Abstract
Herpes simplex virus type I (HSV-1) is the causative agent of several pathologies ranging in severity from the common cold sore to life-threatening encephalitic infection. During productive lytic infection, over 80 viral proteins are expressed in a highly regulated manner, resulting in the replication of viral genomes and assembly of progeny virions. The virion of all herpesviruses consists of an external membrane envelope, a proteinaceous layer called the tegument, and an icosahedral capsid containing the double-stranded linear DNA genome. The capsid shell of HSV-1 is built from four structural proteins: a major capsid protein, VP5, which forms the capsomers (hexons and pentons), the triplex consisting of VP19C and VP23 found between the capsomers, and VP26 which binds to VP5 on hexons but not pentons. In addition, the dodecameric pUL6 portal complex occupies one of the 12 capsid vertices, and the capsid vertex specific component (CVSC), a heterotrimer complex of pUL17, pUL25 and pUL36 binds specifically to the triplexes adjacent to each penton. The capsid is assembled in the nucleus where the viral genome is packaged into newly assembled closed capsid shells. Cleavage and packaging of replicated, concatemeric viral DNA requires the seven viral proteins encoded by the UL6, UL15, UL17, UL25, UL28, UL32, and UL33 genes. Considerable advances have been made in understanding the structure of the herpesvirus capsid and the function of several of the DNA packaging proteins by applying biochemical, genetic, and structural techniques. This review is a summary of recent advances with respect to the structure of the HSV-1 virion capsid and what is known about the function of the seven packaging proteins and their interactions with each other and with the capsid shell.
Keywords: herpesvirus, HSV-1, PRV, capsid, structure, cryo-electron microscopy, triplex, CVSC, DNA packaging
The herpesvirus virion
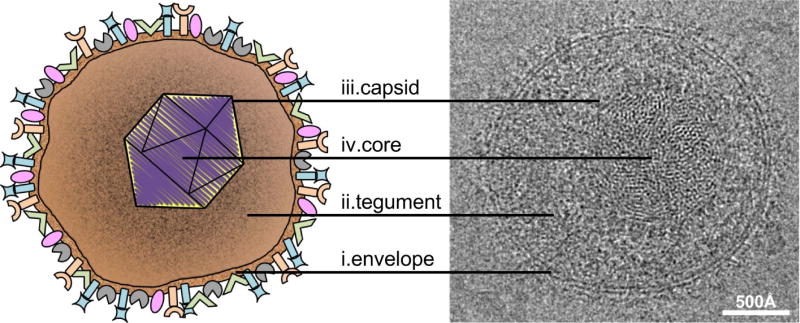
The mature HSV-1 virion is pleomorphic but largely spherical, with an average diameter of 186 nm at the base of the envelope that extends to approximately 225 nm when the glycoprotein spikes are included (Grunewald et al. 2003) (Fig. 1). Like all members of Herpesviridae the HSV-1 virion is composed of four main architectural features: envelope, tegument, capsid, and the viral genome (Pellet and Roizman 2007).
Figure 1. Structure of the HSV-1 virion.
The diagram at left depicts the four major structural components of the HSV-1 virion: (i) the outer envelope studded with various glycoproteins, (ii) the proteinaceous tegument layer, and (iii) the icosahedral capsid that houses (iv) the dsDNA core. Corresponding features in a cryo-electron micrograph of a virion are indicated at right. Bar = 500 Å.
The outer envelope is arranged as a lipid bilayer containing multiple copies of approximately eleven viral glycoproteins that protrude externally and a small number of intrinsic membrane proteins (Eisenberg et al. 2011). The envelope is obtained from the host cell and possesses lipid content similar to that found in the cellular cytoplasmic membrane (Spear and Roizman 1967; van Genderen et al. 1994).
The viral tegument layer is located in the space between the envelope and capsid, and occupies approximately two-thirds of the volume within the virion. Cryo-electron tomography of the HSV-1 virion revealed that the tegument is polar in structure; where at one side of the virion there is approximately 35 nm of tegument between the envelope and the capsid, and at the opposite side the capsid resides in close proximity to the envelope (Grunewald et al. 2003). These studies also showed that the tegument substructure was particulate in appearance and contained short actin-like filaments. The tegument is largely proteinaceous, containing multiple copies of approximately twenty-three viral proteins, but has also been shown to contain viral and cellular gene transcripts (Loret et al. 2008; Sciortino et al. 2001). Mass spectrometry analysis of purified virions has also identified several cellular proteins that may be tegument components; however these results are yet to be verified (Loret et al. 2008).
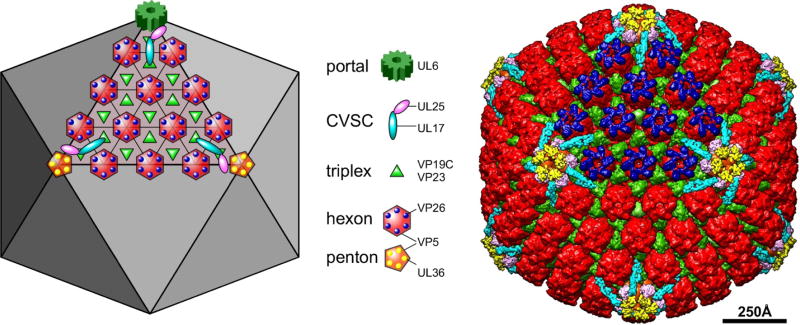
The structure of the HSV-1 capsid has been described in great detail owing to numerous studies utilizing cryo-electron microscopy (cryo-EM) and three-dimensional image reconstruction of isolated capsids, reviewed in: (Brown and Newcomb 2011; Conway and Homa 2011; Homa and Brown 1997) (Fig. 2). The capsid is 125 nm in diameter, with its component proteins positioned on a T=16 icosahedral lattice (Caspar and Klug 1962; Schrag et al. 1989; Wildy et al. 1960). Each capsid is composed of 161 major structural protein subunits termed capsomers, which can be divided more specifically into the 150 hexons that constitute the edges and faces of the icosahedron, and eleven pentons that reside at all but one vertex of the capsid (Newcomb et al. 2001; Wildy et al. 1960). Respectively, the pentamers and hexamers are composed of five and six copies of the major capsid protein, VP5 (Newcomb et al. 1993). The unique capsid vertex not occupied by a VP5 pentamer is the site of the portal complex through which DNA enters or exits the capsid. The portal is cylindrical in geometry and composed of twelve copies of pUL6 (Newcomb et al. 2001). Positioned at the tip of each VP5 protein of every capsid hexamer is one copy of the VP26 protein, which totals 900 copies per capsid (Booy et al. 1994; Newcomb et al. 1993). Located just above the capsid floor at positions of threefold capsomer symmetry is the triplex complex, which functions in linking capsomers during capsid formation (Newcomb et al. 1993; Trus et al. 1996; Zhou et al. 1994). There are 320 triplexes per capsid and each is composed of one subunit of VP19C and two subunits of VP23 (Newcomb et al. 1993). Cryo-EM studies have determined the presence of an additional capsid component that binds specifically to the triplexes adjacent to pentons, termed the capsid vertex specific component (CVSC). Each CVSC is a heterotrimer of the UL17, UL25 and UL36 proteins, and one function of the CVSC is to stabilize the capsid during and after completion of DNA packaging (Cockrell et al. 2011; Conway et al. 2010; Thurlow et al. 2006; Toropova et al. 2011; Trus et al. 2007; Huet et al. 2016; Fan et al. 2015). One final capsid component is the VP24 protease, which cleaves the scaffolding proteins during capsid maturation; however the precise location and function of this protein within virions is not yet known (Loret et al. 2008; Liu and Roizman 1993; Spear and Roizman 1972).
Figure 2. The capsid structural proteins.
The schematic diagram at left and the false-colored cryoEM reconstruction at right depict the locations of major and minor capsid components on one facet of the T=16 icosahedral lattice, as indicated by the legend. The portal complex, a dodecamer of the UL6 protein, occupies a unique penton vertex and functions both to nucleate capsid assembly as well as to provide the entry and exit of the dsDNA genome. It is not seen in cryoEM reconstructions where full icosahedral symmetry is imposed. Five copies of the CVSC molecule are situated around each vertex. The triplex molecule is a heterotrimer comprising one copy of VP19C and two copies of VP23. Hexamers of the major capsid protein, VP5, are capped by the small 12kDa VP26 subunit, while the pentamers bind a tegument protein, UL36, which also interacts with the UL25 subunit of the CVSC.
Cryo-EM analysis of purified HSV virions suggests that the packaged DNA resides in a liquid-crystalline state as a toroid or spool structure, with strands spaced approximately 2.6 nm apart (Booy et al. 1991; Furlong et al. 1972; Zhou et al. 1999) (Fig. 3A). The HSV-1 genome has been sequenced and totals 152,261 base pairs (bp), with a G+C content of 68.3% (Dolan et al. 1998; Kieff et al. 1971; McGeoch et al. 1988). The viral genome consists of covalently linked long and short regions of unique viral sequence (UL and US respectively) that are both flanked by repeated sequences (Fig. 3B). The UL component is bracketed by inverted copies of the b sequence, which differ in size and sequence arrangement from inverted copies of the c sequence that flank the US component (Wadsworth et al. 1975). Repeated a sequences are located at the termini of both the UL and US components and at the junction between both components, and vary in orientation and copy number depending on their position in the genome (Locker and Frenkel 1979; Roizman 1979a, b; Wadsworth et al. 1975; Wagner and Summers 1978). The a sequences are highly conserved and mediate the cleavage and packaging of viral DNA (Umene et al. 2008).
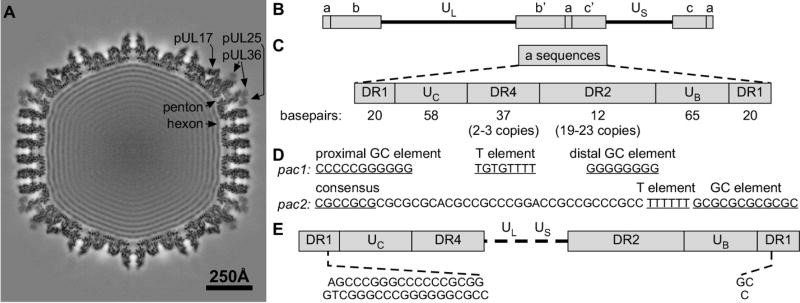
Figure 3. Structure and essential cis-acting elements within the HSV-1 a sequences.
(A) Central thin section section through a virion capsid reconstruction revealing both the CVSC density and the packaged viral genome. (B) Structure and sequence arrangement of the HSV-1 genome. (C) Structure of the a sequence elements. (D) Sequence of the pac1 and pac2 motifs. (E) The sequence of the a sequences at the genome ends. Cleavage within the DR1 element of adjacent a sequences.
HSV-1 DNA replication produces branched, head-to-tail concatemers of viral genomes that must be cleaved and packaged into capsids as individual, unit-length monomers. The specific signals for DNA cleavage are located within the repeated a sequences, which contain all of the necessary cis-acting sequences for genome maturation (Deiss et al. 1986; Deiss and Frenkel 1986; Mocarski and Roizman 1982; Spaete and Frenkel 1982, 1985; Stow and McMonagle 1983; Stow et al. 1983; Varmuza and Smiley 1985; Vlazny et al. 1982) (Fig. 3C). The viral a sequences are located within the inverted repeats that flank the UL and US segments of the viral genome. The UL component is flanked by the repeats ab and b’a’ while the US component is flanked by a’c’ and ca. The number of a sequence repeats located at the UL terminus and at the junction between the UL and US segments vary, while there is only one a sequence at the termini of the US segment (Wadsworth et al. 1975; Wagner and Summers 1978). The a sequences are highly conserved in structure, but contain many variably repeated elements (Umene et al. 2008). Each a sequence consists of directly repeated elements (DR1) at each end that flank unique sequence stretches (UB and UC). Located between the unique sequences are two additional directly repeated elements (DR2 and DR4) that vary widely in their number of copies per a sequence. Due to the variation in copy number of the DR2 and DR4 elements, the size of each a sequence can vary from approximately 465–550 bp (Davison and Wilkie 1981; Mocarski and Roizman 1981). In regions of the genome containing multiple a sequences (ie. the L-S junction), adjacent a sequences share the intervening DR1 element (Mocarski and Roizman 1981).
The cis-acting signals for DNA cleavage have been mapped to specific domains termed pacl and pac2, located within the UB and UC sequences respectively (Deiss et al. 1986; Deiss and Frenkel 1986; Nasseri and Mocarski 1988; Varmuza and Smiley 1985; Mocarski et al. 1985; Brown 2002) (Fig. 3D,E). The pacl domain is characterized by two stretches of 5–8 G nucleotides that are separated by a 3–7 nucleotide T-rich region, while pac2 contains a conserved CGCCGCG motif near a run of 5–10 T nucleotides (Deiss et al. 1986). Cleavage of the dsDNA occurs at a defined distance from both the pacl and pac2 elements (Varmuza and Smiley 1985), making a site-specific cut within DR1 (Deiss et al. 1986). However, it is important to note that although DR1 contains the site of cleavage, this specific sequence is not required; only the defined distance from eitherpac element (Varmuza and Smiley 1985).
Replication of the viral genome produces concatemers where only the UL terminus is exposed (Martinez et al. 1996; Severini et al. 1994; Zhang et al. 1994). DNA packaging initiates at the UL terminus and completes at the US terminus, and in vitro uncoating assays have demonstrated that the US terminus exits the capsid first (Newcomb et al. 2009). Following this model, it is thought that the initial cleavage of the concatemer is directed by pac2, resulting in a truncated DR1 element of 18 base pairs with a 3’ G nucleotide extension within the terminal a sequence at the UL end of the genome (Fig. 3E). Cleavage directed by the pacl site results in a final truncated DR1 element of one base pair with a 3’ C nucleotide overhang within the terminal a sequence at the US end of the genome (Baines and Weller 2005; Mocarski and Roizman 1982). During subsequent rounds of infection, the DR1 overhangs allow for circularization of the viral genome for replication (Mocarski and Roizman 1982).
Capsid assembly
During HSV-1 lytic infection, four types of capsids are formed within the infected cell nucleus. Procapsids are a fragile, precursor form of the more stable A-, B-, and C-capsids (Gibson and Roizman 1972; Newcomb et al. 1996; Rixon and McNab 1999). Each capsid type possesses a distinct morphology when viewed by EM, and the A-, B- and C-capsids can be separated relative to each other by sucrose density gradient ultracentrifugation (Gibson and Roizman 1972; Newcomb et al. 2000; Newcomb et al. 1996). The four capsid types share a similar shell structure, but differ in the minor proteins of the capsid exterior and in the contents of the capsid cavity. Procapsids represent the first completely enclosed structures formed during the capsid assembly process, and possess an outer shell that is porous and largely spherical in shape (Newcomb et al. 1996; Newcomb et al. 1999; Newcomb et al. 1994; Newcomb et al. 2000; Tatman et al. 1994). Procapsids are a precursor form of the other capsid types and have the potential to mature into a more angularized form, package DNA, and assemble into infectious virions (Church and Wilson 1997; Heymann et al. 2003; Preston et al. 1983; Trus et al. 1996). A-capsids are essentially hollow, containing very little DNA or protein content within the capsids cavity, and are thought to form as a result of unsuccessful DNA packaging (Booy et al. 1991; Gibson and Roizman 1972; Schrag et al. 1989; Sherman and Bachenheimer 1988). The cavity of B-capsids possesses a core largely composed of VP22a, the cleaved form of the scaffolding protein, and considerably lower amounts of the UL26 gene products, VP21 and VP24 (Liu and Roizman 1993; Liu and Roizman 1991; Newcomb and Brown 1991). B-capsids are angularized and thought to mature without ever encountering the DNA encapsidation machinery (Gibson and Roizman 1972; Newcomb et al. 1996). C-capsids represent the products of successful DNA packaging events and contain a single, complete HSV-1 genome (Booy et al. 1991; Schrag et al. 1989). C-capsids can exit the nucleus for further assembly into infectious virions, and are similar, if not identical, to the capsids found within mature virions (Booy et al. 1991; Gibson and Roizman 1972; Perdue et al. 1976). Each of the four capsid types are assembled in varying quantities during wild-type HSV-1 infection, but a specific capsid form will accumulate to higher levels within the infected cell nucleus if a virus fails to express one or more of the capsid proteins (Homa and Brown 1997; Newcomb et al. 2000). Studies with these mutants allowed for the isolation of relatively large quantities of the individual capsid types, which has proven invaluable for the determination of capsid structure and elucidation of the capsid assembly process; reviewed in (Conway and Homa 2011; Brown and Newcomb 2011).
In vitro assembly assays utilizing HSV-1 capsid proteins expressed by recombinant baculoviruses have been critical towards unraveling the mechanism of capsid formation [reviewed in (Homa and Brown 1997)]. Using an in vitro assembly system, it was determined that VP5, VP19C, VP23, and either pre-VP22a or the maturational protease (UL26 gene product), were the minimum proteins required for the formation of morphologically normal capsids (Newcomb et al. 1996; Newcomb et al. 1994; Tatman et al. 1994; Thomsen et al. 1994). The in vitro system also identified the formation of intermediate or partial procapsid structures during assembly and identified that HSV-1 utilizes a procapsid structure that is similar to the empty proheads seen during dsDNA bacteriophage assembly [(Newcomb et al. 1996; Newcomb et al. 1999; Newcomb et al. 1994; Trus et al. 1996), reviewed in (Catalano 2005)]. Recent studies demonstrated that the initial complex or protomer that is used in assembly of the procapsid consists of one triplex, surrounded by three major capsid proteins and the closed T=16 procapsid shell is built from 320 copies of the protomer (Aksyuk et al. 2015). The interaction of the protomers is likely to be guided by the scaffold proteins binding to the major capsid protein.
The pUL17 and pUL25 CVSC proteins along with packaging proteins pUL15, pUL28, and pUL33 have been detected on procapsids, suggesting they assemble onto the capsid before the start of DNA encapsidation (Sheaffer et al. 2001; Thurlow et al. 2005). At a time point before, or coinciding with, DNA packaging the scaffold is cleaved from the procapsid interior, resulting in the angularization of the spherical procapsid shell to a mature, icosahedral form (Heymann et al. 2003; Newcomb et al. 1996; Church and Wilson 1997; Perdue et al. 1976). Procapsids that proceed through this structural transformation without encountering the DNA packaging machinery form the B-capsids (Newcomb et al. 1996). DNA packaging results in the expulsion of the cleaved scaffolding proteins from the capsid cavity (Gibson and Roizman 1972; Rixon et al. 1988). However, the cleaved VP24 protease remains within the capsid (Davison et al. 1992; Gibson and Roizman 1972), although its function after scaffold cleavage and DNA encapsidation is not known. Capsids that have initiated DNA packaging but are unstable, or abort the packaging process early, release the viral DNA resulting in the empty A-capsid (Sherman and Bachenheimer 1988). Stable capsids containing a complete viral genome represent the C-capsids that can egress from the nucleus and assemble into mature virions (Gibson and Roizman 1972; Perdue et al. 1976).
DNA packaging
Studies utilizing HSV-1 mutants encoding temperature-sensitive or null mutations have revealed that successful encapsidation of HSV-1 DNA requires the protein products of seven viral genes; UL6, UL15, UL17, UL25, UL28, UL32, and UL33 (Addison et al. 1984; Addison et al. 1990; al-Kobaisi et al. 1991; Baines et al. 1994; Cavalcoli et al. 1993; Lamberti and Weller 1996, 1998; McNab et al. 1998; Patel and MacLean 1995; Patel et al. 1996; Poon and Roizman 1993; Salmon et al. 1998; Schaffer et al. 1973; Sherman and Bachenheimer 1987, 1988; Tengelsen et al. 1993; Weller et al. 1987; Yu et al. 1997). Six of these proteins are required for viral DNA cleavage (pUL6, pUL15, pUL17, pUL28, pUL32, pUL33), and when even one is missing or nonfunctional, concatemeric DNA and B-capsids accumulate within the infected cell nucleus. In the absence of a functional pUL25, cleaved viral genomes and A-capsids accumulate within the infected cell nucleus, indicating a defect in packaging. With the exception of pUL32, each of the essential cleavage and packaging proteins have been identified as minor components of the HSV-1 capsid, and interact in varying amounts with each capsid type (Beard and Baines 2004; Beard et al. 2004; Goshima et al. 2000; McNab et al. 1998; Patel and MacLean 1995; Salmon and Baines 1998; Sheaffer et al. 2001; Thurlow et al. 2005; Wills et al. 2006; Yu and Weller 1998b). Proposed functions for each protein have been ascribed based upon analogy with essential DNA encapsidation proteins utilized by dsDNA bacteriophage (Catalano 2005). More recently the roles of several of the essential HSV-1 cleavage and packaging proteins have been better defined using genetic and biochemical methods, along with electron microscopy. The following sections will detail the current state of knowledge regarding the seven HSV-1 cleavage and packaging proteins and their proposed role in producing a stable DNA containing capsid.
pUL6
Twelve copies of pUL6 form the ring-like portal structure through which viral DNA enters and exits the capsid (Cardone et al. 2007; Chang et al. 2007; Newcomb et al. 2001; Trus et al. 2004).This observation was initially determined from immunoelectron microscopy analysis of portal structures from isolated capsids, and EM examination of portal structures formed in vitro, using soluble pUL6 monomers purified from recombinant baculovirus infected cells (Newcomb et al. 2001). The formation of stable portal ring structures has been shown to require a putative leucine zipper domain within UL6, and disulfide bond formation between pUL6 monomers (Albright et al. 2011; Nellissery et al. 2007). EM analysis has determined that the HSV-1 portal structure is similar to the portals of dsDNA bacteriophage, and that it resides at a single, unique capsid vertex (Bazinet and King 1985; Cardone et al. 2007; Chang et al. 2007; Trus et al. 2004). In vitro capsid assembly assays revealed that pUL6 interacts directly with the pre-VP22a scaffold protein (Newcomb et al. 2005; Newcomb et al. 2003), and further studies using deletion mutants determined that amino acids 143–151 of the scaffold are required for this interaction (Huffman et al. 2008; Singer et al. 2005; Yang and Baines 2008). The in vitro capsid assembly assays also demonstrated that not only is the scaffold/portal interaction required for portal incorporation, but the portal proteins must be present when capsid assembly initiates in order to be incorporated into the capsid (Newcomb et al. 2005; Newcomb et al. 2003). These results suggest that capsid assembly initiates around the portal and that a regulatory mechanism must be in place to ensure that each capsid contains only one portal (Cardone et al. 2007; Chang et al. 2007; Newcomb et al. 2005; Newcomb et al. 2003).
pUL32
Although the UL32 protein is essential for cleavage and packaging, its role during this process is largely unknown. pUL32 is the only one of the seven cleavage packaging proteins that has not been found to associate with capsids. The UL32 protein is a cysteine rich, zinc-binding protein that accumulates in both the cytoplasm and nucleus of infected cells (Chang et al. 1996; Lamberti and Weller 1998). In the absence of pUL32, capsids do not accumulate within replication compartments, but in perinuclear regions near the nuclear membrane, possibly suggesting a role in the transport of assembled capsids to sites for DNA encapsidation. pUL32 contains C-X-X-C motifs that when mutated alter the disulfide bond profiles of several of the capsid and cleavage packaging proteins suggesting that pUL32 may be critical for regulating disulfide bond formation during procapsid assembly, maturation and DNA packaging (Albright et al. 2015).
Terminase complex (pUL15, pUL28, pUL33)
Initial evidence suggesting an interaction between HSV-1 pUL28 and pUL15 came from studies using the closely related herpesvirus, pseudorabies virus (PRV). Working with cell lines stably expressing the PRV UL28 protein it was demonstrated that pUL28 was predominantly cytoplasmic in the absence of other PRV proteins, but entered the nucleus upon PRV infection (Koslowski et al. 1997). The interaction of pUL15 and pUL28 in HSV-1 infected cells was demonstrated by ion-exchange and DNA affinity chromatography of infected cell lysates followed by sucrose gradient centrifugation of the purified proteins (Koslowski et al. 1999). Immunoblotting of gradient fractions for pUL15 and pUL28 revealed that both proteins co-migrated through the gradient as a 1:1 heterodimeric complex. Additional studies have corroborated the interaction between HSV-1 pUL15, pUL28, and pUL33 using a variety of methods including immunofluorescence assay to determine protein localization (Abbotts et al. 2000; Beard et al. 2002; Higgs et al. 2008; Koslowski et al. 1999; Reynolds et al. 2000; White et al. 2003), and coimmunoprecipitation experiments using either proteins expressed from recombinant baculoviruses within infected insect cells (Abbotts et al. 2000; Beard et al. 2002; White et al. 2003) or proteins from HSV-1-infected cells (Beard et al. 2002; Jacobson et al. 2006; Yang and Baines 2006; Yang et al. 2007; Yang et al. 2011). Further confirmation has come from the observed interaction between homologues of HSV-1 pUL15, pUL28, and pUL33 in varicella zoster virus (VZV) (Visalli et al. 2009; Visalli et al. 2007; Vizoso Pinto et al. 2011), HCMV (Wang et al. 2012; Thoma et al. 2006; Borst et al. 2013), and PRV (Fuchs et al. 2009) demonstrating the level of conservation of these genes and implied importance during infection (Davison et al. 2002; Fossum et al. 2009).
The pUL15/pUL28/pUL33 protein complex is essential for virus replication. Studies with pUL28 mutants revealed that pUL15 and pUL33 interact indirectly via their binding with the C-terminus of pUL28 (Jacobson et al. 2006; Yang and Baines 2006) (Fig. 4). Specifically, the C-terminal 44 amino acids of pUL28 appear essential for the interaction of pUL28 with both pUL15 and pUL33 (Jacobson et al. 2006). Mutational analysis of the UL33 gene has also suggested that amino acids 51–74 of pUL33 mediate the interaction with pUL28 (Beilstein et al. 2009), while residues within the C-terminus of pUL15 may be required for the interaction with pUL28 (Abbotts et al. 2000; Yang et al. 2008). The terminase complex was isolated from HSV-1 infected cells by tandem-affinity purification (TAP) using recombinant viruses expressing a full length or a C-terminally truncated NTAP-UL28 fusion protein (Heming et al. 2014). TAP of pUL28 from infected cells, followed by silver staining, Western blotting, and mass spectrometry, identified the pUL15, pUL28, and pUL33 subunits, while TAP of the pUL28 C-terminal truncated mutant confirmed previous findings that the C terminus of pUL28 is required for pUL28 interaction with pUL33 and pUL15. Analysis of the oligomeric state of the purified complexes by sucrose density gradient ultracentrifugation revealed that the three proteins formed a complex with a molecular mass that is consistent with the formation of a pUL15/pUL28/pUL33 heterotrimer (Heming et al. 2014).
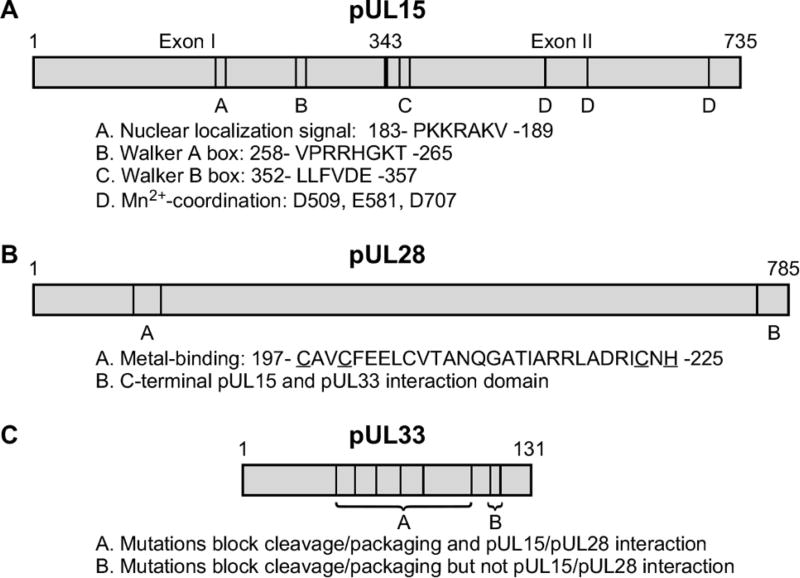
Figure 4.
Conserved amino acid domains and mutations within the HSV-1 (A) pUL15, (B) pUL28 and (C) pUL33 that affect terminase activity.
The lack of an HSV-1 in vitro packaging assay has limited the direct biochemical analysis of the terminase. However, genetic experiments utilizing temperature-sensitive mutants, or viruses bearing deletions or insertions, have identified several critical domains within the individual terminase subunits that are essential for complex formation and function (Baines et al. 1994; Jacobson et al. 2006; Poon and Roizman 1993; Przech et al. 2003; Yang et al. 2007; Yang et al. 2008; Yang et al. 2011; Yu et al. 1997; Yu and Weller 1998a; Baines et al. 1997; Nadal et al. 2010; Underwood et al. 1998; Addison et al. 1990; Cavalcoli et al. 1993; Krosky et al. 1998; Tengelsen et al. 1993; al-Kobaisi et al. 1991; Beilstein et al. 2009). These studies have also been aided greatly by the high degree of sequence conservation between the terminase proteins of the herpesviruses (Davison et al. 2002; Draper and Rao 2007). UL15 is the most highly conserved gene within the family Herpesviridae and contains several protein domains that are proposed, or have been demonstrated, to be critical for the cleavage and packaging of viral DNA (Jacobson et al. 2006; Przech et al. 2003; Yang et al. 2007; Yang et al. 2008; Yang et al. 2011; Yu and Weller 1998a; Nadal et al. 2010; Underwood et al. 1998; Davison 1992; Draper and Rao 2007) (Fig. 4). The UL15 protein is relatively unique within HSV-1 in that it’s expressed from a spliced transcript (Costa et al. 1985). The N-terminal region of pUL15 contains conserved amino acid motifs, such as Walker A and B boxes, that are typically found in proteins that metabolize ATP, therefore implicating this region of pUL15 as the “motor” for the translocation of DNA into capsids during packaging (Davison 1992; Draper and Rao 2007; Walker et al. 1982; Yu and Weller 1998a). A recombinant HSV-1 encoding a point mutation of the conserved glycine residue (G263 A) within the Walker A box was shown to be deficient in cleavage and packaging (Przech et al. 2003; Yu and Weller 1998a).
Nadal et al (Nadal et al. 2010) and Selvarajan Sigamani et al (Selvarajan Sigamani et al. 2013) have purified a soluble fragment of the C-terminal regions of the human cytomegalovirus (HCMV) pUL89 and HSV pUL15, respectively. The crystal structures of both proteins show a fold resembling those of RNase H/integrase-like enzymes with an active site clustered with acidic amino acids most likely required for the metal ion-mediated catalytic activity of both proteins (Fig. 4). In addition, mutational studies revealed that the three acidic residues D509, E581, and D707 (Fig. 4) that form the conserved triad in the UL15 nuclease domain were found to be essential for viral replication (Heming et al. 2014; Nadal et al. 2010). Interestingly, an HSV-1 mutant virus with a deletion that removes pUL15 amino acids 400–420, which are located in a position between the proposed ATPase and nuclease domains, showed only a slight defect in DNA cleavage but DNA packaging efficiency was drastically reduced (Yang et al. 2011). Taken together, these findings suggest that the DNA translocation (N-terminus) and DNA cleavage (C-terminus) functions of pUL15 reside in separate domains of the protein.
The UL28 protein and its HCMV homolog pUL56 have been implicated as the DNA-binding subunits of the terminase complex. The UL28 protein has been shown to bind specific HSV-1 DNA sequences that are required for cleavage and packaging, and this function has also been observed with the HCMV pUL56 (Bogner et al. 1998; Adelman et al. 2001). Studies using purified pUL28 expressed in bacterial cells demonstrated that pUL28 interacted with one strand of the pacl motif suggesting that during packaging viral DNA may adopt novel structures and extrude single-stranded regions that are recognized by pUL28 (Adelman et al. 2001). Studies performed with the purified HCMV pUL56 have also demonstrated an interaction with HCMV pacl sequences (Bogner et al. 1998). A highly conserved domain of pUL28 (C197-X2-C200-X22-C223-X-H225) resembling a putative zinc-finger motif is found in a number of herpesvirus pUL28 homologs (Krosky et al. 1998; Champier et al. 2008) (Fig. 4). This domain is critical for proper terminase function as a recombinant virus encoding a deletion of the putative pUL28 metal-binding domain fails to replicate due to the absence of DNA cleavage and packaging (Heming et al. 2014). pUL28 amino acids C197, C200, C223, and H225 are conserved and most likely correspond to the metal coordinating amino acids within the zinc-finger motif predicted for this region of pUL28. Alanine substitutions of any of the four conserved amino acids were found to block cleavage and packaging (Heming et al. 2014). The importance of this region for HCMV replication was demonstrated with mutants that were resistant to the DNA cleavage inhibitors BDCRB and TCRB. Amino acids changes within the conserved metal-binding domain region were found to confer resistance to these drugs (Krosky et al. 1998).
The role of pUL33 in terminase complex formation and its function in the cleavage and packaging reaction is unknown. It’s interesting to speculate why HSV-1 would utilize a three subunit terminase complex when most of the well-studied dsDNA bacteriophage terminases consist of only two subunits (Catalano 2005). Studies clearly indicate that the interaction between pUL33 and pUL28 is critical for proper terminase function (Beilstein et al. 2009; Jacobson et al. 2006; Yang and Baines 2006; Yang et al. 2008). Genetic experiments have identified two regions of pUL33 that are essential for terminase function (Beilstein et al. 2009; Yang et al. 2008) (Fig. 4). Viruses encoding temperature-sensitive or insertion mutations clustered near the center of the protein precluded the interaction with pUL28, while mutations at the C-terminus allowed complex formation, but in both cases the mutants were deficient in the cleavage and packaging of viral DNA. In a genome-wide yeast-two-hybrid screen of several herpesvirus one of the most consistent interactions was between pUL33 and the nuclear egress proteins, pUL31 and pUL34 (Fossum et al. 2009). Therefore it is possible that pUL33 performs several functions, with terminase-associated pUL33 functioning in encapsidation, while capsid-associated pUL33 molecules play a role in capsid nuclear egress.
CVSC (pUL17, pUL25, pUL36)
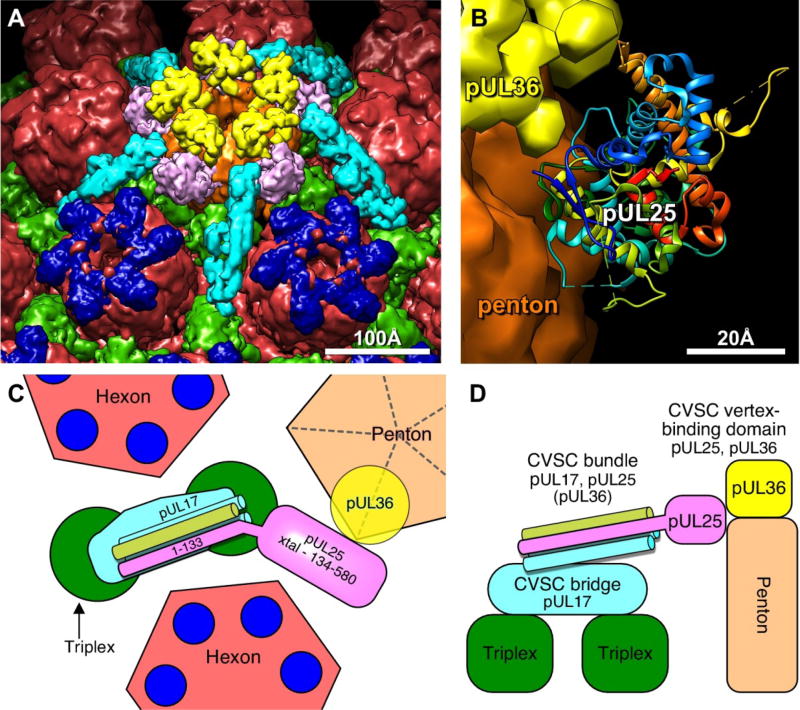
The capsid vertex specific component or CVSC consists of a complex of the HSV-1 pUL17, pUL25, and pUL36 proteins that binds specifically to the triplexes adjacent to each of the eleven pentons (Fan et al. 2015; Huet et al. 2016) (Fig. 2). Five copies of the CVSC form a star-shaped density that extends from the top of the penton to the adjacent triplexes and hexons, and which was originally considered to be part of the tegument. This complex was initially discovered in cryo-EM reconstructions of wild-type C capsids where these elongated densities were found on the capsid exterior surrounding the vertices (Trus et al. 2007). This density was initially termed the “C-capsid-specific component” (CCSC) but it was subsequently observed that this feature is present on reconstructions of A, B, and C capsids, thus resulting in the more general term, CVSC (Toropova et al. 2011). Initial studies indicated that the complex consisted of pUL17 and pUL25. By visualizing GFP-tags on pUL25 and pUL17 the location of the two proteins in the complex was determined with pUL25 in the penton-distal portion of the molecule and pUL17 occupying the region proximal to the capsid vertex (Conway et al. 2010; Homa et al. 2013; Toropova et al. 2011). However, more recent high-resolution reconstructions of Kaposi’s sarcoma-associated herpesvirus (KSHV), HSV-1, and PRV virion capsids produced a different interpretation. These new studies demonstrated that pUL17 is present in the distal part of the CVSC bridging the triplexes, with the major part of pUL25 occupying a position nearest the penton (Dai et al. 2014; Fan et al. 2015; Huet et al. 2016) (Fig 5A). In addition, these studies demonstrated that the UL36 protein is required for the stable association of the CVSC with the capsid vertices (Fan et al. 2015; Huet et al. 2016). Evidence supporting this arrangement was demonstrated in cryoEM reconstructions of capsids isolated from either pUL25 or pUL17 null viruses. In the absence of pUL17 the CVSC density was completely missing, while in the absence of pUL25 the CVSC density that bridges the triplexes was readily apparent in central sections through the native capsid (Thurlow et al. 2006; Huet et al. 2016). The location of pUL25 in the CVSC was demonstrated by fitting the pUL25 crystal structure into the cryoEM density maps which placed the pUL25 C-terminal domain (aa 134–580) in contact with two of the penton VP5 subunits as well as in contact with pUL36 sitting on top of the penton (Dai et al. 2014; Huet et al. 2016) (Fig 5B). The higher resolution CVSC maps revealed a group of helices covering the triplex bridge and extending toward the penton. Sections through this bundle indicated that it was composed of 4–5 helices which were predicted to originate from pUL17, pUL25 and pUL36 (Huet et al. 2016). The new organization of the CVSC demonstrates why pUL17 is required for both pUL25 and pUL36 to bind capsids since it bridges the triplexes and serves as the anchor for stable binding the CVSC to the capsid (Fig 5C,D).
Figure 5. Organization of the CVSC molecule and its binding partners.
(A) Surface view above the penton (brown) including hexons (red), triplex (green), VP26 (dark blue) CVSC trimer consisting of pUL17, the N-terminal domain of pUL25 and the C-terminus of pUL36 (light blue), as well as the C-terminal domain of pUL25 (pink), and the pUL36 density above the penton (yellow). (B) Atomic model of the HSV-1 pUL25 C-terminal fragment (residues 134–580; PDB 2F5U8), fit into the virion capsid cryoEM density map. Note regions of pUL25 that may be contact points with pUL36. Model of the CVSC subunit organization viewed from above (C) and from the side (D) of the penton.
The UL25 protein is unique relative to the six other essential DNA encapsidation proteins in that it is not required for cleavage of viral DNA (Cockrell et al. 2011; Cockrell et al. 2009; Stow 2001). Analysis of replicated viral DNA from pUL25 mutants revealed that the L-terminus was cleaved correctly, while cleavage at the S end of the genome was aberrant or did not occur (Cockrell et al. 2009; Stow 2001). Taken together, these data suggest that pUL25 plays a role in capsid stabilization during DNA packaging not unlike the “head-completion” proteins utilized by dsDNA bacteriophage (Catalano 2005). The UL25 protein is also observed in increasing amounts from procapsids, to B-, A-, then C-capsids, and finally virions, further supporting a role in capsid stabilization, with increasing amounts of pUL25 added as encapsidation progresses (McNab et al. 1998; Newcomb et al. 2006; Sheaffer et al. 2001). In addition to its role in capsid stabilization, the UL25 protein may also be important at a late stages of infection. Analysis of an HSV-1 mutant with a temperature-sensitive lesion within pUL25 demonstrated a viral uncoating defect at the nonpermissive temperature (Preston et al. 2008). Another study revealed an interaction between pUL25 and the large tegument protein, pUL36, at the capsid surface, implicating pUL25 in tegumentation of the viral capsid (Coller et al. 2007).
The UL17 protein is required for viral DNA cleavage and packaging and in its absence only nuclear B capsids are produced (Salmon et al. 1998). This phenotype is shared with null mutants of the three terminase proteins, pUL15, pUL28 and pUL33. In light of the recent assignment of pUL17 to the triplex bridge density as part of the CVSC it’s interesting to speculate if pUL17 binds to triplexes surrounding the portal vertex where it may be required for the assembly of the portal/terminase DNA packaging complex.
Model of HSV DNA packaging
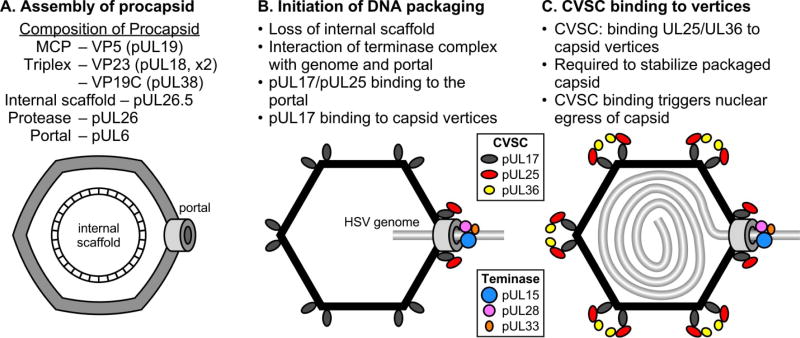
The pathway for assembly of the HSV capsid has been described in several previous reviews (Brown 2002; Brown and Newcomb 2011; Conway and Homa 2011; Homa and Brown 1997). The HSV procapsid assembles from several structural proteins including a scaffold that is the product of the UL26.5 gene (Fig. 6A). The DNA chromosome is incorporated into the procapsid through the ring-shaped portal present at a unique vertex. During infection the UL15, UL28, and UL33 proteins are translated within the cytoplasm of the infected cell. An initial interaction occurs between pUL28 and pUL33, protecting pUL33 from degradation (Jacobson et al. 2006; Yang and Baines 2006). The UL15 protein then interacts with pUL28, and this interaction is enhanced by pUL33 (Jacobson et al. 2006; Yang and Baines 2006; Yang et al. 2008). The assembled terminase complex of pUL15, pUL28, and pUL33 localizes to replication centers within the nucleus via the NLS of pUL15 (Higgs et al. 2008; Yang et al. 2007). The UL28 protein binds the replicated viral DNA concatemer and scans the DNA for specific sequences (Adelman et al. 2001; Bogner et al. 1998). Upon encountering a pac2 site in the correct orientation, the endonuclease activity of pUL15 is triggered and cleaves the DNA within an upstream DR1 element (Hodge and Stow 2001; Nadal et al. 2010; Scheffczik et al. 2002; Varmuza and Smiley 1985). This cleavage generates a free L-terminus for packaging that contains a truncated DR1 element containing a one nucleotide 3’ overhang (Mocarski and Roizman 1982). The terminase, with bound viral DNA, docks at the pUL6 portal of assembled procapsids in an orientation that positions pUL15 in close proximity to the portal (Beard et al. 2004; White et al. 2003; Wills et al. 2006; Yang et al. 2009) (Fig. 6B). This interaction activates the DNA translocation function of pUL15 which begins packaging the free L-terminus into the viral capsid in an ATP-dependent manner (Dasgupta and Wilson 1999; Newcomb et al. 2009; Yu and Weller 1998a). DNA packaging also triggers protease activation and subsequent cleavage of the scaffold protein, resulting in procapsid maturation to the mature, polyhedral form (Heymann et al. 2003; Newcomb et al. 1996; Church and Wilson 1997; Perdue et al. 1976). DNA translocation continues from the L-component, through the junction, and into the S-component (Newcomb et al. 2009) (Fig. 6C). As packaging nears completion, single-stranded regions within the a sequence of the S-component are extruded and the pacl motif is recognized by the pUL28 subunit (Adelman et al. 2001). This triggers the second DNA cleavage by pUL15, producing an S-terminus containing a single a sequence followed by a one nucleotide extension of the DR1 element (Hodge and Stow 2001; Mocarski and Roizman 1982; Nadal et al. 2010; Scheffczik et al. 2002; Varmuza and Smiley 1985). The freed genome end is packaged and the terminase components subsequently disassociate from the viral capsid, possibly to act in additional rounds of cleavage and packaging (Beard et al. 2004; Sheaffer et al. 2001; Taus and Baines 1998; Yu and Weller 1998b).
Figure 6. Steps in the HSV capsid assembly and DNA packaging pathway.
The mechanism is illustrated starting with the assembly of the procapsid (A), the initiation of DNA packaging (B), and the formation of a stable DNA-containing capsid that is primed for nuclear egress (C). The viral proteins associated with each step in the process are indicated.
As mentioned earlier the pUL17 protein is required for DNA cleavage and packaging and since it binds to the triplexes surrounding the pentons it’s likely to be present at the portal vertex (Fig. 6B). The importance of portal-associated pUL17 for DNA cleavage and packaging is supported by the following observations. First, pUL17 is found at the capsid vertices on capsids produced from both pUL25 (Huet et al. 2016) and pUL36 (unpublished data) null viruses; and second, the pUL36 null virus produces abundant nuclear DNA containing capsids while the pUL25 null virus is defective in packaging but not the cleavage reaction. In addition, pUL25 may also be required at the portal where its function would be to prevent loss of DNA from the capsid after the second cleavage reaction. Again, this is supported by the fact that stable DNA containing capsids are found with the pUL36 null virus. At some point during the packaging reaction pUL25 and pUL36 are added to the capsid vertices to complete the CVSC in forming a capsid that is primed to exit the nucleus (Fig. 6C).
Conclusions and Outlook
In summary, additional questions remain regarding the assembly and function of the HSV-1 terminase complex and the role of pUL17 and pUL32 in the DNA packaging reaction. Mass spectrometry, sucrose density gradient centrifugation, and Western blotting analysis all support that the HSV-1 terminase complex consists of interacting pUL15, pUL28, and pUL33 subunits. However, questions remain as to stoichiometry of the complex and whether a multimeric terminase complex is assembled at the portal vertex and how and in what order does the complex interact with DNA and with the portal vertex. Future studies could involve attempting to visualize the terminase complex at the portal vertex on isolated capsids by cryoEM and 3D image reconstruction. However, the difficulty resides in determining and aligning the portal-vertex of each capsid for 3D image reconstruction. The ability to purify endogenous terminase complexes represents a critical step toward establishing an in vitro HSV-1 cleavage and packaging system by combining the purified cleavage packaging proteins with capsids, viral DNA, and ATP. Such a system would allow for the direct biochemical analysis of purified complexes and the role of individual cleavage/packaging proteins in the DNA packaging process.
Acknowledgments
The research in the laboratories of F.L.H. and J.F.C. was supported by National Institutes of Health awards (AI060836).
References
- Abbotts AP, Preston VG, Hughes M, Patel AH, Stow ND. Interaction of the herpes simplex virus type 1 packaging protein UL15 with full-length and deleted forms of the UL28 protein. J Gen Virol. 2000;81(Pt 12):2999–3009. doi: 10.1099/0022-1317-81-12-2999. [DOI] [PubMed] [Google Scholar]
- Addison C, Rixon FJ, Palfreyman JW, O'Hara M, Preston VG. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984;138(2):246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Addison C, Rixon FJ, Preston VG. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J Gen Virol. 1990;71(Pt 10):2377–2384. doi: 10.1099/0022-1317-71-10-2377. [DOI] [PubMed] [Google Scholar]
- Adelman K, Salmon B, Baines JD. Herpes simplex virus DNA packaging sequences adopt novel structures that are specifically recognized by a component of the cleavage and packaging machinery. Proc Natl Acad Sci U S A. 2001;98(6):3086–3091. doi: 10.1073/pnas.061555698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksyuk AA, Newcomb WW, Cheng N, Winkler DC, Fontana J, Heymann JB, Steven AC. Subassemblies and asymmetry in assembly of herpes simplex virus procapsid. MBio. 2015;6(5):e01525–01515. doi: 10.1128/mBio.01525-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Kobaisi MF, Rixon FJ, McDougall I, Preston VG. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology. 1991;180(1):380–388. doi: 10.1016/0042-6822(91)90043-b. [DOI] [PubMed] [Google Scholar]
- Albright BS, Kosinski A, Szczepaniak R, Cook EA, Stow ND, Conway JF, Weller SK. The putative herpes simplex virus 1 chaperone protein UL32 modulates disulfide bond formation during infection. J Virol. 2015;89(1):443–453. doi: 10.1128/JVI.01913-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright BS, Nellissery J, Szczepaniak R, Weller SK. Disulfide bond formation in the herpes simplex virus 1 UL6 protein is required for portal ring formation and genome encapsidation. J Virol. 2011;85(17):8616–8624. doi: 10.1128/JVI.00123-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines JD, Cunningham C, Nalwanga D, Davison A. The U(L)15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the U(L)15 gene product. J Virol. 1997;71(4):2666–2673. doi: 10.1128/jvi.71.4.2666-2673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines JD, Poon AP, Rovnak J, Roizman B. The herpes simplex virus 1 UL15 gene encodes two proteins and is required for cleavage of genomic viral DNA. J Virol. 1994;68(12):8118–8124. doi: 10.1128/jvi.68.12.8118-8124.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines JD, Weller SK. Cleavage and Packaging of Herpes Simplex Virus 1 DNA, Herpesvirus Assembly. In: Catalano CE, editor. Viral Genome Packaging Machines : Genetics, Structure, and Mechanism. Kluwer Academic/Plenum Publishers; New York, N.Y: 2005. pp. 135–150. [Google Scholar]
- Bazinet C, King J. The DNA translocating vertex of dsDNA bacteriophage. Annu Rev Microbiol. 1985;39:109–129. doi: 10.1146/annurev.mi.39.100185.000545. [DOI] [PubMed] [Google Scholar]
- Beard PM, Baines JD. The DNA cleavage and packaging protein encoded by the UL33 gene of herpes simplex virus 1 associates with capsids. Virology. 2004;324(2):475–482. doi: 10.1016/j.virol.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Beard PM, Duffy C, Baines JD. Quantification of the DNA cleavage and packaging proteins U(L)15 and U(L)28 in A and B capsids of herpes simplex virus type 1. J Virol. 2004;78(3):1367–1374. doi: 10.1128/JVI.78.3.1367-1374.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard PM, Taus NS, Baines JD. DNA cleavage and packaging proteins encoded by genes U(L)28, U(L)15, and U(L)33 of herpes simplex virus type 1 form a complex in infected cells. J Virol. 2002;76(10):4785–4791. doi: 10.1128/JVI.76.10.4785-4791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilstein F, Higgs MR, Stow ND. Mutational analysis of the herpes simplex virus type 1 DNA packaging protein UL33. J Virol. 2009;83(17):8938–8945. doi: 10.1128/JVI.01048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner E, Radsak K, Stinski MF. The gene product of human cytomegalovirus open reading frame UL56 binds the pac motif and has specific nuclease activity. J Virol. 1998;72(3):2259–2264. doi: 10.1128/jvi.72.3.2259-2264.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy FP, Newcomb WW, Trus BL, Brown JC, Baker TS, Steven AC. Liquid-crystalline, phage-like packing of encapsidated DNA in herpes simplex virus. Cell. 1991;64(5):1007–1015. doi: 10.1016/0092-8674(91)90324-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy FP, Trus BL, Newcomb WW, Brown JC, Conway JF, Steven AC. Finding a needle in a haystack: detection of a small protein (the 12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus) Proc Natl Acad Sci U S A. 1994;91(12):5652–5656. doi: 10.1073/pnas.91.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst EM, Kleine-Albers J, Gabaev I, Babic M, Wagner K, Binz A, Degenhardt I, Kalesse M, Jonjic S, Bauerfeind R, Messerle M. The human cytomegalovirus UL51 protein is essential for viral genome cleavage-packaging and interacts with the terminase subunits pUL56 and pUL89. J Virol. 2013;87(3):1720–1732. doi: 10.1128/JVI.01955-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, McVoy MA, Homa FL. Packaging DNA into herpesvirus capsids. In: Bogner AHE, editor. Structure–function relationships of human pathogenic viruses. Kluwer Acacdemic/Plenum; New York: 2002. pp. 111–155. [Google Scholar]
- Brown JC, Newcomb WW. Herpesvirus capsid assembly: insights from structural analysis. Curr Opin Virol. 2011;1(2):142–149. doi: 10.1016/j.coviro.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone G, Winkler DC, Trus BL, Cheng N, Heuser JE, Newcomb WW, Brown JC, Steven AC. Visualization of the herpes simplex virus portal in situ by cryo-electron tomography. Virology. 2007;361(2):426–434. doi: 10.1016/j.virol.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar DL, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Catalano CE. Viral Genome Packaging Machines : Genetics, Structure, and Mechanism. Kluwer Academic/Plenum Publishers; New York, N.Y: 2005. [Google Scholar]
- Cavalcoli JD, Baghian A, Homa FL, Kousoulas KG. Resolution of genotypic and phenotypic properties of herpes simplex virus type 1 temperature-sensitive mutant (KOS) tsZ47: evidence for allelic complementation in the UL28 gene. Virology. 1993;197(1):23–34. doi: 10.1006/viro.1993.1563. [DOI] [PubMed] [Google Scholar]
- Champier G, Couvreux A, Hantz S, Rametti A, Mazeron MC, Bouaziz S, Denis F, Alain S. Putative functional domains of human cytomegalovirus pUL56 involved in dimerization and benzimidazole D-ribonucleoside activity. Antivir Ther. 2008;13(5):643–654. [PubMed] [Google Scholar]
- Chang JT, Schmid MF, Rixon FJ, Chiu W. Electron cryotomography reveals the portal in the herpesvirus capsid. J Virol. 2007;81(4):2065–2068. doi: 10.1128/JVI.02053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YE, Poon AP, Roizman B. Properties of the protein encoded by the UL32 open reading frame of herpes simplex virus 1. J Virol. 1996;70(6):3938–3946. doi: 10.1128/jvi.70.6.3938-3946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GA, Wilson DW. Study of herpes simplex virus maturation during a synchronous wave of assembly. J Virol. 1997;71(5):3603–3612. doi: 10.1128/jvi.71.5.3603-3612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell SK, Huffman JB, Toropova K, Conway JF, Homa FL. Residues of the UL25 protein of herpes simplex virus that are required for its stable interaction with capsids. J Virol. 2011;85(10):4875–4887. doi: 10.1128/JVI.00242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell SK, Sanchez ME, Erazo A, Homa FL. Role of the UL25 protein in herpes simplex virus DNA encapsidation. J Virol. 2009;83(1):47–57. doi: 10.1128/JVI.01889-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller KE, Lee JI, Ueda A, Smith GA. The capsid and tegument of the alphaherpesviruses are linked by an interaction between the UL25 and VP1/2 proteins. J Virol. 2007;81(21):11790–11797. doi: 10.1128/JVI.01113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JF, Cockrell SK, Copeland AM, Newcomb WW, Brown JC, Homa FL. Labeling and localization of the herpes simplex virus capsid protein UL25 and its interaction with the two triplexes closest to the penton. J Mol Biol. 2010;397(2):575–586. doi: 10.1016/j.jmb.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JF, Homa FL. Nucleocapsid Structure, Assembly and DNA Packaging of Herpes Simplex Virus. In: Weller SK, editor. Alphaherpesviruses: Molecular Virology. Caister Academic Press; Norfolk, UK: 2011. pp. 175–193. [Google Scholar]
- Costa RH, Draper KG, Kelly TJ, Wagner EK. An unusual spliced herpes simplex virus type 1 transcript with sequence homology to Epstein-Barr virus DNA. J Virol. 1985;54(2):317–328. doi: 10.1128/jvi.54.2.317-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Gong D, Wu TT, Sun R, Zhou ZH. Organization of capsid-associated tegument components in Kaposi's sarcoma-associated herpesvirus. J Virol. 2014;88(21):12694–12702. doi: 10.1128/JVI.01509-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, Wilson DW. ATP depletion blocks herpes simplex virus DNA packaging and capsid maturation. J Virol. 1999;73(3):2006–2015. doi: 10.1128/jvi.73.3.2006-2015.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AJ. Channel catfish virus: a new type of herpesvirus. Virology. 1992;186(1):9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- Davison AJ, Dargan DJ, Stow ND. Fundamental and accessory systems in herpesviruses. Antiviral Res. 2002;56(1):1–11. doi: 10.1016/s0166-3542(02)00107-9. [DOI] [PubMed] [Google Scholar]
- Davison AJ, Wilkie NM. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981;55(Pt 2):315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- Davison MD, Rixon FJ, Davison AJ. Identification of genes encoding two capsid proteins (VP24 and VP26) of herpes simplex virus type 1. J Gen Virol. 1992;73(Pt 10):2709–2713. doi: 10.1099/0022-1317-73-10-2709. [DOI] [PubMed] [Google Scholar]
- Deiss LP, Chou J, Frenkel N. Functional domains within the a sequence involved in the cleavage-packaging of herpes simplex virus DNA. J Virol. 1986;59(3):605–618. doi: 10.1128/jvi.59.3.605-618.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiss LP, Frenkel N. Herpes simplex virus amplicon: cleavage of concatemeric DNA is linked to packaging and involves amplification of the terminally reiterated a sequence. J Virol. 1986;57(3):933–941. doi: 10.1128/jvi.57.3.933-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan A, Jamieson FE, Cunningham C, Barnett BC, McGeoch DJ. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72(3):2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper B, Rao VB. An ATP hydrolysis sensor in the DNA packaging motor from bacteriophage T4 suggests an inchworm-type translocation mechanism. J Mol Biol. 2007;369(1):79–94. doi: 10.1016/j.jmb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Eisenberg RJ, Heldwein EE, Cohen GH, Krummenacher C. Recent Progress in Understanding Herpes Simplex Virus Entry: Relationship of Structure to Function. In: Weller SK, editor. Alphaherpesviruses: Molecular Virology. Caister Academic Press; Norfolk, UK: 2011. pp. 131–152. [Google Scholar]
- Fan WH, Roberts AP, McElwee M, Bhella D, Rixon FJ, Lauder R. The large tegument protein pUL36 is essential for formation of the capsid vertex-specific component at the capsid-tegument interface of herpes simplex virus 1. J Virol. 2015;89(3):1502–1511. doi: 10.1128/JVI.02887-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum E, Friedel CC, Rajagopala SV, Titz B, Baiker A, Schmidt T, Kraus T, Stellberger T, Rutenberg C, Suthram S, Bandyopadhyay S, Rose D, von Brunn A, Uhlmann M, Zeretzke C, Dong YA, Boulet H, Koegl M, Bailer SM, Koszinowski U, Ideker T, Uetz P, Zimmer R, Haas J. Evolutionarily conserved herpesviral protein interaction networks. PLoS Pathog. 2009;5(9):e1000570. doi: 10.1371/journal.ppat.1000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W, Klupp BG, Granzow H, Leege T, Mettenleiter TC. Characterization of pseudorabies virus (PrV) cleavage-encapsidation proteins and functional complementation of PrV pUL32 by the homologous protein of herpes simplex virus type 1. J Virol. 2009;83(8):3930–3943. doi: 10.1128/JVI.02636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong D, Swift H, Roizman B. Arrangement of herpesvirus deoxyribonucleic acid in the core. J Virol. 1972;10(5):1071–1074. doi: 10.1128/jvi.10.5.1071-1074.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W, Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima F, Watanabe D, Takakuwa H, Wada K, Daikoku T, Yamada M, Nishiyama Y. Herpes simplex virus UL17 protein is associated with B capsids and colocalizes with ICP35 and VP5 in infected cells. Arch Virol. 2000;145(2):417–426. doi: 10.1007/s007050050033. [DOI] [PubMed] [Google Scholar]
- Grunewald K, Desai P, Winkler DC, Heymann JB, Belnap DM, Baumeister W, Steven AC. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science. 2003;302(5649):1396–1398. doi: 10.1126/science.1090284. [DOI] [PubMed] [Google Scholar]
- Heming JD, Huffman JB, Jones LM, Homa FL. Isolation and characterization of the herpes simplex virus 1 terminase complex. J Virol. 2014;88(1):225–236. doi: 10.1128/JVI.02632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann JB, Cheng N, Newcomb WW, Trus BL, Brown JC, Steven AC. Dynamics of herpes simplex virus capsid maturation visualized by time-lapse cryo-electron microscopy. Nat Struct Biol. 2003;10(5):334–341. doi: 10.1038/nsb922. [DOI] [PubMed] [Google Scholar]
- Higgs MR, Preston VG, Stow ND. The UL15 protein of herpes simplex virus type 1 is necessary for the localization of the UL28 and UL33 proteins to viral DNA replication centres. J Gen Virol. 2008;89(Pt 7):1709–1715. doi: 10.1099/vir.0.2008/000448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge PD, Stow ND. Effects of mutations within the herpes simplex virus type 1 DNA encapsidation signal on packaging efficiency. J Virol. 2001;75(19):8977–8986. doi: 10.1128/JVI.75.19.8977-8986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa FL, Brown JC. Capsid assembly and DNA packaging in herpes simplex virus. Rev Med Virol. 1997;7(2):107–122. doi: 10.1002/(sici)1099-1654(199707)7:2<107::aid-rmv191>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Homa FL, Huffman JB, Toropova K, Lopez HR, Makhov AM, Conway JF. Structure of the pseudorabies virus capsid: comparison with herpes simplex virus type 1 and differential binding of essential minor proteins. J Mol Biol. 2013;425(18):3415–3428. doi: 10.1016/j.jmb.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet A, Makhov AM, Huffman JB, Vos M, Homa FL, Conway JF. Extensive subunit contacts underpin herpesvirus capsid stability and interior-to-exterior allostery. Nat Struct Mol Biol. 2016 doi: 10.1038/nsmb.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JB, Newcomb WW, Brown JC, Homa FL. Amino acids 143 to 150 of the herpes simplex virus type 1 scaffold protein are required for the formation of portal-containing capsids. J Virol. 2008;82(13):6778–6781. doi: 10.1128/JVI.00473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JG, Yang K, Baines JD, Homa FL. Linker insertion mutations in the herpes simplex virus type 1 UL28 gene: effects on UL28 interaction with UL15 and UL33 and identification of a second-site mutation in the UL15 gene that suppresses a lethal UL28 mutation. J Virol. 2006;80(24):12312–12323. doi: 10.1128/JVI.01766-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff ED, Bachenheimer SL, Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslowski KM, Shaver PR, Casey JT, 2nd, Wilson T, Yamanaka G, Sheaffer AK, Tenney DJ, Pederson NE. Physical and functional interactions between the herpes simplex virus UL15 and UL28 DNA cleavage and packaging proteins. J Virol. 1999;73(2):1704–1707. doi: 10.1128/jvi.73.2.1704-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslowski KM, Shaver PR, Wang XY, Tenney DJ, Pederson NE. The pseudorabies virus UL28 protein enters the nucleus after coexpression with the herpes simplex virus UL15 protein. J Virol. 1997;71(12):9118–9123. doi: 10.1128/jvi.71.12.9118-9123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krosky PM, Underwood MR, Turk SR, Feng KW, Jain RK, Ptak RG, Westerman AC, Biron KK, Townsend LB, Drach JC. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J Virol. 1998;72(6):4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti C, Weller SK. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology. 1996;226(2):403–407. doi: 10.1006/viro.1996.0668. [DOI] [PubMed] [Google Scholar]
- Lamberti C, Weller SK. The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments. J Virol. 1998;72(3):2463–2473. doi: 10.1128/jvi.72.3.2463-2473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Roizman B. Characterization of the protease and other products of amino-terminus-proximal cleavage of the herpes simplex virus 1 UL26 protein. J Virol. 1993;67(3):1300–1309. doi: 10.1128/jvi.67.3.1300-1309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FY, Roizman B. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J Virol. 1991;65(10):5149–5156. doi: 10.1128/jvi.65.10.5149-5156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker H, Frenkel N. BamI, KpnI, and SalI restriction enzyme maps of the DNAs of herpes simplex virus strains Justin and F: occurrence of heterogeneities in defined regions of the viral DNA. J Virol. 1979;32(2):429–441. doi: 10.1128/jvi.32.2.429-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loret S, Guay G, Lippe R. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J Virol. 2008;82(17):8605–8618. doi: 10.1128/JVI.00904-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R, Sarisky RT, Weber PC, Weller SK. Herpes simplex virus type 1 alkaline nuclease is required for efficient processing of viral DNA replication intermediates. J Virol. 1996;70(4):2075–2085. doi: 10.1128/jvi.70.4.2075-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, Perry LJ, Scott JE, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McNab AR, Desai P, Person S, Roof LL, Thomsen DR, Newcomb WW, Brown JC, Homa FL. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J Virol. 1998;72(2):1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES, Deiss LP, Frenkel N. Nucleotide sequence and structural features of a novel US-a junction present in a defective herpes simplex virus genome. J Virol. 1985;55(1):140–146. doi: 10.1128/jvi.55.1.140-146.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES, Roizman B. Site-specific inversion sequence of the herpes simplex virus genome: domain and structural features. Proc Natl Acad Sci U S A. 1981;78(11):7047–7051. doi: 10.1073/pnas.78.11.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES, Roizman B. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell. 1982;31(1):89–97. doi: 10.1016/0092-8674(82)90408-1. [DOI] [PubMed] [Google Scholar]
- Nadal M, Mas PJ, Blanco AG, Arnan C, Sola M, Hart DJ, Coll M. Structure and inhibition of herpesvirus DNA packaging terminase nuclease domain. Proc Natl Acad Sci U S A. 2010;107(37):16078–16083. doi: 10.1073/pnas.1007144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M, Mocarski ES. The cleavage recognition signal is contained within sequences surrounding an a-a junction in herpes simplex virus DNA. Virology. 1988;167(1):25–30. doi: 10.1016/0042-6822(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Nellissery JK, Szczepaniak R, Lamberti C, Weller SK. A putative leucine zipper within the herpes simplex virus type 1 UL6 protein is required for portal ring formation. J Virol. 2007;81(17):8868–8877. doi: 10.1128/JVI.00739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Brown JC. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991;65(2):613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Cockrell SK, Homa FL, Brown JC. Polarized DNA ejection from the herpesvirus capsid. J Mol Biol. 2009;392(4):885–894. doi: 10.1016/j.jmb.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Homa FL, Brown JC. Involvement of the portal at an early step in herpes simplex virus capsid assembly. J Virol. 2005;79(16):10540–10546. doi: 10.1128/JVI.79.16.10540-10546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Homa FL, Brown JC. Herpes simplex virus capsid structure: DNA packaging protein UL25 is located on the external surface of the capsid near the vertices. J Virol. 2006;80(13):6286–6294. doi: 10.1128/JVI.02648-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Homa FL, Thomsen DR, Booy FP, Trus BL, Steven AC, Spencer JV, Brown JC. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J Mol Biol. 1996;263(3):432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- Newcomb WW, Homa FL, Thomsen DR, Trus BL, Cheng N, Steven A, Booy F, Brown JC. Assembly of the herpes simplex virus procapsid from purified components and identification of small complexes containing the major capsid and scaffolding proteins. J Virol. 1999;73(5):4239–4250. doi: 10.1128/jvi.73.5.4239-4250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Homa FL, Thomsen DR, Ye Z, Brown JC. Cell-free assembly of the herpes simplex virus capsid. J Virol. 1994;68(9):6059–6063. doi: 10.1128/jvi.68.9.6059-6063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Juhas RM, Thomsen DR, Homa FL, Burch AD, Weller SK, Brown JC. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J Virol. 2001;75(22):10923–10932. doi: 10.1128/JVI.75.22.10923-10932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Thomsen DR, Homa FL, Brown JC. Assembly of the herpes simplex virus capsid: identification of soluble scaffold-portal complexes and their role in formation of portal-containing capsids. J Virol. 2003;77(18):9862–9871. doi: 10.1128/JVI.77.18.9862-9871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb WW, Trus BL, Booy FP, Steven AC, Wall JS, Brown JC. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J Mol Biol. 1993;232(2):499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- Newcomb WW, Trus BL, Cheng N, Steven AC, Sheaffer AK, Tenney DJ, Weller SK, Brown JC. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J Virol. 2000;74(4):1663–1673. doi: 10.1128/jvi.74.4.1663-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AH, MacLean JB. The product of the UL6 gene of herpes simplex virus type 1 is associated with virus capsids. Virology. 1995;206(1):465–478. doi: 10.1016/s0042-6822(95)80062-x. [DOI] [PubMed] [Google Scholar]
- Patel AH, Rixon FJ, Cunningham C, Davison AJ. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology. 1996;217(1):111–123. doi: 10.1006/viro.1996.0098. [DOI] [PubMed] [Google Scholar]
- Pellet PE, Roizman B. The Family Herpesviridae: A Brief Introduction. In: Knipe DMMHP, editor. Fields Virology. Fifth. II. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2479–2499. [Google Scholar]
- Perdue ML, Cohen JC, Randall CC, O'Callaghan DJ. Biochemical studies of the maturation of herpesvirus nucleocapsid species. Virology. 1976;74(1):194–208. [PubMed] [Google Scholar]
- Poon AP, Roizman B. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J Virol. 1993;67(8):4497–4503. doi: 10.1128/jvi.67.8.4497-4503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston VG, Coates JA, Rixon FJ. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston VG, Murray J, Preston CM, McDougall IM, Stow ND. The UL25 gene product of herpes simplex virus type 1 is involved in uncoating of the viral genome. J Virol. 2008;82(13):6654–6666. doi: 10.1128/JVI.00257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przech AJ, Yu D, Weller SK. Point mutations in exon I of the herpes simplex virus putative terminase subunit, UL15, indicate that the most conserved residues are essential for cleavage and packaging. J Virol. 2003;77(17):9613–9621. doi: 10.1128/JVI.77.17.9613-9621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Fan Y, Baines JD. Characterization of the U(L)33 gene product of herpes simplex virus 1. Virology. 2000;266(2):310–318. doi: 10.1006/viro.1999.0090. [DOI] [PubMed] [Google Scholar]
- Rixon FJ, Cross AM, Addison C, Preston VG. The products of herpes simplex virus type 1 gene UL26 which are involved in DNA packaging are strongly associated with empty but not with full capsids. J Gen Virol. 1988;69(Pt 11):2879–2891. doi: 10.1099/0022-1317-69-11-2879. [DOI] [PubMed] [Google Scholar]
- Rixon FJ, McNab D. Packaging-competent capsids of a herpes simplex virus temperature-sensitive mutant have properties similar to those of in vitro-assembled procapsids. J Virol. 1999;73(7):5714–5721. doi: 10.1128/jvi.73.7.5714-5721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. The organization of the herpes simplex virus genomes. Annu Rev Genet. 1979a;13:25–57. doi: 10.1146/annurev.ge.13.120179.000325. [DOI] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979b;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Salmon B, Baines JD. Herpes simplex virus DNA cleavage and packaging: association of multiple forms of U(L)15-encoded proteins with B capsids requires at least the U(L)6, U(L)17, and U(L)28 genes. J Virol. 1998;72(4):3045–3050. doi: 10.1128/jvi.72.4.3045-3050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon B, Cunningham C, Davison AJ, Harris WJ, Baines JD. The herpes simplex virus type 1 U(L)17 gene encodes virion tegument proteins that are required for cleavage and packaging of viral DNA. J Virol. 1998;72(5):3779–3788. doi: 10.1128/jvi.72.5.3779-3788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer PA, Aron GM, Biswal N, Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Scheffczik H, Savva CG, Holzenburg A, Kolesnikova L, Bogner E. The terminase subunits pUL56 and pUL89 of human cytomegalovirus are DNA-metabolizing proteins with toroidal structure. Nucleic Acids Res. 2002;30(7):1695–1703. doi: 10.1093/nar/30.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag JD, Prasad BV, Rixon FJ, Chiu W. Three-dimensional structure of the HSV1 nucleocapsid. Cell. 1989;56(4):651–660. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- Sciortino MT, Suzuki M, Taddeo B, Roizman B. RNAs extracted from herpes simplex virus 1 virions: apparent selectivity of viral but not cellular RNAs packaged in virions. J Virol. 2001;75(17):8105–8116. doi: 10.1128/JVI.75.17.8105-8116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan Sigamani S, Zhao H, Kamau YN, Baines JD, Tang L. The structure of the herpes simplex virus DNA-packaging terminase pUL15 nuclease domain suggests an evolutionary lineage among eukaryotic and prokaryotic viruses. J Virol. 2013;87(12):7140–7148. doi: 10.1128/JVI.00311-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severini A, Morgan AR, Tovell DR, Tyrrell DL. Study of the structure of replicative intermediates of HSV-1 DNA by pulsed-field gel electrophoresis. Virology. 1994;200(2):428–435. doi: 10.1006/viro.1994.1206. [DOI] [PubMed] [Google Scholar]
- Sheaffer AK, Newcomb WW, Gao M, Yu D, Weller SK, Brown JC, Tenney DJ. Herpes simplex virus DNA cleavage and packaging proteins associate with the procapsid prior to its maturation. J Virol. 2001;75(2):687–698. doi: 10.1128/JVI.75.2.687-698.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman G, Bachenheimer SL. DNA processing in temperature-sensitive morphogenic mutants of HSV-1. Virology. 1987;158(2):427–430. doi: 10.1016/0042-6822(87)90214-5. [DOI] [PubMed] [Google Scholar]
- Sherman G, Bachenheimer SL. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988;163(2):471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- Singer GP, Newcomb WW, Thomsen DR, Homa FL, Brown JC. Identification of a region in the herpes simplex virus scaffolding protein required for interaction with the portal. J Virol. 2005;79(1):132–139. doi: 10.1128/JVI.79.1.132-139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete RR, Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982;30(1):295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- Spaete RR, Frenkel N. The herpes simplex virus amplicon: analyses of cis-acting replication functions. Proc Natl Acad Sci U S A. 1985;82(3):694–698. doi: 10.1073/pnas.82.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PG, Roizman B. Buoyant density of herpes simplex virus in solutions of caesium chloride. Nature. 1967;214(5089):713–714. doi: 10.1038/214713a0. [DOI] [PubMed] [Google Scholar]
- Spear PG, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow ND. Packaging of genomic and amplicon DNA by the herpes simplex virus type 1 UL25-null mutant KUL25NS. J Virol. 2001;75(22):10755–10765. doi: 10.1128/JVI.75.22.10755-10765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow ND, McMonagle EC. Characterization of the TRS/IRS origin of DNA replication of herpes simplex virus type 1. Virology. 1983;130(2):427–438. doi: 10.1016/0042-6822(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Stow ND, McMonagle EC, Davison AJ. Fragments from both termini of the herpes simplex virus type 1 genome contain signals required for the encapsidation of viral DNA. Nucleic Acids Res. 1983;11(23):8205–8220. doi: 10.1093/nar/11.23.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatman JD, Preston VG, Nicholson P, Elliott RM, Rixon FJ. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J Gen Virol. 1994;75(Pt 5):1101–1113. doi: 10.1099/0022-1317-75-5-1101. [DOI] [PubMed] [Google Scholar]
- Taus NS, Baines JD. Herpes simplex virus 1 DNA cleavage/packaging: the UL28 gene encodes a minor component of B capsids. Virology. 1998;252(2):443–449. doi: 10.1006/viro.1998.9475. [DOI] [PubMed] [Google Scholar]
- Tengelsen LA, Pederson NE, Shaver PR, Wathen MW, Homa FL. Herpes simplex virus type 1 DNA cleavage and encapsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J Virol. 1993;67(6):3470–3480. doi: 10.1128/jvi.67.6.3470-3480.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma C, Borst E, Messerle M, Rieger M, Hwang JS, Bogner E. Identification of the interaction domain of the small terminase subunit pUL89 with the large subunit pUL56 of human cytomegalovirus. Biochemistry. 2006;45(29):8855–8863. doi: 10.1021/bi0600796. [DOI] [PubMed] [Google Scholar]
- Thomsen DR, Roof LL, Homa FL. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J Virol. 1994;68(4):2442–2457. doi: 10.1128/jvi.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow JK, Murphy M, Stow ND, Preston VG. Herpes simplex virus type 1 DNA-packaging protein UL17 is required for efficient binding of UL25 to capsids. J Virol. 2006;80(5):2118–2126. doi: 10.1128/JVI.80.5.2118-2126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow JK, Rixon FJ, Murphy M, Targett-Adams P, Hughes M, Preston VG. The herpes simplex virus type 1 DNA packaging protein UL17 is a virion protein that is present in both the capsid and the tegument compartments. J Virol. 2005;79(1):150–158. doi: 10.1128/JVI.79.1.150-158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toropova K, Huffman JB, Homa FL, Conway JF. The herpes simplex virus 1 UL17 protein is the second constituent of the capsid vertex-specific component required for DNA packaging and retention. J Virol. 2011;85(15):7513–7522. doi: 10.1128/JVI.00837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus BL, Booy FP, Newcomb WW, Brown JC, Homa FL, Thomsen DR, Steven AC. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. J Mol Biol. 1996;263(3):447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- Trus BL, Cheng N, Newcomb WW, Homa FL, Brown JC, Steven AC. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. J Virol. 2004;78(22):12668–12671. doi: 10.1128/JVI.78.22.12668-12671.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus BL, Newcomb WW, Cheng N, Cardone G, Marekov L, Homa FL, Brown JC, Steven AC. Allosteric signaling and a nuclear exit strategy: binding of UL25/UL17 heterodimers to DNA-Filled HSV-1 capsids. Mol Cell. 2007;26(4):479–489. doi: 10.1016/j.molcel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umene K, Oohashi S, Yoshida M, Fukumaki Y. Diversity of the a sequence of herpes simplex virus type 1 developed during evolution. J Gen Virol. 2008;89(Pt 4):841–852. doi: 10.1099/vir.0.83467-0. [DOI] [PubMed] [Google Scholar]
- Underwood MR, Harvey RJ, Stanat SC, Hemphill ML, Miller T, Drach JC, Townsend LB, Biron KK. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J Virol. 1998;72(1):717–725. doi: 10.1128/jvi.72.1.717-725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen IL, Brandimarti R, Torrisi MR, Campadelli G, van Meer G. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology. 1994;200(2):831–836. doi: 10.1006/viro.1994.1252. [DOI] [PubMed] [Google Scholar]
- Varmuza SL, Smiley JR. Signals for site-specific cleavage of HSV DNA: maturation involves two separate cleavage events at sites distal to the recognition sequences. Cell. 1985;41(3):793–802. doi: 10.1016/s0092-8674(85)80060-x. [DOI] [PubMed] [Google Scholar]
- Visalli RJ, Knepper J, Goshorn B, Vanover K, Burnside DM, Irven K, McGauley R, Visalli M. Characterization of the Varicella-zoster virus ORF25 gene product: pORF25 interacts with multiple DNA encapsidation proteins. Virus Res. 2009;144(1–2):58–64. doi: 10.1016/j.virusres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalli RJ, Nicolosi DM, Irven KL, Goshorn B, Khan T, Visalli MA. The Varicella-zoster virus DNA encapsidation genes: Identification and characterization of the putative terminase subunits. Virus Res. 2007;129(2):200–211. doi: 10.1016/j.virusres.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizoso Pinto MG, Pothineni VR, Haase R, Woidy M, Lotz-Havla AS, Gersting SW, Muntau AC, Haas J, Sommer M, Arvin AM, Baiker A. Varicella zoster virus ORF25 gene product: an essential hub protein linking encapsidation proteins and the nuclear egress complex. J Proteome Res. 2011;10(12):5374–5382. doi: 10.1021/pr200628s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlazny DA, Kwong A, Frenkel N. Site-specific cleavage/packaging of herpes simplex virus DNA and the selective maturation of nucleocapsids containing full-length viral DNA. Proc Natl Acad Sci U S A. 1982;79(5):1423–1427. doi: 10.1073/pnas.79.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth S, Jacob RJ, Roizman B. Anatomy of herpes simplex virus DNA. II. Size, composition, and arrangement of inverted terminal repetitions. J Virol. 1975;15(6):1487–1497. doi: 10.1128/jvi.15.6.1487-1497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Summers WC. Structure of the joint region and the termini of the DNA of herpes simplex virus type 1. J Virol. 1978;27(2):374–387. doi: 10.1128/jvi.27.2.374-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha-and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Zhu Y, McVoy MA, Parris DS. Changes in subcellular localization reveal interactions between human cytomegalovirus terminase subunits. Virol J. 2012;9:315. doi: 10.1186/1743-422X-9-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller SK, Carmichael EP, Aschman DP, Goldstein DJ, Schaffer PA. Genetic and phenotypic characterization of mutants in four essential genes that map to the left half of HSV-1 UL DNA. Virology. 1987;161(1):198–210. doi: 10.1016/0042-6822(87)90186-3. [DOI] [PubMed] [Google Scholar]
- White CA, Stow ND, Patel AH, Hughes M, Preston VG. Herpes simplex virus type 1 portal protein UL6 interacts with the putative terminase subunits UL15 and UL28. J Virol. 2003;77(11):6351–6358. doi: 10.1128/JVI.77.11.6351-6358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildy P, Russell WC, Horne RW. The morphology of herpes virus. Virology. 1960;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]
- Wills E, Scholtes L, Baines JD. Herpes simplex virus 1 DNA packaging proteins encoded by UL6, UL15, UL17, UL28, and UL33 are located on the external surface of the viral capsid. J Virol. 2006;80(21):10894–10899. doi: 10.1128/JVI.01364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Baines JD. The putative terminase subunit of herpes simplex virus 1 encoded by UL28 is necessary and sufficient to mediate interaction between pUL15 and pUL33. J Virol. 2006;80(12):5733–5739. doi: 10.1128/JVI.00125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Baines JD. Domain within herpes simplex virus 1 scaffold proteins required for interaction with portal protein in infected cells and incorporation of the portal vertex into capsids. J Virol. 2008;82(10):5021–5030. doi: 10.1128/JVI.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Homa F, Baines JD. Putative terminase subunits of herpes simplex virus 1 form a complex in the cytoplasm and interact with portal protein in the nucleus. J Virol. 2007;81(12):6419–6433. doi: 10.1128/JVI.00047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Poon AP, Roizman B, Baines JD. Temperature-sensitive mutations in the putative herpes simplex virus type 1 terminase subunits pUL15 and pUL33 preclude viral DNA cleavage/packaging and interaction with pUL28 at the nonpermissive temperature. J Virol. 2008;82(1):487–494. doi: 10.1128/JVI.01875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Wills E, Baines JD. The putative leucine zipper of the UL6-encoded portal protein of herpes simplex virus 1 is necessary for interaction with pUL15 and pUL28 and their association with capsids. J Virol. 2009;83(9):4557–4564. doi: 10.1128/JVI.00026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Wills EG, Baines JD. A mutation in UL15 of herpes simplex virus 1 that reduces packaging of cleaved genomes. J Virol. 2011;85(22):11972–11980. doi: 10.1128/JVI.00857-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Sheaffer AK, Tenney DJ, Weller SK. Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins. J Virol. 1997;71(4):2656–2665. doi: 10.1128/jvi.71.4.2656-2665.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Weller SK. Genetic analysis of the UL 15 gene locus for the putative terminase of herpes simplex virus type 1. Virology. 1998a;243(1):32–44. doi: 10.1006/viro.1998.9041. [DOI] [PubMed] [Google Scholar]
- Yu D, Weller SK. Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging. J Virol. 1998b;72(9):7428–7439. doi: 10.1128/jvi.72.9.7428-7439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Efstathiou S, Simmons A. Identification of novel herpes simplex virus replicative intermediates by field inversion gel electrophoresis: implications for viral DNA amplification strategies. Virology. 1994;202(2):530–539. doi: 10.1006/viro.1994.1375. [DOI] [PubMed] [Google Scholar]
- Zhou ZH, Chen DH, Jakana J, Rixon FJ, Chiu W. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J Virol. 1999;73(4):3210–3218. doi: 10.1128/jvi.73.4.3210-3218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Prasad BV, Jakana J, Rixon FJ, Chiu W. Protein subunit structures in the herpes simplex virus A-capsid determined from 400 kV spot-scan electron cryomicroscopy. J Mol Biol. 1994;242(4):456–469. doi: 10.1006/jmbi.1994.1594. [DOI] [PubMed] [Google Scholar]