Abstract
Objectives
Depressive symptoms are associated with preterm birth among adults. Pregnant adolescents have high rates of depressive symptoms and low rates of treatment; however, few interventions have targeted this vulnerable group. Objectives are to: (1) examine impact of CenteringPregnancy® Plus group prenatal care on perinatal depressive symptoms compared to individual prenatal care; and (2) determine effects of depressive symptoms on gestational age and preterm birth among pregnant adolescents.
Method
This cluster-randomized controlled trial was conducted in 14 community health centers and hospitals in New York City. Clinical sites were randomized to receive standard individual prenatal care (n=7) or CenteringPregnancy Plus group prenatal care (n=7). Pregnant adolescents (ages 14–21, N=1,135) completed the Center for Epidemiologic Studies Depression Scale during pregnancy (second and third trimesters) and postpartum (6 and 12 months). Gestational age was obtained from medical records, based on ultrasound dating. Intention to treat analyses were used to examine objectives.
Results
Adolescents at clinical sites randomized to Centering Pregnancy Plus experienced greater reductions in perinatal depressive symptoms compared to those at clinical sites randomized to individual care (p=.003). Increased depressive symptoms from second to third pregnancy trimester were associated with shorter gestational age at delivery and preterm birth (<37 weeks gestation). Third trimester depressive symptoms were also associated with shorter gestational age and preterm birth. All p<0.05.
Conclusions
Pregnant adolescents should be screened for depressive symptoms prior to third trimester. Group prenatal care may be an effective non-pharmacological option for reducing depressive symptoms among perinatal adolescents. ClinicalTrials.gov #NCT00628771.
Keywords: Perinatal, depression, preterm birth, group prenatal care, cluster randomized controlled trial
Introduction
Pregnant adolescents and their offspring are at increased risk for poor outcomes, including lower educational attainment, persistent socioeconomic disadvantage, and adverse physical health outcomes (Assini-Meytin & Green, 2015; Jutte et al., 2010; Kane, Morgan, Harris, & Guilkey, 2013; Pirkle, Sousa, Alvarado, Zunzunegui, & For the IMIAS Research Group, 2014). Focusing specifically on psychological correlates, there are high rates of depression among pregnant and postpartum adolescents. As many as 57% experience moderate to severe depressive symptoms; this rate is higher than reports among non-pregnant adolescent females and perinatal adults (Avenevoli, Swendsen, He, Burstein, & Merikangas, 2015; Cunningham et al., 2016; Gavin et al., 2005; Lanzi, Bert, Jacobs, & Centers for the Prevention of Child, 2009; Schmidt, Wiemann, Rickert, & Smith, 2006).
Negative consequences of untreated perinatal depression include adverse birth, neonatal, and infant outcomes (Jeha, Usta, Ghulmiyyah, & Nassar, 2015; Kleiber & Dimidjian, 2014; Stein et al., 2014). Fortunately, evidence-based treatment options exist. Psychotherapeutic interventions delivered during pregnancy result in significant reductions in postpartum depression among adults (Sockol, Epperson, & Barber, 2013) and adolescents (Phipps, Raker, Ware, & Zlotnick, 2013). Evidence of efficacy for antenatal depressive symptom severity is more limited, but promising (Dennis, Ross, & Grigoriadis, 2007; Milgrom et al., 2015; Thomas, Komiti, & Judd, 2014).
The United States Preventive Services Task Force now recommends depression screening for all pregnant and postpartum women (Siu et al., 2016). Unfortunately, fewer than one-half of pregnant adolescents who were screened and referred for depression treatment attended even one appointment with a psychologist (Ashby, Ranadive, Alaniz, St John-Larkin, & Scott, 2016), converging with evidence of low treatment rates among depressed perinatal adults (Flynn, O’Mahen, Massey, & Marcus, 2006) and depressed non-pregnant adolescents (Kessler, Avenevoli, & Ries Merikangas, 2001). Research has elucidated barriers to depression care, including lack of knowledge about depressive symptoms or treatment options, stigma, and practical barriers, difficulties with scheduling, transportation and childcare (Abrams, Dornig, & Curran, 2009; Goodman, 2009; Kopelman et al., 2008). In contrast to low depression treatment rates among pregnant adolescents, it is estimated that nearly all (97%) pregnant adolescents receive prenatal care (United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), & Division of Vital Statistics, 2016). Integrating depression care within prenatal care may reduce barriers to obtaining treatment for depression.
CenteringPregnancy® is an evidence-based prenatal care program provided in a group context and bundled with other services (e.g., nutrition, labor preparation). Evidence suggests CenteringPregnancy improves birth outcomes among pregnant adolescents, including a 33% reduction in risk of preterm birth in a sample of pregnant women ages 14–25 years (Ickovics et al., 2007). In the primary outcomes paper of the current study, participants at CenteringPregnancy group prenatal care sites were less likely to have infants small-for-gestational-age in intention-to- treat analyses, and less likely to have a preterm delivery in as-treated analyses (Ickovics et al., 2016). Group care provides opportunities for social support, and participants learn stress reduction techniques and communication skills, which may improve psychosocial functioning (Ickovics et al., 2011).
Preterm birth complications are the leading cause of death before age five globally (L. Liu et al., 2015). A growing body of evidence points to depression as one psychological risk factor for preterm birth (Grote et al., 2010; Jarde et al., 2016; Liou, Wang, & Cheng, 2016; C. Liu, Cnattingius, Bergstrom, Ostberg, & Hjern, 2016; Pesonen et al., 2016). However, there is a dearth of evidence examining this association among pregnant adolescents. Treating depressive symptoms may reduce risk for preterm birth, but results are mixed for effects of antidepressant medication. One recent observational study found no association between a positive depression screen and preterm birth among pregnant women treated with antidepressant medication (Venkatesh, Riley, Castro, Perlis, & Kaimal, 2016), whereas meta-analytic work indicates that women treated with a selective serotonin reuptake inhibitor (SSRI) had significantly increased risk of preterm birth compared to depressed women not treated with an SSRI (Saccone, Eke, & Berghella, 2016; Wisner et al., 2009). This, in addition to women’s preferences for non- pharmacological treatments for perinatal depression (Goodman, 2009), highlights the need for research on the impact of non-pharmacological approaches for antenatal depression on preterm birth.
The first objective of this secondary data analysis was to examine the impact of CenteringPregnancy Plus group prenatal care on perinatal depressive symptoms compared to standard individual prenatal care. Depressive symptoms were measured during the second and third trimester of pregnancy, and six and twelve months postpartum. We hypothesized that participants at clinical sites randomized to group prenatal care (n=7) would experience significantly greater reductions in perinatal depressive symptoms compared to participants at clinical sites randomized to standard individual prenatal care (n=7). Depressive symptoms were included as a secondary outcome on ClinicalTrials.gov (NCT00628771). The second objective was to determine the association between depressive symptoms and gestational age. Based on research with adults, we hypothesized that depressive symptoms would be associated with shorter gestational age and increased risk of preterm birth among pregnant adolescents. Finally, we examined whether the effect of depressive symptoms on outcomes was moderated by prenatal care type. Hypotheses were planned prior to data analysis.
Method
Participants and Procedures
Participants were from a cluster randomized controlled trial designed to compare the impact of CenteringPregnancy Plus group prenatal care to standard individual prenatal care on birth, neonatal, and reproductive health outcomes (Ickovics et al., 2016; ClinicalTrials.gov #NCT00628771).
Fourteen clinical sites participated, including four community health centers and ten community hospitals serving predominately low-resource women. Participants were recruited in 2008–2012 via provider referral or through research staff. Inclusion criteria were: (1) ages 14–21, (2) pregnancy <24 weeks gestation, (3) pregnancy not considered high risk, (4) ability to speak English or Spanish, and (5) willingness to participate in CenteringPregnancy Plus. All participants consented to receive prenatal care through a group prenatal care format at their site, if available. To reduce selection bias, participants were only un-blinded to whether their site offered group care after study enrollment.
Surveys were completed at four timepoints: second trimester of pregnancy (M = 18.72 weeks gestational age, SD = 3.29), third trimester of pregnancy (M = 29.99 weeks gestational age, SD = 5.28), 6 months postpartum (M = 26.07 weeks postpartum, SD = 5.21), and 12 months postpartum (M = 57.30 weeks postpartum, SD = 13.50). Study interviews were completed in English or Spanish using handheld computers with audio capability. Questions were visually displayed on handheld computers; concurrently, participants heard the questions through headphones via pre-recorded audio files of each question. This enabled participants with low literacy to complete study interview privately. Research assistants provided an orientation on how to complete the study interview and were available to answer questions, conduct the interview one-to-one for low literacy patients, or address technical issues (all of these were rare). Interviews were scheduled at times and places convenient for participants, including the health centers and/or places in their neighborhoods (e.g., home, library).
Extensive locator information was obtained at study recruitment, including phone and mailing addresses for participants, their baby’s father, family, friends, or other individuals who would know how to find them if they moved or changed their phone number. Participants were compensated $20 for completing study interviews at each timepoint. There was no compensation associated with adherence to prenatal care. The study protocol was approved by Institutional Review Boards at Yale University, Clinical Directors Network (CDN) and all clinical sites.
Interventions
Individual Prenatal Care
Individual prenatal care is the standard of care received by most United States women (U.S. Department of Health and Human Services, 2013). The American Academy of Pediatrics and American Congress of Obstetricians and Gynecologists (2012) together established guidelines for perinatal care. Goals are to reduce maternal risk; obtain accurate gestational age estimate; identify patients at risk for complications; prevent health problems; provide patient education; and improve delivery outcomes. Initial intake appointments include a comprehensive medical and psychosocial history, physical exam, laboratory testing and education (typically 1–2 hours). Subsequent visits are brief (10–15 minutes), including physical exam (e.g., fundal height, fetal heart tones) and basic education to address nutrition, weight gain and safety. Laboratory work (e.g., CBC, glucose tolerance) and ultrasounds (e.g., anatomy screen 18–20 weeks) occur during pregnancy per guidelines or as clinically indicated. The schedule of prenatal care typically includes monthly visits before 28 weeks gestation, bi-weekly from 28–36 weeks gestation, and weekly beyond 36 weeks.
CenteringPregnancy Plus
Described in detail previously (Ickovics et al., 2016; Ickovics et al., 2007; Kershaw, Magriples, Westdahl, Rising, & Ickovics, 2009; Massey, Rising, & Ickovics, 2006), CenteringPregnancy Plus begins with a standard individual comprehensive intake visit. Thereafter, all care occurs within group format, except for concerns requiring privacy or urgent medical attention. Each group includes 8 to 12 women around the same gestational age (e.g., all with expected due dates in January). Group care follows the same American College of Obstetricians and Gynecologists (ACOG) clinical guidelines as individual prenatal care. A credentialed provider (e.g., obstetrician, midwife) and co-facilitator (e.g., nurse, medical assistant) facilitate group sessions. No specific mental health training was required or provided to facilitators as part of this study.
At arrival, patients engage in self-care activities, including taking their own weight and blood pressure, charting their progress in health records, and completing a brief self-assessment activity to spark group discussion. Clinicians conduct brief individual medical assessments (e.g., fundal height, heart rate monitoring) with each patient in a private area within the group space. Groups are scheduled to meet for 10 sessions throughout pregnancy, with each session lasting 120 minutes.
Twenty hours of CenteringPregnancy Plus, compared to an average of about two hours for standard individual care, fosters social support and provides more time for in-depth, facilitated discussions on a broad range of issues, including nutrition, physical activity, relaxation techniques, understanding pregnancy problems, communication and self-esteem, comfort measures in pregnancy, sexuality and childbearing, interpersonal violence, parenting and childbirth preparation, infant care and feeding, and postpartum issues (including contraception) (Massey et al., 2006). CenteringPregnancy Plus integrates sexual risk reduction intervention targeting contraception use and HIV/STI prevention. Modules include: contraceptive planning and use, personalization of risk, triggers for risk behaviors, decision making, goal setting, and partner negotiation tactics (Ickovics et al., 2016).
Participants in both study conditions received the mental health screening and services that were standard practice at their clinical site. Study interviews were not used to assess patients for clinical services nor individually reported to the clinical sites. Interviews were confidential; however, protocols were established to reduce risk for adverse outcomes. Specifically, all participants were given information about resources for mental health and social service needs at their healthcare site and in their community. Facilitators followed their clinical site’s protocols for suicidality and other mental health crises. Research staff who administered the interviews were trained to address suicidal ideation, and make a referral or direct handoff to the mental health provider specified by the clinical site. A licensed mental health provider on the study team was available for urgent consultation if needed.
Measures
Depressive symptoms
Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977), which has been used previously in studies with pregnant adolescents (Cunningham et al., 2016; Westdahl et al., 2007). The CES-D contains 20 items. Respondents rate how often they experience affective components of depressed mood (e.g., feelings of failure, guilt, hopelessness) over the past week on a four- point Likert scale ranging from none of the time (0) to all of the time (3). Consistent with prior studies with pregnant women, five psychophysiological items were omitted due to conflation with normative symptoms across the perinatal period (e.g., changes in sleep, appetite). Scores for these 15 affect-only items were summed, with higher scores indicating higher depressive symptom severity (range = 0–45). Cases of probable depression were defined as scores ≥ 12 (adjusted from 16 for the current 15-item version). The CES-D was administered at all four study timepoints. Cronbach’s alphas exceeded 0.85 for each timepoint, indicating good reliability.
Gestational age and preterm birth
Gestational age at delivery was assessed via medical record using the estimated date of delivery based on ultrasound. Structured medical record reviews were conducted by trained research assistants. Gestational age was reported in days. Preterm birth was dichotomized using standard clinical practice, defined as <37 weeks or <259 days.
Control variables
Prenatal care type (CenteringPregnancy Plus versus individual care) was entered as a control variable in regression models examining the impact of depressive symptoms on gestational age and preterm birth. The following variables with documented associations with preterm birth (Jelliffe-Pawlowski et al., 2015) were included as control variables in regression models: maternal age, maternal race/ethnicity, prepregnancy body mass index, parity, history of adverse pregnancy outcome (i.e., miscarriage or stillbirth), and sexually transmitted infection during pregnancy. Control variables were collected as part of the survey and via medical record review. Maternal age was measured continuously in years. Race/Ethnicity were categorized as Latina; Black, not Latina; White/Other, used as reference group. Prepregnancy body mass index was calculated based on participants’ self-report of height and weight. Participants reported the number of previous births, which was dichotomized for nulliparity (yes/no). History of adverse pregnancy outcomes were categorized by history of miscarriage and/or stillbirth (yes/no). Sexually transmitted infection during pregnancy was assessed through medical record review, and included gonorrhea, chlamydia, syphilis, trichomonas, bacterial vaginosis, and herpes (yes/no).
Data Analyses
The analytic sample included participants who had a singleton pregnancy and no history of preterm birth. We followed intention to treat principles whereby participants were included based on randomization regardless of participation/adherence (Detry & Lewis, 2014). Independent samples t-tests and chi-square tests were used to examine differences in sociodemographic and clinical characteristics by prenatal care type, and to explore patterns of missing depressive symptom data. Multiple imputation was used as a robust technique for handling missing data. Control variables and variables related to missingness were included as auxiliary variables in the multiple imputation models (Collins, Schafer, & Kam, 2001), and 25 datasets were imputed and combined (Li, Stuart, & Allison, 2015). All data analyses were performed using SPSS version 23.
Hierarchical linear modeling with the MIXED procedure in SPSS was used to test the hypothesis that participants at clinical sites randomized to CenteringPregnancy Plus would experience significantly greater reductions in perinatal depressive symptoms compared to participants at sites randomized to standard individual prenatal care. Hierarchical linear modeling was used to examine differences in linear rates of depressive symptom change between group and individual prenatal care (Singer & Willett, 2003). The model utilized random intercept and slopes, and unstructured covariance type. Time was unstructured, operationalized as number of days since baseline interview, and included all timepoints (baseline/second trimester, third trimester, 6 and 12 months postpartum). No other variables were included. This statistical approach accommodated repeated measures and unequal spacing between time intervals. Effect size was computed for repeated measures with three or more timepoints for a linear model (Feingold, 2013). Regression coefficient for the Prenatal Care Type × Time interaction was multiplied by study duration, defined by average number of days from baseline to 12 months postpartum and divided by baseline depressive symptom severity standard deviation.
Linear regression models and logistic regression models were used to test the second hypothesis that depressive symptoms would be associated with shorter gestational age and increased risk of preterm birth among pregnant adolescents. Separate models were used to examine depressive symptoms at: (1) baseline/second trimester, (2) third trimester, and (3) residualized change from baseline to third trimester. Residualized change scores were calculated to represent increases in depressive symptoms from baseline/second trimester to third trimester. Unstandardized residuals were computed by regressing third trimester depressive symptoms on baseline depressive symptoms. Positive residualized change scores indicate increased depressive symptoms from baseline to third trimester. In each model, the depressive symptoms variable was entered simultaneously to control variables (i.e., prenatal care type; maternal age; race/ethnicity; prepregnancy BMI; parity; history of adverse pregnancy outcome; STI during pregnancy). Separate models added a two-way interaction between depressive symptoms and prenatal care type to explore whether the effect of depressive symptoms on gestational age or preterm birth was moderated by prenatal care type. A significant interaction would indicate that the effect of depressive symptoms differed between the two prenatal care type conditions. Adjusted R2 values were used as estimates of effect size for linear regression models; values of .02, .13, and .26 correspond with small, medium, and large effect sizes, respectively (Cohen, 1988). Odds ratios were used as a measure of effect size in logistic regression models.
Results
Participant Characteristics
Figure 1 illustrates participant flow using the CONSORT diagram. Seven clinical sites were randomized to CenteringPregnancy Plus and seven clinical sites were randomized to individual prenatal care. The final analytic sample for these analyses included 569 adolescents for CenteringPregnancy Plus and 566 adolescents for individual prenatal care. Table 1 presents participant characteristics by prenatal care type. On average, participants were 18 years old (range=14–21), and more than one-half were Latina. Participants had a prepregnancy BMI within the normal range, and most participants were pregnant with their first child. Approximately 10% of participants delivered preterm infants. The only significant between-group difference in participant characteristics at baseline was for relationship status, such that a higher proportion of group care participants were married or cohabitating with a partner (p = .01).
Figure 1.

CONSORT diagram for cluster randomized controlled trials. aThere were 35 women enrolled at this cluster/clinical site prior to discontinuation. These women were included in all analyses per intent to treat principles.
Table 1.
Sociodemographic and Clinical Characteristics by Prenatal Care Type, Reported as M (SD) or % (n)
| Participant Characteristics | CenteringPregnancy Plus group prenatal care (n=569) |
Individual prenatal care (n=566) |
|---|---|---|
| Sociodemographic Characteristics | ||
| Age | 18.66 (1.78) | 18.62 (1.69) |
| Race/Ethnicity | ||
| Latina | 59.36% (336) | 56.24% (320) |
| Black, Non-Latina | 34.10% (193) | 33.22% (189) |
| Other | 6.54% (37) | 10.54% (60) |
| Married or cohabitating with partner* | 45.60% (259.48) | 38.11% (215.72) |
| Living arrangements | ||
| With parent, siblings, or other relatives (without partner) | 52.53% (298.88) | 55.10% (311.88) |
| With partner (without parent, siblings, or other relatives) | 29.43% (167.48) | 23.13% (130.92) |
| With partner, and parent, siblings, or other relatives | 13.22% (75.24) | 17.11% (96.84) |
| With friends only | 2.02% (11.50) | 2.16% (12.20) |
| Alone | 2.79% (15.90) | 2.51% (14.20) |
| Clinical Characteristics | ||
| Pre-pregnancy body mass index | 24.78 (6.67) | 24.19 (6.31) |
| Nulliparous | 85.11% (484.28) | 86.55% (489.88) |
| Adverse pregnancy history | 10.96% (62.36) | 10.90 (61.68) |
| Sexually transmitted infection, during pregnancy | 17.75% (101.00) | 15.72% (89.00) |
| Received early prenatal care | 53.89% (306.68) | 56.12% (317.64) |
| Number of prenatal care visits** | 9.78 (3.32) | 8.97 (4.12) |
| Gestational age at delivery (days) | 272.89 (19.09) | 273.19 (15.85) |
| Preterm birth | 10.34% (58.84) | 10.25% (58) |
| Depression Characteristics (15-item version of CES-D) | ||
| Depressive symptoms | ||
| Baseline/Second trimester | 13.39 (8.60) | 12.54 (8.81) |
| Third trimester | 11.86 (8.73) | 11.26 (8.82) |
| 6 months postpartum | 11.00 (9.90) | 11.24 (9.31) |
| 12 months postpartum | 10.25 (9.09) | 11.14 (9.21) |
| Above cutoff (adjusted for 15-item version, CES-D ≥ 12) | ||
| Baseline/Second trimester | 52.08% (296.36) | 46.71% (264.36) |
| Third trimester | 47.32% (269.24) | 41.19% (233.12) |
| 6 months postpartum | 41.63% (236.88) | 40.93% (231.68) |
| 12 months postpartum | 36.34% (206.76) | 39.92% (225.92) |
Note. Ns presented have decimal places because data are based on pooled descriptives from imputed dataset. CES-D = Center for Epidemiologic Studies Depression Scale. Preterm birth is defined as <37 weeks gestation at delivery (or < 259 days).
p=0.01,
p<.001
Patterns of Missing Depressive Symptom Data
Note that 92% of adolescents originally enrolled in the study were included in the analytic sample (1135/1233) and 93% of adolescents had a baseline interview and at least one follow-up interview. Nonetheless, in this large community-based randomized controlled trial, there were missing data either as a function of a missed interview or because of refusal to answer individual questions. The six-month postpartum interview had the lowest participation rate with many participants re-engaged twelve months postpartum. Little’s Missing Completely at Random (MCAR) indicated that data were missing at random (X2= 235.27, p = .95). Patterns of missing depressive symptom data were examined with missingness defined as missing at least 20% of the scale (three out of 15 items), per recommendations by Radloff (1977). When examining whether participants who were missing depressive symptoms data at two or more timepoints (n=371) differed from other participants with regards to prenatal care type and the sociodemographic and clinical characteristics listed in Table 1, several significant associations emerged. Missingness was associated with living situation (χ2(4) = 18.33, p=.001, Cramer’s V = 0.13), nulliparity (χ2(1)=5.21, p=.023, Cramer’s V = −0.07), older age (OR = 1.13, p=.002), and lower gestational age at delivery (OR = 0.991, p=.008). Thus, multiple imputation was used to estimate missing values.
Changes in Perinatal Depressive Symptoms by Prenatal Care Type
Cases of probable depression (Table 1) decreased by 31% among pregnant adolescents who were at clinical sites randomized to CenteringPregnancy Plus (52% to 36%) compared to a 15% reduction among those at clinical sites randomized to individual prenatal care (47% to 40%). There was a significant Prenatal Care Type × Time interaction for depressive symptoms, F = 9.26, df (Satterthwaite’s approximation) = 816.08, p=.002, d = 0.21. Tests of the simple slopes revealed that both individual (b=−0.0033, SE=.00074, p<.001) and group (b=−0.0065, SE=.00075, p<.001) prenatal care participants experienced a significant decrease in depressive symptoms over time, but that the rate of change was greater for group care participants. Figure 2 illustrates simple slopes for the Prenatal Care Type × Time interaction. Follow-up tests indicated that the Prenatal Care Type × Time interaction was not significant from baseline to third trimester (p = .44), or baseline to six months postpartum (p = .11); however, it was significant from baseline to 12 months postpartum (p = .002).
Figure 2.
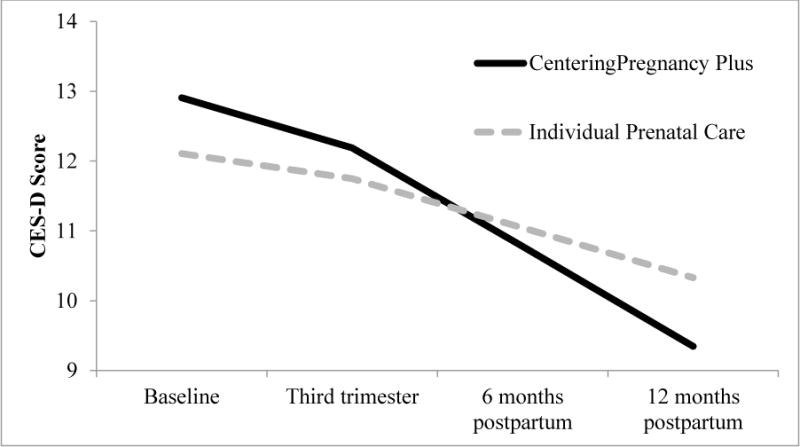
Simple slopes of the Prenatal Care Type × Time interaction for perinatal depressive symptoms. Prototypical time values were used to demonstrate the effect of prenatal care type on the rate of change in depressive symptoms over time (i.e., average number of days since baseline for each timepoint; Singer & Willett, 2003; Shek & Ma, 2011).
Psychological distress is higher among mothers of preterm versus term infants (Henderson, Carson, & Redshaw, 2016; Ionio et al., 2016) and rates of preterm birth may be lower among adolescents who receive group compared to individual prenatal care (Ickovics et al., 2007). Thus, improved depressive outcomes among group prenatal care participants may be due, in part, to their better birth outcomes. Post-hoc analyses were conducted to examine whether the effect of prenatal care type on depressive symptom reductions was attenuated after adjusting for gestational age at delivery. Results did not substantively change when adjusting for gestational age at delivery.
Depressive Symptoms as Predictor of Gestational Length
Gestational age at delivery
Linear regression models testing depressive symptoms as a predictor of gestational age at delivery, adjusting for control variables, are presented in the left panel of Table 2. Depressive symptoms during the second trimester of pregnancy did not significantly predict gestational age at delivery (adjusted R2 = 0.003). Higher depressive symptoms in the third trimester were significantly associated with shorter gestational age (adjusted R2 = 0.02). Each one standard deviation increase in third trimester depressive symptoms (SD = 7.64) was associated with a 2.33-day decrease in gestational age at delivery, holding all other variables constant. Although analyses of change indicated reductions in depressive symptoms over the perinatal period on average, some adolescents experienced increased depressive symptoms from second to third trimester (39% and 42% of group and individual prenatal care participants, respectively). Thus, we examined the effect of increased depressive symptoms on gestational age. Increased depressive symptoms from the second to third trimester of pregnancy were significantly associated with shorter gestational age (adjusted R2 = 0.02). Each one standard deviation increase in change in depressive symptoms (SD = 6.33) was associated with a 2.44-day decrease in gestational age at delivery, holding all other variables constant. No control variables were statistically significant predictors of gestational age at delivery. Effects of depressive symptoms on gestational age at delivery did not vary as a function of prenatal care type, as indicated by non-significant interactions between prenatal care type and depressive symptoms (ps > 0.05).
Table 2.
Linear and Logistic Regression Models Predicting Gestational Length
| Variable | Gestational Age
|
Preterm Birth
|
||
|---|---|---|---|---|
| B | SE | OR | 95% CI | |
| Second trimester depressive symptoms | −0.06 | 0.06 | 1.00 | 0.98, 1.03 |
| Third trimester depressive symptoms | −0.31** | 0.08 | 1.03* | 1.01, 1.06 |
| Residualized change in depressive symptoms | −0.39* | 0.11 | 1.05* | 1.01, 1.08 |
Note. Each model was adjusted for prenatal care type, maternal age, race/ethnicity, prepregnancy body mass index, nulliparity, adverse pregnancy history, and sexually transmitted infection. None of the control variables were statistically significant predictors in any model. Gestational age was measured in days. Preterm birth was defined as < 37 weeks.
p<.05,
p<.001
Preterm birth
Gestational length was examined categorically because of the clinical relevance of categorizing preterm birth (< 37 weeks gestation). The logistic regression models revealed a similar pattern of results (Table 2, right panel). For each one-point increase in third trimester depressive symptoms, the odds of preterm birth increase by a factor of 1.03, or 3%. For adolescents who scored one standard deviation above the mean on third trimester depressive symptoms (SD = 7.64), we would expect to see a 30% increase in the odds of preterm birth. Adjusting for the control variables and prenatal care type, increased depressive symptoms from the second to third trimester was associated with significant increased odds of having a preterm birth. For each one-point increase in depressive symptoms from second to third trimester, the odds of preterm birth increase by a factor of 1.05. For adolescents who scored one standard deviation above the mean on change in depressive symptoms (SD = 6.33), we would expect to see a 35% increase in the odds of preterm birth. No control variables were statistically significant predictors of preterm birth. The effects of depressive symptoms on preterm birth did not vary as a function of prenatal care type, as indicated by non-significant interactions between prenatal care type and depressive symptoms (ps > .05).
Discussion
Results from this cluster randomized controlled trial indicate that CenteringPregnancy Plus group prenatal care may be an effective non-pharmacological approach for reducing perinatal depressive symptoms among pregnant adolescents. Consistent with hypotheses, group prenatal care was associated with greater reductions in depressive symptoms compared to standard individual care. This effect was driven by group differences in depressive symptom reductions from baseline to 12 months postpartum. Findings are of public health relevance because maternal depression is associated with adverse consequences for offspring, ranging from social and emotional difficulties among toddlers to internalizing and externalizing problems among adolescents (Campbell, Morgan-Lopez, Cox, McLoyd, & NICHD Early Childcare Research Network, 2009; Guyon-Harris, Huth-Bocks, Lauterbach, & Janisse, 2016; Hammen & Brennan, 2003).
Findings provide further evidence of the high prevalence of depressive symptoms among perinatal adolescents (Ashby et al., 2016; Colletta, 1983). Approximately one-half of participants had symptom reports indicating probable depression at study entry. Cases decreased by 31% among adolescents who were at clinical sites randomized to group prenatal care, compared to only a 15% reduction among those at clinical sites randomized to individual prenatal care, but cases of probable depression did not differ significantly between prenatal care types at any timepoint.
Results raise important questions for future research about the mechanisms by which group prenatal care improves depressive symptoms. Post-hoc analyses indicated that reductions in depressive symptoms were not attenuated when controlling for gestational length. Participants may have benefited from the social support they received from their peers and health care providers within the group, and also from acquisition of skills designed to improve communication and manage conflict with their partners, family members, and friends. Previous research indicates that social support and conflict independently predict perinatal depressive symptoms (Westdahl et al., 2007). Interventions that similarly target interpersonal role disputes and interpersonal deficits, such as interpersonal psychotherapy, also improve perinatal depression symptoms (Field, Diego, Delgado, & Medina, 2013; Spinelli & Endicott, 2003). Adolescents who received CenteringPregnancy Plus also may have participated in more self-care activities and engaged in health-promoting behaviors associated with improved pregnancy outcomes. Participants learned stress reduction and affect regulation techniques, which may have reduced depressive symptoms. Because healthcare providers spent more time with their patients in group care, identification and referral for depression screening or treatment, and support for receiving ongoing care, may have occurred. Patients may have reduced stigma about perinatal depression and treatment. Finally, it is possible that participants experienced greater reductions in depressive symptoms because of the increased time of the intervention, peer support and contact with health care providers: up to 20 hours in group care versus approximately two hours in individual care. Elucidation of mechanisms will inform development of targeted approaches to yield more robust effects (e.g., greater attention to the role of stress in both the development of depression and risk for preterm birth) (Dunkel Schetter, 2011; Dunkel Schetter & Glynn, 2011; Hammen, 2005).
Approximately 1 in 10 adolescents delivered preterm in this study, slightly lower than prevalence rates among adolescents nationally and adults in New York City (National Center for Health Statistics; Ventura, Hamilton, & Matthews, 2014). This study extends the growing body of research linking depression and preterm birth among adults to adolescents. Findings support our second hypothesis of significant associations between depressive symptoms and gestational length at two of the three timepoints examined. Specifically, higher depressive symptoms during the third trimester of pregnancy, as well as increased symptoms from second to third trimester, were significantly associated with shorter gestational age and increased odds of preterm birth among adolescents. The associations between depressive symptoms and gestational length did not depend on prenatal care type.
The magnitude of effects of depressive symptoms on gestational length were small. Nevertheless, small effects can be meaningful, especially in the context of preventive medicine (Ellis, 2010). Even among term births (>37 weeks gestation), those that occur earlier are associated with increased risk for neonatal morbidity, mortality, and childhood infection, lower infant mental and cognitive development scores, and decreased infant brain maturation (Broekman et al., 2014; Espel, Glynn, Sandman, & Davis, 2014; Miller et al., 2016; Spong, 2013). In other words, when it comes to gestational age, “every day counts” (Seale, 2016).
Depressive symptoms in the second trimester were not associated with shorter gestational age or increased odds of preterm birth among adolescents in this study. Among adults, findings are mixed as to when during pregnancy depressive symptoms confer greatest risk for preterm birth. For example, Liou and colleagues (2016) found that depressive symptoms in the first trimester, but not second or third, were associated with significantly increased odds of preterm birth. In contrast, Venkatesh and colleagues (2016) found that second trimester depressive symptoms were associated with increased odds of preterm birth. Current results, if replicated, may have important clinical and public health implications about when to implement depression screening and intervention programs for pregnant adolescents to avert the impact of depression on gestational length. While there may be particular windows during pregnancy when there is a stronger impact of depression on birth outcomes, there is great individual variability in onset and duration of depression and rapidness of treatment response, making it important to screen adolescents throughout the perinatal period.
As with all studies, the current study has limitations. Medical records did not routinely record whether patients were referred for psychological assessment or treatment, and we did not have data on whether participants were taking anti-depressants or other psychoactive medications. There were several potential limits to generalizability. First, the focus on pregnant adolescents who were primarily racial/ethnic minorities limits generalizability to other patient populations. This vulnerable group is likely most in need for interventions to improve psychosocial, maternal and child health outcomes. Second, approximately one-half of participants received early prenatal care, defined as beginning care at or before 13 weeks gestation, higher than previous reports of pregnant adolescents (<6%; Lynch, Tumin, & Prasad, 2014). Third, many participants were missing depressive symptom data, particularly at six months postpartum, and it appears that these participants differed from other participants on some baseline sociodemographic characteristics. Finally, approximately 20% of participants at sites randomized to CenteringPregnancy Plus never attended any group prenatal care. One site randomized to deliver group prenatal care dropped out after recruiting 35 patients; these patients never had the opportunity to receive a session of group prenatal care, but were included in intention-to-treat analyses. Of the remaining 92 women who did not attend any group care sessions, 33 terminated care because of a fetal demise and 8 were lost to the health center before birth. The remaining 51 patients may have encountered scheduling difficulties or simply may have changed their minds after consent and scheduled individual care for the remainder of their pregnancy. Deeper engagement by the clinical sites may allow for better recruitment and retention of women in group prenatal care.
In conclusion, findings indicate that it is important to screen pregnant adolescents for depressive symptoms prior to the third trimester. Successful screening will depend in part on efforts to reduce late or no prenatal care among adolescents (Child Trends Databank, 2015). Even when detected, depression during pregnancy can be challenging to treat, given patient preferences to avoid medication, potential adverse consequences of antidepressant medication, and the complex individual and systemic barriers to accessing mental health services. Group prenatal care may be an effective non-pharmacological option for reducing depressive symptoms among pregnant adolescents that does not require additional appointments outside of prenatal care. Prior research indicates that group care results in an array of positive maternal and child health outcomes, with no adverse effects. Given the high prevalence of depressive symptoms among pregnant adolescents, results from these analyses support continued research and dissemination of group prenatal care.
Public Health Significance.
Depression is common in perinatal adolescents. Centering Pregnancy Plus group prenatal care reduced depressive symptoms across the perinatal period. Depressive symptoms were associated with shorter gestational age and preterm birth among adolescents.
References
- Abrams LS, Dornig K, Curran L. Barriers to service use for postpartum depression symptoms among low-income ethnic minority mothers in the United States. Qualitative Health Research. 2009;19(4):535–551. doi: 10.1177/1049732309332794. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics & American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 7th. 2012. Retrieved from. [Google Scholar]
- Ashby B, Ranadive N, Alaniz V, St John-Larkin C, Scott S. Implications of comprehensive mental health services embedded in an adolescent obstetric medical home. Maternal and Child Health Journal. 2016;20(6):1258–1265. doi: 10.1007/s10995-016-1927-y. [DOI] [PubMed] [Google Scholar]
- Assini-Meytin LC, Green KM. Long-term consequences of adolescent parenthood among African-American urban youth: A propensity score matching approach. Journal of Adolescent Health. 2015;56(5):529–535. doi: 10.1016/j.jadohealth.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: Prevalence, correlates, and treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54(1):37–44. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman BF, Wang C, Li Y, Rifkin-Graboi A, Saw SM, Chong YS, Group, G. S. Gestational age and neonatal brain microstructure in term born infants: a birth cohort study. Plos One. 2014;9(12):e115229. doi: 10.1371/journal.pone.0115229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SB, Morgan-Lopez AA, Cox MJ, McLoyd VC, NICHD Early Childcare Research Network A latent class analysis of maternal depressive symptoms over 12 years and offspring adjustment in adolescence. Journal of Abnormal Psychology. 2009;118(3):479–493. doi: 10.1037/a0015923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child Trends Databank. Late or no prenatal care. 2015 Retrieved from. [Google Scholar]
- Cohen J. Statistical power analysis for the behavior science. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Colletta ND. At risk for depression: A study of young mothers. Journal of Genetic Psychology. 1983;142:301–310. doi: 10.1080/00221325.1983.10533521. [DOI] [PubMed] [Google Scholar]
- Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods. 2001;6(4):330–351. doi: 10.1037//1082-989X.6.4.330. [DOI] [PubMed] [Google Scholar]
- Cunningham SD, Smith A, Kershaw T, Lewis JB, Cassells A, Tobin JN, Ickovics JR. Prenatal depressive symptoms and postpartum sexual risk among young urban women of color. Journal of Pediatric and Adolescent Gynecology. 2016;29(1):11–17. doi: 10.1016/j.jpag.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858. Artn Cd006309. [DOI] [PubMed] [Google Scholar]
- Detry MA, Lewis RJ. The intention-to-treat principle: How to assess the true effect of choosing a medical treatment. JAMA. 2014;312(1):85–86. doi: 10.1001/jama.2014.7523. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. Vol 62. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, Glynn LM. Stress in pregnancy: Empirical evidence and theoretical issues to guide interdisciplinary researchers. In: Contrada R, Baum A, editors. Handbook of Stress. New York, NY: Springer Publishing Company; 2011. pp. 321–343. [Google Scholar]
- Ellis PD. The essential guide to effect sizes: Statistical power, meta-analysis, and the interpretation of research results. Cambridge: University Press; 2010. [Google Scholar]
- Espel EV, Glynn LM, Sandman CA, Davis EP. Longer Gestation among Children Born Full Term Influences Cognitive and Motor Development. Plos One. 2014;9(11) doi: 10.1371/Journal.Pone.0113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A. A regression framework for effect size assessments in longitudinal modeling of group differences. Review of General Psychology. 2013;17(1):111–121. doi: 10.1037/a0030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Delgado J, Medina L. Peer support and interpersonal psychotherapy groups experienced decreased prenatal depression, anxiety and cortisol. Early Human Development. 2013;89(9):621–624. doi: 10.1016/j.earlhumdev.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn HA, O’Mahen HA, Massey L, Marcus S. The impact of a brief obstetrics clinic-based intervention on treatment use for perinatal depression. Journal of Womens Health. 2006;15(10):1195–1204. doi: 10.1089/jwh.2006.15.1195. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression - A systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106(5):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Goodman JH. Women’s attitudes, preferences, and perceived barriers to treatment for perinatal depression. Birth-Issues in Perinatal Care. 2009;36(1):60–69. doi: 10.1111/j.1523-536X.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of General Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon-Harris K, Huth-Bocks A, Lauterbach D, Janisse H. Trajectories of maternal depressive symptoms across the birth of a child: associations with toddler emotional development. Arch Womens Ment Health. 2016;19(1):153–165. doi: 10.1007/s00737-015-0546-8. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA. Severity, chronicity, and timing of maternal depression and risk for adolescent offspring diagnoses in a community sample. Arch Gen Psychiatry. 2003;60(3):253–258. doi: 10.1001/archpsyc.60.3.253. [DOI] [PubMed] [Google Scholar]
- Henderson J, Carson C, Redshaw M. Impact of preterm birth on maternal well-being and women’s perceptions of their baby: a population-based survey. BMJ Open. 2016;6(10):e012676. doi: 10.1136/bmjopen-2016-012676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Earnshaw V, Lewis JB, Kershaw TS, Magriples U, Stasko E, Tobin JN. Cluster randomized controlled trial of group prenatal care: Perinatal outcomes among adolescents in New York City health centers. American Journal of Public Health. 2016;106(2):359–365. doi: 10.2105/AJPH.2015.302960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Kershaw TS, Westdahl C, Magriples U, Massey Z, Reynolds H, Rising SS. Group prenatal care and perinatal outcomes - A randomized controlled trial. Obstetrics and Gynecology. 2007;110(2):330–339. doi: 10.1097/01.Aog.0000275284.24298.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Reed E, Magriples U, Westdahl C, Rising SS, Kershaw TS. Effects of group prenatal care on psychosocial risk in pregnancy: Results from a randomised controlled trial. Psychology & Health. 2011;26(2):235–250. doi: 10.1080/08870446.2011.531577. Pii 933381052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionio C, Colombo C, Brazzoduro V, Mascheroni E, Confalonieri E, Castoldi F, Lista G. Mothers and Fathers in NICU: The Impact of Preterm Birth on Parental Distress. Eur J Psychol. 2016;12(4):604–621. doi: 10.5964/ejop.v12i4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarde A, Morais M, Kingston D, Giallo R, MacQueen GM, Giglia L, McDonald SD. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: A systematic review and meta-analysis. JAMA Psychiatry. 2016;73(8):826–837. doi: 10.1001/jamapsychiatry.2016.0934. [DOI] [PubMed] [Google Scholar]
- Jeha D, Usta I, Ghulmiyyah L, Nassar A. A review of the risks and consequences of adolescent pregnancy. J Neonatal Perinatal Med. 2015 doi: 10.3233/NPM-15814038. [DOI] [PubMed] [Google Scholar]
- Jelliffe-Pawlowski LL, Baer RJ, Blumenfeld YJ, Ryckman KK, O’Brodovich HM, Gould JB, Currier RJ. Maternal characteristics and mid-pregnancy serum biomarkers as risk factors for subtypes of preterm birth. Bjog-an International Journal of Obstetrics and Gynaecology. 2015;122(11):1484–1493. doi: 10.1111/1471-0528.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutte DP, Roos NP, Brownell MD, Briggs G, MacWilliam L, Roos LL. The ripples of adolescent motherhood: Social, educational, and medical outcomes for children of teen and prior teen mothers. Academic Pediatrics. 2010;10(5):293–301. doi: 10.1016/j.acap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Kane JB, Morgan SP, Harris KM, Guilkey DK. The educational consequences of teen childbearing. Demography. 2013;50(6):2129–2150. doi: 10.1007/s13524-013-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw TS, Magriples U, Westdahl C, Rising SS, Ickovics J. Pregnancy as a window of opportunity for HIV prevention: Effects of an HIV intervention delivered within prenatal care. American Journal of Public Health. 2009;99(11):2079–2086. doi: 10.2105/AJPH.2008.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kleiber BV, Dimidjian S. Postpartum depression among adolescent mothers: A comprehensive review of prevalence, course, correlates, consequences, and interventions. Clinical Psychology-Science and Practice. 2014;21(1):48–66. doi: 10.1111/Cpsp.12055. [DOI] [Google Scholar]
- Kopelman RC, Moel J, Mertens C, Stuart S, Arndt S, O’Hara MW. Barriers to care for antenatal depression. Psychiatric Services. 2008;59(4):429–432. doi: 10.1176/Appi.Ps.59.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzi RG, Bert SC, Jacobs BK, Centers for the Prevention of Child, N. Depression among a sample of first-time adolescent and adult mothers. J Child Adolesc Psychiatr Nurs. 2009;22(4):194–202. doi: 10.1111/j.1744-6171.2009.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Stuart EA, Allison DB. Multiple imputation: A flexible tool for handling missing data. JAMA. 2015;314(18):1966–1967. doi: 10.1001/jama.2015.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou SR, Wang P, Cheng CY. Effects of prenatal maternal mental distress on birth outcomes. Women Birth. 2016 doi: 10.1016/j.wombi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Liu C, Cnattingius S, Bergstrom M, Ostberg V, Hjern A. Prenatal parental depression and preterm birth: a national cohort study. BJOG. 2016 doi: 10.1111/1471-0528.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post- 2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Tumin R, Prasad MR. Association Between Body Mass Index and the Timing of Pregnancy Recognition and Entry Into Prenatal Care. Obstetrics and Gynecology. 2014;124(5):911–918. doi: 10.1097/AOG.0000000000000516. [DOI] [PubMed] [Google Scholar]
- Massey Z, Rising SS, Ickovics J. CenteringPregnancy group prenatal care: Promoting relationship-centered care. Journal of Obstetric Gynecologic and Neonatal Nursing. 2006;35(2):286–294. doi: 10.1111/J.1552-6909.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Holt C, Holt CJ, Ross J, Ericksen J, Gemmill AW. Feasibility study and pilot randomised trial of an antenatal depression treatment with infant follow-up. Archives of Womens Mental Health. 2015;18(5):717–730. doi: 10.1007/s00737-015-0512-5. [DOI] [PubMed] [Google Scholar]
- Miller JE, Hammond GC, Strunk T, Moore HC, Leonard H, Carter KW, Burgner DP. Association of gestational age and growth measures at birth with infection-related admissions to hospital throughout childhood: a population-based, data-linkage study from Western Australia. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)00150-X. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. VitalStats - Births. Centers for Disease Control and Prevention; http://www.cdc.gov/nchs/vitalstats.htm. [Google Scholar]
- Pesonen AK, Lahti M, Kuusinen T, Tuovinen S, Villa P, Hamalainen E, Raikkonen K. Maternal prenatal positive affect, depressive and anxiety symptoms and birth outcomes: The PREDO Study. Plos One. 2016;11(2) doi: 10.1371/journal.pone.0150058. ARTN e0150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps MG, Raker CA, Ware CF, Zlotnick C. Randomized controlled trial to prevent postpartum depression in adolescent mothers. American Journal of Obstetrics and Gynecology. 2013;208(3) doi: 10.1016/j.ajog.2012.12.036. doi:ARTN 192.e110.1016/j.ajog.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkle CM, Sousa ACPD, Alvarado B, Zunzunegui MV, For the IMIAS Research Group Early maternal age at first birth is associated with chronic diseases and poor physical performance in older age: Cross-sectional analysis from the International Mobility in Aging Study. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Saccone G, Eke AC, Berghella V. Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and risk of preterm birth: a systematic review and metaanalysis. American Journal of Obstetrics and Gynecology. 2016;214(1):S443–S443. doi: 10.1111/1471-0528.14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RM, Wiemann CM, Rickert VI, Smith EO. Moderate to severe depressive symptoms among adolescent mothers followed four years postpartum. Journal of Adolescent Health. 2006;38(6):712–718. doi: 10.1016/j.jadohealth.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Seale AC. Every day and every gram counts at birth. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)00154-7. [DOI] [PubMed] [Google Scholar]
- Shek DTL, Ma CMS. Longitudinal data analyses using linear mixed models in SPSS: Concepts, procedures and illustrations. The scientific world journal. 2011;11:42–76. doi: 10.1100/tsw.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Siu AL, Force, U. S. P. S. T. Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Pignone MP. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380–387. doi: 10.1001/jama.2015.18392. [DOI] [PubMed] [Google Scholar]
- Sockol LE, Epperson CN, Barber JP. Preventing postpartum depression: A meta-analytic review. Clinical Psychology Review. 2013;33(8):1205–1217. doi: 10.1016/J.Cpr.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. American Journal of Psychiatry. 2003;160(3):555–562. doi: 10.1176/appi.ajp.160.3.555. [DOI] [PubMed] [Google Scholar]
- Spong CY. Defining “term” pregnancy recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309(23):2445–2446. doi: 10.1001/jama.2013.6235. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Thomas N, Komiti A, Judd F. Pilot early intervention antenatal group program for pregnant women with anxiety and depression. Archives of Womens Mental Health. 2014;17(6):503–509. doi: 10.1007/s00737-014-0447-2. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Health Resources and Services Administration, & Maternal and Child Health Bureaus. Child Health USA 2013. Rockville, MD: 2013. Retrieved from. [Google Scholar]
- United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), & Division of Vital Statistics. Natality public-use data 2007–2014. 2016 Retrieved from CDC WONDER Online Database: http://wonder.cdc.gov/natality-current.html.
- Venkatesh KK, Riley L, Castro VM, Perlis RH, Kaimal AJ. Association of antenatal depression symptoms and antidepressant treatment with preterm birth. Obstetrics and Gynecology. 2016;127(5):926–933. doi: 10.1097/AOG.0000000000001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura SJ, Hamilton BE, Matthews TJ. National and state patterns of teen births in the United States, 1940–2013. Natl Vital Stat Rep. 2014;63(4):1–34. [PubMed] [Google Scholar]
- Westdahl C, Milan S, Magriples U, Kershaw TS, Rising SS, Ickovics JR. Social support and social conflict as predictors of prenatal depression. Obstetrics and Gynecology. 2007;110(1):134–140. doi: 10.1097/01.Aog.0000265352.61822.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Sit DKY, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, Singer LT. Major depression and antidepressant treatment: Impact on pregnancy and neonatal outcomes. American Journal of Psychiatry. 2009;166(5):557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]


