Abstract
A growing literature exists on neural correlates of treatment outcome. However, different types - or components of - treatment have distinct theorized mechanisms of action. And, it is not yet known how changes in neural activity across treatment relate to engagement in different treatment components. Participants with cocaine-use disorders in a randomized clinical trial received cognitive behavioral therapy (CBT) plus, in a 2×2 design, contingency management (CM) or no-CM, and disulfiram or placebo. Participants performed an fMRI Stroop Task, a measure of cognitive-control, at the beginning of and after the 12-week treatment. Analyses assessed changes in Stroop-related neural activity within the sample overall and assessed how changes in Stroop-related activity correlated with measures of treatment process specific to each form of treatment (i.e., participation in CBT sessions, receipt of CM prizes, administration of disulfiram pills). Within the sample overall, compared to beginning-of-treatment, post-treatment Stroop-related neural activity was diminished in hippocampus, thalamus, cingulate, precentral, post/precentral gyrus, and precuneus and culmen regions (pFWE<.05). In separate whole-brain correlation analyses, greater reductions in Stroop-related activity were associated with more treatment engagement: ‘CBT sessions’ with precentral gyrus, inferior parietal lobule, middle and medial frontal gyrus; ‘CM prizes’ with postcentral frontal gyrus. Disulfiram ‘medication days’ were not associated with changes in Stroop-related activity. Findings suggest key process indicators of CBT and CM may be associated with functional changes in cognitive-control-related neurocircuitry.
Keywords: fMRI, cognitive behavioral therapy, contingency management, disulfiram, cognitive-control
Introduction
Cocaine-use disorder (CUD) is associated with significant societal cost. Even with the best available treatments, many individuals with CUD cannot achieve or maintain abstinence (SAMHSA, 2014), highlighting the need for advancements in treatments. An understanding of the mechanism of action of treatments, including neurocognitive mechanisms, could lead to improvements in existing treatments or development of new treatments (Chung et al., 2016; Insel & Gogtay, 2014). The aim of the current study is to make a preliminary attempt to relate changes in functional neural activity from beginning-of-treatment- to post-treatment to engagement with different components of treatment for cocaine use. The treatment offered in this study was a mix of cognitive behavioral therapy (CBT), contingency management (CM) and the medication disulfiram.
The treatments offered in this study are thought to have complementary clinical strengths, to address different aspects of substance use disorders (SUDs) and are hypothesized to have different mechanisms of actions. Combining several such treatments is widely accepted to improve clinical outcomes (Anton et al., 2006; NIDA, 2012; Sammons & Schmidt, 2001). For example, CBT (Carroll et al., 2004; Carroll, Nich, Ball, McCance, & Rounsavile, 1998), CM (Petry et al., 2005) and disulfiram (Carroll et al., 2004; Carroll et al., 1998) have each demonstrated enhancements in treatment efficacy relative to standard care alone, behavioral treatment controls, or placebo conditions in randomized clinical trials, and are thought to have complementary strengths and mechanisms. Specifically, CBT promotes abstinence by enhancing behavioral and cognitive coping skills as well as functional analyses of factors that contribute to continued drug use (Carroll, 1998). A strength of CBT is its durability (i.e., persistent efficacy after treatment ceases), perhaps due to the focus on skill-building and greater cognitive-control over craving and drug-use behaviors (Carroll, Nich, Ball, et al., 2000). CM’s strengths and limitations are complementary to CBT’s. CM promotes treatment adherence and abstinence initiation by reinforcing target behaviors (e.g., treatment attendance, medication administration, cocaine abstinence) with money or prizes (Petry et al., 2005). As such, CM is a useful adjunct to enhance treatment adherence as well as initiate abstinence. However, CM’s effects tend to weaken to some extent after the reinforcement schedule has ended. While no pharmacotherapies have FDA-indication for the treatment of cocaine-use disorder, disulfiram has shown promise in some trials (Carroll et al., 2004; Carroll, Nich, Ball, et al., 2000; Carroll, Nich, Shi, Eagan, & Ball, 2012; George et al., 2000; Kosten et al., 2013; Petrakis et al., 2000; Shorter et al., 2013). Although the mechanism underlying improved cocaine-use outcomes with disulfiram is unknown, several potential mechanisms have been proposed (Barth & Malcolm, 2010; Carroll et al., 2004; Gaval-Cruz & Weinshenker, 2009) such as increasing plasma levels of dopamine by slowing the conversion of dopamine into noradrenaline via disulfiram’s inhibition of dopamine- β-hydroxylase (DβH) (Karamanakos, Pappas, Stephanou, & Marselos, 2001; Vaccari, Saba, Ruiu, Collu, & Devoto, 1996), reducing alcohol-precipitated relapses by inducing aversive responses to alcohol (Jorgensen, Pedersen, & Tonnesen, 2011) and altering cocaine’s subjective reinforcing effects (Baker, Jatlow, & McCance-Katz, 2007).
One step towards investigating neurocognitive mechanisms of treatments for cocaine use could be to incorporate functional magnetic resonance imaging (fMRI) measures into clinical trials for CUD. The specific fMRI task chosen should tap a specific cognitive domain, such as cognitive control, related to CUD. Disrupted cognitive-control has been proposed to contribute to initiation and maintenance of CUD through poor attentional control, attentional biases towards drug-related stimuli, impaired response inhibition, and reduced ability to regulate craving (Garavan, Brennan, Hester, & Whelan, 2013; Garavan & Hester, 2007; Goldstein & Volkow, 2011; Lopez, Onyemekwu, Hart, Ochsner, & Kober, 2015). However, the pattern of drug-induced alterations in cognitive-control-related-activity in SUDs is somewhat complex. Specifically, individuals with stimulant-use disorders differ from non-substance-using individuals on task-related functional activity in a manner that is task- and process-specific; that is, the direction of difference (hypo- versus hyper-activation) and affected anatomical regions vary by task (for review (Aron & Paulus, 2007; Crunelle, Veltman, Booij, Emmerik-van Oortmerssen, & van den Brink, 2012)). For example, cognitive-control-related neural activity differs between active cocaine users and healthy comparison groups. Two separate studies comparing short-term abstinent (≤72 hrs.) cocaine users with healthy controls showed relatively decreased activity in cocaine users (N=15) in anterior cingulate and right prefrontal regions and relatively increased activity in cerebellar regions while performing a go/no-go task with a working memory component (Hester & Garavan, 2004), while cocaine users (N=13) performed worse on a go/no-go task and showed relatively lower activity in the anterior cingulate and right insula during successful stops and greater recruitment of medial frontal gyrus, left insula and left inferior frontal gyrus (IFG) during errors (Kaufman, Ross, Stein, & Garavan, 2003). Individuals with CUD (N=14) with confirmed short-term abstinence (>72 hrs.), performing a multi-sensory Stroop task, showed greater recruitment of left dorsolateral and ventrolateral PFC (dlPFC, vlPFC) and bilateral basal ganglia and less deactivation of bilateral ventromedial PFC (vmPFC), relative to non-users (N=16) (Mayer, Wilcox, Teshiba, Ling, & Yang, 2013).
In addition, cognitive-control-related neural activity has been shown to be altered by acute cocaine administration (e.g., (Garavan, Kaufman, & Hester, 2008)) and may vary across abstinence duration (e.g.,(Connolly, Foxe, Nierenberg, Shpaner, & Garavan, 2012; Garavan et al., 2013)). Finally, impairments on cognitive-control-related neuropsychological tasks may remain following abstinence (van Holst & Schilt, 2011). This may be consistent with some irreversible substance-use-related neural insult, or with pre-morbid vulnerabilities in brain systems (which may have preceded substance use) remaining following prolonged cessation of substance use. Taken together, these findings highlight the sensitivity of cognitive-control-related neural activity to CUD, cocaine use, and cocaine abstinence, but also illustrate the complexity of interpreting findings. In turn, this underscores the relevance of investigating these effects in the context of treatment.
Few studies have applied neuroimaging in the context of treatment for SUDs (for review see (Zilverstand, Parvaz, Moeller, & Goldstein, 2016)), and several of those have demonstrated relationships between pre-treatment cognitive-control-task-related brain activations and clinical outcomes (e.g., substance use during treatment or follow-up). For example, Color-Word-Stroop-related activity in CUD individuals (N=20) initiating a range of treatments (sample combined from several randomized controlled trials (RCTs)) was associated with treatment outcome, such that greater pre-treatment Stroop-related activity in the vmPFC, left posterior cingulate cortex and right striatum correlated with longer duration of continuous abstinence during treatment (Brewer, Worhunsky, Carroll, Rounsaville, & Potenza, 2008). In addition, higher pre-treatment Stroop-related activity in the striatum correlated with higher percent of drug-free urines during treatment, and lower pre-treatment Stroop-related activity in the dlPFC correlated with longer treatment retention (Brewer et al., 2008). In another study, males with cannabis use disorder (N=20) scanned prior to CBT and/or CM treatment showed diminished Stroop-related activity in regions including dlPFC and ventral striatum relative to non-users (N=20) (Kober, DeVito, DeLeone, Carroll, & Potenza, 2014). Furthermore, higher pre-treatment Stroop-related activity in dorsal anterior cingulate cortex (ACC) and ventral striatum was associated with less cannabis use during treatment and 1-year follow-up, respectively (Kober et al., 2014). In individuals with CUD assessed at the beginning of inpatient detoxification treatment, higher right dorsal ACC activity during an attentional bias task (Drug Stroop; N=34;(Marhe, Luijten, van de Wetering, Smits, & Franken, 2013)) and lower error-related negativity (an electrophysiological measure of cognitive control; N=49;(Marhe, van de Wetering, & Franken, 2013)) were each associated with more cocaine use at 3-month follow-up. In sum, this research relating pre-treatment functional neural activity to drug use outcomes is important, but, it does not directly address changes across treatment or relationships between neural activity and exposure to different treatment components.
A limited body of work has assessed changes in fMRI activity across treatment in substance users. One prior study from our group assessed change across behavioral treatment in a mixed substance-using outpatient sample (N=12), receiving CBT or treatment as usual (DeVito et al., 2012). We found reduced Stroop-related activity after treatment relative to the beginning-of-treatment, in regions including the thalamus, midbrain, subthalamic nucleus, IFG, MFG and ACC(DeVito et al., 2012). In that study, the sample size and study design limited our ability to relate changes in neural activity to components of treatment received. However, exploratory analyses within the ROIs showing significant Stroop-related change across treatment suggested associations between greater reductions in Stroop-related activity (in left middle temporal gyrus and right MFG ROIs) and more exposure to CBT sessions (Supplemental Materials in (DeVito et al., 2012)). In another study in CUD, increases in Drug-Stroop-related activity (a measure of attentional bias to drug stimuli) in the midbrain from baseline to 6-month follow-up (N=15) was associated with fewer choices to view cocaine-related stimuli at follow-up (Moeller et al., 2012); however, since subjects were recruited from a range of treatment programs, the relative contribution of differential treatment components or types were not assessed.
Studies that have linked functional changes in stimulant users with specific pharmacotherapy have included laboratory one-day trials of the medication, rather than full clinical courses. For example, changes in Color-Word-Stroop-related activity were observed following administration of a single dose of methylphenidate (versus placebo). In individuals with CUDs (N=16) and non-users (N=15), methylphenidate reduced Stroop-related activity in dorsal ACC and also reduced activity in dlPFC in the CUD group only (Moeller et al., 2014). Individuals with methamphetamine use disorders (N=15) exhibited increased Stroop-related activity, including in dlPFC, parietal and occipital regions relative to non-users (N=18), and methylphenidate further increased already hyperactive (relative to controls) Stroop-related activity in dlPFC, but reduced activity in parietal and occipital regions (Jan et al., 2014). These findings suggest that the impact of treatment on Stroop-related activity may vary across different substance using and healthy comparison groups. Taken together the existing research supports the clinical relevance of cognitive-control-related functional activity by demonstrating its associations with treatment outcome and ability to change in response to treatment for SUDs. Focusing on changes over the treatment course that relate to exposure to components of specific treatments is a novel approach to understanding mechanisms of action (Morgenstern, Naqvi, Debellis, & Breiter, 2013). This is important because effective therapies are hypothesized to change substance use by first changing brain-based cognitive processes related to substance use, including cognitive control. Prior work has demonstrated the efficacies of CBT, CM, disulfiram or combinations of these treatments on reducing cocaine use in some individuals (Benishek et al., 2014; Dutra et al., 2008; Pani et al., 2010). However, no studies have directly investigated i) how changes in cognitive-control-related neural functioning over treatment may be associated with exposure to different treatment components; and, ii) whether these treatment-component-related changes are distinct from functional brain changes associated with cocaine use outcomes.
The current study’s goal was to extend prior work and relate exposure to different components of treatment to changes in cognitive-control-related fMRI activity from beginning-of-treatment to post-treatment, in individuals with CUD. The Color-Word Stroop color-word task, a measure of cognitive control, was chosen based on the proposed centrality of this cognitive construct to SUDs and abstinence. Data were drawn from participants in an RCT, all of whom received CBT as a platform treatment. Participants were also randomized to CM vs. no-CM and to disulfiram vs. placebo. The primary goal of the analyses was to identify how exposure to components of treatment was associated with changes in cognitive-control-related brain activity across treatment. First, we used a whole-brain analysis to assessed how Stroop-related brain activity changed at post-treatment versus beginning-of-treatment in the sample overall. Second, we used separate whole brain correlation analyses to assess how changes in Stroop-related brain activity at post-treatment versus beginning-of-treatment related to specific treatment components (number of CBT sessions, CM prizes or days of disulfiram medication) and cocaine-use measures (percent days cocaine abstinence during treatment; percent cocaine-negative urines during treatment). Based on our prior work reviewed above in a mixed substance-using sample receiving CBT or treatment as usual which showed reduced Stroop-related activity at post- relative to pre-treatment in the thalamus, midbrain, subthalamic nucleus, IFG, MFG and ACC (DeVito et al., 2012), we hypothesized that patients would show diminished Stroop-related brain activity at post-treatment relative to beginning-of-treatment in these regions. We hypothesized that greater reductions in Stroop-related activity would be observed in association with exposure to active ingredients of each treatment type as operationalized by process indicators as follows (see Table 1): (1) exposure to CBT skills training (CBT sessions attended), (2) access to reinforcement through CM (CM prizes drawn for abstinence or adherence to medication) and (3) exposure to disulfiram (total days of medication doses taken). Finally, we hypothesized, based on the structure and goals of the respective treatments (Potenza, Sofuoglu, Carroll, & Rounsaville, 2011), that CBT engagement specifically would be associated with greater efficiency (as indicated by lower Stroop-related activity at post-treatment vs. beginning-of-treatment) in regions associated with ‘top-down’ regulatory control (e.g., ACC, IFG, MFG), and that CM engagement (i.e., randomization to CM group; more CM prizes drawn) would be associated with greater efficiency in regions which are involved in ‘bottom-up’ circuitry (i.e., regions implicated in reward-processing and valuation, including striatum, ventromedial prefrontal cortex) which are also engaged by Stroop and other cognitive-control tasks.
Table 1.
Definition of Treatment Engagement Variables
| Treatment Component | Treatment Engagement Variable | Operational Definition of Treatment Variable |
|---|---|---|
| CBT Exposure | CBT Sessions | Number of CBT sessions attended during the 12-week treatment protocol. All subjects were invited to attend one 50-min CBT sessions per week during the 12 week protocol (i.e., total of up to 12 CBT sessions). CBT sessions were held individually with a clinician trained in CBT. Subjects could still remain active in the RCT if they failed to attend CBT sessions, so CBT sessions are not fully predicted by the number of weeks in treatment. |
| CM Exposure | CM Prizes | Number of CM prizes drawn during the RCT protocol, within the group randomized to CM treatment. Prizes were awarded for submission of urines as scheduled (thrice weekly) that were negative for cocaine and in-person observed administration of study pills as scheduled (thrice weekly). The number of prize draws increased for consecutive cocaine-negative urines and pill adherences and decreased following failure to provide a scheduled cocaine-negative urine sample (see Methods and Supplemental Materials for details). |
| Disulfiram Exposure | Disulfiram Medication Days | Number of days of observed (during in person visit days) or self-reported (during take-home pill days) adherence to the medication protocol, within individuals randomized to disulfiram. During thrice-weekly sessions, subjects were observed taking their assigned pills and were given take-home doses of medications and asked to self-report their adherence to prior take-home medication days since last appointment. |
Note. CBT: Cognitive Behavioral Therapy; CM: Contingency Management; RCT: Randomized Clinical Trial.
Materials and Methods
Participants
Treatment-seeking participants were recruited to the fMRI study prior to treatment randomization in an RCT for CUD (Carroll et al., 2016). Participants were 18 years or older, recruited from a community-based outpatient treatment center, met DSM-IV criteria for current cocaine dependence and did not meet current dependence criteria for other illicit drugs. Other exclusion criteria included lifetime psychotic or bipolar disorder, current suicidal or homicidal ideation or current medical condition that would contraindicate disulfiram treatment (e.g. hepatic or cardiac problems, hypertension, pregnancy). RCT participants were offered participation in the fMRI component if they did not report claustrophobia, colorblindness, history of severe head trauma with loss of consciousness, or metallic implants contraindicated in MRI. Of 99 RCT participants, 35 completed fMRI scans at in the beginning-of-treatment and following treatment. 26 of whom were included in the current fMRI analyses (N=9 excluded due to delayed timing of scans relative to beginning-of-treatment or post-treatment, or to insufficient treatment exposure; for details see Supplementary Materials). This study was approved by a human subjects Institutional Review Board and participants provided written informed consent prior to participation.
Treatment and Clinical Assessments
RCT methods are reported in full elsewhere (Carroll et al., 2016). Briefly, treatment lasted 12 weeks, and all participants were offered CBT. In addition, all participants included in the fMRI analyses were randomized to either disulfiram (N=10) or placebo (N=16) and CM (N=14) or no-CM (N=12) in a factorial design, resulting in four treatment conditions: 1) CBT+ CM + disulfiram (N=4), 2) CBT + CM + placebo (N=10), 3) CBT + disulfiram (N=6), 4) CBT + placebo (N=6). Participants were asked to attend the clinic three times per week during the 12-week protocol; medication was dispensed and urine specimens were collected at each clinic visit. All in-person treatment delivery, tracking of treatment adherence and tracking monitoring of substance use occurred in those thrice-weekly visits. Due to the limited sample sizes for the four treatment cells within the fMRI sample, analyses focused on the entire sample or comparing disulfiram versus placebo, or CM versus no-CM.
Cognitive Behavioral Therapy (CBT)
Weekly 50-minute individual CBT sessions were offered as per the CBT manual (Carroll, 1998), delivered by doctoral-level clinicians with CBT experience and demonstrated competence (Carroll, Nich, Sifry, et al., 2000). The primary process indicator for CBT was number of CBT sessions attended (see Table 1). CBT aims to promote abstinence by teaching and promoting practice of behavioral and cognitive control strategies (e.g., coping with craving, improving decision making skills). CBT homework, assigned at each session, offers the opportunity to practice applying the skills discussed in CBT sessions.
Contingency Management (CM)
All participants were randomly assigned to receive contingency management (CM group) or not (no-CM group). Those assigned to CM could draw at least one prize chance from a bowl each time they demonstrated abstinence (submitted a cocaine-free urine specimen) or pill adherence (staff witnessed ingestion of study capsule). Consistent with previously established procedures, the number of CM draws per reinforced behavior (abstinence, pill adherence) escalated (up to a maximum of 7 draws) with each consecutive demonstration of abstinence or adherence. If patients missed a scheduled visit or failed to submit a cocaine-negative urine, the number of prize draws for subsequent reinforced behaviors would drop back down to one (for CM methods details, see Supplementary Methods and (Carroll et al., 2016; Petry, 2000; Petry et al., 2005). The primary process indicator for exposure to CM in these analyses was the sum of total prizes drawn across treatment (‘number of CM prizes’; Table 1).
Disulfiram
All participants were randomly assigned to either disulfiram (250 mg daily) or identical placebo capsules, administered in a double-blind manner. This disulfiram dose was associated with reduced cocaine use in previous trials (Carroll et al., 2004). Medication or placebo pill adherence was tracked by observed medication administration at thrice-weekly visits, plus patient self-report for take-home doses. This combination of observed and self-reported adherence was used as the primary process indicator (‘days of medication’; Table 1) for disulfiram treatment within the group randomized to disulfiram. A riboflavin tracer in the pills indicated high consistency with self-report (Carroll et al., 2016). All participants were warned of negative consequences of drinking alcohol on disulfiram, strongly discouraged from drinking alcohol during the study, and told that their capsules would be withheld if their breath samples tested positive for alcohol.
Substance-use assessments
Baseline assessments included the Addiction Severity Index (ASI; (McLellan et al., 1992). Thrice weekly during treatment, participants were assessed with urine toxicology and alcohol-breath screens, had study capsules dispensed (disulfiram or placebo) and clinical symptoms monitored. Self-reports of day-by-day use of cocaine, alcohol, and other drugs were collected weekly during treatment, using the Timeline Followback method (Robinson, Sobell, Sobell, & Leo, 2014; Sobell & Sobell, 1992). When sessions were missed or the participant did not complete treatment, self-report data were collected for the missed data-collection days at subsequent sessions. Adverse events and blood pressure were tracked weekly during treatment. Consistent with our prior work (Carroll et al., 2014; Carroll et al., 2016), primary clinical outcome variables were percent of cocaine-negative urines and self-reported days of abstinence during treatment.
Clinical Data Analyses
Indicators of cocaine use within-treatment were assessed with ANOVAs including medication (disulfiram, placebo) and CM (CM, no-CM) as between-subject factors (see Table 2). Clinical outcomes for the parent RCT sample are reported elsewhere (Carroll et al., 2016). Briefly, there were consistent effects favoring CM over no-CM, with mixed findings for disulfiram.
Table 2.
Baseline demographic, psychiatric, and substance-use characteristics and Stroop behavior by treatment group
| Total Sample (n=26) |
Contingency Management (n=14) | No Contingency Management (n=12) | Statistics (F(p)) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Placebo (n=10) | Disulfiram (n=4) | Placebo (n=6) | Disulfiram (n=6) | CM Status | Disulfiram Status | CM Status × Disulfiram Status | ||
|
| ||||||||
| Demographics | ||||||||
| Sex, N (%) Female | 10 (38.5) | 3 (30.0) | 3 (75.0) | 2 (33.3) | 2 (33.3) | 0.02 (0.89) | 0.00 (1.00) | 1.14 (0.29) |
| Age | 40.27 (7.55) | 41.00 (6.90) | 42.00 (2.94) | 38.33 (11.26) | 39.83 (7.71) | 0.54 (0.47) | 0.15 (0.71) | 0.01 (0.94) |
| Race, N (%) | ||||||||
| Caucasian | 10 (38.5) | 2 (20.0) | 2 (50.0) | 3 (50.0) | 3 (50.0) | |||
| African-American | 12 (46.2) | 6 (60.0) | 2 (50.0) | 2 (33.3) | 2 (33.3) | |||
| Hispanic | 2 (7.7) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (16.7) | |||
| Multiracial/Other | 2 (7.7) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Estimated IQ (SILS) | 97.80 (10.77) | 98.33 (10.33) | 93.25 (8.77) | 96.33 (13.84) | 101.50 (10.60) | 0.30 (0.59) | 0.02 (0.88) | 2.12 (0.16) |
| Number of months incarcerated, lifetime | 25.38 (52.88) | 37.20 (65.94) | 3.00 (6.00) | 5.83 (11.94) | 40.17 (67.75) | 0.02 (0.90) | 0.00 (0.99) | 2.40 (0.14) |
| Other Lifetime Psychiatric Diagnoses, N(%) | ||||||||
| Alcohol-Use Disorder | 16 (61.5) | 5 (50.0) | 3 (75.0) | 4 (66.7) | 6 (100.0) | 0.42 (0.52) | 0.00 (1.00) | 0.00 (1.00) |
| Major Depression | 7 (26.9) | 7 (70.0) | 0 (0.0) | 1 (16.7) | 3 (50.0) | 0.35 (0.56) | 1.39 (0.24) | 0.00 (1.00) |
| Anxiety Disorder | 0(0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) |
| Antisocial-Personality Disorder | 2 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) |
| Days of Use in Month Prior to Treatment1 | ||||||||
| Cocaine | 14.88 (7.80) | 13.50 (7.69) | 17.50 (8.81) | 16.83 (8.95) | 13.50 (7.40) | 0.01 (0.92) | 0.01 (0.92) | 1.20 (2.90) |
| Alcohol | 7.35 (8.38) | 5.80 (7.22) | 14.25 (11.90) | 6.67 (9.75) | 6.00 (5.62) | 1.15 (0.30) | 1.28 (0.27) | 1.75 (0.20) |
| Cannabis | 2.12 (5.55) | 0.40 (0.97) | 7.00 (14.00) | 2.67 (2.66) | 1.17 (1.17) | 0.64 (0.43) | 1.31 (0.26) | 3.31 (0.08) |
| Cigarettes | 20.73 (11.89) | 18.80 (13.21) | 21.00 (14.00) | 23.33 (11.43) | 21.17 (11.36) | 0.21 (0.66) | 0.00 (1.00) | 0.18 (0.68) |
| Lifetime Years of Regular Use | ||||||||
| Cocaine | 9.31 (6.18) | 7.60 (4.25) | 10.75 (6.99) | 9.33 (8.89) | 11.17 (6.18) | 0.17 (0.69) | 089 (0.36) | 0.06 (0.81) |
| Alcohol | 8.69 (9.01) | 3.90 (4.95) | 9.00 (8.60) | 13.67 (11.60) | 11.50 (9.85) | 3.02 (0.10) | 0.17 (0.68) | 1.01 (0.31) |
| Cannabis | 5.81 (7.38) | 2.30 (3.37) | 1.50 (2.38) | 7.17 (6.37) | 13.17 (10.05) | 10.65 (0.004) | 1.05 (0.32) | 1.80 (0.19) |
| Treatment Engagement | ||||||||
| Days in Treatment | 59.77 (30.22) | 44.60 (29.14) | 80.50 (7.00) | 49.67 (38.51) | 81.33 (6.53) | 0.07 (0.79) | 9.54 (0.005) | 0.04 (0.85) |
| Number of CBT Sessions Attended | 6.73 (4.28) | 5.20 (4.24) | 8.25 (2.87) | 6.17 (5.42) | 8.83 (3.55) | 0.20 (0.66) | 2.66 (0.12) | 0.01 (0.91) |
| Number of Days of Disulfiram Doses Taken | N/A | N/A | 39.70 (25.81) | N/A | 45.00 (34.18) | 0.13 (0.73) | N/A | N/A |
| Number of CM Prizes Drawn | N/A | 82.30 (88.87) | 120.50 (84.03) | N/A | N/A | N/A | 0.54 (0.48) | N/A |
| Treatment Outcome | ||||||||
| % Days Self-reported Abstinence Within Treatment Period | 81.50 (22.50) | 92.62 (7.48) | 59.23 (45.00) | 76.39 (19.39) | 82.94 (12.73) | 0.19 (0.66) | 2.50 (0.13) | 5.54 (0.03) |
| % Urines Negative for Cocaine Within Treatment Period | 44.87 (39.78) | 67.74 (34.19) | 35.64 (45.24) | 16.50 (27.53) | 41.29 (41.55) | 2.30 (0.14) | 0.06 (0.81) | 3.58 (0.07) |
| Stroop Task Behavior2 | ||||||||
| Beginning-of-Treatment | ||||||||
| Mean Congruent Trial RT (ms) | 508.88 (124.40) | 537.27 (120.59) | 436.51 (125.42) | 506.07 (121.09) | 521.59 (157.83) | . | . | . |
| Mean Incongruent Trial RT (ms) | 637.30 (208.80) | 691.27 (209.99) | 574.74 (221.48) | 597.74 (187.61) | 642.76 (304.05) | . | . | . |
| Percent Incongruent Errors | 22.58 (19.64) | 28.14 (26.12) | 15.71 (16.41) | 23.33 (19.24) | 17.14 (5.71) | . | . | . |
| Post-Treatment | ||||||||
| Mean Congruent Trial RT (ms) | 517.27 (132.25) | 477.15 (113.64) | 463.05 (53.16) | 560.90 (137.53) | 577.23 (177.49) | . | . | . |
| Mean Incongruent Trial RT (ms) | 639.87 (211.44) | 593.14 (210.29) | 547.90 (116.71) | 701.69 (240.86) | 719.79 (241.08) | . | . | . |
| Percent Incongruent Errors | 23.10 (23.02) | 30.21 (33.00) | 15.00 (15.36) | 18.57 (8.62) | 21.19 (17.06) | . | . | . |
Results are reported as mean (SD) unless otherwise noted, in which case it is reported as N (percent)
SILS=Shipley Institute of Living Scale; RT= response time; ms =milliseconds
Days of use in month prior to treatment was out of a possible total of 28 days (i.e., past four weeks)
Stroop Task Behavior analysed with mixed model ANOVAs including session (beginning-of-treatment, post-treatment) and trial type (incongruent, congruent) as within-subject factors and medication (disulfiram, placebo) and CM (CM, no-CM) conditions as between-subject factors (Trial type (F=58.99, p<.001); no other significant main or interactive effects). Stroop errors on incongruent trials were analyzed with mixed-model ANOVAs including session as a within-subject factor and medication and CM conditions as between-subject factors (no significant main or interactive effects).
fMRI Methods
Participants were administered a measure of cognitive-control, the event-related fMRI Color-Word Stroop task (DeVito et al., 2012; Kober et al., 2014), on two occasions: prior to or in the beginning-of-treatment (days between start of study treatment and ‘beginning-of-treatment’ scan: M=3 days, SD=5, range= 6 days prior to treatment start −12 days into treatment) and following the end of the 12-week treatment (i.e., post-treatment and prior to 3-month follow-up (days between end of treatment and post-treatment scan: M=20 days, SD=20, range=1–61 days). On each trial, participants were asked to name the ink color of color-words presented in congruent (e.g., “RED” in red ink) or incongruent colors (e.g., “RED” in blue ink; see Supplemental Methods for task details).
Stroop mean response times, collected out-of-scanner at time of scanning, were analyzed in SPSS with mixed model ANOVAs including session (beginning-of-treatment, post-treatment) and trial type (incongruent, congruent) as within-subject factors and medication (disulfiram, placebo) and CM (CM, no-CM) conditions as between-subject factors. Stroop errors on incongruent trials were analyzed with mixed-model ANOVAs including session as a within-subject factor and medication and CM conditions as between-subject factors.
fMRI data acquisition and pre-processing steps were consistent with our prior work (e.g., Kober et al., 2014; see Supplemental Methods for details). For second-level random-effects analyses, the contrast of interest was the ‘change in Stroop-effect’ calculated as [(Incongruentpost>Congruentpost)>(Incongruentpre>Congruentpre)], which assessed changes in Stroop-effect-related activity at post-treatment versus beginning-of-treatment (DeVito et al., 2012). fMRI results were family-wise-error-corrected at two-tailed pFWE<0.05.
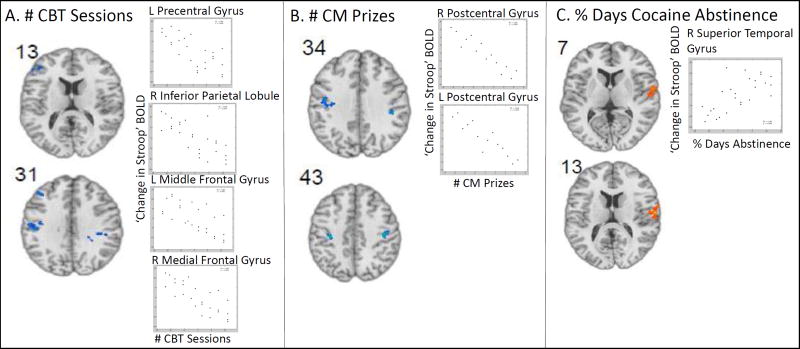
To address the primary research question regarding how neural correlates of cognitive-control change across treatment and how these changes relate to engagement with different treatment components, the following approach was taken. First, changes in fMRI Stroop effect were assessed at the whole brain level (Table 3A). Second, separate whole-brain correlation analyses were carried out between the ‘change in Stroop effect’ contrast and the following indicators of treatment engagement: a) CBT sessions (entire sample, N=26); b) CM prizes drawn (subsample randomized to CM, N=14); and c) days of study medication taken (subsample randomized to disulfiram, N=10) (description of variables Table 1; results Table 3B). All analyses were familywise error corrected for multiple comparisons (pFWE = .05)
Table 3.
fMRI Stroop Changes At Post-Treatment vs. Beginning-of-Treatment and Associations With Treatment Components
| Peak Coordinates | Statistics (t-value) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cluster Label (Peak) | Regions of Activation | R/L | x | y | z | k | Maximum Voxel | Cluster Mean |
| A. Change in fMRI Stroop Effect at Post-Treatment vs. Beginning-of-Treatment (Trial Type (Inc, Con) × Session (Post, Beginning)) (N=26) | ||||||||
| Culmen | L Culmen, L Parahippocampal Gyrus | L | −9 | −36 | −9 | 188 | −5.45 | −3.29 |
| L Precuneus, L Lingual Gyrus, L Posterior | L | −9 | −60 | 24 | ||||
| Precuneus | Cingulate | 269 | −5.05 | −3.29 | ||||
| Cingulate Gyrus | R/L Cingulate Gyrus | R | 9 | 9 | 42 | 144 | −4.63 | −3.30 |
| Postcentral Gyrus | R Postcentral Gyrus, R Precentral Gyrus | R | 39 | −18 | 33 | 83 | −4.57 | −3.14 |
| L Angular Gyrus, L Middle Temporal Gyrus, L | ||||||||
| Angular Gyrus | Superior Temporal Gyrus | L | −33 | −75 | 36 | 77 | −4.52 | −3.27 |
| Thalamus | L and R Thalamus, R Caudate | L | −3 | 0 | 0 | 139 | −4.51 | −3.20 |
| Hippocampus | R Hippocampus, R Amygdala, R Parahippocampal | R | 30 | −21 | −15 | 109 | −4.28 | −3.23 |
| Precentral Gyrus | R Precentral Gyrus | R | 60 | 6 | 3 | 96 | −3.99 | −3.14 |
| B. Whole Brain Correlations between ‘Change in fMRI Stroop Effect’ and Treatment Engagement and Outcome | ||||||||
| i. Correlation between ‘Change in fMRI Stroop Effect’ and Number of CBT Sessions (N=26) | ||||||||
| Precentral Gyrus | L Precentral Gyrus | L | 118 | −36 | −15 | 48 | −0.75 | −0.55 |
| Inferior Parietal Lobule | R Inferior Parietal Lobule | R | 74 | 33 | −36 | 39 | −0.73 | −0.56 |
| Middle Frontal Gyrus | L Middle Frontal Gyrus | L | 61 | −42 | 33 | 27 | −0.72 | −0.57 |
| Medial Frontal Gyrus | R Medial Frontal Gyrus, L/R Precentral Gyrus, L/R | R | 194 | 6 | −15 | 69 | −0.70 | −0.55 |
| ii. Correlation ‘Change in fMRI Stroop Effect’ and Total Number of Contingency Management Prizes (within CM Group, N=14) | ||||||||
| Postcentral | R Postcentral | R | 51 | 45 | −18 | 39 | −0.93 | −0.73 |
| Postcentral | L Postcentral, L Precentral Gyrus | L | 58 | −33 | −24 | 45 | −0.91 | −0.73 |
| iii. Correlation between ‘Change in fMRI Stroop Effect’ and Number of Days of Disulfiram Treatment Taken (N=10) | ||||||||
| No Significant Clusters | ||||||||
| iv. Correlation between ‘Change in fMRI Stroop Effect’ and Percent Days Cocaine Abstinent During Treatment Period (N=26) | ||||||||
| Superior Temporal Gyrus | R Superior Temporal Gyrus, R Transverse Temporal | R | 63 | −12 | 12 | 72 | 0.72 | 0.55 |
Note. All results presented survive family wise error correction for multiple comparisons at the whole brain level to two-tailed pFWE<.05.
Section A shows clusters that survived corrections for mutliple comparisons that showed a change in ‘Stroop effect’ related activity in the sample overall at post-treatment versus beginning-of-treatment (N=26).
Section B shows clusters that survived corrections for multiple comparisons from separate whole-brain rank-order non-parametric correlations with the change in Stroop effect contrast and measures of treatment engagement (i. Number of Cognitive Behavioral Therapy (CBT) Sessions (N=26); ii. Total Number of Contingency Management (CM) Prizes within those randomized to CM (N=14); iii. Number of Days of Disulfiram Treatment Taken within those randomized to medication (N=10)); and within-treatment cocaine use (iv. Percent Days Cocaine Abstinent During the Treatment Period (N=26)).
Abbreviations: Inc= incongruent trials, Con=congruent trials, Beginning= beginning-of-treatment, Post=post-treatment, R=right, L=left, k=cluster size (in voxels), CM=contingency management; CBT= cognitive behavioral therapy.
The figures which correspond to the these results are as follows: For section A, see Figure 1A, extracted cluster means in Figure 1B, and “whole-brain” slice-out images in Supplemental Figure 2 (as well as full slice-out images for the beginning of treatment Stroop effect and post-treatment Stroop effect contrasts separately in Supplemental Figure 1). For section B, see Figure 2 and “whole-brain” slice-out images in Supplemental Figure 3.
Since level of cocaine abstinence during treatment could affect ‘change in Stroop-effect’ across treatment, a separate whole-brain correlation was run between the ‘change in Stroop effect’ contrast and cocaine use within-treatment (i.e., percent days self-reported abstinence during treatment; percent cocaine-negative urines during treatment) (Table 3B). To check whether the regions associated with engagement in different aspects of treatment overlapped or were simply reflections of the same regions associated with cocaine abstinence during treatment, the separate correlation analyses were entered into formal conjunction analyses (see Supplemental Material for detailed methods). For all fMRI correlation analyses, if variables were not normally distributed, rank-order correlations were used as a non-parametric alternative.
Additional analyses addressed whether ‘change in Stroop-effect’ differed by randomly assigned treatment condition in separate analyses: a) CM versus no-CM groups, b) disulfiram versus placebo groups. Since treatment group differences in ‘change in Stroop-effect’ could be influenced by beginning-of-treatment group differences, potentially confounding beginning-of-treatment differences between treatment groups were assessed by comparing beginning-of-treatment Stroop-effect (Incongruentpre >Congruentpre trials) by CM (CM>no-CM) and medication (disulfiram>placebo) randomization status.
Results
Beginning-of-Treatment Clinical Characteristics, Treatment Engagement and Cocaine-Use Outcomes
There were no significant treatment group differences on demographic, baseline clinical or treatment engagement measures, with two exceptions: there were more years of cannabis use in no-CM vs. CM groups, and more days in treatment in the disulfiram versus placebo groups (see Table 2). Despite no main effects of group on cocaine-use outcomes in this fMRI subsample, a significant CM by medication condition interaction on ‘percent days self-reported cocaine abstinence’ during treatment indicated best cocaine use outcomes in the CM/placebo group, worst outcomes in the CM/disulfiram group and intermediate outcomes in the no-CM groups (see Table 2). This pattern is slightly different than that of the full RCT sample wherein cocaine-use outcomes were best in the CM/placebo group, worst in the no-CM/placebo group and intermediate in the disulfiram groups (Carroll et al., 2016).
Stroop Behavior
The expected behavioral Stroop-effect was indicated by a main effect of trial type on response times, reflecting slower correct response times for incongruent versus congruent trials (trial type: F(1,15)= 58.99, p<.001). There were no main or interactive effects of group (CM, No CM; disulfiram, placebo) or session (post-treatment, beginning-of-treatment) on response times errors during incongruent trials (see Table 2).
Changes in fMRI Stroop and Relationship to Treatment Engagement and Outcome
Within the whole sample (across treatment groups; N=26), there were significant reductions in Stroop-related neural activity from beginning-of-treatment to post-treatment in: hippocampus, thalamus, cingulate, precentral gyrus, postcentral gyrus, precuneus and culmen clusters (see Table 3A, Figure 1A–B; Supplementary Figure 2 for additional views; and Supplementary Figure 1 for Stroop-related activity at beginning-of-treatment and post-treatment). This is consistent with our prior work (DeVito et al., 2012).
Figure 1. Reduced Stroop-related Activity at Post-Treatment vs. Beginning-of-Treatment.
A. Changes in Stroop effect-related activity (incongruent>congruent trials) at post-treatment versus beginning-of-treatment in the sample overall (N=26). Blue indicates regions with lower Stroop-related BOLD signal at post-treatment relative to beginning-of-treatment. fMRI results are family-wise-error-corrected for multiple comparisons at pFWE<.05. For additional details see Table 3A. For full results, see Supplementary Figure 2.
B. The mean extracted betas from significant clusters (significant clusters shown in section A, in full Supplementary Figure 2 and reported in Table 3A). Mean betas are presented for each trial type (incongruent, congruent) and time point (beginning-of-treatment, post-treatment), relative to all unmodeled baseline. L= left, R= right. Bars are in following order: Congruent (Beginning-of-Treatment), Incongruent (Beginning-of-Treatment), Congruent (Post-Treatment), Incongruent (Post-Treatment). Fill color indicates trial type (white= congruent; grey= incongruent). Border color indicates time point (black=Beginning-of-Treatment; red= Post-Treatment). Error bars represent +/− 1 SEM.
Whole-brain correlations between ‘change in Stroop effect’ and treatment engagement revealed negative correlations, showing that greater reductions in Stroop-related activity at post-treatment (relative to beginning-of-treatment), were associated with greater treatment engagement. Importantly, this pattern was apparent in different regions, depending on the specific treatment engagement measure (Table 3B). Specifically, attendance at more CBT sessions was associated with a greater reduction in Stroop-related activity in the precentral gyrus, IPL, MFG and medial frontal gyrus and more earned CM prizes were associated with greater reduction in postcentral gyrus. In contrast, days of disulfiram medication taken was not significantly associated with changes in Stroop fMRI. Better cocaine use outcomes during treatment (higher percent days of cocaine abstinence during treatment) was positively associated with increases in Stroop-related activity in the superior temporal gyrus. There were no significant correlations between changes in Stroop fMRI and cocaine negative urines. Conjunction analyses revealed that the regions associated with cocaine-use outcomes did not overlap with regions associated with treatment engagement measures and regions associated with different aspects of treatment engagement (CBT, CM or disulfiram) did not overlap (see Supplementary Materials for detailed results).
Analyses comparing changes in Stroop effect from beginning-of-treatment to post-treatment by treatment group did not reveal any significant differences between CM vs. no-CM or disulfiram vs. placebo groups. Further, there were no significant differences in fMRI Stroop-related neural activity at beginning-of-treatment by CM or disulfiram group status, which was investigated as a potentially confounding factor.
Discussion
Cognitive-control is considered a key process in SUDs (Garavan et al., 2013; Garavan & Hester, 2007; Potenza et al., 2011; Sofuoglu, DeVito, Waters, & Carroll, 2013). This study is the first to investigate relationships between cognitive-control-related neural activity and indicators of exposure to putative active ingredients of treatment in individuals with CUDs. Findings partially supported our hypotheses. Consistent with our hypotheses for the first analytic approach, greater treatment engagement was associated with greater reduction in Stroop-related activity. Importantly, engagement with different treatment components (e.g., exposure to CBT or CM components) was correlated with reductions in Stroop-related activity across different cognitive-control-related regions - consistent with the conceptualization of these components as theoretically and mechanistically distinct. However, engagement with disulfiram was not associated with change in Stroop-related activity in these regions. The patterns of associations between treatment engagement and changes in Stroop-related activity were in distinct regions from those associated with cocaine use during treatment, suggesting these associations were not simply a reflection of cocaine-use outcomes.
In contrast with our hypotheses for the second analytic approach, no treatment group (CM vs. no-CM; disulfiram vs. placebo) differences in ‘change in Stroop effect’ survived corrections for multiple comparisons at the whole brain level. Out-of-scanner behavioral Stroop performance did not significantly change across treatment. Although this lack of significant improvement could be seen as a limitation, it is also a strength in allowing interpretation of improved neural efficiency in the context of relatively stable behavioral performance. Designing fMRI studies to match groups behaviorally such that the neural activity can be directly compared is a well-established method when cognitive impairment is expected in one group (e.g., (Casey, Tottenham, Liston, & Durston, 2005)).
In the sample overall, we found diminished Stroop-related activity post treatment compared to beginning-of-treatment. This pattern of change is consistent with our prior findings in a mixed substance-abusing sample receiving CBT or treatment as usual (DeVito et al., 2012). Regions showing reduced Stroop-related activity in the overall sample have previously been shown to exhibit task-related functional differences between individuals with CUD and non-substance-using comparisons, using tasks tapping similar cognitive constructs as the Stroop (Elton et al., 2012; Hester & Garavan, 2004; Kaufman et al., 2003). Prior research provides context for interpreting such diminished activity following treatment.
Decreased task-related activity following treatment may reflect improved efficiency. For example, while increased task difficulty and attentional load have been associated with increased task-related activity, practice is known to reduce task-related activity, which suggests improved efficiency (e.g., (Tomasi, Ernst, Caparelli, & Chang, 2004). Furthermore, practice effects are associated with decreased precuneus activation, and individuals with CUD show less practice-effect-related deactivation than healthy-comparison subjects, suggesting greater practice-related decreases in functional activity may be more optimal (Goldstein et al., 2007).
Changes in task-related activity following treatment may also relate to changes in cocaine use since cognitive-control-related functional activity is altered by acute cocaine administration and across cocaine abstinence. For example, acute intravenous administration of cocaine prior to Go/No-Go increased fMRI task-related neural activity in a manner that partially ‘normalized’ functional activity in active cocaine users (Garavan et al., 2008). More specifically, following intravenous injection of cocaine (relative to intravenous saline) active cocaine users had higher successful-inhibition-related activity in right insula/IFG and right middle frontal gyrus (MFG) and higher error-related activity in right posterior cingulate/lingual gyrus, culmen of vermis/left lingual gyrus, left inferior parietal lobule (IPL) and right middle frontal gyrus, and decreased activity in left posterior cingulate on a Go/No-Go task. When compared with healthy participants from a prior study and using the same ROIs that had previously shown reduced activity in cocaine users (Kaufman et al., 2003), the findings were replicated in this group of cocaine users on saline indicating that cocaine users showed Go/No-Go-related hypo-activation following IV saline, relative to healthy controls, and that IV cocaine increased task-related activity in overlapping regions and abolished the significant hypoactive differences (relative to healthy controls)(Garavan et al., 2008). Furthermore, response-inhibition-related activity is altered non-uniformly throughout abstinence (Garavan et al., 2013) and prior findings from cross-sectional studies remain somewhat mixed. For example, in a cross-sectional study comparing healthy non-users, shorter-term abstinent (1–5weeks) and longer-term abstinent (40–120 weeks) cocaine users, fMRI Go/No-Go task-related activity differed across groups in several brain regions (Connolly et al., 2012). However, longer duration of abstinence was not necessarily associated with a change towards normalization (i.e., becoming closer to the healthy control group); rather, neural activity in some regions was more ‘abnormal’ in the longer-term abstinence group, which might reflect compensation (Connolly et al., 2012). Furthermore, one study found no statistically significant group differences in fMRI Go/No-Go between healthy controls (N=45) and cocaine-abstinent individuals (average abstinence 32.3 weeks; range <1 −100 weeks) who were recruited from intensive inpatient treatment for cocaine-use disorder (N=27) (Bell, Foxe, Ross, & Garavan, 2014). In contrast, a separate Go/No-Go fMRI study found no group differences during successful inhibitions, but during commission errors found that both current (N=30) and former cocaine users (N=29; average duration of abstinence 51.2 weeks) differed from healthy controls (N=35), with former cocaine users showing differences from healthy controls in more a priori regions of interest than current cocaine users(Castelluccio, Meda, Muska, Stevens, & Pearlson, 2014). However, cocaine use or abstinence did not appear to be substantial factors driving the current findings since changes in Stroop-related BOLD-signal measures within treatment did not correlate with activity in the same regions as did measures of cocaine abstinence during treatment.
When interpreting the pattern of change in task-related activity following treatment, it may be important to note that clinical improvements may not always be accompanied by functional changes in the direction of ‘normalization’. Rather, substance-use treatments may act through both adaptation and ‘normalization’ of functional activity. For example, modafinil further increased hyperactive task-related functional activity in the ACC in cocaine users, relative to non-substance-using comparisons, and this increased activity was associated with diminished cocaine-craving (Goudriaan, Veltman, van den Brink, Dom, & Schmaal, 2013). Furthermore, duration of abstinence is not necessarily associated with a change towards normalization (i.e., becoming closer to the healthy control group) (Connolly et al., 2012). , This may reflect the complex interplay between pre-existing vulnerabilities and drug-induced neuroadaptation in substance users. Namely, pre-morbid functional abnormalities may confer vulnerability to SUDs and drugs may have acute and prolonged effects on brain function, and some drug-induced abnormalities may persist through prolonged abstinence. Therefore, abstinence may not return individuals to pre-substance-use-disorder baselines, and pre-morbid baselines may be suboptimal treatment targets as they may not represent ‘normalizations’ of functional activity relative to healthy non-drug-users with low SUD-vulnerability (see (Moeller, Bederson, Alia-Klein, & Goldstein, 2016) for review of changes with substance-use onset and predictors of treatment outcome). These complexities underline the limits of using healthy case-comparisons to derive treatment targets and reinforce the importance of testing treatment-related change within substance abusers and relating these changes to treatment mechanisms. However, taken together, greater diminishment of Stroop-related activity across treatment and its association with exposure to treatment components could be consistent with greater improvements in efficiency with more treatment exposure.
Clinical Implications and Mechanisms of Action
Within the thalamus, failed-inhibition-related activity is greater in individuals with CUD in earlier versus later stages of abstinence and positively associated with subjective feelings of loss of control (Li et al., 2010). Thalamo-cortical connectivity is important in ‘bottom-up’ cognitive-control in CUD (Worhunsky et al., 2013). Performance-monitoring, including error-processing and behavioral adjustment, is crucial to successful cognitive-control (Taylor, Stern, & Gehring, 2007) and impaired in cocaine-dependent individuals (Garavan & Stout, 2005). The ACC has been implicated in cocaine craving (Garavan et al., 2000) and has a well-established role in response conflict monitoring (Barch et al., 2001). Thus, our findings of reduced Stroop-effect-related thalamic and cingulate activity at post-treatment relative to beginning-of-treatment in the sample overall may be consistent with greater efficiency of cognitive-control and performance-monitoring following treatment.
The medial frontal gyrus has been implicated in the cognitive regulation of craving (Kober et al., 2010). Training in recognizing and coping with craving is central to CBT. Therefore, correlations between greater reductions in Stroop-related activity in the medial frontal gyrus and more CBT sessions attended, but not other treatment components, may be consistent with ‘top-down’ regulation of attention-related processes as a treatment mechanism of CBT (Potenza et al., 2011)
Disulfiram doses did not correlate with change in cognitive-control-related activity in any clusters. This may reflect the weak therapeutic efficacy of disulfiram in this RCT (Carroll et al., 2016) and moderate efficacy in prior trials (Pani et al., 2010) and may suggest that disulfiram’s therapeutic mechanism of action is not likely related to cognitive-control, although a larger sample of individuals receiving disulfiram is needed to make more definitive statements.
Limitations
Strengths of this manuscript included availability of beginning of and post-treatment fMRI data from a factorial RCTs testing three well-systematized evidence-based therapies, which also included objective indices of treatment process. The principal limitation is the small sample size, which limited power and precluded a full mediational analysis linking beginning-of-treatment to post-treatment changes in neural activity to exposure to treatment components and cocaine-use outcomes. In addition, only a subset of RCT participants participated in the optional fMRI component of the RCT. This limitation is mitigated by matching of groups on important baseline variables in the RCT and fMRI samples. Although the RCT included a full-factorial design, fMRI subgroup sample sizes prohibited analysis of CM-by-disulfiram or sex/gender-by-treatment type interactions. Future research is needed since disulfiram may less effectively treat cocaine-use in women than men (DeVito, Babuscio, Nich, Ball, & Carroll, 2014) and cognitive-control-related neural activity may differentially relate to treatment outcomes across sex/gender (Luo et al., 2013). The current study focused on cognitive-control-related brain function and was therefore only sensitive to treatment-related changes associated with this construct. Future research should investigate relationship between treatment components and non-cognitive-control-related mechanisms, using tasks tapping other cognitive constructs with relevance to SUDs (e.g., reward sensitivity, craving). As with all treatment trials, it is not possible to control for all potential sources of variance and other measures (e.g., motivation, self-efficacy, social factors) which may fluctuate across treatment and may also theoretically impact changes in fMRI Stroop effects. While inclusion of a healthy control test-retest group would allow for some control of time and practice effects, these effects cannot be presumed to be identical between SUD and healthy samples for reasons discussed above.
Conclusions
This study is the first to assess indicators of treatment process and exposure in relation to changes in fMRI during treatment for CUD. It represents an important, albeit preliminary, step in understanding neurocognitive mechanisms of action for well-established treatments for CUD, particularly CM. Specifically, enhanced cognitive control may be an important treatment target for SUD treatments, as indicated by the relationships seen here between exposure to components of effective therapies (CM and CBT) and post-treatment versus beginning-of-treatment changes in areas related to cognitive-control as assessed by Stroop. Our approach to these analyses may also prove a step forward in utilizing fMRI to understand how empirically-validated therapies may exert their effects. That is, we attempted to go beyond analyses of whether specific patterns of neural activity are associated with post-treatment drug use outcomes, which have yielded inconsistent findings across studies and have limited ability to parse out effects of specific treatment, time, or chronic or acute effects of drug use on those relationships. Rather, in these analyses we linked changes in neural activity associated with cognitive-control to putative indicators of treatment exposure for three specific treatment conditions, while separately assessing associations with drug use during the trial. This approach, which focuses on comparing effects of multiple treatments with known efficacy, reliable indicators of treatment exposure, and a well-established cognitive task (Stroop) associated with a likely common mechanism of treatment response in addicted populations, may be a path toward understanding how effective therapies affect complex conditions such as SUDs.
Supplementary Material
Stroop effect-related activity (incongruent>congruent trials) at beginning-of-treatment and post-treatment in the sample overall (N=26). Red indicates regions with higher BOLD signal for Incongruent vs. Congruent trials. Blue indicates regions with lower BOLD signal at Incongruent vs. Congruent trials. Results are family-wise-error-corrected for multiple comparisons at pFWE<.05.
Full slice-out of results presented in Figure 1. Changes in Stroop effect-related activity (incongruent>congruent trials) at post-treatment versus beginning-of-treatment in the sample overall (N=26). Blue indicates regions with lower Stroop-related BOLD signal at post-treatment relative to beginning-of-treatment. fMRI results are family-wise-error-corrected for multiple comparisons at pFWE<.05. For additional details see Table 3A.
Full slice-out of results presented in Figure 2 and Table 3B. Rank-order whole brain correlations between change in Stroop effect-related activity at post-treatment versus beginning-of-treatment ((post incongruent- post congruent)-(pre incongruent- pre congruent)) and (A) number of cognitive behavioral therapy (CBT) sessions attended (N=26); (B) number of contingency management (CM) prizes received, within group randomized to CM (N=14); (C) percent days of self-reported days of cocaine abstinence during treatment. Blue regions indicate inverse correlations showing lower Stroop-related activity at post- versus beginning-of-treatment associated with more treatment engagement. Red regions indicate positive correlations showing higher Stroop-related activity at post- versus beginning-of-treatment associated with more cocaine abstinence. fMRI results are family-wise-error-corrected for multiple comparisons at pFWE<.05.
Figure 2. Correlation between Change in Stroop effect and Treatment Engagement and Outcome.
Rank-order whole brain correlations between change in Stroop effect-related activity at post-treatment versus beginning-of-treatment ((post incongruent- post congruent)-(pre incongruent- pre congruent)) and (A) number of cognitive behavioral therapy (CBT) sessions attended (N=26); (B) number of contingency management (CM) prizes received, within group randomized to CM (N=14); (C) percent days of self-reported days of cocaine abstinence during treatment. Blue regions indicate inverse correlations showing lower Stroop-related activity at post- vs. beginning-of-treatment associated with more treatment engagement. Red regions indicate positive correlations showing higher Stroop-related activity at post- versus beginning-of-treatment associated with more cocaine abstinence. Scatter plots show extracted rank order correlations from each significant cluster (see Table 3B). fMRI results are family-wise-error-corrected for multiple comparisons at pFWE<.05. For full sliceout of results, see Supplementary Figure 3.
Acknowledgments
Support for the study was provided by National Institute on Drug Abuse (NIDA) grants R01 DA019078 (PI: Carroll), R01 DA020908 (PI: Potenza), R01 DA035058 (PI: Potenza and Carroll), P50 DA09241 (PI: Carroll), and the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC). In addition, Dr. DeVito was supported by BIRCWH K12 DA031050 (PI: Mazure) from NIDA, Office of Research on Women’s Health (ORWH), National Institute on Alcohol Abuse and Alcoholism (NIAAA) and National Institutes of Health (OD); Dr. Dong was supported by 31371023 from the National Science Foundation of China, Dr. Kober was supported by K12 DA00167 (PI: Potenza and O’Malley) from NIDA; Dr. Xu was supported by K01 DA027750 (PI: Xu) from NIDA; Dr. Potenza was supported by CASAColumbia. The funding agencies did not provide input or comment on the content of the manuscript. The manuscript reflects the contributions and thoughts of the authors and not necessarily the views of the funding agencies.
We acknowledge Dan Marino and Monica Solorzano for neuroimaging data collection; Elizabeth Vollono for clinical data collection; Karen Hunkele, Theresa Babuscio, and Tami Frankforter for management of the clinical data; Joanne Corvino for research staff training and quality assurance; David Iamkis for assistance with medication preparation and delivery; and Noah Konkus for assistance with manuscript formatting. We thank the staff and patients at the APT Foundation and its Central Medical Unit.
Footnotes
Aspects of the data included in this manuscript were presented in a poster at the Annual Meeting of The College on Problems of Drug Dependence (CPDD) (Palm Springs, California, 13 June 2016), and in a talk as part of the Yale Division of Substance Abuse Seminar series (West Haven, Connecticut, 16 March 2016).
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Group CSR. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;(102 Suppl 1):33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. doi:S1053-8119(05)00110-2 [pii] [DOI] [PubMed] [Google Scholar]
- Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87(2–3):202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11(9):837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Barth KS, Malcolm RJ. Disulfiram: an old therapeutic with new applications. CNS Neurol Disord Drug Targets. 2010;9(1):5–12. doi: 10.2174/187152710790966678. [DOI] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Ross LA, Garavan H. Intact inhibitory control processes in abstinent drug abusers (I): a functional neuroimaging study in former cocaine addicts. Neuropharmacology. 2014;82:143–150. doi: 10.1016/j.neuropharm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, Festinger DS. Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction. 2014;109(9):1426–1436. doi: 10.1111/add.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST. A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction. Rockville, MD: NIDA; 1998. [Google Scholar]
- Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Rockville, Maryland: NIDA; 1998. [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61(3):264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, DeVito EE, Decker S, LaPaglia D, Ball SA. Toward empirical identification of a clinically meaningful indicator of treatment outcome: features of candidate indicators and evaluation of sensitivity to treatment effects and relationship to one year follow up cocaine use outcomes. Drug Alcohol Depend. 2014;137:3–19. doi: 10.1016/j.drugalcdep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ. One-year follow-up of disulfiram and psychotherapy for cocaine-alcohol users: sustained effects of treatment. Addiction. 2000;95(9):1335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93(5):713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Petry NM, Eagan DA, Shi JM, Ball SA. A randomized factorial trial of disulfiram and contingency management to enhance cognitive behavioral therapy for cocaine dependence. Drug Alcohol Depend. 2016;160:135–142. doi: 10.1016/j.drugalcdep.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Shi JM, Eagan D, Ball SA. Efficacy of disulfiram and Twelve Step Facilitation in cocaine-dependent individuals maintained on methadone: a randomized placebo-controlled trial. Drug Alcohol Depend. 2012;126(1–2):224–231. doi: 10.1016/j.drugalcdep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend. 2000;57(3):225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Castelluccio BC, Meda SA, Muska CE, Stevens MC, Pearlson GD. Error processing in current and former cocaine users. Brain Imaging Behav. 2014;8(1):87–96. doi: 10.1007/s11682-013-9247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Noronha A, Carroll KM, Potenza MN, Hutchison K, Calhoun VD, Feldstein Ewing SW. Brain mechanisms of Change in Addictions Treatment: Models, Methods, and Emerging Findings. Curr Addict Rep. 2016;3(3):332–342. doi: 10.1007/s40429-016-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2012;121(1–2):45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelle CL, Veltman DJ, Booij J, Emmerik-van Oortmerssen K, van den Brink W. Substrates of neuropsychological functioning in stimulant dependence: a review of functional neuroimaging research. Brain Behav. 2012;2(4):499–523. doi: 10.1002/brb3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Babuscio TA, Nich C, Ball SA, Carroll KM. Gender differences in clinical outcomes for cocaine dependence: Randomized clinical trials of behavioral therapy and disulfiram. Drug Alcohol Depend. 2014;145:156–167. doi: 10.1016/j.drugalcdep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug Alcohol Depend. 2012;122(3):228–235. doi: 10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Elton A, Young J, Smitherman S, Gross RE, Mletzko T, Kilts CD. Neural network activation during a stop-signal task discriminates cocaine-dependent from non-drug-abusing men. Addict Biol. 2012;19(3):427–438. doi: 10.1111/adb.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Brennan KL, Hester R, Whelan R. The neurobiology of successful abstinence. Curr Opin Neurobiol. 2013;23(4):668–674. doi: 10.1016/j.conb.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17(3):337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9(4):195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D. mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse. Mol Interv. 2009;9(4):175–187. doi: 10.1124/mi.9.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry. 2000;47(12):1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. Neuroimage. 2007;35(1):194–206. doi: 10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict Behav. 2013;38(2):1509–1517. doi: 10.1016/j.addbeh.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Gogtay N. National Institute of Mental Health clinical trials: new opportunities, new expectations. JAMA Psychiatry. 2014;71(7):745–746. doi: 10.1001/jamapsychiatry.2014.426. [DOI] [PubMed] [Google Scholar]
- Jan RK, Lin JC, McLaren DG, Kirk IJ, Kydd RR, Russell BR. The effects of methylphenidate on cognitive control in active methamphetamine dependence using functional magnetic resonance imaging. Front Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen CH, Pedersen B, Tonnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749–1758. doi: 10.1111/j.1530-0277.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- Karamanakos PN, Pappas P, Stephanou P, Marselos M. Differentiation of disulfiram effects on central catecholamines and hepatic ethanol metabolism. Pharmacol Toxicol. 2001;88(2):106–110. [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23(21):7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, DeVito EE, DeLeone CM, Carroll KM, Potenza MN. Cannabis abstinence during treatment and one-year follow-up: relationship to neural activity in men. Neuropsychopharmacology. 2014;39(10):2288–2298. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107(33):14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biol Psychiatry. 2013;73(3):219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Luo X, Sinha R, Rounsaville BJ, Carroll KM, Malison RT, Ide JS. Increased error-related thalamic activity during early compared to late cocaine abstinence. Drug Alcohol Depend. 2010;109(1–3):181–189. doi: 10.1016/j.drugalcdep.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RB, Onyemekwu C, Hart CL, Ochsner KN, Kober H. Boundary conditions of methamphetamine craving. Exp Clin Psychopharmacol. 2015;23(6):436–444. doi: 10.1037/pha0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, Li CS. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136(Pt 4):1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, Luijten M, van de Wetering BJ, Smits M, Franken IH. Individual differences in anterior cingulate activation associated with attentional bias predict cocaine use after treatment. Neuropsychopharmacology. 2013;38(6):1085–1093. doi: 10.1038/npp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, van de Wetering BJ, Franken IH. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. Biol Psychiatry. 2013;73(8):782–788. doi: 10.1016/j.biopsych.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Wilcox CE, Teshiba TM, Ling JM, Yang Z. Hyperactivation of the cognitive control network in cocaine use disorders during a multisensory Stroop task. Drug Alcohol Depend. 2013;133(1):235–241. doi: 10.1016/j.drugalcdep.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Bederson L, Alia-Klein N, Goldstein RZ. Neuroscience of inhibition for addiction medicine: from prediction of initiation to prediction of relapse. Prog Brain Res. 2016;223:165–188. doi: 10.1016/bs.pbr.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Konova AB, Parvaz MA, Tomasi D, Lane RD, Fort C, Goldstein RZ. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry. 2014;71(1):61–70. doi: 10.1001/jamapsychiatry.2013.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Woicik PA, Maloney T, Alia-Klein N, Honorio J, Goldstein RZ. Enhanced midbrain response at 6-month follow-up in cocaine addiction, association with reduced drug-related choice. Addict Biol. 2012;17(6):1013–1025. doi: 10.1111/j.1369-1600.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Naqvi NH, Debellis R, Breiter HC. The contributions of cognitive neuroscience and neuroimaging to understanding mechanisms of behavior change in addiction. Psychol Addict Behav. 2013;27(2):336–350. doi: 10.1037/a0032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA. Principles of drug addiction treatment: A research based guide. National Institute on Drug Abuse; 2012. [Google Scholar]
- Pani PP, Trogu E, Vacca R, Amato L, Vecchi S, Davoli M. Disulfiram for the treatment of cocaine dependence. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD007024.pub2. CD007024. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95(2):219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend. 2000;58(1–2):9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol. 2007;75(6):983–991. doi: 10.1037/0022-006X.75.6.983. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68(2):250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2005;62(10):1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Petry NM, Petrakis I, Trevisan L, Wiredu G, Boutros NN, Martin B, Kosten TR. Contingency management interventions: from research to practice. Am J Psychiatry. 2001;158(5):694–702. doi: 10.1176/appi.ajp.158.5.694. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69(4):695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National findings. (HHS Publication No. (SMA) 14–4863) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Sammons MT, Schmidt NB. Combined treatment for mental disorders: A guide to psychological and pharmacological interventions: American Psychological Association 2001 [Google Scholar]
- Shorter D, Nielsen DA, Huang W, Harding MJ, Hamon SC, Kosten TR. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and alpha1A–adrenoceptor gene variation. Eur Neuropsychopharmacol. 2013;23(11):1401–1407. doi: 10.1016/j.euroneuro.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell KM, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. New Jersey: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13(2):160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. Neuroimage. 2004;23(4):1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Vaccari A, Saba PL, Ruiu S, Collu M, Devoto P. Disulfiram and diethyldithiocarbamate intoxication affects the storage and release of striatal dopamine. Toxicol Appl Pharmacol. 1996;139(1):102–108. doi: 10.1006/taap.1996.0147. [DOI] [PubMed] [Google Scholar]
- van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev. 2011;4(1):42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, Potenza MN. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol Addict Behav. 2013;27(2):477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Moeller SJ, Goldstein RZ. Cognitive interventions for addiction medicine: Understanding the underlying neurobiological mechanisms. Prog Brain Res. 2016;224:285–304. doi: 10.1016/bs.pbr.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stroop effect-related activity (incongruent>congruent trials) at beginning-of-treatment and post-treatment in the sample overall (N=26). Red indicates regions with higher BOLD signal for Incongruent vs. Congruent trials. Blue indicates regions with lower BOLD signal at Incongruent vs. Congruent trials. Results are family-wise-error-corrected for multiple comparisons at pFWE<.05.
Full slice-out of results presented in Figure 1. Changes in Stroop effect-related activity (incongruent>congruent trials) at post-treatment versus beginning-of-treatment in the sample overall (N=26). Blue indicates regions with lower Stroop-related BOLD signal at post-treatment relative to beginning-of-treatment. fMRI results are family-wise-error-corrected for multiple comparisons at pFWE<.05. For additional details see Table 3A.
Full slice-out of results presented in Figure 2 and Table 3B. Rank-order whole brain correlations between change in Stroop effect-related activity at post-treatment versus beginning-of-treatment ((post incongruent- post congruent)-(pre incongruent- pre congruent)) and (A) number of cognitive behavioral therapy (CBT) sessions attended (N=26); (B) number of contingency management (CM) prizes received, within group randomized to CM (N=14); (C) percent days of self-reported days of cocaine abstinence during treatment. Blue regions indicate inverse correlations showing lower Stroop-related activity at post- versus beginning-of-treatment associated with more treatment engagement. Red regions indicate positive correlations showing higher Stroop-related activity at post- versus beginning-of-treatment associated with more cocaine abstinence. fMRI results are family-wise-error-corrected for multiple comparisons at pFWE<.05.