous adaptation of AVF also depends on coordinated synthesis, secretion, and degradation of ECM [42]. The matrix metalloproteinase (MMP) family regulates ECM remodeling and allows cell migration through degradation of collagen and elastin. MMP activity is stimulated by a variety of factors present during vein graft adaptation including flow, stretch, mechanical injury, inflammation, and oxidative stress [65–69]. In AVF, MMP-2 and MMP-9 expression are upregulated, and a high serum ratio of MMP-2 to TIMP predicts AVF maturation [61, 65, 66, 68].
During AVF venous limb maturation, the process of ECM expression, synthesis, secretion, and deposition occurs in distinct temporal phases (Figure 2) [70]. Initial ECM degradation occurs early after AVF creation, coincident with early increased expression of MMP and tissue inhibitor of metalloproteinase 1 (TIMP-1). By day 7 there is increased expression of many collagen subunits as well as changing patterns of MMP expression. By day 21, a later phase is characterized by reduced MMP expression and increased expression of larger structural and non-collagenous matrix proteins.
Figure 2.
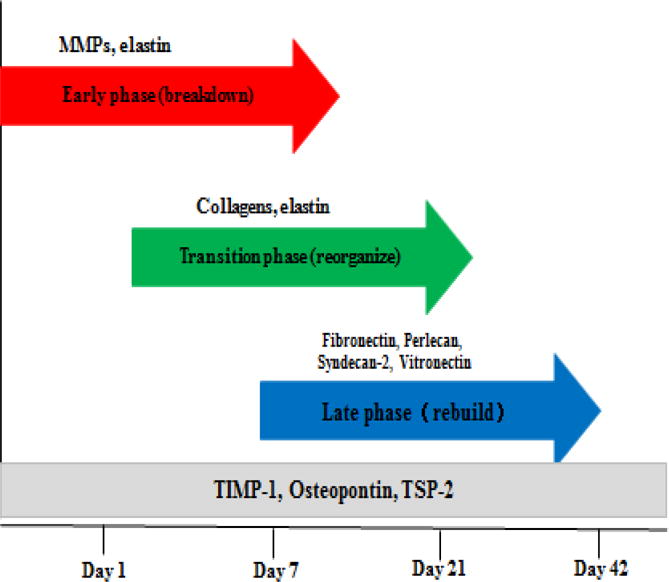
Diagram depicting the 3 phases of ECM changes during the adaptive process of AVF maturation. There is an early phase of ECM breakdown from MMP degradation. A transition phase follows with collagen and elastin reorganization of the venous scaffold. A later rebuilding phase strengthens the matrix of the AVF with larger non-collagenous and glycoproteins. TIMP-1, osteopontin and thrombospondin-2 (TSP-2) are highly expressed throughout AVF maturation suggesting regulatory roles for these proteins.
Matrix degradation is regulated by MMP whereas matrix deposition is regulated by transforming growth factor-β (TGF-β); TGF-β is produced by a variety of cell types present in the venous wall, including endothelial, smooth muscle, and inflammatory cells, potentially contributing significantly to intimal and medial thickening [63, 71, 72]. TGF-β is upregulated at both early and later time points after AVF formation, depending on the model, and this expression correlates with ECM accumulation [61, 71, 73, 74].
1.5 Adventitia and perivascular cells
With growing recognition of the importance of the adventitia and perivascular cells to vascular remodeling, the role of adventitial fibroblasts has gained attention. Recent data suggests that NIH consists of smooth muscle alpha-actin-positive, vimentin-positive and desmin-negative myofibroblasts that have probably migrated from the adventitial layer [52, 75]. AVF failure is associated with an increased adventitial fibrosis, myofibroblast activation and capillary rarefaction [76].
Molecular signals originating from the adventitia and perivascular cells play essential roles in regulation of vascular development, physiology, vascular wall remodeling, immune surveillance, and vascular disease. The adventitia contains many different interacting cell types including fibroblasts, microvascular endothelium, nerves, resident macrophages, T cells, B cells, mast cells, and dendritic cells; the adventitia is also the home to resident vascular progenitor cells [77]. Perivascular adipose tissue (PVAT) plays multiple roles in vascular physiology and remodeling including production of vasorelaxing and anticontractile factors [78, 79]. One important component of this adipose tissue–derived anticontractile activity is adiponectin. Adiponectin is an adipocyte-derived 244 amino acid long peptide hormone that regulates metabolic processes such as fatty acid oxidation, and also mediates vasorelaxation. Adiponectin receptors on VSMC activate calcium-sensitive potassium channels (BKca) leading to stimulation of eNOS activity and production of NO [80]. A similar pathway exists in endothelial cells. Together, the production of NO from endothelial cells and SMC mediates the anticontractile effects of PVAT-derived adiponectin [79].
Mechanisms of AVF failure
1. Early thrombosis
Blood, flow and the vessel wall, components of Virchow’s triad, are traditionally considered to be the three critical components of thrombosis [81]. Patients with ESRD show both a bleeding risk and an increased risk of thrombosis. The bleeding episodes involve platelet dysfunction, impaired platelet–vessel wall interactions and anemia [82–84], in addition to the use of anticoagulants during hemodialysis. Several clinical trials have also shown increased risk of both spontaneous venous and arterial thrombosis along the entire spectrum of CKD, beginning from CKD stage 2 to stage 5 patients [85–87].
ESRD is characterized by a number of metabolic abnormalities that alter the balance of pro- and anti-thrombotic factors that affect both thrombosis and hemostasis [88]. This is likely to be an important contributor to the increased risk of thrombosis in patients with ESRD. Several pro-thrombotic hemostatic mediators are elevated in CKD patients, including fibrinogen, which directly contributes to a hypercoagulable state [89], soluble thrombomodulin [90], soluble tissue factor (TF), thrombin-anti-thrombin (TAT) [91], von Willebrand factor (vWF) [91], factor VIII and C-reactive protein (CRP) [91, 92]. The generalized inflammatory state, endothelial dysfunction and possibly poor clearance of some of the thrombotic mediators may account for this metabolic derangement [89]. CKD and ESRD patients have a disrupted endothelial glycocalyx, which also contributes to the increased risk of thrombosis [93, 94]. The endothelial cells of uremic patients express elevated levels of tissue factor, a crucial procoagulant activating the extrinsic coagulation cascade [95, 96]. Uremic endothelial cells also release small extracellular vesicles, called microparticles, loaded with TF to augment thrombosis.[96–98].
In addition, hypercoagulable states also predispose to an increased risk of early thrombosis. Factor V Leiden polymorphism has been inconsistently implicated [99, 100]. High levels of phospholipid antibodies, probably due to the uremic state and high levels of low density lipoprotein have similarly shown an association [101, 102].
In the setting of the pro-thrombotic state of ESRD, lack of surgical experience and insufficient preoperative vessel mapping can result in early thrombosis. A study from the Hemodialysis Fistula Maturation Study group showed that factors that contribute to early thrombosis include: female sex, use of forearm AVF, smaller arterial size, draining vein diameter of 2 to 3 mm, and protamine use [26]. Patient selection, sufficient preoperative mapping and an appropriately experienced surgical team are important to prevent early thrombosis.
2. AVF failure to mature
Effects of Hemodynamic Flow and Shear Stress
In response to the hemodynamic changes after AVF creation, an AVF will undergo adaptive remodeling with outward remodeling and increased wall thickness. But in the setting of pre-existent vasculopathy and systemic abnormalities, an AVF will fail to mature either because of aggressive NIH or impaired outward remodeling, or both. Although most research on the pathophysiology of AVF maturation failure focuses on NIH, the role of vascular outward remodeling should be also highlighted [103].
The increased hemodynamics of arterial flow that increases vessel wall shear stress (WSS) is likely to be a critical event after AVF creation that promotes AVF adaptation [104–106]. In a patient-specific side-to-end fistula, image-based computational fluid dynamics studies showed laminar flow within the arterial limbs and a complex multidirectional and reciprocating flow field on the inner side of the swing segment in the proximal venous limb [106]. NIH is predisposed to occur in the inner wall of the venous segment near the anastomosis, and has a strong inverse correlation with magnitudes of shear stress, but is also related to flow patterns [107].
In contrast, disturbed flow, with low and reciprocating WSS, induces selective expression of atherogenic and thrombogenic genes (pro-oxidant, proinflammatory, procoagulant, and proapoptotic) in endothelial cells [108]. Dai et al. compared the effects of two different atheroprone and atheroprotective shear stress patterns in vitro and demonstrated an important effect of low and oscillating WSS on the EC cytoskeleton, interleukin production, and nuclear translocation of transcription factor NF-κB to enhance expression of adhesion molecules [109]. Disturbed flow promotes an inflammatory and thrombotic phenotype in arterial ECs, increasing binding of monocyte chemotactic protein-1 (MCP-1) to the cysteine-cysteine receptor and stimulating SMC migration and proliferation, all of which may enhance NIH [110, 111].
Using pulsatile computational fluid dynamics simulation with idealized models of AVF, Ene-Iordache et al found that despite the high flow rate after AVF creation, WSS in the areas of the juxta-anastomotic vein was low and oscillating, both in end-to-side and end-to-end anastomosis configurations [39]. Due to the pulsatility of flow during the cardiac cycle, recirculation and reattachment flow with low velocity develops near the wall, inducing disturbed flow with low and reciprocating WSS on the inner surface of the juxta-anastomotic segment and on the distal artery. In a parametric idealized model of end-to-side AVF, Ene-Iordache et al further studied whether the anastomosis angle influences the pattern of disturbed flow. Quantification of these areas showed that the smaller the angle, the smaller the area of low and oscillating WSS, as quantified by the relative residence time. These results suggest that an acute anastomosis angle (30°) should be preferred to minimize the risk of NIH in the juxta-anastomotic vein [112]. They also demonstrated that in hemodialysis patients, the peak shear stress rather than the average shear stress, is the key factor in driving vessel dilatation to increase blood volume flow [113].
Bharat et al. performed a clinical study in patients undergoing radiocephalic AVF comparing three different anastomotic techniques. A novel technique of vascular anastomosis, the piggyback straight-line onlay technique, was characterized by a very small anastomosis angle and resulted in very few juxta-anastomotic stenoses compared to the traditional end-to-side and side-to-side techniques [114]. By using the piggyback straight-line onlay technique, disturbed flow is reduced due to the acute angle and the venous wall injury produced by the traditional torsion of the juxta-anastomotic vein is minimized [114]. Recently, Sadaghianloo et al reported that radial-cephalic fistulae with angles <30° have reduced primary and secondary patency and increased numbers of interventions, suggesting that, if possible, surgeons should avoid an anastomotic angle of <30° when creating radial-cephalic fistulae [115]. Chemla et al reported that AVF created with the VasQ™, an external support device, showed a high unassisted maturation and patency rate, possibly by minimizing flow disturbance around the area of anastomosis [116]. All of these data suggest that research based on anastomotic geometry may optimize the flow state and potentially be used for preoperative surgical planning.
Responses to injury
Endothelial and vascular injury results in the activation, proliferation and migration of fibroblasts, smooth muscle cells, and myofibroblasts from the media and/or adventitia to the intima; complex interactions of adhesion molecules, inflammatory mediators and chemokines results in venous NIH [117]. During this process, inflammation, oxidative stress, cellular phenotype change and migration of vascular cells and ECM remodeling play important roles.
Inflammation
In the inflammatory state of the uremic environment, the injury of AVF creation and local hypoxia is characterized by the presence of CD68-positive macrophages and CD3-positive lymphocytes. This infiltration is more significant in the setting of CKD [118]. Some inflammatory cytokines are upregulated such as IL-6, IL-8, MCP-1, and PAI-1 [61, 63, 119], and these mediators are associated with fistula failure [120]. IL-6 and TNF-α are more highly expressed in thrombosed AVF, and both CRP and fibrinogen are associated with AVF failure [121, 122].
CD68- and CD3- positive cells have been found in increased numbers in stenotic vessels. Macrophage migration inhibitory factor (MMIF) is hypothesized to play an important role in this local inflammatory response, potentiating neointimal thickening by driving inflammatory cells toward the neointima and leading to the proliferation of medial and intimal cells [123, 124]. MMIF has been identified in clinical and experimental models of vascular access. MMIF acts through the CD74 receptor, chemokine receptor 2, and chemokine receptor 4 [123]. These in turn act through extracellular signal-regulated and p38 mitogen-activated protein kinase pathways that up-regulate vascular endothelial growth factor (VEGF)-A, interleukin (IL)-8, and monocyte chemotactic protein-1 (MCP-1) [124].
MCP-1 is a potent chemokine that plays an important role in atherosclerosis and other vascular diseases through promoting chemotaxis of monocytes and macrophages, activation and migration of endothelial cells, proliferation and migration of smooth muscle cells, and induction of procoagulant mediators [125–128]. Expression of MCP-1 increased 1 week after AVF creation in mice, and was localized within the endothelium, smooth muscle cells, and leukocytes in a rodent AVF model. The MCP-1 knockout mouse model showed reduced NIH [129]. Moreover, in the murine model of CKD with AVF, there is an increase in gene expression of arginase-1, a marker for proinflammatory macrophages, followed by an increase in inducible nitric oxide, a marker for reparative macrophages [130]. Thus, MCP-1 appears to be upregulated very early after AVF creation and serves as a mediator for AVF dysfunction and failure.
Oxidative stress
Patients with ESRD have systemic inflammation and oxidative stress; the hemodynamic changes and local injury of the AVF procedure may further increase oxidative stress in the AVF wall. Oxidative stress and injury stimulates synthesis and secretion of ROS that in turn stimulate numerous signaling pathways, regulating diverse processes such as smooth muscle cell migration and proliferation as well as activating latent MMP, potentially mediating many aspects of venous remodeling [64, 131]. For example, superoxide can deplete NO, resulting in disruption of numerous pathways with resultant endothelial cell and general vascular dysfunction.
Recent investigations have shown that heme oxygenase (HO) production is related to AVF function [132–136]. HO is a cytoprotective and rate-limiting enzyme responsible for heme degradation, generating free iron, biliverdin, and carbon monoxide; biliverdin is subsequently converted to bilirubin by biliverdin reductase, and free iron is rapidly sequestered by ferritin [134]. Bilirubin is a free radical scavenger that blocks lipid peroxidation [137]. Carbon monoxide is a physiologically important vasodilator, acting via cyclic guanosine monophosphate (cGMP) [138]. HO exerts antioxidant, anti-inflammatory, antiapoptotic, and angiogenic functions through its reactive products [134, 137, 138]. HO-1 is the inducible isoform, whereas HO-2 is expressed constitutively. Patients with HO-1 gene polymorphisms characterized by long GT repeats, resulting in less HO-1 production, were more likely to have worse AVF patency [132, 133]. In murine AVF, HO-1 gene expression is markedly induced in the vascular smooth muscle cells; HO-1 knockout mice how reduced patency with thinner vein walls and increased luminal area, as well as increased expression of proinflammatory and pro-oxidant mediators such as MCP-1, MMP-2 and MMP-9 [139]. A functional AVF also requires HO-2 [135]. Shear stress can regulate HO-1 activity, with high flow inducing HO-1 to generate NO and mitochondria-derived hydrogen peroxide; low flow induces lower levels of HO-1 that lead to macrophage infiltration and superoxide production within the vessel wall, suggesting an important role for HO in promoting outward remodeling and preventing NIH [140].
Cellular phenotype change and migration of vascular cells
The development of NIH is a complex process that requires activation, phenotype change and migration of vascular cells; NIH that forms at the venous anastomosis of a dialysis access graft or fistula is comprised primarily of smooth muscle alpha-actin-positive, synthetic VSMC phenotype or vimentin-positive and desmin-negative myofibroblasts that probably migrated from the media or adventitia layers in response to vascular injury [52, 75]. Other studies have suggested that bone marrow–derived cells are capable of differentiation into smooth muscle cells and may be another potential origin of neointimal cells [54, 141]. Interestingly, VSMC from the proximal artery may contribute substantially to venous intimal hyperplasia; in a murine AVF model increased Notch signaling can drive migration of these cells to the venous outflow tract [142].
In addition to increased cell numbers, there is synthesis of new ECM within the intima to form the lesion of NIH [21, 103, 117]. VSMC also become resistant to NO, decreasing SMC relaxation and preventing AVF maturation by reducing the ability to successfully outward remodel [143].
These data show that the entire vessel wall is involved in the processes that lead to access failure, and suggest additional targets for potential therapy; recent studies have demonstrated the potential to target and treat the adventitial space to prevent the adventitial response to injury and prevent AVF failure [56, 144, 145].
Growth Factors and Cell Adhesion Molecules
Numerous growth factors and cytokines play roles during AVF maturation, particularly by regulating pathways that control ECM synthesis, secretion, and degradation, as well as through control of cell proliferation and migration [42].
Local inflammation with monocyte infiltration into the AVF increases expression of TGF-β1 and insulin-like growth factor-1 (IGF-1) [74]. TGF-β1, despite its classically having anti-inflammatory properties, leads to ECM deposition, potentially causing thrombosis [74]. Differences in TGF-β1 polymorphisms, result in differing levels of TGF-β1 production, have been correlated with AVF patency [71]. TGF-β is expressed within stenotic AVF, and correlates with areas of ECM deposition; TGF-β1 also activates the fibroblast transition to the myofibroblast phenotype [146]; myofibroblasts are the major cellular component found in AVF stenoses, producing ECM proteins and MMP [52]. Sustained TGF-β expression abolishes myofibroblast disappearance and leads to NIH [63]. In toto, increased expression of TGF-β is associated with decreased AVF patency, likely due to increased deposition of ECM [73, 74].
Other growth factors participate in AVF maturation. IGF-1 also induces ECM synthesis, smooth muscle proliferation and migration, and inhibits apoptosis [74]. Platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) also play significant roles in stimulating cell proliferation and migration. Both PDGF-α/β and IGF-1 expression are upregulated in AVF [61, 64]. Vascular endothelial growth factor (VEGF) plays several roles in vascular remodeling, including stimulation of endothelial proliferation and differentiation, modulation of smooth muscle cell proliferation and migration, angiogenesis, ECM deposition, and modulation of the inflammatory response. VEGF may also play an inhibitory role in AVF adaptation, since overexpression of VEGF-A contributes to negative remodeling and NIH, whereas inhibition of VEGF-A is associated with increased lumen area and decreased inward remodeling [56].
Selectins facilitate leukocyte adhesion. P-selectin is present on endothelial cells and platelets, and E-selectin is present on endothelial cells; ICAM and VCAM facilitate additional binding and migration [147]. P-selectin and E-selectin expression are both upregulated early after AVF creation, followed by decreased P-selectin expression after 1 month [61]. VCAM-1, but not ICAM-1, is highly expressed in thrombosed and stenotic AVF [121]. β-catenin and c-Myc expression are increased 1 week after AVF creation, correlating with decreased N-cadherin and associated with smooth muscle cell proliferation [148].
ECM remodeling
ECM remodeling involves coordinated synthesis, secretion, and degradation of ECM, which plays an important role both in normal AVF maturation as well as in the development of neointima and AVF failure [20, 21, 149]. ECM degradation occurs early after AVF creation, coincident with early increased expression of MMP and TIMP-1 [70]. Outward remodeling in response to the increased arterial flow requires early expression of MMP-2 and MMP-9 to degrade cell basement membranes and the internal elastic lamina, allowing vessel enlargement [150]. A high serum ratio of MMP-2 to TIMP predicts AVF maturation [65, 66, 68]. Other elastases such as cathepsin S and cathepsin K are also upregulated in the AVF and may be associated with degradation of the internal elastic lamina [66]. Diminished elastin results in enhanced outward remodeling, suggesting that elastin degradation might be an option to improve AVF maturation [151].
However, the disruption of the elastic lamina and loss of integrity of this structural barrier may allow migration of medial VSMC or adventitial fibroblasts into the intima. Moreover, elastin degradation products can act as chemo-attractants for VSMC. MMP-2 and MMP-9 are also elevated in AVF stenoses and may play a role in thrombosis. Decreased expression of MMP-1, MMP-3, and MMP-9 have been linked to increased AVF failure and stenosis secondary to accumulation of ECM and impaired wall remodeling [152]. Although the role of TIMP is not clear [65–67], an unbalance of MMP and TIMP may contribute to the failure of AVF maturation. ADAMTS-1 is a matrix metalloproteinase that is expressed during AVF maturation and plays a role similar to MMP-2 and MMP-9; reducing ADAMTS1 expression leads to positive vascular remodeling [144].
3. Late failure in the mature AVF
Although a mature AVF can support HD, in the setting of uremia and other systemic abnormalities, compounded with local injury due to the anastomotic configuration as well as repeated needle puncture, even the mature AVF is predisposed to eventual failure. NIH worsens with time, typically leading to stenosis of the AVF venous limb. This later failure usually requires interventional treatment or surgical revision to maintain the access for functional HD.
3.1 Systemic abnormalities
ESRD patients have systemic abnormalities, such as uremia, systemic inflammation, endothelial dysfunction, lipid abnormalities, hyperparathyroidism, hyperphosphatemia and hypercalcemia [21, 153–155]. These abnormalities may predispose the vessel wall to inward remodeling and stenoses after AVF creation.
Uremia
The inherent uremia of ESRD increases inflammation and oxidative stress [154, 155]. This oxidative stress is further increased by HD, which causes activation of phagocytes, release of oxygen radicals, peroxidation of lipids and ultimately depletion of the patient’s antioxidant protectants [154, 156]. Certain cytokines implicated in the formation of NIH, such as IL-6, TGF-β and TNF-α, are elevated in uremia [120]. Uremia adversely affects endothelial function resulting in a prothrombotic state, increasing the tendency for calcific uremic arteriopathy (CUA) [88, 157]. CUA is associated with multiple histologic abnormalities that collectively result in medial calcification, stenoses, fibrosis, proinflammatory and prothrombogenic arterioles that are compatible with a calcific obliterative vasculopathy. The mechanism is thought to be initiated by the interaction of uremic hyperphosphatemia, multiple uremic toxins, and reactive oxygen species (ROS) with decreased local vascular calcification inhibitory proteins such as Matrix Gla protein (MGP) and the systemic globulin: fetuin-A—(a2-Heremans-Schmid glycoprotein) AHSG [158].
Systemic inflammation
Inflammation is a typical prominent feature of ESRD and contributes to the uremic phenotype in advanced stages of CKD [155]. Systemic concentrations of both pro- and anti-inflammatory cytokines are often several fold higher than in healthy individuals.[159, 160]. Persistent inflammation is also a major cause of vascular aging and vascular calcification [159, 161].
Endothelial cell dysfunction
Due to the negative impacts of uremia and oxidative stress on the endothelial cells, flow-mediated, endothelium-dependent vasodilation is markedly reduced in uremic patients compared with normal control patients [162, 163]. The reasons for this endothelial dysfunction include increased oxidative stress, the presence of NO inhibitors such as asymmetric dimethylargine (AMDA) and a reduced number and function of endothelial progenitor cells [164]. AMDA accumulates with progressive CKD, and high levels are associated with aggressive restenosis after angioplasty [165]. AMDA and glycation end products (AGE) lead to decreased NO bioavailability, impairing arterial dilation as well as impairing NO-related signaling [166].
Other abnormalities
Dyslipidemia is a well-established traditional risk factor for atherosclerosis in the general population in addition to patients with CKD and may actively participate in the increased cardiovascular morbidity [153]. Hyperphosphatemia and hypercalcemia, typical features of advanced CKD, are often accompanied by dysregulation of parathyroid hormone (PTH), contributing to the inflammatory state [167]. They also induce vascular calcification and stiffness [161].
3.2 Pre-existent vascular pathology
The role of pre-existent arterial and venous vasculopathy in uremic patients has been gathering increased attention. Vessel morphology and function seen with preoperative duplex ultrasound mapping correlate with AVF maturation and patency [168]. The systemic abnormalities in ESRD patients induce accelerated atherosclerosis, vessel thickening, vascular calcification and stiffness [166, 169]. Whereas atherosclerosis is associated with intimal calcification, calcification of the media occurs independently of atherosclerotic plaque formation and is commonly observed in all-diameter arteries in CKD patients [170]. This arterial vasculopathy impairs the vessels ability to expand upon exposure to high-flow.
The detrimental effects of CKD on the arterial system may affect veins in a similar manner [171]. Marked pre-existing segmental venous disease is frequently present in patients with ESRD prior to vascular access surgery [172, 173]. Lee et al. reported extensive calcification in the intima and media of venous segments that were harvested at the time of vascular access surgery [173]. Venous calcification is likely to reduce venous compliance, as it does in arteries, potentially limiting the ability of the vein to dilate and outward remodel for successful AVF maturation.
Although minimum vein diameter (MVD) has been reported as a unique clinical factor associated with both AVF maturation and long-term patency [174], other studies suggest that forearm venous distensibility is a better predictor of successful AVF maturation [175], which is consistent with the increased outward remodeling of the venous AVF limb compared with the feeding artery [37]. Additional studies are needed to determine the impact of pre-existing venous calcification on AVF maturation failure and whether this is a potentially modifiable factor for clinical treatment [168].
Future Approaches to Treatment
With only 26–58% of arteriovenous fistulae functional at 1 year various therapies have been pursued to treat access failure and improve long-term patency. Many medical treatments using different drugs aimed at decreasing access failure and improving patency have been examined in patients using an AVF or AVG for HD. A recent systematic review and meta-analysis reported the effects of aspirin, ticlopidine, dipyridamole, dipyridamole plus aspirin, warfarin, fish oil, clopidogrel, and sulfinpyrazone. The study showed that three trials compared the platelet aggregation inhibitor ticlopidine versus placebo and favored active treatment (OR 0.45, 95% CI 0.25 to 0.82; p = 0.009); three RCT assessed aspirin versus placebo and did not show a statistical benefit (OR 0.40, 95% CI 0.07–2.25; p = 0.30); two trials compared clopidogrel with placebo and did not favor treatment (OR 0.40, 95% CI 0.13 to 1.19; p = 0.10); two RCT assessed fish oil and did not favor treatment (OR 0.24, 95% CI 0.03–1.95; p = 0.18); and single trials comparing dipyridamole alone, dipyridamole plus aspirin, and sulfinpyrazone against placebo favored active treatment but a meta-analysis could not be undertaken; a single trial of warfarin versus placebo found warfarin resulted in increased bleeding complications and worse patency rates [176]. Despite the overall quality of evidence being low with short follow-up, this systematic review showed that there currently is no adjuvant treatment showing increased AVF or graft long term patency [176].
Table 2 summarizes the most popular current approaches to treatment of the failing AVF, as well as some treatments that may become more popular in the future. Despite surgical revision having traditionally been the most effective treatment of local disease, the current standard treatment for arteriovenous stenosis is percutaneous transluminal angioplasty (PTA) [177] [12]. Although PTA can improve patency and function in some cases of thrombosis and stenosis, PTA is not the optimal treatment for many lesions including resistant or recurrent stenosis [178]. This section of the review focuses on treatment of AVF access failures, broadly dividing them into stimulatory and inhibitory treatments, with further division into endovascular approaches, perivascular approaches and internal/external support devices.
Table 2.
Current and future approaches to treatment of the failing AVF.
| Gold Standard: percutaneous transluminal angioplasty (PTA) or surgical revision | ||
|---|---|---|
| Stimulatory Approaches: promote dilation and/or wall thickening | ||
| Application | Limitation | |
| Balloon Assisted Maturation | Promotes maturation in an AVF with limited outward remodeling | Injures intima and media, increasing likelihood of NIH and recurrent stenosis; vessel rupture |
| Cutting Balloon Angioplasty | Dilate stenoses with reduced wall trauma | Some wall trauma; long term durability |
| Angioplasty with Stent | Dilate stenoses | Optimal stent type and design not established; long term durability |
| Elastase Therapy | Facilitate vessel dilation | Dose and efficacy not established; requires topical delivery |
| Inhibitory Approaches: prevent or inhibit NIH | ||
| Drug Eluting Angioplasty | Inhibit VSMC proliferation | Optimal drug and dose not established; single treatment |
| Cryoplasty | Induce VSMC apoptosis | Variability in cell survival after freeze-thaw; more painful than angioplasty |
| Brachytherapy | Inhibit VSMC proliferation and migration | Dose and efficacy not established |
| Adventitial Wraps | Inhibit VSMC proliferation | Wrap design and toxicity; optimal drug and dose not established |
| Mechanical Support Devices | Optimize AVF geometry | Limited data |
| Gene Therapy | Identifiable patient risk factors | Optimal targets not established; ethical considerations for human trials |
PTA, percutaneous transluminal angioplasty; AVF, arteriovenous fistula; BAM, balloon assisted maturation; NIH, neointimal hyperplasia; VSMC, vascular smooth muscle cell
Stimulatory Treatments
Stimulatory treatments recapitulate AVF maturation by promoting dilation and/or wall thickening, encouraging the cells of the intima, media or adventitia to proliferate or differentiate into useful phenotypes that will allow the fistula to mature or become usable after stenosis or occlusion. The two commonly used stimulatory endovascular approaches are currently balloon-assisted maturation (BAM) and angioplasty with stent placement. Cutting balloon angioplasty remains popular as well.
Balloon Assisted Maturation
The BAM technique uses repeated balloon angioplasty to disrupt the venous wall and sequentially dilate the vein to a larger diameter useable fistula; with this technique, it is possible to use even smaller diameter veins for access [179, 180]. However, the concern with this technique lies in its very nature; angioplasty injures the intima and media to produce NIH [181]. Although balloon injury has been described most commonly in the context of arteries, it is likely that veins show a similar response to angioplasty, especially in the uremic environment of renal failure. Diabetes, the most common etiology for renal failure, is also implicated in endothelial dysfunction. The clinical question is how to modulate the NIH response to allow this technique to achieve long term durability and successful use of the access site.
Although there is a relative paucity of randomized data on this technique, there are several positive short term studies. One report of 53 patients showed 85% secondary patency at 1 year; complications did include occlusion, conversion to a graft, and ligation due to steal [179]. Another report of 42 fistulae with maturation failure treated with 1.45 ± 0.57 balloon angioplasties showed a success rate of 46.2% at 1 year; however, there was no significant difference in AVF flow ratio between the successful and failure groups [182]. Miller et al matured 118 out of 122 fistulae requiring 1.5 interventions per access year with a secondary patency of 75% at 1 year; however 14 patients with upper arm fistulae required stents in the cephalic arch to maintain patency [183]. Gallagher et al performed 185 BAM in 45 patients (mean 3.7 procedures per patient); all cases except one were successfully dilated but 7 patients failed to mature due to cephalic arch and subclavian vein stenosis, with maturation after angioplasty of the venous outflow [184].
Interestingly, BAM is frequently complicated by vessel rupture but rupture does not necessarily cause fistula failure. Derderian et al performed 139 BAM in 30 patients with 74 hematomas post procedure but still noted a statistically significant increase in flow [185]. Although BAM has appeal and early reports suggest that it might be a useful procedure, as yet no blinded randomized clinical trial exists to allow for conclusions on its clinical use.
Cutting Balloon Angioplasty
Cutting balloon angioplasty is used to dilate stenoses, and like BAM, creates trauma to the vessel wall, but attempts to limit the trauma; longitudinal incisions along the stenosis are made in a controlled fashion, at lower pressures than the conventional balloon, potentially also reducing risk of vessel rupture. 3 or 4 blades are mounted longitudinally on a non-compliant balloon that after inflation create incisions and release hoop pressure; the lower pressure and decreased force is thought to reduce the risk of a neoproliferative response and restenosis [186], and may limit dissection [187]. Several studies have reported improved patency at up to 6 months [188–192]. Prospective randomized trials have had heterogeneous data but not conclusively shown long term durability [193–195].
Stenting
Angioplasty with stenting of failing fistulae is another tool to treat resistant stenoses; multiple studies have reported higher primary and secondary patency rates after stent placement, in both fistulae and grafts. One prospective multicenter randomized trial tested self-expanding nitinol stents covered with PTFE and showed a higher 6-month patency rate in the stent graft group compared to balloon angioplasty alone (51% vs. 23%, p<0.001); there was also greater freedom from subsequent interventions (32% vs. 16%, p=0.03) and less restenosis (28% vs. 78%, p<0.001) [196]. A meta-analysis of 10 studies suggested there was improved primary patency at 6 months in those treated with nitinol stents compared to angioplasty; however, bare metal stents showed no significant increase in patency [197]. Covered stents can also be used to treat pseudoaneurysms that develop within the access [198]. Other issues with stents include how to locate sites for needle cannulation of the access in relationship to the stent, as well as the potential for infection of the foreign body.
Elastase Therapy
Elastin within the vessel wall provides elastic recoil and resting vessel tone. In animal AVF models, delivery of recombinant human type 1 pancreatic elastase resulted in vessel dilation, inhibition of NIH and improved patency [199]. These findings suggest that use of elastase in humans could improve AVF dilation and maturation; elastase must be delivered topically over the fistula adventitia as elastase is inactivated in blood.
A randomized, double blinded, placebo controlled dose escalation study suggested improved primary patency at low doses [200]. A second double-blinded, randomized, placebo controlled trial suggested improved unassisted maturation but without increased primary patency [201, 202]. Without meeting the primary efficacy end point, it is not clear whether additional trials will be performed.
Inhibitory Treatments
Inhibitory treatments seek to inhibit NIH. Treatments can be applied primarily to prevent initial access failure and possibly promote maturation, or treatments can be applied secondarily, to directly treat a failed AVF or to prevent secondary restenosis associated with a stimulatory treatment such as angioplasty. Several delivery strategies can be used including direct delivery to the endothelium, delivery to the adventitia, or delivery to the entire vessel wall; mechanical support devices have also been used.
Drug Eluting Angioplasty
Drug eluting angioplasty has shown encouraging results in the management of coronary artery in-stent restenosis and peripheral arterial stenosis; as such there has been recent interest to use this technique to treat failing AVF, with paclitaxel being the most commonly reported drug. Katsanos et al treated 20 patients with failing arteriovenous fistula with paclitaxel coated balloon angioplasty; there was an overall statistically significant improvement in primary patency at 6 months (70% vs 25%); however only 35% had autologous fistulae and in these cases there was a 45% device success rate with patients requiring high-pressure post procedure dilatation [203]. Lai et al treated radiocephalic fistulae with either plain balloon angioplasty or plain balloon angioplasty followed by paclitaxel-coated balloon; there was increased 6-month patency in the paclitaxel group, but not at 12 months [204]. Kitrou et al showed a numerical improvement with paclitaxel coated balloon angioplasty compared to plain angioplasty but there was no statistical significance in outcome; however, there were only 7 patients in each group [205]. A meta-analysis of 6 studies, 2 RCT and 4 cohort studies, reported encouraging 6-month patency with drug eluting balloon angioplasty (70–97% vs. 0–26%), although in these studies the numbers of patients were small and heterogeneous [206]. Infection also remains a concern for paxlitaxel [207].
Dual antiplatelet therapy (DAPT) is beneficial and currently recommended for patients with coronary stenosis after placement of a DES [208], and also beneficial for patients with peripheral arterial diseases to reduce major adverse cardiovascular events and death [209], although a recent meta-analysis reported lack of evidence for DAPT after endovascular arterial procedures [210]. Although some studies used DAPT or clopidogrel after implantation a DES to treat AVF stenosis [203, 211], there is no study of DAPT on the outcome of AVF patency after DES treatment.
Cryoplasty
Cryoplasty uses liquid nitrous oxide to fill the balloon during inflation, cooling the vessel wall to −10 degrees Celsius, with the goal of inducing smooth muscle cell apoptosis. Subzero temperatures induce ice crystal nucleation in the vessel wall extracellular fluid to produce a hypertonic environment, since ice does not incorporate solutes, resulting in osmotic dehydration; upon removal of the cold source, the extracellular fluid thaws and osmolality returns to normal resulting in rehydration of the smooth muscle cells and induction of apoptosis. Cell survival is dependent on the rate of freezing and thawing cycles, the lowest temperature reached and the length of time at subzero temperature.
In porcine PTFE grafts there was no significant difference in intimal hyperplasia but a significant difference in media to intima thickness ratio at 4 weeks [212]. Rifkin et al treated 5 patients with perianastamotic PTFE graft-vein stenoses after 3 failed balloon angioplasty; 3 patients had no recurrence of stenosis at 12 weeks [213]. Gray et al treated 20 patients (AVG 18 patients; AVF 2 patients), with 80% needing immediate post cryotherapy angioplasty to achieve anatomical success; 3-month patency rates were equivocal at 3 months but only 16 and 25% at 6 months. Cryotherapy was also more painful than angioplasty [214].
Brachytherapy
Endovascular brachytherapy delivers beta radiation to the vessel wall, typically using either high doses over a short period of time or low doses over a long period using beta-particle emitting stents. Brachytherapy decreases vascular smooth muscle cell proliferation and migration [215].
In a canine PTFE graft model, brachytherapy was associated with reduced NIH at up to 9 months [216]. An early study treating 5 patients with restenosis showed only 2 patients with a clinically patent fistula at 6 months [217]. Waksman et al treated 18 grafts with restenosis with 11 sites remaining patent at 44 weeks [218]. The BRAVO 2 trial aimed to randomize patients into brachytherapy or sham treatment after the promising results of the BRAVO pilot, but this trial was halted preliminarily [219, 220].
Adventitial Wraps
The role of adventitial wraps to deliver sirolimus or paclitaxel has been extensively investigated; rat, pig, dog and sheep models using paclitaxel wraps showed decreased NIH [207, 221–224]. A significant concern with perivascular delivery however, is leak of the impregnated drug to surrounding tissues; a clinical trial was stopped early due to a 25% increase in infections [207]. This trial, however, used mesh for delivery to PTFE grafts.
Sanders et al used a non-porous polymer barrier laminated with a drug loaded hydrogel to allow unidirectional release towards the target area. In a porcine model there was no detection of the drug within any surrounding tissues; addition of a polylactide-co-glycolide wrap showed acceptable degradation over time and was undetectable by day 35 [224].
An interesting study described a sirolimus-eluting collagen membrane (Coll-R). In a small clinical trial of 12 patients, there was minimal toxicity and primary patency rates were 76% and 38% at 12 and 24 months respectively [225]. A phase 3 trial is currently in progress.
Mechanical Support Devices
Mechanical support devices can be used to optimize the geometry of the fistula anastomosis to prevent or delay NIH. The Optiflow™ device has been the most studied; in a human pilot study safety and technical success were achieved in 10 patients [226]. A follow up study of 41 patients showed unassisted patency of 78% at 90 days with no device related adverse events [227]. Similarly, the VasQ™ device provides an external support to control the geometry and flow, with a small study reporting maturation rates of 74% at 6 months [116].
Gene Therapy
Gene therapy continues to be of interest, with research directed towards understanding what patient risk factors predispose to access failure and thus might be suitable for appropriate targeted gene therapy. Several polymorphisms have been associated with vascular access thrombosis including methylenetetrahydrofolate (MTHFR), HO-1, factor V, TGFβ-1 and klotho (KL) [71, 133, 177, 228–230]. Polymorphisms of NOS have also been implicated in arterial restenosis [231]. Single nucleotide polymorphisms in the gene for Factor V were significantly associated with increased risk of access failure despite treatment with antiplatelet agents [232].
Gene therapy can alter luminal area and stenosis in large animal PTFE graft models. Rotmans et al used C-Natriuretic Peptide to increase lumen area and the intima/media ratio [233]. Luo et al. showed reduced NIH with beta-adrenergic receptor kinase C-terminus [234].
Gene therapy targeting VEGF-A may be promising. VEGF-A is necessary at low concentrations to promote endothelial cell health, nitric oxide and prostacyclin production, vasodilatation, antithrombosis and suppression of smooth muscle cell proliferation; at high concentrations VEGF-A promotes angiogenesis and vasculogenesis. Systemic VEGF receptor gene transfer in rats decreased carotid artery restenosis, suggesting the utility of targeting this pathway [235]. Increased VEGF expression is associated with early AVF thrombosis in human patients [236], and patients with the VEGF-936C/C gene polymorphism have a 5.54 increased risk of fistula thrombosis [237]. Lentivirus inhibiting adventitial VEGF-A expression decreased cellular proliferation and constrictive remodeling and increased patency in a mouse model [56]. However, a human trial administering VEGF-D was stopped early due to poor recruitment[238].
Future Directions
Successful hemodialysis requires a strong collagen tube that can be punctured repetitively to support the high flows necessary for efficient dialysis exchange. Veins are typically preferred, as arterial use can lead to ischemia and prosthetics have reduced patency and increased infection. However, the thin walled veins must successfully adapt to the arterial environment with a combination of diameter expansion and wall thickening. Failure to mature successfully is an important mechanism of access failure, just as NIH is an important mechanism of late failure. Accordingly, therapy to promote maturation, e.g. diameter expansion and wall thickening, is an important component of providing successful access; however, promoting wall thickening for strength, without exuberant thickening and NIH, is a current challenge.
The role of hemodynamics such as shear stress in the development of NIH is recognized, especially in the pathogenesis of juxta-anastomotic stenosis that frequently develops along the inner wall of the swing segment. Both pharmacological as well as mechanical approaches may be applicable for therapy. The new RADAR technique to create AVF, e.g. minimizing venous handling and potential for wall ischemia, alters hemodynamics and may be a simple and low-cost method to improve access patency [239].
An interesting alternative that may obviate fistulae is the use of tissue engineered blood vessels. Although still in development and trials, tissue engineered grafts can resist high pressures, providing an endothelial-lined tube that supports dialysis [240, 241]. Continued advances in tissue engineering and 3D printing may show that tissue engineered vessels might replace the current gold standard fistula.
Acknowledgments
Supported in part by the National Institutes of Health (R01-HL128406 and R56-HL095498 [to A.D.]); the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Program (Merit Review Award I01-BX002336 [to A.D.]); a Sarnoff Cardiovascular Foundation Fellowship (to J.M.S.); as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet (London, England) 2013 Jul 20;382(9888):260–72. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2015 Jan 10;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas. 7th. Brussels, Belgium: .pdf. [Google Scholar]
- 4.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. The New England journal of medicine. 2010 Mar 25;362(12):1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 5.Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World journal of diabetes. 2015 Jun 25;6(6):850–67. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah VN, Mohan V. Diabetes in India: what is different? Current opinion in endocrinology, diabetes, and obesity. 2015 Aug;22(4):283–9. doi: 10.1097/MED.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 7.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014 Oct;64(4):510–33. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt RJ. Informing our elders about dialysis: is an age-attuned approach warranted? Clinical journal of the American Society of Nephrology: CJASN. 2012 Jan;7(1):185–91. doi: 10.2215/CJN.10401011. [DOI] [PubMed] [Google Scholar]
- 9.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. Journal of the American Society of Nephrology: JASN. 2013 Feb;24(3):465–73. doi: 10.1681/ASN.2012070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lok CE, Foley R. Vascular access morbidity and mortality: trends of the last decade. Clinical journal of the American Society of Nephrology: CJASN. 2013 Jul;8(7):1213–9. doi: 10.2215/CJN.01690213. [DOI] [PubMed] [Google Scholar]
- 11.Santoro D, Benedetto F, Mondello P, Pipito N, Barilla D, Spinelli F, et al. Vascular access for hemodialysis: current perspectives. International journal of nephrology and renovascular disease. 2014;7:281–94. doi: 10.2147/IJNRD.S46643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical practice guidelines for vascular access. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006 Jul;48(Suppl 1):S176–247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Donca IZ, Wish JB. Systemic barriers to optimal hemodialysis access. Seminars in nephrology. 2012 Nov;32(6):519–29. doi: 10.1016/j.semnephrol.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Rayner HC, Pisoni RL, Bommer J, Canaud B, Hecking E, Locatelli F, et al. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2004 Jan;19(1):108–20. doi: 10.1093/ndt/gfg483. [DOI] [PubMed] [Google Scholar]
- 15.Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, et al. Establishment and maintenance of vascular access in incident hemodialysis patients: a prospective cost analysis. Journal of the American Society of Nephrology: JASN. 2005 Jan;16(1):201–9. doi: 10.1681/ASN.2004050355. [DOI] [PubMed] [Google Scholar]
- 16.Murad MH, Elamin MB, Sidawy AN, Malaga G, Rizvi AZ, Flynn DN, et al. Autogenous versus prosthetic vascular access for hemodialysis: a systematic review and meta-analysis. Journal of vascular surgery. 2008 Nov;48(5 Suppl):34s–47s. doi: 10.1016/j.jvs.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 17.Almasri J, Alsawas M, Mainou M, Mustafa RA, Wang Z, Woo K, et al. Outcomes of vascular access for hemodialysis: A systematic review and meta-analysis. Journal of vascular surgery. 2016 Jul;64(1):236–43. doi: 10.1016/j.jvs.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 18.Manook M, Calder F. Practical aspects of arteriovenous fistula formation in the pediatric population. Pediatric nephrology (Berlin, Germany) 2013 Jun;28(6):885–93. doi: 10.1007/s00467-012-2328-0. [DOI] [PubMed] [Google Scholar]
- 19.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008 May 14;299(18):2164–71. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon BS. Why don’t fistulas mature? Kidney international. 2006 Oct;70(8):1413–22. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 21.Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P. Biology of arteriovenous fistula failure. Journal of nephrology. 2007 Mar-Apr;20(2):150–63. [PubMed] [Google Scholar]
- 22.Wilmink T, Hollingworth L, Powers S, Allen C, Dasgupta I. Natural History of Common Autologous Arteriovenous Fistulae: Consequences for Planning of Dialysis. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2016 Jan;51(1):134–40. doi: 10.1016/j.ejvs.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014 Mar;63(3):464–78. doi: 10.1053/j.ajkd.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Dunn J, Herscu G, Woo K. Factors influencing maturation time of native arteriovenous fistulas. Annals of vascular surgery. 2015;29(4):704–7. doi: 10.1016/j.avsg.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney international. 2003 Jan;63(1):346–52. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 26.Farber A, Imrey PB, Huber TS, Kaufman JM, Kraiss LW, Larive B, et al. Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. Journal of vascular surgery. 2016 Jan;63(1):163–70.e6. doi: 10.1016/j.jvs.2015.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vachharajani TJ, Moist LM, Glickman MH, Vazquez MA, Polkinghorne KR, Lok CE, et al. Elderly patients with CKD–dilemmas in dialysis therapy and vascular access. Nature reviews Nephrology. 2014 Feb;10(2):116–22. doi: 10.1038/nrneph.2013.256. [DOI] [PubMed] [Google Scholar]
- 28.Tordoir JH, Bode AS, van Loon MM. Preferred strategy for hemodialysis access creation in elderly patients. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2015 Jun;49(6):738–43. doi: 10.1016/j.ejvs.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Olsha O, Hijazi J, Goldin I, Shemesh D. Vascular access in hemodialysis patients older than 80 years. Journal of vascular surgery. 2015 Jan;61(1):177–83. doi: 10.1016/j.jvs.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Drew DA, Lok CE. Strategies for planning the optimal dialysis access for an individual patient. Current opinion in nephrology and hypertension. 2014 May;23(3):314–20. doi: 10.1097/01.mnh.0000444815.49755.d9. [DOI] [PubMed] [Google Scholar]
- 31.Lee T, Mokrzycki M, Moist L, Maya I, Vazquez M, Lok CE. Standardized definitions for hemodialysis vascular access. Seminars in dialysis. 2011 Sep-Oct;24(5):515–24. doi: 10.1111/j.1525-139X.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosa SD, Al-Jaishi AA, Moist L, Lok CE. Preoperative vascular access evaluation for haemodialysis patients. The Cochrane database of systematic reviews. 2015;(9):Cd007013. doi: 10.1002/14651858.CD007013.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lomonte C, Meola M, Petrucci I, Casucci F, Basile C. The key role of color Doppler ultrasound in the work-up of hemodialysis vascular access. Seminars in dialysis. 2015 Mar-Apr;28(2):211–5. doi: 10.1111/sdi.12312. [DOI] [PubMed] [Google Scholar]
- 34.Bashar K, Conlon PJ, Kheirelseid EA, Aherne T, Walsh SR, Leahy A. Arteriovenous fistula in dialysis patients: Factors implicated in early and late AVF maturation failure. The surgeon: journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2016 Mar 14; doi: 10.1016/j.surge.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Albayrak R, Yuksel S, Colbay M, Degirmenci B, Acarturk G, Haktanir A, et al. Hemodynamic changes in the cephalic vein of patients with hemodialysis arteriovenous fistula. J Clin Ultrasound. 2007 Mar-Apr;35(3):133–7. doi: 10.1002/jcu.20307. [DOI] [PubMed] [Google Scholar]
- 36.Dixon BS, Novak L, Fangman J. Hemodialysis vascular access survival: upper-arm native arteriovenous fistula. Am J Kidney Dis. 2002 Jan;39(1):92–101. doi: 10.1053/ajkd.2002.29886. [DOI] [PubMed] [Google Scholar]
- 37.Corpataux JM, Haesler E, Silacci P, Ris HB, Hayoz D. Low-pressure environment and remodelling of the forearm vein in Brescia-Cimino haemodialysis access. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2002 Jun;17(6):1057–62. doi: 10.1093/ndt/17.6.1057. [DOI] [PubMed] [Google Scholar]
- 38.Krishnamoorthy MK, Banerjee RK, Wang Y, Zhang J, Roy AS, Khoury SF, et al. Hemodynamic wall shear stress profiles influence the magnitude and pattern of stenosis in a pig AV fistula. Kidney Int. 2008 Dec;74(11):1410–9. doi: 10.1038/ki.2008.379. [DOI] [PubMed] [Google Scholar]
- 39.Ene-Iordache B, Remuzzi A. Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: low and oscillating shear stress locates the sites of stenosis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2012 Jan;27(1):358–68. doi: 10.1093/ndt/gfr342. [DOI] [PubMed] [Google Scholar]
- 40.Manning E, Skartsis N, Orta AM, Velazquez OC, Liu ZJ, Asif A, et al. A new arteriovenous fistula model to study the development of neointimal hyperplasia. J Vasc Res. 2012;49(2):123–31. doi: 10.1159/000332327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ojha M, Cobbold RS, Johnston KW. Influence of angle on wall shear stress distribution for an end-to-side anastomosis. J Vasc Surg. 1994 Jun;19(6):1067–73. doi: 10.1016/s0741-5214(94)70219-5. [DOI] [PubMed] [Google Scholar]
- 42.Lu DY, Chen EY, Wong DJ, Yamamoto K, Protack CD, Williams WT, et al. Vein graft adaptation and fistula maturation in the arterial environment. The Journal of surgical research. 2014 May 1;188(1):162–73. doi: 10.1016/j.jss.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz LB, O’Donohoe MK, Purut CM, Mikat EM, Hagen PO, McCann RL. Myointimal thickening in experimental vein grafts is dependent on wall tension. J Vasc Surg. 1992 Jan;15(1):176–86. doi: 10.1067/mva.1992.33805. [DOI] [PubMed] [Google Scholar]
- 44.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: clinical implications. J Vasc Surg. 2010 Mar;51(3):736–46. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arteriosclerosis, thrombosis, and vascular biology. 2011 Nov;31(11):2391–6. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 46.Gasper WJ, Owens CD, Kim JM, Hills N, Belkin M, Creager MA, et al. Thirty-day vein remodeling is predictive of midterm graft patency after lower extremity bypass. J Vasc Surg. 2013 Jan;57(1):9–18. doi: 10.1016/j.jvs.2012.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K. Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis. 2003 Nov;42(5):1000–12. doi: 10.1016/j.ajkd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007 Dec;46(6):1180–90. doi: 10.1016/j.jvs.2007.08.033. discussion 90. [DOI] [PubMed] [Google Scholar]
- 49.Lin SL, Chen HS, Huang CH, Yen TS. Predicting the outcome of hemodialysis arteriovenous fistulae using duplex ultrasonography. J Formos Med Assoc. 1997 Nov;96(11):864–8. [PubMed] [Google Scholar]
- 50.Lin SL, Huang CH, Chen HS, Hsu WA, Yen CJ, Yen TS. Effects of age and diabetes on blood flow rate and primary outcome of newly created hemodialysis arteriovenous fistulas. Am J Nephrol. 1998;18(2):96–100. doi: 10.1159/000013315. [DOI] [PubMed] [Google Scholar]
- 51.Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circulation journal: official journal of the Japanese Circulation Society. 2010 Aug;74(8):1501–12. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, et al. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2009 Sep;24(9):2786–91. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castier Y, Lehoux S, Hu Y, Foteinos G, Tedgui A, Xu Q. Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. Kidney international. 2006 Jul;70(2):315–20. doi: 10.1038/sj.ki.5001569. [DOI] [PubMed] [Google Scholar]
- 54.Caplice NM, Wang S, Tracz M, Croatt AJ, Grande JP, Katusic ZS, et al. Neoangiogenesis and the presence of progenitor cells in the venous limb of an arteriovenous fistula in the rat. American journal of physiology Renal physiology. 2007 Aug;293(2):F470–5. doi: 10.1152/ajprenal.00067.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duque JC, Vazquez-Padron RI. Myofibroblasts: the ideal target to prevent arteriovenous fistula failure? Kidney international. 2014 Feb;85(2):234–6. doi: 10.1038/ki.2013.384. [DOI] [PubMed] [Google Scholar]
- 56.Yang B, Janardhanan R, Vohra P, Greene EL, Bhattacharya S, Withers S, et al. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney international. 2014 Feb;85(2):289–306. doi: 10.1038/ki.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. The Journal of clinical investigation. 1991 Nov;88(5):1663–71. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996 Oct;16(10):1256–62. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- 59.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. The New England journal of medicine. 1993 Dec 30;329(27):2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 60.Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006 Apr;84(2):115–24. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 61.Croatt AJ, Grande JP, Hernandez MC, Ackerman AW, Katusic ZS, Nath KA. Characterization of a model of an arteriovenous fistula in the rat: the effect of L-NAME. The American journal of pathology. 2010 May;176(5):2530–41. doi: 10.2353/ajpath.2010.090649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones GT, van Rij AM, Packer SG, Walker RJ, Stehbens WE. Venous endothelial changes in therapeutic arteriovenous fistulae. Atherosclerosis. 1998 Mar;137(1):149–56. doi: 10.1016/s0021-9150(97)00271-2. [DOI] [PubMed] [Google Scholar]
- 63.Nath KA, Kanakiriya SK, Grande JP, Croatt AJ, Katusic ZS. Increased venous proinflammatory gene expression and intimal hyperplasia in an aorto-caval fistula model in the rat. The American journal of pathology. 2003 Jun;162(6):2079–90. doi: 10.1016/S0002-9440(10)64339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss MF, Scivittaro V, Anderson JM. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2001 May;37(5):970–80. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 65.Chan CY, Chen YS, Ma MC, Chen CF. Remodeling of experimental arteriovenous fistula with increased matrix metalloproteinase expression in rats. Journal of vascular surgery. 2007 Apr;45(4):804–11. doi: 10.1016/j.jvs.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 66.Chang CJ, Chen CC, Hsu LA, Chang GJ, Ko YH, Chen CF, et al. Degradation of the internal elastic laminae in vein grafts of rats with aortocaval fistulae: potential impact on graft vasculopathy. The American journal of pathology. 2009 May;174(5):1837–46. doi: 10.2353/ajpath.2009.080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Misra S, Fu AA, Anderson JL, Sethi S, Glockner JF, McKusick MA, et al. The rat femoral arteriovenous fistula model: increased expression of matrix metalloproteinase-2 and -9 at the venous stenosis. Journal of vascular and interventional radiology: JVIR. 2008 Apr;19(4):587–94. doi: 10.1016/j.jvir.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Lee ES, Shen Q, Pitts RL, Guo M, Wu MH, Sun SC, et al. Serum metalloproteinases MMP-2, MMP-9, and metalloproteinase tissue inhibitors in patients are associated with arteriovenous fistula maturation. Journal of vascular surgery. 2011 Aug;54(2):454–9. doi: 10.1016/j.jvs.2011.02.056. discussion 9–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Southgate KM, Mehta D, Izzat MB, Newby AC, Angelini GD. Increased secretion of basement membrane-degrading metalloproteinases in pig saphenous vein into carotid artery interposition grafts. Arterioscler Thromb Vasc Biol. 1999 Jul;19(7):1640–9. doi: 10.1161/01.atv.19.7.1640. [DOI] [PubMed] [Google Scholar]
- 70.Hall MR, Yamamoto K, Protack CD, Tsuneki M, Kuwahara G, Assi R, et al. Temporal regulation of venous extracellular matrix components during arteriovenous fistula maturation. The journal of vascular access. 2015 Mar-Apr;16(2):93–106. doi: 10.5301/jva.5000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heine GH, Ulrich C, Sester U, Sester M, Kohler H, Girndt M. Transforming growth factor beta1 genotype polymorphisms determine AV fistula patency in hemodialysis patients. Kidney international. 2003 Sep;64(3):1101–7. doi: 10.1046/j.1523-1755.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- 72.Wolff RA, Ryomoto M, Stark VE, Malinowski R, Tomas JJ, Stinauer MA, et al. Antisense to transforming growth factor-beta1 messenger RNA reduces vein graft intimal hyperplasia and monocyte chemotactic protein 1. J Vasc Surg. 2005 Mar;41(3):498–508. doi: 10.1016/j.jvs.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 73.Ikegaya N, Yamamoto T, Takeshita A, Watanabe T, Yonemura K, Miyaji T, et al. Elevated erythropoietin receptor and transforming growth factor-beta1 expression in stenotic arteriovenous fistulae used for hemodialysis. Journal of the American Society of Nephrology: JASN. 2000 May;11(5):928–35. doi: 10.1681/ASN.V115928. [DOI] [PubMed] [Google Scholar]
- 74.Stracke S, Konner K, Kostlin I, Friedl R, Jehle PM, Hombach V, et al. Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney international. 2002 Mar;61(3):1011–9. doi: 10.1046/j.1523-1755.2002.00191.x. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, et al. Venous stenosis in a pig arteriovenous fistula model–anatomy, mechanisms and cellular phenotypes. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2008 Feb;23(2):525–33. doi: 10.1093/ndt/gfm547. [DOI] [PubMed] [Google Scholar]
- 76.Simone S, Loverre A, Cariello M, Divella C, Castellano G, Gesualdo L, et al. Arteriovenous fistula stenosis in hemodialysis patients is characterized by an increased adventitial fibrosis. Journal of nephrology. 2014 Oct;27(5):555–62. doi: 10.1007/s40620-014-0050-7. [DOI] [PubMed] [Google Scholar]
- 77.Majesky MW. Adventitia and perivascular cells. Arterioscler Thromb Vasc Biol. 2015 Aug;35(8):e31–5. doi: 10.1161/ATVBAHA.115.306088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clinical and experimental hypertension Part A, Theory and practice. 1991;13(2):277–96. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 79.Withers SB, Bussey CE, Saxton SN, Melrose HM, Watkins AE, Heagerty AM. Mechanisms of adiponectin-associated perivascular function in vascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2014 Aug;34(8):1637–42. doi: 10.1161/ATVBAHA.114.303031. [DOI] [PubMed] [Google Scholar]
- 80.Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, et al. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. American journal of physiology Heart and circulatory physiology. 2013 Mar 15;304(6):H786–95. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furie B, Furie BC. Mechanisms of thrombus formation. The New England journal of medicine. 2008 Aug 28;359(9):938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 82.Glorieux G, Cohen G, Jankowski J, Vanholder R. Platelet/Leukocyte activation, inflammation, and uremia. Seminars in dialysis. 2009 Jul-Aug;22(4):423–7. doi: 10.1111/j.1525-139X.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 83.Sreedhara R, Itagaki I, Hakim RM. Uremic patients have decreased shear-induced platelet aggregation mediated by decreased availability of glycoprotein IIb-IIIa receptors. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1996 Mar;27(3):355–64. doi: 10.1016/s0272-6386(96)90358-3. [DOI] [PubMed] [Google Scholar]
- 84.Lutz J, Menke J, Sollinger D, Schinzel H, Thurmel K. Haemostasis in chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2014 Jan;29(1):29–40. doi: 10.1093/ndt/gft209. [DOI] [PubMed] [Google Scholar]
- 85.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. Journal of the American Society of Nephrology: JASN. 2008 Jan;19(1):135–40. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ocak G, Verduijn M, Vossen CY, Lijfering WM, Dekker FW, Rosendaal FR, et al. Chronic kidney disease stages 1–3 increase the risk of venous thrombosis. Journal of thrombosis and haemostasis: JTH. 2010 Nov;8(11):2428–35. doi: 10.1111/j.1538-7836.2010.04048.x. [DOI] [PubMed] [Google Scholar]
- 87.Lambert ND, Sacrinty MT, Ketch TR, Turner SJ, Santos RM, Daniel KR, et al. Chronic kidney disease and dipstick proteinuria are risk factors for stent thrombosis in patients with myocardial infarction. American heart journal. 2009 Apr;157(4):688–94. doi: 10.1016/j.ahj.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 88.Shashar M, Francis J, Chitalia V. Thrombosis in the uremic milieu–emerging role of “thrombolome”. Seminars in dialysis. 2015 Mar-Apr;28(2):198–205. doi: 10.1111/sdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003 Jan 7;107(1):87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 90.Segarra A, Chacon P, Martinez-Eyarre C, Argelaguer X, Vila J, Ruiz P, et al. Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: biochemical correlations and role as independent predictors of coronary artery stenosis. Journal of the American Society of Nephrology: JASN. 2001 Jun;12(6):1255–63. doi: 10.1681/ASN.V1261255. [DOI] [PubMed] [Google Scholar]
- 91.Milburn JA, Ford I, Mutch NJ, Fluck N, Brittenden J. Thrombin-anti-thrombin levels and patency of arterio-venous fistula in patients undergoing haemodialysis compared to healthy volunteers: a prospective analysis. PloS one. 2013;8(7):e67799. doi: 10.1371/journal.pone.0067799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sioulis A, Malindretos P, Makedou A, Makris P, Grekas D. Coagulation factors as biological risk markers of endothelial dysfunction. Association with the thrombotic episodes of chronic hemodialysis patients. Hippokratia. 2009 Oct;13(4):237–41. [PMC free article] [PubMed] [Google Scholar]
- 93.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. Journal of the American Society of Nephrology: JASN. 2012 Nov;23(11):1900–8. doi: 10.1681/ASN.2011121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. Journal of the American Society of Nephrology: JASN. 2012 Aug;23(8):1339–50. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Serradell M, Diaz-Ricart M, Cases A, Zurbano MJ, Lopez-Pedret J, Arranz O, et al. Uremic medium causes expression, redistribution and shedding of adhesion molecules in cultured endothelial cells. Haematologica. 2002 Oct;87(10):1053–61. [PubMed] [Google Scholar]
- 96.Gondouin B, Cerini C, Dou L, Sallee M, Duval-Sabatier A, Pletinck A, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney international. 2013 Oct;84(4):733–44. doi: 10.1038/ki.2013.133. [DOI] [PubMed] [Google Scholar]
- 97.Ando M, Iwata A, Ozeki Y, Tsuchiya K, Akiba T, Nihei H. Circulating platelet-derived microparticles with procoagulant activity may be a potential cause of thrombosis in uremic patients. Kidney international. 2002 Nov;62(5):1757–63. doi: 10.1046/j.1523-1755.2002.00627.x. [DOI] [PubMed] [Google Scholar]
- 98.Amabile N, Guerin AP, Tedgui A, Boulanger CM, London GM. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2012 May;27(5):1873–80. doi: 10.1093/ndt/gfr573. [DOI] [PubMed] [Google Scholar]
- 99.Rios DR, Fernandes AP, Carvalho MG, Figueiredo RC, Guimaraes DA, Reis DR, et al. Hemodialysis vascular access thrombosis: The role of factor V Leiden, prothrombin gene mutation and ABO blood groups. Clinica chimica acta; international journal of clinical chemistry. 2011 Feb 20;412(5–6):425–9. doi: 10.1016/j.cca.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 100.Verschuren JJ, Ocak G, Dekker FW, Rabelink TJ, Jukema JW, Rotmans JI. Candidate gene analysis of arteriovenous fistula failure in hemodialysis patients. Clinical journal of the American Society of Nephrology: CJASN. 2013 Aug;8(8):1358–66. doi: 10.2215/CJN.11091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serrano A, Garcia F, Serrano M, Ramirez E, Alfaro FJ, Lora D, et al. IgA antibodies against beta2 glycoprotein I in hemodialysis patients are an independent risk factor for mortality. Kidney international. 2012 Jun;81(12):1239–44. doi: 10.1038/ki.2011.477. [DOI] [PubMed] [Google Scholar]
- 102.Fischer MJ, Rauch J, Levine JS. The antiphospholipid syndrome. Seminars in nephrology. 2007 Jan;27(1):35–46. doi: 10.1016/j.semnephrol.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rothuizen TC, Wong C, Quax PH, van Zonneveld AJ, Rabelink TJ, Rotmans JI. Arteriovenous access failure: more than just intimal hyperplasia? Nephrol Dial Transplant. 2013 May;28(5):1085–92. doi: 10.1093/ndt/gft068. [DOI] [PubMed] [Google Scholar]
- 104.Remuzzi A, Ene-Iordache B. Novel paradigms for dialysis vascular access: upstream hemodynamics and vascular remodeling in dialysis access stenosis. Clinical journal of the American Society of Nephrology: CJASN. 2013 Dec;8(12):2186–93. doi: 10.2215/CJN.03450413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fitts MK, Pike DB, Anderson K, Shiu YT. Hemodynamic Shear Stress and Endothelial Dysfunction in Hemodialysis Access. The open urology & nephrology journal. 2014;7(Suppl 1 M5):33–44. doi: 10.2174/1874303X01407010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Browne LD, Bashar K, Griffin P, Kavanagh EG, Walsh SR, Walsh MT. The Role of Shear Stress in Arteriovenous Fistula Maturation and Failure: A Systematic Review. PloS one. 2015;10(12):e0145795. doi: 10.1371/journal.pone.0145795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jia L, Wang L, Wei F, Yu H, Dong H, Wang B, et al. Effects of wall shear stress in venous neointimal hyperplasia of arteriovenous fistulae. Nephrology (Carlton, Vic) 2015 May;20(5):335–42. doi: 10.1111/nep.12394. [DOI] [PubMed] [Google Scholar]
- 108.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiological reviews. 2011 Jan;91(1):327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proceedings of the National Academy of Sciences of the United States of America. 2004 Oct 12;101(41):14871–6. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fan L, Karino T. Effect of a disturbed flow on proliferation of the cells of a hybrid vascular graft. Biorheology. 2010;47(1):31–8. doi: 10.3233/BIR-2010-0561. [DOI] [PubMed] [Google Scholar]
- 111.Fan L, Sakai J, Bessho S, Wada S, Karino T. Effect of a disturbed flow on adhesion of monocytes to a model of an arterial wall. Biorheology. 2010;47(1):15–29. doi: 10.3233/BIR-2010-0562. [DOI] [PubMed] [Google Scholar]
- 112.Ene-Iordache B, Cattaneo L, Dubini G, Remuzzi A. Effect of anastomosis angle on the localization of disturbed flow in ‘side-to-end’ fistulae for haemodialysis access. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2013 Apr;28(4):997–1005. doi: 10.1093/ndt/gfs298. [DOI] [PubMed] [Google Scholar]
- 113.Manini S, Passera K, Huberts W, Botti L, Antiga L, Remuzzi A. Computational model for simulation of vascular adaptation following vascular access surgery in haemodialysis patients. Computer methods in biomechanics and biomedical engineering. 2014;17(12):1358–67. doi: 10.1080/10255842.2012.745857. [DOI] [PubMed] [Google Scholar]
- 114.Bharat A, Jaenicke M, Shenoy S. A novel technique of vascular anastomosis to prevent juxta-anastomotic stenosis following arteriovenous fistula creation. Journal of vascular surgery. 2012 Jan;55(1):274–80. doi: 10.1016/j.jvs.2011.07.090. [DOI] [PubMed] [Google Scholar]
- 115.Sadaghianloo N, Jean-Baptiste E, Rajhi K, Francois E, Declemy S, Dardik A, et al. Increased reintervention in radial-cephalic arteriovenous fistulas with anastomotic angles of less than 30 degrees. Journal of vascular surgery. 2015 Dec;62(6):1583–9. doi: 10.1016/j.jvs.2015.07.074. [DOI] [PubMed] [Google Scholar]
- 116.Chemla E, Velazquez CC, D’Abate F, Ramachandran V, Maytham G. Arteriovenous fistula construction with the VasQ external support device: a pilot study. The journal of vascular access. 2016 May 7;17(3):243–8. doi: 10.5301/jva.5000527. [DOI] [PubMed] [Google Scholar]
- 117.Lee T. Novel paradigms for dialysis vascular access: downstream vascular biology–is there a final common pathway? Clinical journal of the American Society of Nephrology: CJASN. 2013 Dec;8(12):2194–201. doi: 10.2215/CJN.03490413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liang A, Wang Y, Han G, Truong L, Cheng J. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. American journal of physiology Renal physiology. 2013 Jun 15;304(12):F1413–20. doi: 10.1152/ajprenal.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Marchi S, Falleti E, Giacomello R, Stel G, Cecchin E, Sepiacci G, et al. Risk factors for vascular disease and arteriovenous fistula dysfunction in hemodialysis patients. Journal of the American Society of Nephrology: JASN. 1996 Aug;7(8):1169–77. doi: 10.1681/ASN.V781169. [DOI] [PubMed] [Google Scholar]
- 120.Wasse H, Huang R, Naqvi N, Smith E, Wang D, Husain A. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. The journal of vascular access. 2012 Apr-Jun;13(2):168–74. doi: 10.5301/jva.5000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang CJ, Ko YS, Ko PJ, Hsu LA, Chen CF, Yang CW, et al. Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney international. 2005 Sep;68(3):1312–9. doi: 10.1111/j.1523-1755.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 122.Kaygin MA, Halici U, Aydin A, Dag O, Binici DN, Limandal HK, et al. The relationship between arteriovenous fistula success and inflammation. Ren Fail. 2013 Sep;35(8):1085–8. doi: 10.3109/0886022X.2013.815100. [DOI] [PubMed] [Google Scholar]
- 123.Asare Y, Schmitt M, Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thrombosis and haemostasis. 2013 Mar;109(3):391–8. doi: 10.1160/TH12-11-0831. [DOI] [PubMed] [Google Scholar]
- 124.Misra S, Fu AA, Rajan DK, Juncos LA, McKusick MA, Bjarnason H, et al. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. Journal of vascular and interventional radiology: JVIR. 2008 Feb;19(2 Pt 1):252–9. doi: 10.1016/j.jvir.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 125.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2009 Jun;29(6):313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation (New York, NY: 1994) 2003 Jun;10(3–4):247–57. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- 127.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. The New England journal of medicine. 2006 Feb 9;354(6):610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 128.Schober A. Chemokines in vascular dysfunction and remodeling. Arteriosclerosis, thrombosis, and vascular biology. 2008 Nov;28(11):1950–9. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 129.Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, et al. MCP-1 contributes to arteriovenous fistula failure. Journal of the American Society of Nephrology: JASN. 2011 Jan;22(1):43–8. doi: 10.1681/ASN.2010040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miki K, Kumar A, Yang R, Killeen ME, Delude RL. Extracellular activation of arginase-1 decreases enterocyte inducible nitric oxide synthase activity during systemic inflammation. American journal of physiology Gastrointestinal and liver physiology. 2009 Oct;297(4):G840–8. doi: 10.1152/ajpgi.90716.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. Journal of vascular research. 2012;49(6):463–78. doi: 10.1159/000339151. [DOI] [PubMed] [Google Scholar]
- 132.Lin CC, Chung MY, Yang WC, Lin SJ, Lee PC. Length polymorphisms of heme oxygenase-1 determine the effect of far-infrared therapy on the function of arteriovenous fistula in hemodialysis patients: a novel physicogenomic study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2013 May;28(5):1284–93. doi: 10.1093/ndt/gfs608. [DOI] [PubMed] [Google Scholar]
- 133.Lin CC, Yang WC, Lin SJ, Chen TW, Lee WS, Chang CF, et al. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney international. 2006 Jan;69(1):165–72. doi: 10.1038/sj.ki.5000019. [DOI] [PubMed] [Google Scholar]
- 134.Lever JM, Boddu R, George JF, Agarwal A. Heme Oxygenase-1 in Kidney Health and Disease. Antioxidants & redox signaling. 2016 May 26; doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang L, Grande JP, Farrugia G, Croatt AJ, Katusic ZS, Nath KA. Functioning of an arteriovenous fistula requires heme oxygenase-2. American journal of physiology Renal physiology. 2013 Aug 15;305(4):F545–52. doi: 10.1152/ajprenal.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang L, Grande JP, Hillestad ML, Croatt AJ, Barry MA, Katusic ZS, et al. A new model of an arteriovenous fistula in chronic kidney disease in the mouse: beneficial effects of upregulated heme oxygenase-1. American journal of physiology Renal physiology. 2016 Mar 15;310(6):F466–76. doi: 10.1152/ajprenal.00288.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adin CA, Croker BP, Agarwal A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. American journal of physiology Renal physiology. 2005 Apr;288(4):F778–84. doi: 10.1152/ajprenal.00215.2004. [DOI] [PubMed] [Google Scholar]
- 138.Kim YM, Pae HO, Park JE, Lee YC, Woo JM, Kim NH, et al. Heme oxygenase in the regulation of vascular biology: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling. 2011 Jan 1;14(1):137–67. doi: 10.1089/ars.2010.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, et al. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney international. 2008 Jul;74(1):47–51. doi: 10.1038/ki.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freidja ML, Toutain B, Caillon A, Desquiret V, Lambert D, Loufrani L, et al. Heme oxygenase 1 is differentially involved in blood flow-dependent arterial remodeling: role of inflammation, oxidative stress, and nitric oxide. Hypertension. 2011 Aug;58(2):225–31. doi: 10.1161/HYPERTENSIONAHA.111.170266. [DOI] [PubMed] [Google Scholar]
- 141.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nature communications. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liang M, Wang Y, Liang A, Mitch WE, Roy-Chaudhury P, Han G, et al. Migration of smooth muscle cells from the arterial anastomosis of arteriovenous fistulas requires Notch activation to form neointima. Kidney international. 2015 Sep;88(3):490–502. doi: 10.1038/ki.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Geenen IL, Kolk FF, Molin DG, Wagenaar A, Compeer MG, Tordoir JH, et al. Nitric Oxide Resistance Reduces Arteriovenous Fistula Maturation in Chronic Kidney Disease in Rats. PloS one. 2016;11(1):e0146212. doi: 10.1371/journal.pone.0146212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nieves Torres EC, Yang B, Roy B, Janardhanan R, Brahmbhatt A, Leof E, et al. Adventitial delivery of lentivirus-shRNA-ADAMTS-1 reduces venous stenosis formation in arteriovenous fistula. PloS one. 2014;9(4):e94510. doi: 10.1371/journal.pone.0094510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang B, Brahmbhatt A, Nieves Torres E, Thielen B, McCall DL, Engel S, et al. Tracking and Therapeutic Value of Human Adipose Tissue-derived Mesenchymal Stem Cell Transplantation in Reducing Venous Neointimal Hyperplasia Associated with Arteriovenous Fistula. Radiology. 2016 May;279(2):513–22. doi: 10.1148/radiol.2015150947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, et al. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. American journal of physiology Heart and circulatory physiology. 2007 Jul;293(1):H482–8. doi: 10.1152/ajpheart.01372.2006. [DOI] [PubMed] [Google Scholar]
- 147.Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, et al. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006 Sep;26(9):2063–9. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- 148.Nath KA, Grande JP, Kang L, Juncos JP, Ackerman AW, Croatt AJ, et al. ss-Catenin is markedly induced in a murine model of an arteriovenous fistula: the effect of metalloproteinase inhibition. Am J Physiol Renal Physiol. 2010 Dec;299(6):F1270–7. doi: 10.1152/ajprenal.00488.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Loftus IM, Porter K, Peterson M, Boyle J, London NJ, Bell PR, et al. MMP inhibition reduces intimal hyperplasia in a human vein graft stenosis model. Ann N Y Acad Sci. 1999 Jun 30;878:547–50. doi: 10.1111/j.1749-6632.1999.tb07723.x. [DOI] [PubMed] [Google Scholar]
- 150.Sho E, Sho M, Singh TM, Nanjo H, Komatsu M, Xu C, et al. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Experimental and molecular pathology. 2002 Oct;73(2):142–53. doi: 10.1006/exmp.2002.2457. [DOI] [PubMed] [Google Scholar]
- 151.Wong CY, Rothuizen TC, de Vries MR, Rabelink TJ, Hamming JF, van Zonneveld AJ, et al. Elastin is a key regulator of outward remodeling in arteriovenous fistulas. Eur J Vasc Endovasc Surg. 2015 Apr;49(4):480–6. doi: 10.1016/j.ejvs.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 152.Lin CC, Yang WC, Chung MY, Lee PC. Functional polymorphisms in matrix metalloproteinases-1, -3, -9 are associated with arteriovenous fistula patency in hemodialysis patients. Clinical journal of the American Society of Nephrology: CJASN. 2010 Oct;5(10):1805–14. doi: 10.2215/CJN.01500210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wahl P, Ducasa GM, Fornoni A. Systemic and renal lipids in kidney disease development and progression. American journal of physiology Renal physiology. 2016 Mar 15;310(6):F433–45. doi: 10.1152/ajprenal.00375.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tucker PS, Dalbo VJ, Han T, Kingsley MI. Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2013 Mar;18(2):103–15. doi: 10.3109/1354750X.2012.749302. [DOI] [PubMed] [Google Scholar]
- 155.Machowska A, Carrero JJ, Lindholm B, Stenvinkel P. Therapeutics targeting persistent inflammation in chronic kidney disease. Translational research: the journal of laboratory and clinical medicine. 2016 Jan;167(1):204–13. doi: 10.1016/j.trsl.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 156.Aveles PR, Criminacio CR, Goncalves S, Bignelli AT, Claro LM, Siqueira SS, et al. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clinical practice. 2010;116(4):c294–9. doi: 10.1159/000318792. [DOI] [PubMed] [Google Scholar]
- 157.Hayden MR. Calciphylaxis and the cardiometabolic syndrome: the emerging role of sodium thiosulfate as a novel treatment option. Journal of the cardiometabolic syndrome. 2008 Winter;3(1):55–9. doi: 10.1111/j.1559-4572.2008.08261.x. [DOI] [PubMed] [Google Scholar]
- 158.Sowers KM, Hayden MR. Calcific uremic arteriolopathy: pathophysiology, reactive oxygen species and therapeutic approaches. Oxidative medicine and cellular longevity. 2010 Mar-Apr;3(2):109–21. doi: 10.4161/oxim.3.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sezer S, Ozdemir FN, Arat Z, Turan M, Haberal M. Triad of malnutrition, inflammation, and atherosclerosis in hemodialysis patients. Nephron. 2002 Jul;91(3):456–62. doi: 10.1159/000064287. [DOI] [PubMed] [Google Scholar]
- 160.Liu BC, Li L, Gao M, Wang YL, Yu JR. Microinflammation is involved in the dysfunction of arteriovenous fistula in patients with maintenance hemodialysis. Chinese medical journal. 2008 Nov 5;121(21):2157–61. [PubMed] [Google Scholar]
- 161.Schlieper G, Schurgers L, Brandenburg V, Reutelingsperger C, Floege J. Vascular calcification in chronic kidney disease: an update. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2016 Jan;31(1):31–9. doi: 10.1093/ndt/gfv111. [DOI] [PubMed] [Google Scholar]
- 162.Joannides R, Bakkali EH, Le Roy F, Rivault O, Godin M, Moore N, et al. Altered flow-dependent vasodilatation of conduit arteries in maintenance haemodialysis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 1997 Dec;12(12):2623–8. doi: 10.1093/ndt/12.12.2623. [DOI] [PubMed] [Google Scholar]
- 163.Lee MJ, Han SH, Lee JE, Choi HY, Yoon CY, Kim EJ, et al. Endothelial dysfunction is associated with major adverse cardiovascular events in peritoneal dialysis patients. Medicine. 2014 Sep;93(11):e73. doi: 10.1097/MD.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients–protein-bound uremic toxins and endothelial dysfunction. Seminars in dialysis. 2011 May-Jun;24(3):327–37. doi: 10.1111/j.1525-139X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 165.Wu CC, Wen SC, Yang CW, Pu SY, Tsai KC, Chen JW. Plasma ADMA predicts restenosis of arteriovenous fistula. Journal of the American Society of Nephrology: JASN. 2009 Jan;20(1):213–22. doi: 10.1681/ASN.2008050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brunet P, Gondouin B, Duval-Sabatier A, Dou L, Cerini C, Dignat-George F, et al. Does uremia cause vascular dysfunction? Kidney & blood pressure research. 2011;34(4):284–90. doi: 10.1159/000327131. [DOI] [PubMed] [Google Scholar]
- 167.Hruska KA, Seifert M, Sugatani T. Pathophysiology of the chronic kidney disease-mineral bone disorder. Current opinion in nephrology and hypertension. 2015 Jul;24(4):303–9. doi: 10.1097/MNH.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Allon M, Greene T, Dember LM, Vita JA, Cheung AK, Hamburg NM, et al. Association between Preoperative Vascular Function and Postoperative Arteriovenous Fistula Development. Journal of the American Society of Nephrology: JASN. 2016 May 9; doi: 10.1681/ASN.2015020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001 Oct;38(4):938–42. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 170.O’Neill WC, Lomashvili KA. Recent progress in the treatment of vascular calcification. Kidney international. 2010 Dec;78(12):1232–9. doi: 10.1038/ki.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kokubo T, Ishikawa N, Uchida H, Chasnoff SE, Xie X, Mathew S, et al. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. Journal of the American Society of Nephrology: JASN. 2009 Jun;20(6):1236–45. doi: 10.1681/ASN.2007121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wali MA, Eid RA, Dewan M, Al-Homrany MA. Pre-existing histopathological changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. Annals of thoracic and cardiovascular surgery: official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2006 Oct;12(5):341–8. [PubMed] [Google Scholar]
- 173.Lee T, Safdar N, Mistry MJ, Wang Y, Chauhan V, Campos B, et al. Preexisting venous calcification prior to dialysis vascular access surgery. Seminars in dialysis. 2012 Sep-Oct;25(5):592–5. doi: 10.1111/j.1525-139X.2012.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dageforde LA, Harms KA, Feurer ID, Shaffer D. Increased minimum vein diameter on preoperative mapping with duplex ultrasound is associated with arteriovenous fistula maturation and secondary patency. Journal of vascular surgery. 2015 Jan;61(1):170–6. doi: 10.1016/j.jvs.2014.06.092. [DOI] [PubMed] [Google Scholar]
- 175.van der Linden J, Lameris TW, van den Meiracker AH, de Smet AA, Blankestijn PJ, van den Dorpel MA. Forearm venous distensibility predicts successful arteriovenous fistula. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006 Jun;47(6):1013–9. doi: 10.1053/j.ajkd.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 176.Tanner NC, da Silva AF. Medical Adjuvant Treatment to Improve the Patency of Arteriovenous Fistulae and Grafts: A Systematic Review and Meta-analysis. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2016 Jun 8; doi: 10.1016/j.ejvs.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 177.Kim Y, Jeong SJ, Lee HS, Kim EJ, Song YR, Kim SG, et al. Polymorphism in the promoter region of the klotho gene (G-395A) is associated with early dysfunction in vascular access in hemodialysis patients. The Korean journal of internal medicine. 2008 Dec;23(4):201–7. doi: 10.3904/kjim.2008.23.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Portugaller RH, Kalmar PI, Deutschmann H. The eternal tale of dialysis access vessels and restenosis: are drug-eluting balloons the solution? The journal of vascular access. 2014 Nov-Dec;15(6):439–47. doi: 10.5301/jva.5000271. [DOI] [PubMed] [Google Scholar]
- 179.De Marco Garcia LP, Davila-Santini LR, Feng Q, Calderin J, Krishnasastry KV, Panetta TF. Primary balloon angioplasty plus balloon angioplasty maturation to upgrade small-caliber veins (<3 mm) for arteriovenous fistulas. Journal of vascular surgery. 2010 Jul;52(1):139–44. doi: 10.1016/j.jvs.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 180.Veroux P, Giaquinta A, Tallarita T, Sinagra N, Virgilio C, Zerbo D, et al. Primary balloon angioplasty of small (</=2 mm) cephalic veins improves primary patency of arteriovenous fistulae and decreases reintervention rates. Journal of vascular surgery. 2013 Jan;57(1):131–6. doi: 10.1016/j.jvs.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 181.Roy-Chaudhury P, Lee T, Woodle B, Wadehra D, Campos-Naciff B, Munda R. Balloon-assisted maturation (BAM) of the arteriovenous fistula: the good, the bad, and the ugly. Seminars in nephrology. 2012 Nov;32(6):558–63. doi: 10.1016/j.semnephrol.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Park SC, Ko SY, Kim JI, Moon IS, Kim SD. Balloon-assisted maturation for arteriovenous fistula maturation failure: an early period experience. Annals of surgical treatment and research. 2016 May;90(5):272–8. doi: 10.4174/astr.2016.90.5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miller GA, Friedman A, Khariton A, Preddie DC, Savransky Y. Access flow reduction and recurrent symptomatic cephalic arch stenosis in brachiocephalic hemodialysis arteriovenous fistulas. The journal of vascular access. 2010 Oct-Dec;11(4):281–7. doi: 10.5301/jva.2010.592. [DOI] [PubMed] [Google Scholar]
- 184.Gallagher JJ, Boniscavage P, Ascher E, Hingorani A, Marks N, Shiferson A, et al. Clinical experience with office-based duplex-guided balloon-assisted maturation of arteriovenous fistulas for hemodialysis. Annals of vascular surgery. 2012 Oct;26(7):982–4. doi: 10.1016/j.avsg.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 185.DerDerian T, Hingorani A, Boniviscage P, Carollo A, Ascher E. Acute complications after balloon-assisted maturation. Annals of vascular surgery. 2014 Jul;28(5):1275–9. doi: 10.1016/j.avsg.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 186.Lee MS, Singh V, Nero TJ, Wilentz JR. Cutting balloon angioplasty. The Journal of invasive cardiology. 2002 Sep;14(9):552–6. [PubMed] [Google Scholar]
- 187.Rabbi JF, Kiran RP, Gersten G, Dudrick SJ, Dardik A. Early results with infrainguinal cutting balloon angioplasty limits distal dissection. Annals of vascular surgery. 2004 Nov;18(6):640–3. doi: 10.1007/s10016-004-0103-9. [DOI] [PubMed] [Google Scholar]
- 188.Heerwagen ST, Lonn L, Schroeder TV, Hansen MA. Cephalic arch stenosis in autogenous brachiocephalic hemodialysis fistulas: results of cutting balloon angioplasty. The journal of vascular access. 2010 Jan-Mar;11(1):41–5. doi: 10.1177/112972981001100109. [DOI] [PubMed] [Google Scholar]
- 189.Wu CC, Lin MC, Pu SY, Tsai KC, Wen SC. Comparison of cutting balloon versus high-pressure balloon angioplasty for resistant venous stenoses of native hemodialysis fistulas. Journal of vascular and interventional radiology: JVIR. 2008 Jun;19(6):877–83. doi: 10.1016/j.jvir.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 190.Bhat R, McBride K, Chakraverty S, Vikram R, Severn A. Primary cutting balloon angioplasty for treatment of venous stenoses in native hemodialysis fistulas: long-term results from three centers. Cardiovascular and interventional radiology. 2007 Nov-Dec;30(6):1166–70. doi: 10.1007/s00270-007-9143-1. discussion 71–2. [DOI] [PubMed] [Google Scholar]
- 191.Kariya S, Tanigawa N, Kojima H, Komemushi A, Shomura Y, Shiraishi T, et al. Primary patency with cutting and conventional balloon angioplasty for different types of hemodialysis access stenosis. Radiology. 2007 May;243(2):578–87. doi: 10.1148/radiol.2432051232. [DOI] [PubMed] [Google Scholar]
- 192.Peregrin JH, Rocek M. Results of a peripheral cutting balloon prospective multicenter European registry in hemodialysis vascular access. Cardiovascular and interventional radiology. 2007;30(2):212–5. doi: 10.1007/s00270-006-0020-0. Mar–Apr. [DOI] [PubMed] [Google Scholar]
- 193.Neuen BL, Gunnarsson R, Webster AC, Baer RA, Golledge J, Mantha ML. Predictors of patency after balloon angioplasty in hemodialysis fistulas: a systematic review. Journal of vascular and interventional radiology: JVIR. 2014 Jun;25(6):917–24. doi: 10.1016/j.jvir.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 194.Aftab SA, Tay KH, Irani FG, Gong Lo RH, Gogna A, Haaland B, et al. Randomized clinical trial of cutting balloon angioplasty versus high-pressure balloon angioplasty in hemodialysis arteriovenous fistula stenoses resistant to conventional balloon angioplasty. Journal of vascular and interventional radiology: JVIR. 2014 Feb;25(2):190–8. doi: 10.1016/j.jvir.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 195.Kundu S, Clemens R, Aziza J, Tam P, Nagai G, You J, et al. Ultrahigh-pressure angioplasty versus the Peripheral Cutting Balloon for treatment of stenoses in autogenous fistulas: comparison of immediate results. The journal of vascular access. 2010 Oct-Dec;11(4):303–11. doi: 10.5301/jva.2010.101. [DOI] [PubMed] [Google Scholar]
- 196.Haskal ZJ, Trerotola S, Dolmatch B, Schuman E, Altman S, Mietling S, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. The New England journal of medicine. 2010 Feb 11;362(6):494–503. doi: 10.1056/NEJMoa0902045. [DOI] [PubMed] [Google Scholar]
- 197.Fu N, Joachim E, Yevzlin AS, Shin JI, Astor BC, Chan MR. A Meta-analysis of Stent Placement vs. Angioplasty for Dialysis Vascular Access Stenosis. Seminars in dialysis. 2015 May-Jun;28(3):311–7. doi: 10.1111/sdi.12314. [DOI] [PubMed] [Google Scholar]
- 198.Barshes NR, Annambhotla S, Bechara C, Kougias P, Huynh TT, Dardik A, et al. Endovascular repair of hemodialysis graft-related pseudoaneurysm: an alternative treatment strategy in salvaging failing dialysis access. Vascular and endovascular surgery. 2008 Jun-Jul;42(3):228–34. doi: 10.1177/1538574408314443. [DOI] [PubMed] [Google Scholar]
- 199.Burke SK, Bingham K, Moss E, Gottlieb DP, Wong MD, Bland KS, et al. Recombinant Human Elastase Alters the Compliance of Atherosclerotic Tibial Arteries After Ex Vivo Angioplasty. Journal of cardiovascular pharmacology. 2016 Apr;67(4):305–11. doi: 10.1097/FJC.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Peden EK, Leeser DB, Dixon BS, El-Khatib MT, Roy-Chaudhury P, Lawson JH, et al. A multi-center, dose-escalation study of human type I pancreatic elastase (PRT-201) administered after arteriovenous fistula creation. The journal of vascular access. 2013 Apr-Jun;14(2):143–51. doi: 10.5301/jva.5000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dwivedi AJ, Roy-Chaudhury P, Peden EK, Browne BJ, Ladenheim ED, Scavo VA, et al. Application of human type I pancreatic elastase (PRT-201) to the venous anastomosis of arteriovenous grafts in patients with chronic kidney disease. The journal of vascular access. 2014 Sep-Oct;15(5):376–84. doi: 10.5301/jva.5000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hye RJ, Peden EK, O’Connor TP, Browne BJ, Dixon BS, Schanzer AS, et al. Human type I pancreatic elastase treatment of arteriovenous fistulas in patients with chronic kidney disease. Journal of vascular surgery. 2014 Aug;60(2):454–61.e1. doi: 10.1016/j.jvs.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 203.Katsanos K, Karnabatidis D, Kitrou P, Spiliopoulos S, Christeas N, Siablis D. Paclitaxel-coated balloon angioplasty vs. plain balloon dilation for the treatment of failing dialysis access: 6-month interim results from a prospective randomized controlled trial. Journal of endovascular therapy: an official journal of the International Society of Endovascular Specialists. 2012 Apr;19(2):263–72. doi: 10.1583/11-3690.1. [DOI] [PubMed] [Google Scholar]
- 204.Lai CC, Fang HC, Tseng CJ, Liu CP, Mar GY. Percutaneous angioplasty using a paclitaxel-coated balloon improves target lesion restenosis on inflow lesions of autogenous radiocephalic fistulas: a pilot study. Journal of vascular and interventional radiology: JVIR. 2014 Apr;25(4):535–41. doi: 10.1016/j.jvir.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 205.Kitrou PM, Katsanos K, Spiliopoulos S, Karnabatidis D, Siablis D. Drug-eluting versus plain balloon angioplasty for the treatment of failing dialysis access: final results and cost-effectiveness analysis from a prospective randomized controlled trial ( NCT01174472) European journal of radiology. 2015 Mar;84(3):418–23. doi: 10.1016/j.ejrad.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 206.Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG. Systematic review of drug eluting balloon angioplasty for arteriovenous haemodialysis access stenosis. The journal of vascular access. 2016 Mar 9;17(2):103–10. doi: 10.5301/jva.5000508. [DOI] [PubMed] [Google Scholar]
- 207.Kelly B, Melhem M, Zhang J, Kasting G, Li J, Krishnamoorthy M, et al. Perivascular paclitaxel wraps block arteriovenous graft stenosis in a pig model. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2006 Sep;21(9):2425–31. doi: 10.1093/ndt/gfl250. [DOI] [PubMed] [Google Scholar]
- 208.Lee CW, Ahn JM, Park DW, Kang SJ, Lee SW, Kim YH, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014 Jan 21;129(3):304–12. doi: 10.1161/CIRCULATIONAHA.113.003303. [DOI] [PubMed] [Google Scholar]
- 209.Armstrong EJ, Anderson DR, Yeo KK, Singh GD, Bang H, Amsterdam EA, et al. Association of dual-antiplatelet therapy with reduced major adverse cardiovascular events in patients with symptomatic peripheral arterial disease. Journal of vascular surgery. 2015 Jul;62(1):157–65.e1. doi: 10.1016/j.jvs.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 210.Peeters Weem SM, van Haelst ST, den Ruijter HM, Moll FL, de Borst GJ. Lack of Evidence for Dual Antiplatelet Therapy after Endovascular Arterial Procedures: A Meta-analysis. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2016 Aug;52(2):253–62. doi: 10.1016/j.ejvs.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 211.Bjorkman P, Peltola E, Alback A, Venermo M. Peripheral Vascular Restenosis: A Retrospective Study on the Use of Drug-Eluting Balloons in Native Arteries, Vein Grafts and Dialysis Accesses. Scandinavian journal of surgery: SJS: official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2016 Jun 7; doi: 10.1177/1457496916654098. [DOI] [PubMed] [Google Scholar]
- 212.Huijbregts HJ, de Borst GJ, Veldhuis WB, Verhagen HJ, Velema E, Pasterkamp G, et al. Cryoplasty of the venous anastomosis for prevention of intimal hyperplasia in a validated porcine arteriovenous graft model. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2010 May;39(5):620–6. doi: 10.1016/j.ejvs.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 213.Rifkin BS, Brewster UC, Aruny JE, Perazella MA. Percutaneous balloon cryoplasty: a new therapy for rapidly recurrent anastomotic venous stenoses of hemodialysis grafts? American journal of kidney diseases: the official journal of the National Kidney Foundation. 2005 Feb;45(2):e27–32. doi: 10.1053/j.ajkd.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 214.Gray RJ, Varma JD, Cho SS, Brown LC. Pilot study of cryoplasty with use of PolarCath peripheral balloon catheter system for dialysis access. Journal of vascular and interventional radiology: JVIR. 2008 Oct;19(10):1460–6. doi: 10.1016/j.jvir.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 215.Fareh J, Martel R, Kermani P, Leclerc G. Cellular effects of beta-particle delivery on vascular smooth muscle cells and endothelial cells: a dose-response study. Circulation. 1999 Mar 23;99(11):1477–84. doi: 10.1161/01.cir.99.11.1477. [DOI] [PubMed] [Google Scholar]
- 216.Trerotola SO, Carmody TJ, Timmerman RD, Bergan KA, Dreesen RG, Frost SV, et al. Brachytherapy for the prevention of stenosis in a canine hemodialysis graft model: preliminary observations. Radiology. 1999 Sep;212(3):748–54. doi: 10.1148/radiology.212.3.r99se28748. [DOI] [PubMed] [Google Scholar]
- 217.Manninen HI, Kaukanen ET, Ikaheimo R, Karhapaa P, Lahtinen T, Matsi P, et al. Brachial arterial access: endovascular treatment of failing Brescia-Cimino hemodialysis fistulas–initial success and long-term results. Radiology. 2001 Mar;218(3):711–8. doi: 10.1148/radiology.218.3.r01mr38711. [DOI] [PubMed] [Google Scholar]
- 218.69th Scientific Sessions of the American Heart Association. New Orleans, Louisiana, November 10–13, 1996. Abstracts. Circulation. 1996 Oct 15;94(8 Suppl):I1–904. [PubMed] [Google Scholar]
- 219.Roy-Chaudhury P, Arnold P, Seigel J, Misra S. From basic biology to randomized clinical trial: the Beta Radiation for Arteriovenous Graft Outflow Stenosis (BRAVO II) Seminars in dialysis. 2013 Mar-Apr;26(2):227–32. doi: 10.1111/sdi.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Misra S, Bonan R, Pflederer T, Roy-Chaudhury P. BRAVO I: A pilot study of vascular brachytherapy in polytetrafluoroethylene dialysis access grafts. Kidney international. 2006 Dec;70(11):2006–13. doi: 10.1038/sj.ki.5001869. [DOI] [PubMed] [Google Scholar]
- 221.Masaki T, Rathi R, Zentner G, Leypoldt JK, Mohammad SF, Burns GL, et al. Inhibition of neointimal hyperplasia in vascular grafts by sustained perivascular delivery of paclitaxel. Kidney international. 2004 Nov;66(5):2061–9. doi: 10.1111/j.1523-1755.2004.00985.x. [DOI] [PubMed] [Google Scholar]
- 222.Kohler TR, Toleikis PM, Gravett DM, Avelar RL. Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable Vascular Wrap paclitaxel-eluting mesh. Journal of vascular surgery. 2007 May;45(5):1029–37. doi: 10.1016/j.jvs.2007.01.057. discussion 37–8. [DOI] [PubMed] [Google Scholar]
- 223.Lee BH, Lee JE, Lee KW, Nam HY, Jeon HJ, Sung YJ, et al. Coating with paclitaxel improves graft survival in a porcine model of haemodialysis graft stenosis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2007 Oct;22(10):2800–4. doi: 10.1093/ndt/gfm438. [DOI] [PubMed] [Google Scholar]
- 224.Sanders WG, Hogrebe PC, Grainger DW, Cheung AK, Terry CM. A biodegradable perivascular wrap for controlled, local and directed drug delivery. Journal of controlled release: official journal of the Controlled Release Society. 2012 Jul 10;161(1):81–9. doi: 10.1016/j.jconrel.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Paulson WD, Kipshidze N, Kipiani K, Beridze N, DeVita MV, Shenoy S, et al. Safety and efficacy of local periadventitial delivery of sirolimus for improving hemodialysis graft patency: first human experience with a sirolimus-eluting collagen membrane (Coll-R) Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2012 Mar;27(3):1219–24. doi: 10.1093/ndt/gfr667. [DOI] [PubMed] [Google Scholar]
- 226.Manson RJ, Ebner A, Gallo S, Chemla E, Mantell M, Deaton D, et al. Arteriovenous fistula creation using the Optiflow vascular anastomosis device: a first in man pilot study. Seminars in dialysis. 2013 Jan-Feb;26(1):97–9. doi: 10.1111/j.1525-139X.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 227.Nikam M, Chemla ES, Evans J, Summers A, Brenchley P, Tavakoli A, et al. Prospective controlled pilot study of arteriovenous fistula placement using the novel Optiflow device. Journal of vascular surgery. 2015 Apr;61(4):1020–5. doi: 10.1016/j.jvs.2014.11.082. [DOI] [PubMed] [Google Scholar]
- 228.Fukasawa M, Matsushita K, Kamiyama M, Mikami Y, Araki I, Yamagata Z, et al. The methylentetrahydrofolate reductase C677T point mutation is a risk factor for vascular access thrombosis in hemodialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2003 Mar;41(3):637–42. doi: 10.1053/ajkd.2003.50125. [DOI] [PubMed] [Google Scholar]
- 229.Knoll GA, Wells PS, Young D, Perkins SL, Pilkey RM, Clinch JJ, et al. Thrombophilia and the risk for hemodialysis vascular access thrombosis. Journal of the American Society of Nephrology: JASN. 2005 Apr;16(4):1108–14. doi: 10.1681/ASN.2004110999. [DOI] [PubMed] [Google Scholar]
- 230.Lazo-Langner A, Knoll GA, Wells PS, Carson N, Rodger MA. The risk of dialysis access thrombosis is related to the transforming growth factor-beta1 production haplotype and is modified by polymorphisms in the plasminogen activator inhibitor-type 1 gene. Blood. 2006 Dec 15;108(13):4052–8. doi: 10.1182/blood-2006-06-028902. [DOI] [PubMed] [Google Scholar]
- 231.Petrovic D, Peterlin B. Genetic markers of restenosis after coronary angioplasty and after stent implantation. Medical science monitor: international medical journal of experimental and clinical research. 2005 Apr;11(4):Ra127–35. [PubMed] [Google Scholar]
- 232.Allon M, Zhang L, Maya ID, Bray MS, Fernandez JR. Association of factor V gene polymorphism with arteriovenous graft failure. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012 May;59(5):682–8. doi: 10.1053/j.ajkd.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rotmans JI, Verhagen HJ, Velema E, de Kleijn DP, van den Heuvel M, Kastelein JJ, et al. Local overexpression of C-type natriuretic peptide ameliorates vascular adaptation of porcine hemodialysis grafts. Kidney international. 2004 May;65(5):1897–905. doi: 10.1111/j.1523-1755.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- 234.Luo Z, Akita GY, Date T, Treleaven C, Vincent KA, Woodcock D, et al. Adenovirus-mediated expression of beta-adrenergic receptor kinase C-terminus reduces intimal hyperplasia and luminal stenosis of arteriovenous polytetrafluoroethylene grafts in pigs. Circulation. 2005 Apr 5;111(13):1679–84. doi: 10.1161/01.CIR.0000160357.80517.92. [DOI] [PubMed] [Google Scholar]
- 235.Ohtani K, Egashira K, Hiasa K, Zhao Q, Kitamoto S, Ishibashi M, et al. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004 Oct 19;110(16):2444–52. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 236.Zohny SF, Abd el-Fattah M. Evaluation of circulating vascular endothelial growth factor and soluble adhesion molecules as reliable predictors of native arteriovenous fistula thrombosis in chronic hemodialysis patients. Clinical biochemistry. 2008 Oct;41(14–15):1175–80. doi: 10.1016/j.clinbiochem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 237.Candan F, Yildiz G, Kayatas M. Role of the VEGF 936 gene polymorphism and VEGF-A levels in the late-term arteriovenous fistula thrombosis in patients undergoing hemodialysis. International urology and nephrology. 2014 Sep;46(9):1815–23. doi: 10.1007/s11255-014-0711-4. [DOI] [PubMed] [Google Scholar]
- 238.Fuster V, Charlton P, Boyd A. Clinical protocol. A phase IIb, randomized, multicenter, double-blind study of the efficacy and safety of Trinam (EG004) in stenosis prevention at the graft-vein anastomosis site in dialysis patients. Human gene therapy. 2001 Nov 1;12(16):2025–7. [PubMed] [Google Scholar]
- 239.Sadaghianloo N, Declemy S, Jean-Baptiste E, Haudebourg P, Robino C, Islam MS, et al. Radial artery deviation and reimplantation inhibits venous juxta-anastomotic stenosis and increases primary patency of radial-cephalic fistulas for hemodialysis. Journal of vascular surgery. 2016 Jul 15; doi: 10.1016/j.jvs.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 240.Peck M, Gebhart D, Dusserre N, McAllister TN, L’Heureux N. The evolution of vascular tissue engineering and current state of the art. Cells, tissues, organs. 2012;195(1–2):144–58. doi: 10.1159/000331406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet (London, England) 2016 May 14;387(10032):2026–34. doi: 10.1016/S0140-6736(16)00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


