Abstract
The AML1-ETO fusion protein, generated by the t(8;21) in acute myeloid leukemia (AML), exerts dominant-negative functions and a variety of gains of function, including a positive effect on the growth of primary human CD34+ hematopoietic stem/progenitor cells. We now show that AML1-ETO expression up-regulates the level of TRKA mRNA and protein in these cells and that AML1-ETO-expressing CD34+ hematopoietic cells grown in the presence of five early-acting hematopoietic cytokines further proliferate in response to nerve growth factor (NGF). These cells also show a unique response to NGF and IL-3; namely, they expand in liquid culture. To determine the biological relevance of our findings, we analyzed 262 primary AML patient samples using real-time RT-PCR and found that t(8;21)-positive AML samples express significantly higher levels of TRKA mRNA than other subtypes of AML. NGF, which is normally expressed by bone marrow stromal cells, could provide important proliferative or survival signals to AML1-ETO-expressing leukemic or preleukemic cells, and the NGF/TRKA signaling pathway may be a suitable target for therapeutic approaches to AML.
Keywords: target gene, leukemia, t(8;21), cellular context, cytokine receptor
The core-binding factor (CBF) transcription factor complex [composed of AML1 (also known as RUNX1) and CBFβ] is frequently affected by chromosomal translocations in acute leukemia, leading to the formation of the AML1-ETO (RUNX1-CBFA2T1) [t(8;21)], RUNX1/MDS/EVI1 [t(3;21)], ETV6-RUNX1 [t(12;21)], and CBFβ/SMMHC [inv(16)] fusion proteins (reviewed in refs. 1–3). Mutations in the AML1 gene are also found in undifferentiated leukemias (4, 5) and therapy-related acute myeloid leukemia (AML) or myelodysplastic syndrome (6, 7). Haploinsufficiency of AML1 plays a role in the familial leukemia predisposition syndrome FPD/AML (8), suggesting that dominant-negative forms of AML1 proteins may not be required to promote leukemogenesis.
AML1 and its heterodimeric partner CBFβ are essential for definitive hematopoiesis, because mice lacking either AML1 or CBFβ die at ≈11.5 days postcoitum because of CNS hemorrhage and the absence of definitive hematopoiesis (9–13). AML1 appears to function primarily as a transcriptional activator (reviewed in ref. 1), but it also acts as a transcriptional repressor in certain cellular and promoter contexts (14, 15). AML1-ETO functions mainly as a transcriptional repressor (16–18), binding corepressor molecules (e.g., N-COR and mSIN3) and recruiting histone deacetylases (19–21). AML1-ETO can also act as a transcriptional activator in certain cellular contexts, perhaps by up-regulating c-JUN activity (22), by blocking repression by the promyelocytic leukemia zinc finger protein (23), or by other not-yet-characterized mechanisms (24, 25). AML1-ETO target genes have been identified by scrutiny of promoter regions for AML1 consensus-binding motifs (26) and by overexpression experiments in leukemia cell lines (27–29). However, the overlap between these target gene lists has been poor, and the contribution of a particular gene to AML1-ETO leukemogenesis has been difficult to assess, because the cells used for these analyses are often already leukemic.
Using human CD34+ hematopoietic cells, we demonstrated that AML1-ETO promotes hematopoietic stem/progenitor cell (HSPC) proliferation while having inhibitory effects on more committed progenitor cells (e.g., colony-forming units) (30). Furthermore, expression of AML1-ETO in human HSPC consistently leads to the generation of factor-dependent, clonal outgrowths of CD34+ cells that retain significant in vitro differentiating activity (31). However, AML1-ETO does not appear to be sufficient for leukemogenesis in either human or murine hematopoietic cells (32–37).
To understand the basis for the effects of AML1-ETO in human CD34+ hematopoietic cells, we used Affymetrix oligonucleotide gene arrays and identified the tyrosine receptor kinase A (TRKA) nerve growth factor (NGF) receptor gene (NTRK1) as a target gene increased by the expression of AML1-ETO. Although NGF/TRKA signaling has been most intensively studied in the nervous system, it also participates in hematopoiesis, prostate cancer cell behavior, and angiogenesis (38–40). NGF is normally expressed by bone marrow stromal cells (41), whereas TRKA is expressed in hematopoietic progenitor cells (42).
We examined the NGF/TRKA pathway in regulating the behavior of AML1-ETO-expressing human hematopoietic cells and found that physiologic concentrations of NGF increase the proliferation of AML1-ETO-positive cells, even in the presence of five early-acting hematopoietic cytokines. Additionally, the combination of NGF and IL-3 promotes the in vitro growth of AML1-ETO-expressing CD34+ hematopoietic cells but not the expansion of empty-vector-transduced CD34+ cells. To define the clinical relevance of these in vitro findings, we examined a large number of primary AML samples and found that those containing the t(8;21) translocation express significantly higher levels of TRKA mRNA than the AML samples without the t(8;21). The involvement of the NGF/TRKA signaling pathway in human leukemogenesis may represent a new therapeutic target for AML.
Materials and Methods
Retroviral Production and CD34 Transduction. MIGRI, pEQ-PAM3(-E), and pSV-A-MLV-env plasmids were transiently transfected into 293T cells, and the viral supernatant was used to transduce human CD34+ cells [isolated from mobilized peripheral blood progenitor cells (PBPCs) or cord blood (CB) cells] as described (30). CD34+ cells were selected by using StemSep CD34 magnetic beads (StemCell Technologies, Vancouver) and Miltenyi MACS CD34 isolation columns (Miltenyi Biotec, Auburn, CA).
Transcript profiling of transduced human CB or peripheral blood cells was conducted by using Affymetrix U95Av2 gene chips. RNA extraction, labeling, and the array processing, image, and data analysis were performed as described (43).
Primary AML Patient Samples. After informed consent, bone marrow aspirates or peripheral blood samples were taken at diagnosis from 285 patients with untreated AML enrolled on HOVON protocols. Total RNA was isolated from purified blast cells and normal CD34+ cells (isolated from three healthy volunteers) as described (44); 10 μg total RNA and Affymetrix U133A gene chips were used for expression profiling. Primer sets that span intron–exon junctions and generate ≈100-bp cDNA amplicons were chosen for all quantitative RT-PCR (qPCR) amplifications.
Supervised analyses were performed by using significance analysis of microarrays (45), calculating a score for each gene based on the change in gene expression relative to the SD of all 285 measurements.
Real-Time RT-PCR Analysis. To quantify the expression of the TRKA mRNA, qPCR amplification was carried out by using the 7700 Sequence detector ABI, and the PCR products were detected using either Sybr green I chemistry or TaqMan methodology (PE Applied Biosystems, Norwalk, CT). For details of the primers and methodologies used, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Protein Detection by Using Immunoprecipitation (IP), Immunoblotting, and Flow Cytometry. The methods used for washing cells and preparing proteins and the antibodies used to perform immunoblotting, IP, and flow cytometry are described in detail in Supporting Materials and Methods.
Cell Proliferation Assay. Cells were plated into 96-well flat-bottom microtiter plates at 1–4 × 104 cells per well in 0.1 ml of total volume in triplicate. The indicated cytokines were added, cells were cultured for 3–4 days, and 0.01 ml of WST-1 reagent was added as recommended by the manufacturer (Roche Applied Science). After 3–6 h, absorbance was measured at 450 nm by using a microplate reader (Molecular Devices, Sunnyvale CA).
Results
The TRKA Oncogene Is Up-Regulated by the AML1-ETO Fusion Protein. To identify molecular targets of AML1-ETO expression, we compared the transcriptional profiles of human CD34+ cells transduced with an AML1-ETO-expressing retrovirus or with the control retroviral vector using Affymetrix Hu95A2 oligonucleotide microarrays. We identified the TRKA oncogene as a highly induced mRNA in human CD34+ cells (2.1- to 9.8-fold) and showed similar induction of TRKA mRNA after the expression of AML1-ETO in the human AML cell line MO91 (≈4- to 5-fold). The early up-regulation of TRKA was confirmed in independent experiments, using qPCR for the CD34+ CB cells (6.5-fold as shown in Fig. 1a) and the MO91 cells (data not shown). Microarray data for the primary human cell experiments can be accessed at www.ncbi.nlm.nih.gov/geo (accession no. GSE2049).
Fig. 1.
AML1-ETO-positive cells overexpress TRKA mRNA. (a) TRKA expression measured by using qPCR for GFP- and CD34-selected MIGR1 control and AML1-ETO-expressing CB cells. TRKA expression was determined by using TaqMan technology. (b) CD34-selected and unselected cells were analyzed for TRKA expression by qPCR, using Sybr green technology, and expression was normalized using C-ABL staining. w, week; d, day. (c) Quiescent CB cells and mobilized peripheral blood CD34+ cells, and day 3-stimulated cultures from the same samples were analyzed for TRKA mRNA expression. The week 9 AML1-ETO-expressing cell line AE9 was also included for comparison.
Increased TRKA expression could be detected in AML1-ETO-expressing cells immediately after transduction (Fig. 1a) and in cells growing in culture for as short a period of time as 4 weeks (Fig. 1 b and c). We also examined the level of TRKA mRNA in highly purified populations of clonal, cytokine-dependent CD34+ AML1-ETO-expressing cells. We found 2- to 30-fold more TRKA mRNA in the long-term AML1-ETO-positive cultures (Fig. 1b) than in several control CB preparations (in culture for 6–36 days) by qPCR. The CD34+-selected cells expressed less TRKA than the total population of AML1-ETO-expressing cells growing in culture, and TRKA expression is substantially lower in normal CD34+ PBPCs or CB cells than in the AML1-ETO-expressing cells. TRKA was expressed in both noncycling and cycling CD34+ cells (compare day 0 and day 3 in cytokines; Fig. 1d). TRKA mRNA expression is clearly increased by the introduction of AML1-ETO.
Functional TRKA Protein Expression in AML1-ETO-Positive Hematopoietic Cells. To examine the levels of TRKA protein, we performed Western blot analysis that showed increased expression of the 140-kDa form of TRKA in three AML1-ETO-positive cultures compared with the three control, CD34+ cell cultures (Fig. 2a). Furthermore, TRKA protein was readily detectable on the surface of the AML1-ETO-expressing cells by flow cytometry, using an antibody specific for a TRKA extracellular epitope (data not shown).
Fig. 2.
A functional TRKA protein is up-regulated in AML1-ETO-positive cells. (a) Protein lysates from three AML1-ETO-positive cell cultures (lanes 4–6) and three control cultures (lanes 1–3) were analyzed for expression of TRKA. The CB cells and the three AML1-ETO-positive cell cultures were selected for CD34+ cells. The two PBPC cultures were already predominantly CD34+. 293T and 293T-TRKA were included as controls. (b) Cells from an actively growing AE9 culture were rested overnight without cytokines, and then treated with 50 ng/ml NGF. Cell lysates were subjected to IP with an anti-TRKA antibody, and Western blots were probed for anti-phosphotyrosine (4G10) and anti-TRKA. (c) The protein lysates were probed by Western blot for activated ERK and activated AKT, which became phosphorylated in response to NGF.
To determine whether the TRKA receptor was functional in the AML1-ETO-expressing cells, we added NGF for 10 min after overnight cytokine starvation. The 140-kDa TRKA receptor was tyrosine phosphorylated (Fig. 2b), as were two downstream kinases, extracellular signal-regulated kinase (ERK) and AKT (Fig. 2c), reflecting activation of the mitogen-activated protein kinase (MAPK) and PI3K signaling pathways, respectively. Thus, TRKA signaling pathways are functional in AML1-ETO-positive cells.
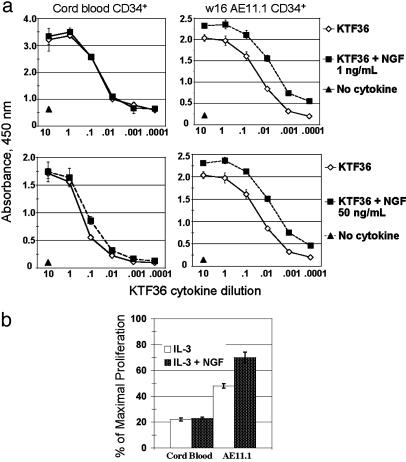
NGF Cooperates with Other Cytokines to Regulate the Proliferation and Survival of AML1-ETO-Expressing Cells. AML1-ETO-expressing human CD34+ HSPCs will grow in liquid culture for many months in a five-cytokine mix, maintaining 10–60% CD34 positivity and the capacity to differentiate into various lineages (31). Given the up-regulation of TRKA expression in AML1-ETO-expressing CD34+ cells, we examined whether NGF had proliferative or survival effects on these cells. Whereas NGF alone had little effect on the proliferation of these cells, the addition of NGF [at 1 ng/ml (Fig. 3a Upper) or 50 ng/ml (Fig. 3a Lower)] to the five-cytokine mix (stem cell factor, megakaryocyte growth and differentiation factor, FLT3 ligand, IL-3, and IL-6) increased cellular proliferative capacity compared with the five-cytokine mix alone (Fig. 3a). This finding implies that NGF may activate signaling pathways in addition to those activated by these five cytokines. No cooperative effect was detected when AML1-ETO was absent, (i.e., in the control, CB samples under the same conditions), although NGF (at 50 but not 1 ng/ml) reproducibly enhanced CB cell proliferation at the 0.1× dilution (Fig. 3a Left Lower).
Fig. 3.
NGF cooperates in the proliferation of AML1-ETO-positive CD34+ cells but not of normal CD34+ cells. (a) CD34+ cells were purified from proliferating cultures by using Miltenyi MACS columns. Cells were grown in 96-well plates at 1–4 × 104 cells per well in 100 μl of total volume in 10-fold dilutions of full-cytokine mix (KTF36 = stem cell factor, MDGF, Flt3L, IL-3, and IL-6) ± 1ng/ml NGF (Upper) or 50 ng/ml NGF (Lower). After 3–4 days, 10 μlof cell proliferation reagent WST-1 was added to each well; the plates were incubated for an additional 4–6 h, and absorbance was measured at 450 nm. Results are the average and SD of triplicate wells. The experiment was repeated twice with similar results. (b) The results of similar experiments where the cells were grown in the presence of 10 ng/ml IL-3 with or without 1ng/ml NGF.
IL-3 alone can temporarily support the survival of AML1-ETO-positive CD34+ cells in culture, although it does not promote their expansion (Fig. 3b and ref. 33). We found that IL-3 and NGF cooperatively promote the proliferation of AML1-ETO-expressing cells ≈70% as effectively as the full-cytokine mix, whereas neither IL-3 alone nor the IL-3/NGF combination had an appreciable effect on the control CD34+ cells (Fig. 3b). This response to IL-3 and NGF appears to be unique to the AML1-ETO-expressing cells.
Because the proliferative potential of the AML1-ETO-expressing cells resides predominantly in the CD34+ subset (31), we examined whether NGF could support the expansion of CD34+ AML1-ETO-positive cells. Although neither NGF nor IL-3 alone supports the expansion of these cells (Fig. 4a and data not shown), CD34+ cell expansion was observed in response to IL-3 plus NGF (Fig. 4a). In contrast, normal control stem/progenitor cells showed only a transient, 1-week expansion of CD34+ cells under full-cytokine conditions; neither IL-3, NGF, nor their combination stimulated the proliferation of normal CD34+ CB cells (Fig. 4a). We also cultured the AML1-ETO-positive cells under full-cytokine conditions in the presence and absence of NGF (at 20 or 1 ng/ml); we observed a 2- to 8-fold expansion of total cells and CD34+ cells over a period of 10 weeks (Table 1). Similar experiments performed with normal CD34+ cells showed no effect of NGF on CD34+ expansion (data not shown).
Fig. 4.
NGF and IL-3 synergistically promote the expansion of CD34+ cells expressing AML1-ETO. (a) CD34+ cells were purified from proliferating cultures by using Miltenyi MACS columns. Liquid cultures were established by using 2 × 105 cells per ml in the presence of the indicated cytokines. The percent viable cells were determined weekly by Trypan blue dye exclusion, and CD34 expression was analyzed by flow cytometry. Expansion of CD34+ cells is plotted over time. NGF alone gave no expansion and was not included in the AE11.1 graph. Results for primary CD34+ cells are the average and SD of three separate experiments, two experiments using PBPCs, and one experiment using CB CD34+ cells as starting material. Results for AE11.1 are the average and SD of three separate experiments. (b) Cumulative fold change in total numbers of cells growing in liquid culture. CB CD34+ cells were transduced with AML-1-ETO, TRKA, or control MIGR1 retrovirus, and the CD34+GFP+-sorted cells were plated in liquid culture containing KTF36. Viable cell number was determined weekly by Trypan blue dye exclusion. (Inset) The expansion of CD34+ cells in these cultures over the same time period, by using flow cytometry to calculate the percent of CD34 positivity. The values are representative of several independent experiments.
Table 1. NGF synergizes with the five-cytokine mix (KTF36) to promote expansion of AML1-ETO-positive cells.
| Total cell expansion × 1013
|
CD34+ cell expansion × 1013
|
|||||||
|---|---|---|---|---|---|---|---|---|
| KTF36 | KTF36 + NGF(20) | KTF36 + NGF(1) | Fold increase | KTF36 | KTF36 + NGF(20) | KTF36 + NGF(1) | Fold increase | |
| Week 9 AE9 | 2.3 | 17.9 | 7.8 | 1.3 | 10.3 | 8.2 | ||
| Week 9 AE11.1 | 6.5 | 22.6 | 3.5 | 1.9 | 7.2 | 3.7 | ||
| Week 10 AE13 | 2.5 | 9.3 | 3.8 | 1.1 | 3.8 | 3.6 | ||
| Week 11 AE11.1 | 6.1 | 17.6 | 2.9 | 2.2 | 4.6 | 2.1 | ||
The AML1-ETO-positive cells were CD34-selected at the indicated week of growth and expanded in liquid culture using the five-cytokine mixture KTF36 or additionally supplemented with 20 ng/ml NGF or 1 ng/ml NGF. Cells were counted weekly by Trypan blue dye exclusion and stained for expression of CD34. The numbers indicate growth over a 10-week period. Control CB and PBPC CD34+ cells were cultured under similar conditions, with no effect of NGF on total or CD34+ cell expansion (data not shown).
To determine whether TRKA overexpression recapitulates the effects of expressing AML1-ETO in CD34+ cells, we transduced normal CB cells with a retrovirus expressing human TRKA. TRKA expression in the CD34+ cells did not significantly alter their cellular expansion in liquid culture, compared with the empty MIGR1 vector (Fig. 4b), whereas AML1-ETO-expressing cells show significant cellular expansion, as reported. Whereas expression of AML1-ETO clearly leads to the expansion of CD34+ cells (see Fig. 4b Inset), TRKA expression itself is insufficient enough to do so.
Intact TRKA Signaling in AML1-ETO-Expressing Cells. We performed IP and Western blot analysis of the TRKA receptor to determine its phosphorylation status. After an overnight rest in the absence of cytokines, cells were pulsed for 10 min with the indicated cytokines. NGF, but not IL-3, or the full complement of cytokines, led to strong tyrosine phosphorylation of TRKA in all three AML1-ETO-positive cells tested (Fig. 5a). Similar results were found by using control PBPCs (Fig. 5a). Little constitutive phosphorylation of TRKA is detected after the overnight rest; although the receptor is highly expressed on the AML1-ETO-positive cells, addition of ligand is required for its full activation (Fig. 5a).
Fig. 5.
NGF/TRKA signaling in AML1-ETO-positive cells. (a) Cells were rested overnight without cytokines and pulsed the next day with the indicated cytokine(s) for 10 min. Actively growing cell cultures were included as controls. Protein lysates were subjected to IP with an anti-TRKA antibody, and then a phosphotyrosine-specific antibody (PY99) was used for Western blot analysis. The amount of TRKA in the lysates was determined by using an anti-TRKA antibody for Western blotting. Actin was used as a loading control. (b) Cells were treated as described for a, and protein lysates were prepared and analyzed by Western blot for the level of ERK, AKT, STAT3, and STAT5 and the level of activation of p42/44 ERK, AKT, STAT3, and STAT5 by using phosphospecific antibodies for each of these proteins. A representative experiment is shown.
To understand the biochemical basis for the growth of these cultures better, we analyzed the signaling pathways that are activated in response to IL-3 and/or NGF in normal CD34+ cells and in the AML1-ETO-positive cell cultures by using Western blot analysis to detect activation of the PI3K, MAPK, or Janus kinase-signal transducers and activators of transcription (JAK/STAT) signaling pathways. In the AML1-ETO-positive cells, constitutive activation (i.e., phosphorylation) of STAT3, STAT5, AKT, and ERK was not detected (Fig. 5b). Thus far, aberrant activation of signaling molecules has not been detected in response to NGF.
TRKA Expression Is Significantly Higher in t(8;21)+ AML Primary Patient Samples than in Other AML Samples. To better understand the physiologic relevance of TRKA up-regulation to human t(8;21)+ AML, we determined the expression of TRKA in 285 cases of primary, untreated AML by gene expression profiling (ref. 44 and Table 3, which is published as supporting information on the PNAS web site). Although most AML samples showed TRKA expression similar to the normal CD34+ cells, certain samples had markedly increased levels of TRKA; the largest subset of such samples occurred in the AML t(8;21) subclass (cluster 13). In a significant proportion of t(8;21) AMLs, TRKA expression was 10- to 20-fold up-regulated (compared with most AMLs and the normal CD34+ cells) and significance analysis of microarrays analysis confirmed that TRKA was significantly differentially expressed in the cluster 13 samples. The differences in TRKA levels cannot be explained by differences in secondary mutations such as in the c-KIT or FLT3 kinases or the N- or K-RAS GTPases, which were assessed in these cases. High TRKA expression was sometimes seen in samples that contained such mutations.
Because the intensity of hybridization to the TRKA probe sets on the U133A GeneChip were quite low (mean of 11 ± 21 and a target intensity of 100), we confirmed these results by using real-time RT-PCR, examining 262 of the 285 samples. Overall, 48 of the 262 clustered samples (18.3%) had levels of TRKA expression that were >2-fold greater than that seen in the normal human CD34+ bone marrow cells, and 19 of 262 (7.3%) had expression that were >5-fold greater. For the 2-fold threshold, cluster 3 (9 of 18; 50%), cluster 9 (10 of 21; 47.6%), and cluster 13 (10 of 21; 47.6%) had significantly higher positive proportions than would be expected. For the 5-fold threshold, only cluster 13 (7 of 21; 33.3%) had significantly higher TRKA mRNA expression. Thus, the level of TRKA expression determined by qPCR (shown in Table 2) confirmed the microarray data with respect to the AML1-ETO-positive clinical samples (cluster 13).
Table 2. Real-time qRT-PCR analysis of TRKA mRNA levels in primary AML patient samples.
| Cluster | Nos. in cluster | Nos. >2-fold | Proportion >2-fold | P value for 2-fold | Nos. >5-fold | Proportion >5-fold | P value for 5-fold |
|---|---|---|---|---|---|---|---|
| 1 | 12 | 1 | 0.083 | 1 | 1 | 0.083 | 1 |
| 2 | 17 | 3 | 0.176 | 1 | 0 | 0.000 | 1 |
| 3 | 18 | 9 | 0.500 | 0.0032 | 2 | 0.111 | 0.9989 |
| 4 | 15 | 2 | 0.133 | 1 | 0 | 0.000 | 1 |
| 5 | 42 | 0 | 0.000 | 1 | 0 | 0.000 | 1 |
| 6 | 8 | 1 | 0.125 | 1 | 0 | 0.000 | 1 |
| 7 | 18 | 1 | 0.056 | 1 | 0 | 0.000 | 1 |
| 8 | 12 | 0 | 0.000 | 1 | 0 | 0.000 | 1 |
| 9 | 21 | 10 | 0.476 | 0.0027 | 4 | 0.190 | 0.2675 |
| 10 | 23 | 5 | 0.217 | 0.9999 | 1 | 0.043 | 1 |
| 11 | 9 | 0 | 0.000 | 1 | 0 | 0.000 | 1 |
| 12 | 19 | 6 | 0.316 | 0.7945 | 3 | 0.158 | 0.5398 |
| 13 | 21 | 10 | 0.476 | 0.0027 | 7 | 0.333 | 0.0005 |
| 14 | 10 | 0 | 0.000 | 1 | 0 | 0.000 | 1 |
| 15 | 8 | 1 | 0.125 | 1 | 1 | 0.125 | 1 |
| 16 | 11 | 0 | 0.000 | 1 | 0 | 0.000 | 1 |
Numbers in cluster are the number of patient samples in each cluster. Numbers >2-fold (or 5-fold) are number of sample with TRKA mRNA levels ≥2-fold (or 5-fold) the level of expression in CD34 selected bone marrow cells. Proportion >2-fold (or 5-fold) is the percentage of samples in that cluster with >2-fold (or 5-fold) TRKA mRNA levels, relative to CD34+ bone marrow cells. P values are provided for the proportion of samples with a >2-fold (or >5-fold) increase in TRKA mRNA levels.
Discussion
AML is characterized by the accumulation of immature, nonfunctional hematopoietic cells (myeloblasts) that proliferate but are blocked in their differentiation. This behavior is thought to require two or more distinct genetic lesions, because leukemia-associated fusion transcription factors such as AML1-ETO generally are not able to reproducibly generate acute leukemia by themselves in mice (33–37, 46). We have shown that AML1-ETO expression in human CD34+ hematopoietic cells maintains these cells in culture, allowing their expansion and the generation of factor-dependent, clonal, preleukemic cell cultures (30, 31). The downstream pathways through which AML1-ETO exerts its effects are currently unknown. We have investigated the transcriptional profiles of primary human hematopoietic progenitor cells transduced with AML1-ETO by using Affymetrix oligonucleotide gene arrays, and have demonstrated that the TRKA oncogene is reproducibly up-regulated at both the RNA and protein level. This up-regulation of TRKA results in a proliferative response to NGF that is not apparent by using normal CD34+ cells. This response is seen even in the presence of the five cytokines typically used to grow these cells, implying that a signaling cascade is initiated by NGF, in the presence of AML1-ETO, which is not provided by saturating amounts of stem cell factor, megakaryocyte growth and differentiation factor, FLT3 ligand, IL-6, or IL-3.
Whereas several groups have systematically analyzed potential downstream targets of this fusion protein, by using established cell lines for their studies (27–29), we used primary human CD34+ cells for the identification of AML1-ETO-regulated genes, and identified TRKA as a potentially important component of AML1-ETO function. The retroviral transduction methodology does not allow us to define whether TRKA is a direct or indirect target gene of AML1-ETO, although it did allow us to show its biological relevance. Whereas chromatin IP studies may determine whether the AML1 binding site(s) in the 5′-flanking region of the TRKA gene are occupied by AML1-ETO, other regulatory regions in the gene may be more critical to its regulation, and technical difficulties may also limit interpretation of such studies.
The involvement of TRK family members in acute myeloid leukemia has been well established (47–50). From our data and from previous studies, TRKA mRNA is expressed in both human and murine hematopoietic stem/progenitor cells, and the protein is competent to respond to NGF ligand (42). Our biochemical analyses indicate that NGF activates the MAPK and PI3K signaling pathways in primary human CD34+ cells, and similarly in AML1-ETO-positive cells. Clearly, the functional consequences of TRKA activation are significantly different in the AML1-ETO-expressing cells. Neither primary CD34+ cells, nor those expressing AML1-ETO, proliferate in response to NGF alone. Yet, cells expressing AML1-ETO will respond to the combination of NGF and IL-3 with an expansion of CD34+ cells, but control CD34+ cells will not.
We analyzed the basal and inducible tyrosine phosphorylation pattern in AML1-ETO-positive cells and in normal human CD34+ cells by Western blot using an anti-phosphotyrosine specific antibody. Multiple constitutively tyrosine phosphorylated proteins of ≈45, 50–60, and >200 kDa were reproducibly detected in the two AML1-ETO-positive cells tested; they were not seen in normal CD34+ cells (data not shown). Whether these signaling molecules play a role in the unique response of the AML1-ETO cells to the combination of IL-3 and NGF awaits the identification of these proteins. Whereas the lack of expression of NGF by the AML1-ETO-expressing cells demonstrates that the NGF/TRKA signaling pathway is not essential for the in vitro effects of AML1-ETO on human CD34+ cells, the increased TRKA expression seen in the primary t(8;21)-positive AML patient samples certainly supports the biological relevance of our findings. Furthermore, the inv(16) samples (cluster 9), another subset of the CBF leukemias that contain the CBFβ-SMMHC fusion protein also show increased TrkA expression (≥2-fold; see Table 2).
From all available data, it is clear that AML1-ETO does not impart a strong proliferative effect on its own, because mouse or human hematopoietic cells expressing the fusion protein strictly depend on cytokines for continued growth (31, 51). However, stromal cells in the bone marrow microenvironment secrete NGF and other cytokines (41); thus, the enhanced expression of the TRKA receptor on AML1-ETO-positive cells could impart a selective growth advantage. The recent finding that AML1-ETO deregulates genes involved in DNA repair underscores the importance of promoting preleukemic stem cell growth to allow for the acquisition of cooperating mutations that could lead to a fully transformed state (27).
In summary, our data identify a functionally relevant target gene of AML1-ETO in the clinically relevant CD34+ HSPCs and may be the first example of using primary human cells to identify a downstream target gene of an oncogene. The up-regulation of TRKA by AML1-ETO in human HSPCs and the unique functional response to NGF suggest that TRKA could serve as a potential target for therapeutic intervention in t(8;21) positive leukemias.
Supplementary Material
Acknowledgments
We thank Joseph Scandura for technical and intellectual contribution to this work; Agnes Viale for assistance with transcript profiling; Adam Olshen for statistical analysis of the primary patient sample data; Claudia A. J. Erpelinck for technical assistance; Ellie Park for assistance in preparing this manuscript; and Amgen, Kirin, and Immunex for providing cytokines. This work was supported by a Leukemia and Lymphoma Society Specialized Center of Research Grant (to S.D.N., Agnes Viale, H.Z., and V.J.), National Institutes of Health R01 Grant DK 52621 (to S.D.N.), the Renny Saltzman Leukemia Research Fund (to S.D.N., J.C., and V.J.), National Institutes of Health Grant CA90370 (to J.C.M.), and the Translational Research Initiative at Cincinnati Children's Hospital Medical Center (to J.C.M.).
Author contributions: J.C.M. and S.D.N. designed research; J.C.M., V.J., M.W., J.C., O.K., and P.J.M.V. performed research; V.J. and R.D. contributed new reagents/analytic tools; J.C.M., V.J., M.W., R.D., J.C., H.Z., P.J.M.V., B.L., and S.D.N. analyzed data; J.C.M., V.J., B.L., and S.D.N. wrote the paper; and R.D. and P.J.M.V. analyzed the AML patient samples and their patient sample database.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AML, acute myeloid leukemia; CBF, core-binding factor; HSPC, hematopoietic stem/progenitor cell; NGF, nerve growth factor; PBPC, peripheral blood progenitor cell; qPCR, quantitative RT-PCR; CB, cord blood; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; IP, immunoprecipitation; STAT, signal transducers and activators of transcription; TRKA, tyrosine receptor kinase A.
Data deposition: The sequence reported in this paper has been deposited in the AML-1-ETO target genes database (accession no. GSE2049).
References
- 1.Downing, J. R., Higuchi, M., Lenny, N. & Yeoh, A. E. (2000) Semi. Cell. Dev. Biol. 11, 347–360. [DOI] [PubMed] [Google Scholar]
- 2.Speck, N. A. (2001) Curr. Opin. Hematol. 8, 192–196. [DOI] [PubMed] [Google Scholar]
- 3.Scandura, J. M., Boccuni, P., Cammenga, J. & Nimer, S. D. (2002) Oncogene 21, 3422–3444. [DOI] [PubMed] [Google Scholar]
- 4.Osato, M., Asou, N., Abdalla, E., Hoshino, K., Yamasaki, H., Okubo, T., Suzushima, H., Takatsuki, K., Kanno, T., Shigesada, K. & Ito, Y. (1999) Blood 93, 1817–1824. [PubMed] [Google Scholar]
- 5.Preudhomme, C., Warot-Loze, D., Roumier, C., Grardel-Duflos, N., Garand, R., Lai, J. L., Dastugue, N., Macintyre, E., Denis, C., Bauters, F., et al. (2000) Blood 96, 2862–2869. [PubMed] [Google Scholar]
- 6.Harada, H., Harada, Y., Tanaka, H., Kimura, A. & Inaba, T. (2003) Blood 101, 673–680. [DOI] [PubMed] [Google Scholar]
- 7.Harada, H., Harada, Y., Niimi, H., Kyo, T., Kimura, A. & Inaba, T. (2004) Blood 103, 2316–2324. [DOI] [PubMed] [Google Scholar]
- 8.Song, W. J., Sullivan, M. G., Legare, R. D., Hutchings, S., Tan, X., Kufrin, D., Ratajczak, J., Resende, I. C., Haworth, C., Hock, R., et al. (1999) Nat. Genet. 23, 166–175. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki, K., Yagi, H., Bronson, R. T., Tominaga, K., Matsunashi, T., Deguchi, K., Tani, Y., Kishimoto, T. & Komori, T. (1996) Proc. Natl. Acad. Sci. USA 93, 12359–12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuda, T., van Deursen, J., Hiebert, S. W., Grosveld, G. & Downing, J. R. (1996) Cell 84, 321–330. [DOI] [PubMed] [Google Scholar]
- 11.Wang, Q., Stacy, T., Binder, M., Marin-Padilla, M., Sharpe, A. H. & Speck, N. A. (1996) Proc. Natl. Acad. Sci. USA 93, 3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, Q., Stacy, T., Miller, J. D., Lewis, A. F., Gu, T. L., Huang, X., Bushweller, J. H., Bories, J. C., Alt, F. W., Ryan, G., et al. (1996) Cell 87, 697–708. [DOI] [PubMed] [Google Scholar]
- 13.Niki, M., Okada, H., Takano, H., Kuno, J., Tani, K., Hibino, H., Asano, S., Ito, Y., Satake, M. & Noda, T. (1997) Proc. Natl. Acad. Sci. USA 94, 5697–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniuchi, I., Osato, M., Egawa, T., Sunshine, M. J., Bae, S. C., Komori, T., Ito, Y. & Littman, D. R. (2002) Cell 111, 621–633. [DOI] [PubMed] [Google Scholar]
- 15.Lutterbach, B., Westendorf, J. J., Linggi, B., Isaac, S., Seto, E. & Hiebert, S. W. (2000) J. Biol. Chem. 275, 651–656. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi, H., Kozu, T., Shimizu, K., Enomoto, K., Maseki, N., Kaneko, Y., Kamada, N. & Ohki, M. (1993) EMBO J. 12, 2715–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank, R., Zhang, J., Uchida, H., Meyers, S., Hiebert, S. W. & Nimer, S. D. (1995) Oncogene 11, 2667–2674. [PubMed] [Google Scholar]
- 18.Meyers, S., Lenny, N. & Hiebert, S. W. (1995) Mol. Cell. Biol. 15, 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelmetti, V., Zhang, J., Fanelli, M., Minucci, S., Pelicci, P. G. & Lazar, M. A. (1998) Mol. Cell. Biol. 18, 7185–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutterbach, B., Westendorf, J. J., Linggi, B., Patten, A., Moniwa, M., Davie, J. R., Huynh, K. D., Bardwell, V. J., Lavinsky, R. M., Rosenfeld, M. G., et al. (1998) Mol. Cell. Biol. 18, 7176–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, J., Hoshino, T., Redner, R. L., Kajigaya, S. & Liu, J. M. (1998) Proc. Natl. Acad. Sci. USA 95, 10860–10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank, R. C., Sun, X., Berguido, F. J., Jakubowiak, A. & Nimer, S. D. (1999) Oncogene 18, 1701–1710. [DOI] [PubMed] [Google Scholar]
- 23.Melnick, A. M., Westendorf, J. J., Polinger, A., Carlile, G. W., Arai, S., Ball, H. J., Lutterbach, B., Hiebert, S. W. & Licht, J. D. (2000) Mol. Cell. Biol. 20, 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klampfer, L., Zhang, J., Zelenetz, A. O., Uchida, H. & Nimer, S. D. (1996) Proc. Natl. Acad. Sci. USA 93, 14059–14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhoades, K. L., Hetherington, C. J., Rowley, J. D., Hiebert, S. W., Nucifora, G., Tenen, D. G. & Zhang, D. E. (1996) Proc. Natl. Acad. Sci. USA 93, 11895–11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linggi, B., Muller-Tidow, C., van de Locht, L., Hu, M., Nip, J., Serve, H., Berdel, W. E., van der Reijden, B., Quelle, D. E., Rowley, J. D., et al. (2002) Nat. Med. 8, 743–750. [DOI] [PubMed] [Google Scholar]
- 27.Alcalay, M., Meani, N., Gelmetti, V., Fantozzi, A., Fagioli, M., Orleth, A., Riganelli, D., Sebastiani, C., Cappelli, E., Casciari, C., et al. (2003) J. Clin. Invest. 112, 1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada, H., Ichikawa, H., Nakamura, S., Katsu, R., Iwasa, M., Kitabayashi, I. & Ohki, M. (2000) Blood 96, 655–663. [PubMed] [Google Scholar]
- 29.Muller-Tidow, C., Steffen, B., Cauvet, T., Tickenbrock, L., Ji, P., Diederichs, S., Sargin, B., Kohler, G., Stelljes, M., Puccetti, E., et al. (2004) Mol. Cell. Biol. 24, 2890–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulloy, J. C., Cammenga, J., MacKenzie, K. L., Berguido, F. J., Moore, M. A. & Nimer, S. D. (2002) Blood 99, 15–23. [DOI] [PubMed] [Google Scholar]
- 31.Mulloy, J. C., Cammenga, J., Berguido, F. J., Wu, K., Zhou, P., Comenzo, R. L., Jhanwar, S., Moore, M. A. & Nimer, S. D. (2003) Blood 102, 4369–4376. [DOI] [PubMed] [Google Scholar]
- 32.Nucifora, G., Larson, R. A. & Rowley, J. D. (1993) Blood 82, 712–715. [PubMed] [Google Scholar]
- 33.Higuchi, M., O'Brien, D., Kumaravelu, P., Lenny, N., Yeoh, E. J. & Downing, J. R. (2002) Cancer Cell 1, 63–74. [DOI] [PubMed] [Google Scholar]
- 34.Yuan, Y., Zhou, L., Miyamoto, T., Iwasaki, H., Harakawa, N., Hetherington, C. J., Burel, S. A., Lagasse, E., Weissman, I. L., Akashi, K. & Zhang, D. E. (2001) Proc. Natl. Acad. Sci. USA 98, 10398–10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Guzman, C. G., Warren, A. J., Zhang, Z., Gartland, L., Erickson, P., Drabkin, H., Hiebert, S. W. & Klug, C. A. (2002) Mol. Cell. Biol. 22, 5506–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhoades, K. L., Hetherington, C. J., Harakawa, N., Yergeau, D. A., Zhou, L., Liu, L. Q., Little, M. T., Tenen, D. G. & Zhang, D. E. (2000) Blood 96, 2108–2115. [PubMed] [Google Scholar]
- 37.Schwieger, M., Lohler, J., Friel, J., Scheller, M., Horak, I. & Stocking, C. (2002) J. Exp. Med. 196, 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auffray, I., Chevalier, S., Froger, J., Izac, B., Vainchenker, W., Gascan, H. & Coulombel, L. (1996) Blood 88, 1608–1618. [PubMed] [Google Scholar]
- 39.Delsite, R. & Djakiew, D. (1996) J. Androl. 17, 481–490. [PubMed] [Google Scholar]
- 40.Kraemer, R. & Hempstead, B. L. (2003) Front. Biosci. 8, s1181–s1186. [DOI] [PubMed] [Google Scholar]
- 41.Labouyrie, E., Dubus, P., Groppi, A., Mahon, F. X., Ferrer, J., Parrens, M., Reiffers, J., de Mascarel, A. & Merlio, J. P. (1999) Am. J. Pathol. 154, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chevalier, S., Praloran, V., Smith, C., MacGrogan, D., Ip, N. Y., Yancopoulos, G. D., Brachet, P., Pouplard, A. & Gascan, H. (1994) Blood 83, 1479–1485. [PubMed] [Google Scholar]
- 43.Cammenga, J., Mulloy, J. C., Berguido, F. J., MacGrogan, D., Viale, A. & Nimer, S. D. (2003) Blood 101, 2206–2214. [DOI] [PubMed] [Google Scholar]
- 44.Valk, P. J., Verhaak, R. G., Beijen, M. A., Erpelinck, C. A., Barjesteh van Waalwijk van Doorn-Khosrovani, S., Boer, J. M., Beverloo, H. B., Moorhouse, M. J., van der Spek, P. J., Lowenberg, B. & Delwel, R. (2004) N. Engl. J. Med. 350, 1617–1628. [DOI] [PubMed] [Google Scholar]
- 45.Tusher, V. G., Tibshirani, R. & Chu, G. (2001) Proc. Natl. Acad. Sci. USA 98, 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grisolano, J. L., O'Neal, J., Cain, J. & Tomasson, M. H. (2003) Proc. Natl. Acad. Sci. USA 100, 9506–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eguchi, M., Eguchi-Ishimae, M., Tojo, A., Morishita, K., Suzuki, K., Sato, Y., Kudoh, S., Tanaka, K., Setoyama, M., Nagamura, F., et al. (1999) Blood 93, 1355–1363. [PubMed] [Google Scholar]
- 48.Reuther, G. W., Lambert, Q. T., Caligiuri, M. A. & Der, C. J. (2000) Mol. Cell. Biol. 20, 8655–8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu, Q., Schwaller, J., Kutok, J., Cain, D., Aster, J. C., Williams, I. R. & Gilliland, D. G. (2000) EMBO J. 19, 1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, Z., Dullmann, J., Schiedlmeier, B., Schmidt, M., von Kalle, C., Meyer, J., Forster, M., Stocking, C., Wahlers, A., Frank, O., et al. (2002) Science 296, 497. [DOI] [PubMed] [Google Scholar]
- 51.Okuda, T., Cai, Z., Yang, S., Lenny, N., Lyu, C. J., van Deursen, J. M., Harada, H. & Downing, J. R. (1998) Blood 91, 3134–3143. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.