Abstract
Matrix metalloproteinases (MMPs) are zinc-dependent proteolytic enzymes that degrade various proteins in the extracellular matrix (ECM). MMPs may also regulate the activity of membrane receptors and post-receptor signaling mechanisms, and thereby affect cell function. The MMP family includes collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs and other MMPs. Inactive proMMPs are cleaved by other MMPs or proteases into active MMPs, which interact with various protein substrates in ECM and cell surface. MMPs regulate important biological processes such as vascular remodeling and angiogenesis, and may be involved in the pathogenesis of cardiovascular disorders such as hypertension, atherosclerosis, and aneurysm. The role of MMPs is often assessed by measuring their mRNA expression, protein levels, and proteolytic activity using gel zymography. MMP inhibitors are also used to assess the role of MMPs in different biological processes and pathological conditions. MMP activity is regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs), and the MMP/TIMP balance could determine the net MMP activity, ECM turnover, and tissue remodeling. Also, several synthetic MMP inhibitors have been developed. Synthetic MMP inhibitors include a large number of zinc binding globulins (ZBGs), in addition to non-ZBGs and mechanism-based inhibitors. MMP inhibitors have been proposed as potential tools in the management of osteoarthritis, cancer, and cardiovascular disorders. However, most MMP inhibitors have broad-spectrum actions on multiple MMPs and could cause undesirable musculoskeletal side effects. Currently, doxycycline is the only MMP inhibitor approved by the Food and Drug Administration. New generation biological and synthetic MMP inhibitors may show greater MMP specificity and fewer side-effects, and could be useful in targeting specific MMPs, reducing unrestrained tissue remodeling, and the management of MMP-related pathological disorders.
Keywords: aneurysm, angiogenesis, atherosclerosis, extracellular matrix, hypertension, remodeling, TIMP
1. INTRODUCTION
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that degrade various proteins in the extracellular matrix (ECM). The first MMP was discovered in 1962 as a collagen proteolytic activity during the degradation of ECM proteins and resorption of the tadpole tail.1 The MMP family has now grown to at least 28 enzymes. With the exception of MMP-7, MMP-23 and MMP-26, most members of the MMP family share sequence homology with MMP-1 (collagenase 1), and a common core structure typically consisting of a propeptide, a catalytic metalloproteinase domain, a hinge region, and a hemopexin domain.2–5 MMPs are commonly classified on the basis of their domain organization and substrate preference into collagenases, gelatinases, stromelysins, matrilysins, membrane-type (MT)-MMPs and other MMPs.6,7
MMPs degrade various ECM substrates including collagen, elastin and laminin. MMPs may also interact with various bioactive molecules on the cell surface and G-protein coupled receptors (GPCRs), and thereby affect the cellular environment and signaling.8,9 MMPs play a role in cell proliferation, migration (adhesion/dispersion), differentiation, angiogenesis, and tissue healing and repair. MMPs may also be involved in cell apoptosis, and the inflammatory and immune response.10
MMPs are regulated at different levels including mRNA expression, post-translational modification of the MMP protein, and stimulation of their enzymatic activity by various endogenous and exogenous activators. The proteolytic activity of MMPs is also regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs).7 MMP/TIMP imbalance could affect the net MMP activity, ECM turnover, and tissue remodeling, and could lead to metabolic and immune diseases, cancer, and cardiovascular disorders such as hypertension, atherosclerosis, and aneurysm.6
Changes in MMP expression/activity have been proposed as potential biomarkers for the diagnosis and prognosis of certain pathological disorders. MMP inhibitors have also been utilized to reverse the effects of MMPs and to assess whether MMPs play a role in a specific biological process or pathological condition. MMP inhibitors have also been evaluated as potential pharmacological tools in the management of osteoarthritis, cancer, and cardiovascular disorders. In addition to endogenous TIMPs, synthetic MMP inhibitors have been developed and include broad-spectrum and relatively specific MMP inhibitors.
In this chapter, we will discuss reports from Pubmed and other databases to provide an overview of the different MMP inhibitors and their potential effects in various biological processes and pathological conditions. We will briefly describe the MMP-substrate interaction as it would help to understand the interaction between TIMP and other inhibitors with the MMP molecule. We will then describe the different categories of MMP inhibitors. We will provide examples of the role of MMPs in tissue remodeling and biological processes in humans, experimental animals, blood vessels and vascular cells, and discuss how MMP inhibitors could be used to assess the role of MMPs in these processes, and as potential pharmacological tools in the management of cardiovascular disease, and other disorders such as cancer
2. MMP–SUBSTRATE INTERACTION
ECM segregates tissues from each other, provides anchorage and support for cells, regulates cell migration and intercellular communication, and provides a local depot for growth factors released by different cells. ECM proteins and other components provide a structural scaffold for tissue support, cell migration, differentiation and signaling, as well as epithelialization and wound repair. ECM has three main components; fibers, proteoglycans and polysaccharides. Fibers are largely glycoproteins that include collagen, which is the main ECM protein, and elastin, which is not glycosylated and provides plasticity and flexibility to certain tissues such as the arteries, lungs and skin. Laminin is a glycoprotein localized in the basal lamina of the epithelium. Fibronectin is a glycoprotein used by cells to bind to ECM, and can modulate the cytoskeleton to facilitate or hinder cell movement. Proteoglycans have more carbohydrates than proteins, and attract water to keep the ECM hydrated. Proteoglycans also facilitate binding of growth factors to the ECM milieu. Syndecan-1 is a proteoglycan and integral transmembrane protein that binds chemotactic cytokines during the inflammatory process. Other ECM proteins include glycoproteins such as vitronectin, aggrecan, entactin, fibrin and tenascin, and polysaccharides such as hyaluronic acid.11
MMPs regulate tissue remodeling and promote degradation of various ECM proteins. Collagen and elastin are essential for structural integrity of the vascular wall and are major MMP substrates. Collagen has various subtypes including collagen I, II, III, IV, V, VI, VII, VIII, IX, X, and XIV. Different MMPs break down various collagen subtypes with different efficacies. Other MMP substrates include aggrecan, entactin, fibronectin, gelatin, laminin, tenascin, and vitronectin. MMPs can also degrade myelin basic protein and casein. Casein is digested by different proteinases, and like gelatin, is used to measure MMP activity in gel zymography assays.11
MMPs catalytic activity requires zinc (Zn2+) and a water molecule flanked by three conserved histidine residues and a conserved glutamate, with a conserved methionine acting as a hydrophobic base to support the structure surrounding the catalytic Zn2+. During MMP-substrate interaction, Zn2+ is penta-coordinated with a substrate's carbonyl oxygen atom, one oxygen atom from the MMP glutamate-bound water, and the three MMP conserved histidines. This forms an oxy-anion transition state that can polarize the glutamic acid's oxygen atom, proximate the substrate scissile C-N bond, and induce it to act as reversible electron donor. This allows the substrate scissile bond to break, releasing the N-terminal portion of the substrate and forming an MMP-carboxylate complex. Another free H2O is taken up, releasing the remaining carboxylate portion of the substrate and the free MMP.12–16 Collectively, upon binding of the substrate, the Zn2+-bound water attacks the substrate carbonyl group, and the transfer of protons through the conserved glutamate to the nitrogen of the scissile bond results in peptide cleavage.17,18 Alternatively, Zn2+ may be penta-coordinated with a substrate's carbonyl oxygen atom, two oxygens from the MMP conserved glutamate, and two of the three conserved histidines. One oxygen from glutamate then performs a nucleophilic attack and breakdown of the substrate.19
The specificity of the MMP-substrate interaction depends on specific subsites or pockets (S) within the MMP molecule that interact with corresponding substituents (P) in the substrate. The MMP S1, S2, S3, …Sn pockets on the right side of Zn2+ and the primed S1’, S2’, S3’, …Sn’ pockets on the left side of Zn2+ confer binding specificity to the substrate P1, P2, P3, … Pn and primed P1’, P2’, P3’, … Pn’ substituents, respectively.16 The MMP S1’ pocket is the most critical for substrate specificity and binding. Among different MMPs, the MMP S1’ pocket is extremely variable, and may be shallow (e.g. MMP-1 and MMP-7), intermediate (e.g. MMP-2, MMP-9, and MMP-13), or deep (e.g. MMP-3, MMP-8, and MMP-12).12–14 The MMP S2’ and S3’ pockets are shallower than the S1’ pocket, and, therefore, more exposed to solvents.14 Second to the S1’ pocket, the MMP S3 pocket is also important for substrate specificity.2
3. REGULATION OF MMP EXPRESSION/ACTIVITY
MMPs are regulated at multiple levels including transcription, secretion, activation of the zymogen proMMP form, inhibition by tissue inhibitors of metalloproteinases (TIMPs) and internalization by endocytosis. Hypoxia promotes MMP-2 and MMP-9 mRNA expression.20 Extracellular MMP inducer (EMMPRIN, CD147, Basigin) is a widely expressed membrane protein of the immunoglobulin superfamily that has been implicated in tissue remodeling and in pathological conditions such as atherosclerosis, aneurysm, heart failure, osteoarthritis and cancer. High volume mechanical ventilation causes acute lung injury and is associated with upregulation of MMP-2, MMP-9, MT1-MMP and EMMPRIN mRNA expresion.21 EMMPRIN, MMP-2, MT1-MMP and MT2-MMP are also overexpressed in venous leg ulcers, where unrestrained activation of MMPs could lead to excessive degradation of ECM proteins.22
MMPs are synthesized as pre-proenzymes and the signal peptide is removed during translation to generate proMMPs. ProMMPs have a ‘cysteine switch’ motif PRCGXPD in which the cysteine residue coordinates with the Zn2+ ion in the catalytic domain, keeping the proMMP in the inactive form.23 Activation of proMMPs often takes place extracellularly by other MMPs or other proteases. For example, MMP-3 can transform proMMP-1 into active MMP-1.24 ProMMP-2 activation takes place on the cell surface by most MT-MMPs, but not MT4-MMP,25 a process that also requires TIMP-2.26,27 ProMMP-2 forms a complex with TIMP-2 through their C-terminal domains, thus permitting the N-terminal inhibitory domain of TIMP-2 to bind to MT1-MMP on the cell surface. The cell surface-bound proMMP-2 is then activated by another MT1-MMP molecule that is free of TIMP-2. The MT1-MMP bound to TIMP-2 can act as a "receptor" for proMMP-2. The MT1-MMP-TIMP-2-proMMP-2 complex is then presented to an adjacent free MT1-MMP for activation.28 The level of TIMP-2 may determine whether MT1-MMP cleaves its own substrate or activates proMMP-2.29 Other MMPs such as membrane-bound MMP-11, MMP-23, and MMP-28 may be activated intracellularly via the endopeptidase furin, which selectively cleaves paired base residues.30–33
Oxidants produced by leukocytes and other cells can activate MMPs by oxidation of the prodomain thiol followed by autolytic cleavage. ProMMPs can be activated by reactive oxygen species (ROS).34–37 ROS derived from foam cells can activate proMMP-2. Also, nitric oxide (NO) may activate proMMP-9 during cerebral ischemia by reacting with the thiol group of the cysteine switch and forming an S-nitrosylated derivative.37 MMPs can be activated by thiol-modifying agents such as 4-aminophenylmercuric acetate, mercury chloride, and N-ethylmaleimide, oxidized glutathione, sodium dodecyl sulfate, and chaotropic agents by disturbing the cysteine-Zn2+ interaction at the cysteine switch. MMPs can also be activated by low pH and warm temperature.38 Other MMPs such as MMP-9 are activated mainly by plasmin.39 MMP-7 is activated by both MMP-3 and hypochlorous acid, a product of myeloperoxidase in macrophages of atherosclerotic plaques. MMP-7 can in turn activate MMP-1.34,40
4. TISSUE INHIBITORS OF METALLOPROTEINASES (TIMPs)
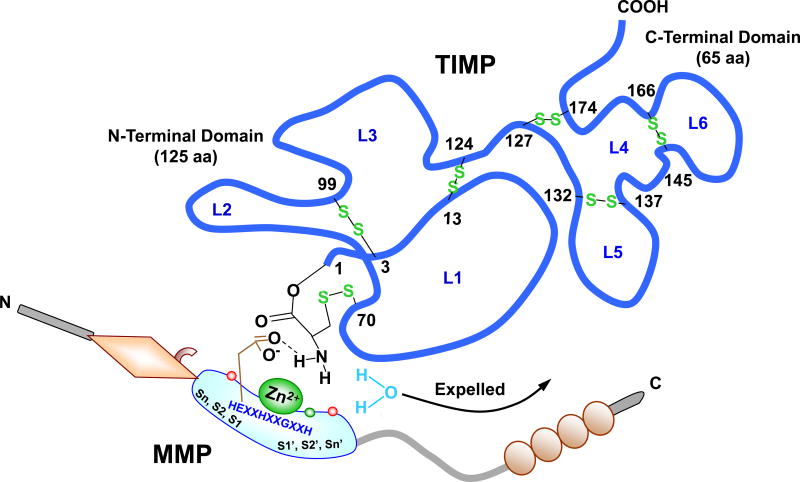
MMPs are inhibited by both endogenous and exogenous inhibitors. TIMPs are endogenous MMP inhibitors that bind MMPs in a 1:1 stoichiometry (Fig. 1).2,12 TIMPs have an N-terminal domain (125 aa) and C-terminal domain (65 aa); each containing 3 disulfide bonds. The N-terminal domain folds as a separate unit and is capable of inhibiting MMPs.41,42 The Cys1 is important for chelating the active site Zn2+ with its N-terminal α-amino group and carbonyl group, thereby expelling the water molecule bound to the catalytic Zn2+. The TIMP molecule wedges into the active-site cleft of MMP in a manner similar to that of the substrate (Fig. 2). Four homologous TIMPs have been identified and termed as TIMP-1, TIMP-2, TIMP-3 and TIMP-4. TIMP-1 and TIMP-3 are glycoproteins, while TIMP-2 and TIMP-4 do not contain carbohydrates. TIMPs can inhibit multiple MMPs with different efficacies. For example, TIMP-2 and -3 inhibit MT1-MMP and MT2-MMP, whereas TIMP-1 is a poor inhibitor of MT1-MMP, MT3-MMP, MT5-MMP and MMP-19.43 Also, while TIMP-1 and TIMP-2 bind MMP-10 (stromelysin-2), the binding is 10-fold weaker than that to MMP-3 (stromelysin-1).44 TIMP-1 has a threonine-2 (Thr2) residue that interacts with the MMP S1’ pocket in a manner similar to that of a substrate P1’ substituent, largely determining the affinity to MMP-3. Substitutions at Thr2 affect the stability of the TIMP-MMP complex and the TIMP specificity to different MMPs. For instance, substitution of Thr2 by alanine results in a 17-fold decrease in the ability of TIMP-1 to bind MMP-1 compared with MMP-3.45
Fig. 1.
TIMP-MMP Interaction. TIMP is a ~190 aa protein, with an N-terminal domain (loops L1, 2, and 3) and C-terminal domain (loops L4, 5 and 6), which fold independently as a result of 6 disulfide bonds between 12 specific Cys residues. The N-terminal Cys1-Thr-Cys-Val4 and Glu67-Ser-Val-Cys70 are connected via a disulfide bond between Cys1 and Cys70 and are essential for MMP inhibition, as they enter the MMP active site and bidentately chelate the MMP Zn2+. The carbonyl oxygen and α-amino nitrogen in the TIMP Cys1 coordinate with the MMP Zn2+, which is localized in the MMP molecule via the 3 histidines in the HEXXHXXGXXH motif. The TIMP α-amino group then expels Zn2+-bound H2O by binding the MMP H2O binding site and forming a H bond with carboxylate oxygen from conserved MMP Glu202 (E in the HEXXHXXGXXH sequence). TIMP-1 and MMP-3 are used as prototypes. The amino acids involved in Zn2+- and pocket-binding may vary with different MMPs and TIMPs.
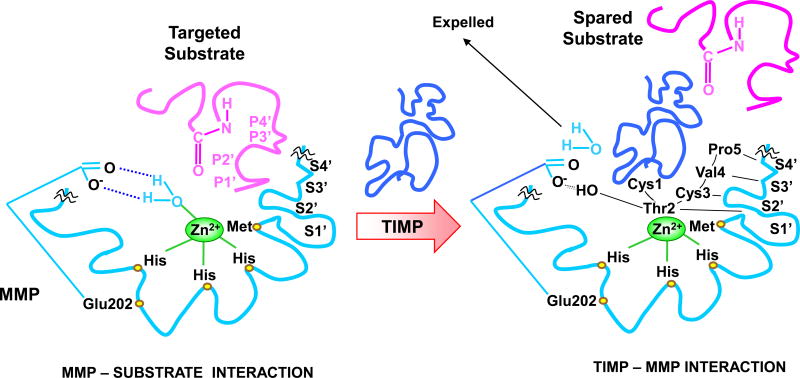
Fig. 2.
MMP Inhibition by TIMP. TIMP Thr2 side chains enter the MMP S1’ pocket in a manner similar to that of a substrate P1’ substituent, largely determining the affinity to MMP. Thr2 –OH group could also interact with Glu202, further contributing to expelling Zn2+-bound H2O and preventing substrate degradation. Additionally, the TIMP Cys3, Val4 and Pro5 interact with MMP S2’, S3’, and S4’ pockets in a P2’, P3’, and P4’-like manner, further preventing substrate binding or degradation. TIMP-1 and MMP-3 are used as prototypes. The amino acids involved in Zn2+- and pocket-binding may vary with different MMPs and TIMPs.
TIMPs are widely distributed in many tissues and organs. A change in either MMP or TIMP levels could alter the MMP/TIMP ratio and cause a net change in specific MMP activity. MMP inhibition by TIMPs would decrease degradation of ECM proteins. On the other hand, serine proteinases such as neutrophil elastase could inactivate TIMPs, spare MMPs from inhibition by TIMPs, and in turn favor breakdown of ECM proteins.46,47
In addition to inhibiting MMPs, TIMPs can inhibit a broader spectrum of metalloproteinases. TIMP-1 inhibits a disintegrin and metalloproteinase-10 (ADAM-10) while TIMP-2 inhibits ADAM-12.48,49 TIMP-3 has a much broader metalloproteinase inhibition profile including ADAM-10, ADAM-12, and ADAM-17 as well as a disintegrin and metalloproteinase with thrombospondin motif ADAMTS-1, ADAMST-2, ADAMST-4 and ADAMST-5.49–52 This broad-spectrum metalloproteinase inhibition by TIMP-3 is best illustrated by the observation that TIMP-3 ablation in mice is associated with emphysema-like alveolar damage and faster apoptosis of mammary epithelial cells after weaning, whereas TIMP-1 or TIMP-2-null mice do not exhibit such abnormalities.53,54
5. OTHER BIOLOGICAL AND PLEIOTROPIC INHIBITORS OF MMPS
In addition to endogenous TIMPs, α2-Macroglobulin is another endogenous MMP inhibitor found in blood and tissue fluids. MMP activity is partly regulated by α2-macroglobulin and related proteins. Human α2-Macroglobulin is a glycoprotein consisting of four identical subunits. α2-Macroglobulin is a wide-spectrum proteinase inhibitor that inhibits most endopeptidases including MMPs, by entrapping them within the macroglobulin. The complex is then rapidly internalized and cleared by endocytosis via low density lipoprotein receptor-related protein-1.55
Other proteinase inhibitors may inhibit specific MMPs, although their mechanism of action is unclear.56 For instance, a secreted form of β-amyloid precursor protein or a C-terminal fragment of procollagen C-proteinase enhancer protein can inhibit MMP-2. Reversion-inducing-cysteine-rich protein with kazal motifs (RECK) is a glycosyl phosphatidylinositol (GPI)-anchored glycoprotein expressed in many cells including vascular smooth muscle cells (VSMCs), and has been shown to inhibit MMP-2, MMP-9 and MMP-14 when expressed in transfected human fibrosarcoma-derived cell line HT1080.57 Tissue factor pathway inhibitor-2 is a serine proteinase inhibitor that can inhibit MMP-1 and MMP-2.58
Monoclonal antibodies have high specificity and affinity for specific MMPs and can detect MMPs in the body fluids and tissues.6 Monoclonal antibodies REGA-3G12 and REGA-2D9 react specifically with MMP-9, and do not cross-react with MMP-2. MMP inhibition by REGA-3G12 involves the catalytic domain and not the Zn2+ binding region or the fibronectin region. REGA-1G8 is less specific and cross reacts with serum albumin. Patients with Crohn's disease suffer from recurring fistulae, and MMP-9, a type IV collagenase, is upregulated in crypt abscesses and around fistulae, suggesting a role of MMP-9 in fistula formation. Interestingly, in a mouse heterotopic xenograft model of intestinal fibrosis, treatment with anti-MMP-9 monoclonal antibody reduced collagen deposition and hydroxyproline content in day-14 intestinal grafts, suggesting reduced fibrosis. Anti-MMP-9 antibody may be a promising therapeutic strategy for fibrosis-related complications of inflammatory bowel disease.59
The hemopexin domain could also be a potential target for MMP antibodies. The hemopexin domain of MMP-1 is essential for the specificity of its catalytic domain to cleave collagen. Also, MMP-2 is localized at extracellular sites by its fibronectin domains and MT1-MMP (MMP-14) requires the hemopexin domain for cell surface clustering and ability to activate proMMP-2.60 The hemopexin domain can also be used to target specific MMP substrates, and prevent their degradation by MMPs. Studies have generated glutathione-S-transferase (GST) fusion proteins containing MMP-9 hemopexin domain or truncated forms corresponding to specific structural blades (B1–B4) of the MMP-9 hemopexin domain. GST-MMP-9 hemopexin domain inhibited MMP-9-dependent degradation of gelatin, but not other MMP-9 substrates such as a fluorogenic peptide, αB crystalline, or nonmuscular actin. The MMP-9 hemopexin domain may shield gelatin and specifically prevent its binding to and degradation by MMP-9. Of note, GST-MMP-9 hemopexin domain also abolishes the degradation of gelatin by MMP-2, confirming that it is not an MMP-9 antagonist. ELISA assays demonstrated that GST-B4 and GST-B1 specifically bound to gelatin. These findings suggest new functions of MMP-9 hemopexin domain attributed to blades B4 and B1 and should help in designing specific inhibitors of gelatin degradation.61
Small interference RNA (siRNA) specific to certain MMPs have been developed and can be used in assessing the role of a specific MMP in a biological process. For instance, MMP-2 siRNA inhibits the transcriptional product of MMP-2.62 Targeted delivery of MMP siRNA could decrease MMP expression and unrestrained ECM turnover and tissue remodeling in localized pathological conditions such as aneurysm, varicose veins, osteoarthritis and tumors. For instance, specific inhibition of either MMP-2 or MT1-MMP by specific shRNAs hampers melanoma cell migration and invasion.63 Gene therapy has shown some success in animal models, and with the design of efficient and safe gene delivery into target tissues downregulation of MMPs using siRNA or overexpression of TIMPs may have clinical applications.64
Sulodexide (SDX) is a highly purified glycosaminoglycan containing fast-moving heparin fraction (80%) and dermatan sulfate (20%). SDX has pro-fibrinolytic, anti-thrombotic, anti-inflammatory and endothelial protective activity in the vascular system that could be partly related to its effects on MMPs. SDX decreases MMP-9 secretion from white blood cells without MMP prodomain displacement,65 and may specifically inhibit proteases with cysteine residues such as MMP-2 and MMP-9.66
The intracellular signaling pathways and the upstream inducers and downstream transcription factors that affect MMP or TIMP mRNA expression may serve as potential targets for MMP inhibition. Studies have generated an anti-EMMPRIN antibody directed against a specific epitope that successfully inhibited the production of MMP-9 in tumor cell-macrophage in vitro co-culture systems. The EMMPRIN antibody also inhibited in vivo tumor progression in both the RENCA renal cell carcinoma and CT26 colon carcinoma subcutaneous tumor models, and reduced tumor size and number of metastatic foci in the 4T1 orthotopic model. This was achieved by inhibiting angiogenesis as assessed by immunohistochemical staining for the endothelial marker CD31, by inhibiting tumor cell proliferation as assessed by the staining for Ki-67, and by enhancing tumor cell apoptosis as assessed by the TUNEL assay. The EMMPRIN antibody also recruited more macrophages into the tumor, and skewed the tumor microenvironment for macrophages from TGF-β-dominated anti-inflammatory microenvironment to a less immunosuppressive one, thus allowing improved ability of stimulated macrophages to perform antibody-dependent cell cytotoxicity and to kill tumor cells. These findings suggest that EMMPRIN antibody maps the epitope capable of inducing MMPs, and place EMMPRIN as a potential target to modulate MMPs in cancer therapy.67 Blockade of mitogen-activated protein kinase (MAPK), NF-κB or activator protein (AP)-1 has shown some efficacy in vitro and in animal models of arthritis, and these effects may be partly due to changes in MMP expression.68 Also, biologics may block inflammatory cytokines and reduce MMP expression in different tissues. Statins may inhibit MMPs through pleiotropic effects. For instance, atorvastatin inhibits MMP-1, MMP-2, and MMP-9 expression in human retinal pigment epithelial cells,69 and MMP-1, MMP-2, MMP-3, and MMP-9 secretion from rabbit macrophages and cultured rabbit aortic and human saphenous vein VSMCs.70 Also, in a rat model of heart failure, pravastatin suppressed the increase in myocardial MMP-2 and MMP-9 activity.71
6. SYNTHETIC MMP INHIBITORS
Divalent ions can influence MMP release and activity. Cu2+ ion decreases the secretion of MMP-2.72 Deep sea water components such as Cu2+, Mg2+, and Mn2+ inhibit proliferation and migration of cultured rat aortic smooth muscle cells (RASMCs) by inhibiting not only extracellular signal–regulated kinase (ERK1/2) and MAPK kinase (MEK) phosphorylation, but also MMP-2 activity,73 a mechanism that may involve interference with Zn2+ binding at the MMP catalytic active site. Zn2+ chelators deprive MMPs from the Zn2+ ion critical for their activity.74 MMP inhibition can also be achieved via a Zn2+ binding group, e.g. hydroxamic acid, carboxylic acid, or sulfhydryl group. Other approaches to inhibit MMPs are through non-covalent interaction with sites on the MMP backbone such as the S1’, S2’, S3’, and S4’ pockets to which the MMP inhibitor side chains bind in a fashion similar to that of the substrate P1’, P2’, P3’, and P4’ substituents. The efficacy and specificity of inhibition are determined by which pockets are blocked for a given MMP.75 Several synthetic MMP inhibitors have been developed and some of them have been evaluated as investigational or therapeutic tools for degenerative diseases and vascular disorders (Table 1).14 However, because of the inherent flexibility in the MMP active-site, accurate modeling of specific MMP–inhibitor complexes has been severely limited.76
Table 1.
MMP inhibitors, their specificity to MMPs (Ki or IC50 <1 nM to 10 µM), and their effects in preclinical trials.
| MMP Inhibitor Category, Number, Chemistry, Other Name |
MMP Specificity, IC50 or Ki | Preclinical Trial | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| <1 nM | 1–10 nM | 11–100 nM | 0.1–1 µM | 1–10 µM | |||
|
| |||||||
| 1 | ZBGs, Hydroxamic Acids | Glioblastoma, breast, lung, ovarian, and prostate cancer | |||||
| Succinyl Hydroxamate | |||||||
| Batimastat (BB-94) | 1, 2, 8, 9 | 3 | |||||
| Marimastat (BB-2516) | 1, 2, 9, 14 | 7 | |||||
| Ilomastat (GM6001, Galardin) | 1, 2, 8, 9, 26 | 7, 12 | 3, 14 | ||||
|
| |||||||
| 2 | Sulfonamide Hydroxamate, AG3340, Prinomastat | 2, 3, 9, 13, 14 | 1 | 7 | Neovascularization, lung and prostate cancer, uveal melanoma, gliomas | ||
|
| |||||||
| 3 | Succinyl Hydroxamate | 1 | |||||
|
| |||||||
| 4 | RS-104966 | 13 | 1 | ||||
|
| |||||||
| 7 | Sulfonamide Hydroxamate | 2 | 8, 9, 14 | 1, 3 | 7 | Decrease tumor invasion | |
|
| |||||||
| 8 | Sulfonamide Hydroxamate | 3 | 2 | 9 | Chronic non-healing wounds | ||
|
| |||||||
| 10 | 2, 3 | 1 | |||||
|
| |||||||
| 12 | 3 | ||||||
|
| |||||||
| 5 | Carboxylic Acids | 13 | 3, 8 | 2 | 7, 9, 14 | Osteoarthritis | |
|
| |||||||
| 6 | 11 | 3, 12 | 1, 9, 14 | Chronic obstructive pulmonary disease | |||
|
| |||||||
| 9 | Sulfonylhydrazides | 2, 9 | 1 | 7 | 3 | ||
|
| |||||||
| 16 | Thiol- and Cyclic Mercaptosulfides | 9 | 2 | 1, 7, 14 | 3 | ||
|
| |||||||
| 19 | Aminomethyl Benzimidazoles | 11 | 2, 9, 13 | ||||
|
| |||||||
| Phosphorous-Based | |||||||
| 17 | Sulfonamide Phosphonate | 8 | 2 | 3 | |||
|
| |||||||
| 18 | Sulfonamide Phosphonate | 8 | 2, 9, 13, 14 | 1, 3 | 7 | Liver disease, multiple sclerosis, breast cancer | |
|
| |||||||
| 20 | Carbamoyl Phosphonate | 2 | Melanoma | ||||
|
| |||||||
| 21 | Carbamoyl Phosphonate | 2 | Melanoma, prostate cancer | ||||
|
| |||||||
| Nitrogen-Based | |||||||
| 22 | Oxazoline | 11 | |||||
|
| |||||||
| 23 | Dionethiones and Pyrimidine-2,4,6 triones, Ro-28-2653 | 2, 14 | 8, 9 | 3 | Anti-angiogenic and anti-invasive in tumor models | ||
|
| |||||||
| 24 | Dionethiones and Pyrimidine-2,4,6 triones | 13 | 2, 9, 12 | Osteoarthritis | |||
|
| |||||||
| Heterocyclic Bidentate Chelators | |||||||
| 25 | Terphenyl Backbone, AM-6 | 3 | |||||
|
| |||||||
| 26 | Biphenyl Backbone, 1,2 -HOPO-2 | 8, 12 | 2, 3 | 13 | Cardiac ischemia /reperfusion injury | ||
|
| |||||||
| 27 | Diphenyl Ether Backbone | 2, 9, 13 | 3 | 1 | Brain edema following ischemia/reperfusion | ||
|
| |||||||
| 28 | Biphenyl Backbone, Pyrone-based | 3, 9, 12 | 8 | 2, 13 | |||
|
| |||||||
| 29 | Biphenyl Backbone, Hydroxypyridinone Derivative | 8, 12 | |||||
|
| |||||||
| 30 | Biphenyl Backbone, AM-2 | 8, 12 | −3 | −2 | |||
|
| |||||||
| 31 | Biphenyl Backbone | 2, 8, 12, 13 | |||||
|
| |||||||
| 34 | Non-ZBGs | 13 | Osteoarthritis | ||||
|
| |||||||
| 35 | 12 | 2, 8, 13 | 3, 9 | ||||
|
| |||||||
| 36 | 2, 8, 13 | ||||||
|
| |||||||
| 37 | 13 | Osteoarthritis | |||||
|
| |||||||
| 38 | Pyrimidine Dicarboxamide | 13 | |||||
|
| |||||||
| 39 | Pyrimidine Dicarboxamide | 13 | |||||
|
| |||||||
| Mechanism-Based | |||||||
| 40 | Diphenyl Ether Backbone, SB-3CT | 2 | 9, 14 | 3 | Inhibits liver metastasis in T-cell lymphoma and bone metastasis in prostate cancer | ||
|
| |||||||
| 42 | Diphenyl Ether Backbone, Thiol-Containing | 2, 9 | 14 | 3 | |||
|
| |||||||
| 43 | Diphenyl Ether Backbone | 2 | 14 | 9 | 3 | ||
|
| |||||||
| 45 | Diphenyl Ether Backbone | 9 | 2 | 3, 14 | |||
The crystal structure of the MMP-11 catalytic domain during the interaction with a phosphinic inhibitor mimicking a D,L-peptide has suggested that the MMP-11 S1' pocket forms a tunnel running through the enzyme. This open channel is filled by the MMP inhibitor P1' group which adopts a constrained conformation to fit the MMP-11 S1’ pocket, together with two water molecules interacting with the MMP-11 specific residue Gln215. The presence of a water molecule interacting with one oxygen atom of the MMP inhibitor phosphinyl group and the proline residue of the MMP Met-turn suggests how the intermediate formed during proteolysis may be stabilized. Furthermore, the hydrogen bond distance observed between the methyl of the phosphinic group and the carbonyl group of Ala182 mimics the interaction between this carbonyl group and the amide group of the cleaved peptidic bond. This crystal structure provides a good model to study the mechanism of proteolysis by MMPs.77
The following sections provide brief description of different classes of MMP inhibitors. For detailed information and the original references regarding the different categories of MMP inhibitors, their MMP specificity, IC50 or Ki, and their potential use in certain pathological conditions, the reader is referred to other reviews.11,78
6.1. Derivatives of Early ZBGs
Because MMPs require catalytic Zn2+ for their activation and cleavage of their substrates, the design of MMP inhibitor has traditionally utilized Zn2+ binding globulin (ZBG) (Table 1). ZBGs displace the Zn2+-bound water molecule and inactivate the MMP enzyme.79 ZBG also acts as an anchor to lock the MMP inhibitor in the MMP active site and direct the backbone of the inhibitor to enter the MMP substrate-binding pockets.14
Early MMP inhibitors included hydroxamic acids (ZBG1), carboxylates (ZBG2), thiols, and phosphonic acids (phosphorus-based ZBGs).17 Hydroxamic acids derivatives were preferred because of the relative ease of their synthesis, and their strong binding to MMPs.80–83 The effectiveness of hydroxamates stems from the hydrogen bonding between the heteroatoms of the ZBG and the neighboring conserved amino acids in the MMP active site. Some hydroxamate- and carboxylate-based MMP inhibitors show some selectivity to certain MMPs.84–88 However, while hydroxamates are potent MMP inhibitors, they have poor oral bioavailability, inhibit multiple MMPs, and therefore cause musculoskeletal side effects.89,90
Hydroxamic acid derivatives include succinyl, sulfonamide, and phosphinamide hydroxamates.75,91,92 Batimastat (BB-94), marimastat (BB-2516), and ilomastat (GM6001) are broad spectrum succinyl hydroxamates with a collagen mimicking structure that inhibits MMPs by bidentate chelation of the active site Zn2+.75,93 Other ZBGs include carboxylic acids, sulfonylhydrazides, thiols, aminomethyl benzimidazole-containing ZBGs, phosphorous-based ZBGs, nitrogen-based ZBGs, and heterocyclic bidentate chelators.14,17,94
Hydrazide (ZBG3) and sulfonylhydrazide (ZBG4) analogs of the hydroxamate MMP inhibitor illomastat have been developed (Table 1).95 Sulfonylhydrazide 9 is a potent inhibitor of MMP-1, MMP-2, and MMP-9.96 Mercaptosulfide inhibitors (ZBG8) target MMP-14. MMP inhibitors with phosphorus-based ZBGs show improved MMP selectivity. Inhibitor 18 is a potent phosphonate inhibitor with relative selectivity for MMP-8 (neutrophil collagenase).97 Other phosphorus-based ZBGs include carbamoyl phosphonate ZBG (ZBG9).14
ZBGs have a net negative charge that prevents them from penetrating the cell and restricts their actions to the extracellular space, and therefore reduces their cell toxicity.98 Some ZBGs have shown relative selectivity for MMP-2 and have been evaluated in tissue and animal models of angiogenesis and tumor invasion. Compound 20 is more specific to MMP-2 than MMP-1, MMP-3, MMP-8, and MMP-9. In a murine model of metastatic melanoma, intraperitoneal administration of compound 20 at 50 mg/kg/day for three weeks caused 55% reduction in lung metastasis.99
Compound 21 is a carbamoyl phosphonate MMP inhibitor with greater selectivity to MMP-2 and MMP-9 than MMP-1, MMP-3, MMP-8, MMP-12, and MMP-13. Compound 21 inhibits cell invasion in Matrigel assay in a concentration-dependent fashion, and prevents tumor colonization in a murine melanoma model when administered orally or intraperitoneally. Compound 21 reduces tumor growth and metastasis in a murine model produced by implantation of human prostate tumor cells in immunodeficient mice. Compound 21 is water soluble at physiological pH and does not cause acute toxic effects at the doses used in the murine models.98
Research has been directed toward the development of MMP inhibitors with increased selectivity toward specific MMPs. The development of highly specific synthetic active-site-directed MMP inhibitors necessitates identifying the specific structural features of each individual MMP that can be exploited to obtain the desired selectivity. Some ZBGs could have the potential to be used clinically if their potency and selectivity toward specific MMPs are enhanced and their targets are better-defined using site-specific delivery.100 For example, a series of biphenyl sulfonamide carboxylate MMP inhibitors with high selectivity for MMP-13 were designed for treatment of osteoarthritis.100 Also, the carboxylic acid scaffold of those MMP inhibitors was used to develop selective MMP-12 inhibitors for treatment of chronic obstructive pulmonary disease,101 Selective hydroxamic acid inhibitors of MMP-2 have been developed as potent anti-angiogenic agents, and inhibitor 7 is the most selective MMP-2 inhibitor of this series.86 Another hydroxamate MMP inhibitor with specificity towards MMP-3 was designed for treatment of chronic non-healing wounds.88 Other ZBGs have been developed to improve selectivity, bioavailability, and pharmacokinetics, and include oxygen, nitrogen, and sulfur donor–atom ligands and monodentate, bidentate, and tridentate chelators.
6.2. Nitrogen-based ZBGs
Nitrogen-based ZBGs (ZBG10–16) have binding preference to late transition metals and improved selectivity towards Zn2+-dependent enzymes.102,103 Compound 22 is an adequate inhibitor of MMP-9 with little effects on MMP-1, MMP-2, and MMP-12. Nitrogen-based ZBGs such as the pyrimidine-2,4,6-trione and dionethione inhibitors have been studied extensively. The pyrimidine-2,4,6-trione group is found in many FDA-approved drugs including barbiturates, and the metabolic disposition and bioavailability of these compounds have been well-studied.104 Pyrimidine-2,4,6-trione MMP inhibitors have shown relative specificity toward gelatinases and potential usefulness as anticancer drugs.105 As part of the development of osteoarthritis drugs, pyrimidine-2,4,6-trione MMP inhibitors have been optimized to inhibit MMP-13,106–109 and have shown 100-fold selectivity for MMP-13 over MMP-2, MMP-8, and MMP-12.108
Compound 23 was evaluated for its anti-angiogenic, anti-invasive, and anti-tumorigenic, activity. At concentrations as low as 10 nM, compound 23 shows anti-cancer efficacy in both in vitro and in vivo models, and inhibits tumor invasion by 85%.110
6.3. Heterocyclic bidentate ZBGS
Heterocyclic bidentate chelators ZBG20–30 were developed as MMP inhibitors.94 Compared with hydroxamic acids. heterocyclic bidentate ZBGs have better biostability and tighter Zn2+ binding due to ligand rigidity and, in some cases, the presence of sulfur donor atoms.111,112 Heterocyclic bidentate ZBGs are more potent in inhibiting MMP-1, MMP-2, and MMP-3 than acetohydroxamic acid,94 and show low toxicity in cell viability assays.113 Compound 25 is a pyrone-based MMP inhibitor that is more selective toward MMP-3 than MMP-1 and -2.114 Compound 26 is a potent inhibitor of MMP-2, MMP-3, MMP-8 and MMP-12, with less effects on MMP-1, MMP-7, MMP-9, and MMP-13.115 In a rat model of cardiac ischemia/reperfusion injury, treatment of the heart with compound 26 (5 µM) recovered more than 80% of the heart’s original contractile function compared with 50% in control nontreated hearts.115
Other ZBGs include 6-, 7-, and 8-membered heterocyclic chelators as 1-hydroxy-2-piperidinone, 1-hydroxyazepan-2-1, 1-hydroxyazocan-2-1, and 1-hydroxy-1,4-diazepan-2-1.116 Compound 27 is highly selective to MMP-1 and moderately selective to MMP-3. Compound 27 has a 47 hour half-life when administered intravenously at 2 mg/kg in rats, and has been shown to reduce brain edema in a mouse model of cerebral ischemia/reperfusion injury produced by transient occlusion of mid-cerebral artery.116
Just as changes in the ZBG can alter the MMP selectivity, changes in the point of attachment of the ZBG to the backbone of the MMP inhibitor can also change its potency and selectivity. For instance, compound 30 has an IC50 of 240 nM against MMP-3,114,115 while its structural isomer compound 32 shows weaker ~30% inhibition even at 100 µM concentration.117
6.4. Tetracycline-based MMP inhibitors
Tetracyclines are antibiotics that can chelate Zn2+ ion and thereby inhibit MMP activity.75 Doxycycline is a semi-synthetic tetracycline that inhibits MMP-2 and MMP-9.118 Chemically modified tetracyclines have been developed to inhibit MMP activity.118 Chemically modified tetracyclines are preferred over conventional tetracyclines because they reach higher plasma levels for prolonged periods of time, and therefore require less frequent administration, and cause less gastrointestinal side effects when administered orally for a chronic disorder. COL-3 or metastat is a chemically modified tetracycline that has a tetracycline scaffold with unsubstituted positions C4-C9, and is a potent MMP inhibitor.119 Although tetracyclines are relatively weak Zn2+ chelators and inhibitors of MMP activity, they could affect MMP expression,119 and their effects on MMP synthesis may contribute to their potential benefits in rheumatoid arthritis.120
6.5. Non-zinc-binding MMP inhibitors
Some MMP inhibitors do not have a ZBG and hence do not bind the catalytic Zn2+ ion (Table 1).121–128 Because the Zn2+ active site is the most conserved feature in all MMPs, it has been thought that minimizing the interaction with the catalytic Zn2+ ion would improve the inhibitor selectivity toward different MMPs. Non-zinc-binding MMP inhibitors show a noncompetitive mechanism of inhibition.125 These MMP inhibitors bind to and lock the MMP active site into a specific conformation that is less favorable for substrate binding. Non-zinc-binding MMP inhibitors show high selectivity to MMP-13 and have shown therapeutic potential in animal models of osteoarthritis.121,128 Compound 37 inhibits MMP-13, but not MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-12, MMP-14, or MMP-17. The flexibility of the MMP-13 molecule relative to other MMPs may provide a favorable and accessible conformation for interaction with compound 37 that is not available in other MMPs.123,128 Although these MMP inhibitors show high degree of MMP selectivity that could minimize the side effects associated with broad-spectrum MMP inhibitors, it is not clear whether their selectivity is due to their non-ZBG properties or other factor(s).123,128
Of note, non-zinc binding MMP inhibitors are hydrophobic. The hydrophobicity of these MMP inhibitors is important for maintaining sufficient inhibitor-MMP interaction to produce high potency. However, hydrophobicity also decreases the water solubility of the MMP inhibitor. Because most of these non-zinc-binding MMP inhibitors are highly potent and show relative selectivity, studies have attempted to improve their water solubility and other biochemical properties.121 Derivatives have been developed to modify the solvent-exposed portions of the MMP inhibitor while maintaining its hydrophobic core structure.124
Compound 37 has shown promising results in animal models of osteoarthritis. In MMP-13-induced rat model of knee joint cartilage damage, compound 37 was effective at doses as low as 0.1 mg/kg. Also, in rat model of surgically induced knee cartilage damage, compound 37 administered orally twice daily at 30 mg/kg resulted in a 68% reduction in the cartilage lesion. Fibroplasias were absent in joints of rats treated with compound 37, but were observed in rats treated with broad-spectrum MMP inhibitors.
6.6. Mechanism-Based MMP Inhibitors
Mechanism-based MMP inhibitors such as SB-3CT (compound 40) coordinate with the MMP Zn2+, thus allowing the conserved MMP Glu202 to perform a nucleophilic attack and form a covalent bond with the inhibitor.14 When compared with the traditional Zn2+ chelating MMP inhibitors, the covalent bond prevents dissociation of the MMP inhibitor, and therefore decreases the rate of catalytic turnover and the amount of MMP inhibitor needed to saturate the MMP active site.129 SB-3CT and its successors have shown therapeutic potential, and more selective MMP inhibitors may be developed through covalent modifications with the MMP active site.14
SB-3CT is a selective inhibitor of MMP-2 and MMP-9. The structure of SB-3CT is relatively simple, as reflected by its low molecular weight. The mechanism of MMP inhibition by SB-3CT is similar to that of a “suicide substrate” in which a functional group is activated, leading to covalent modification of the MMP active site.129 SB-3CT shows slow-binding kinetics with MMP-2, MMP-3, and MMP-9, that reach equilibrium between the MMP, the inhibitor and the MMP–inhibitor complex within seconds to minutes. Slow-binding inhibition also contributes to slow dissociation rate of the MMP-inhibitor complex.130 Of note, following 95% inhibition, MMP-2 regains 50% of its activity after 3 days dialysis, indicating some degree of reversibility and thus distinguishes SB-3CT from the irreversible mechanism of a true suicide inhibitor.129,130 The selectivity of SB-3CT stems from the difference in the binding kinetics for various MMPs, and may be related to its inhibition of MMP-2 and MMP-9 via a slow-binding mechanism and inhibition of MMP-14 through competitive inhibition.131
In preclinical studies, SB-3CT has shown potential benefits in reducing brain damage caused by cerebral ischemia. SB-3CT showed anti-cancer effects in T-cell lymphoma and prostate cancer models.132–134 In in vitro Matrigel tests SB-3CT at 1 µM concentration reduced the invasion ability of human prostate cancer cells by 30%.133 SB-3CT also reduced angiogenesis and intraosseous tumor growth in a bone metastasis model of prostate cancer.133 In a mouse model of T-cell lymphoma, SB-3CT caused dose-dependent reduction in the number of liver metastases.135 At 50 mg/kg/day, SB-3CT inhibited liver metastases by 73% and reduced the colony size of the metastases, while treatment with the broad-spectrum MMP inhibitor batimastat was associated with increased metastasis in the same tumor model. SB-3CT also provided neuronal protection in a murine model of stroke.136 In mice treated with SB-3CT either prior to or 2 h following ischemia induced by occlusion of right middle cerebral artery, the infarct volume was decreased to 30% of the control. Administration of SB-3CT was protective up to 6 hours after the ischemic event in mice. Also, neurological behavioral scores evaluated 24 hours after reperfusion showed improvement in SB-3CT -treated compared with control non-treated mice, and the improvement was correlated with the reduction in the brain infarct volume.
Although SB-3CT (compound 40) shows marked in vivo activity, it undergoes rapid metabolism, and a metabolite of the parent compound may be responsible for its in vivo activity.137,138 Compound 43 shows slow-binding kinetics with MMP-2, MMP-9, and MMP-14 and is a more potent inhibitor of MMP-2, MMP-3, MMP-7, MMP-9, and MMP-14 than compound 40.139 Analysis of the different MMP inhibitor metabolites led to the design of derivatives with better in vivo stability and prolonged systemic effects.139 Compound 45 is a slow-binding inhibitor of MMP-2 and MMP-9, but a competitive inhibitor of other MMPs. Compound 45 is more potent for MMP-9 than MMP-2, and its metabolites are 75% more stable and show longer systemic effects than those of compound 40.
SB-3CT and its successors may have clinical potential, and the use of mechanism-based, slow-binding inhibitors may provide a new approach to improve selectivity of MMP inhibitors. Other covalent modifications in the MMP active site may lead to better MMP selectivity.48
Given the accessibility of secreted MMPs such as MMP-2 and membrane-tethered MMPs such as MT1-MMP, they represent ideal targets for specific inhibition by small molecules. Thiirane-based ND-322 is a novel small-molecule and selective MMP-2/MT1-MMP inhibitor that has been shown to reduce melanoma cell growth, migration and invasion, and to delay metastatic dissemination. ND-322 may represent a new inhibitor in the repertoire of treatments of melanoma.63
Even with the marked improvements in the design of MMP inhibitors, doxycycline remains the only FDA-approved MMP inhibitor.89,90,140,141 Another major limitation of MMP inhibitors is that they cause musculoskeletal side effects in the form of joint stiffness, pain, inflammation, and tendinitis.14,90,142,143
7. MMP Inhibitors as Investigational Tools in Biological Processes
MMPs play a role in many biological processes including tissue remodeling and growth as well as tissue defense mechanisms and immune response. Increased expression of MMPs has been documented during different stages of mammalian development, from embryonic implantation144 to the morphogenesis of different tissues including lung, bone and mammary gland.145,146 Other biological processes such as tissue repair and wound healing are associated with increased expression of MMPs.147 The role of MMPs in these biological processes has been supported by reversal of the effects of MMPs by MMP inhibitors.
7.1. MMP Inhibitors and Role of MMPs in Smooth Muscle Relaxation
Studies have suggested that MMPs via PI3K and ATP synthesis may transactivate EGFR and contribute to the α-adrenergic receptor-induced vascular tone. Inhibition of the expression of MMP-2 or MMP-7 blunted the phosphorylation of Akt by PI3K and thus inhibited the response to phenylephrine in rat mesenteric artery.148 We have shown that phenylephrine-induced contraction of rat aorta is inhibited ~50% by MMP-2 and ~70% by MMP-9.149 The inhibitory effects of MMP-2 and MMP-9 on phenylephrine contraction were reversible upon washing out the MMPs, supporting specificity of the effects of MMPs. The MMP-induced inhibition of aortic contraction was concentration- and time-dependent, and reversible suggesting that the actions of MMPs are not solely due to irreversible degradation of ECM protein. Also, the inhibitory effects of MMPs on VSM contraction are not likely due to degradation of phenylephrine or the α-adrenergic receptors because MMPs also inhibit prostaglandin F2α-induced contraction, suggesting that the effects of MMPs are not specific to a particular agonist/receptor, but likely involve direct effects on common VSM contraction pathway(s) downstream from receptor activation.
VSM contraction is triggered by increases in Ca2+ release from the intracellular stores and Ca2+ entry from the extracellular space. MMPs do not inhibit phenylephrine-induced contraction in Ca2+-free solution, suggesting that they do not inhibit the Ca2+ release mechanism from the intracellular stores. On the other hand, MMPs inhibit phenylephrine-induced Ca2+ influx in rat aortic rings.149 The mechanism by which MMPs inhibit Ca2+ entry could involve direct effects on the Ca2+ channels. MMPs may also affect K+ channels. MMP-2 causes relaxation of rat inferior vena cava (IVC) that is abolished by blockers of the large conductance Ca2+-activated K+ channels such as iberiotoxin, suggesting a role of VSM hyperpolarization.150 MMPs are known to induce collagen degradation and produce Arg-Gly-Asp (RGD)-containing peptides, which could bind to αvβ3 integrin receptors and inhibit Ca2+ entry into VSM.151 MMPs may also stimulate protease-activated receptors (PARs) and activate signaling pathways that could lead to blockade of VSM Ca2+ channels.152 This is supported by reports that proteases such as thrombin activate PARs and promote endothelium-dependent VSM relaxation by inhibiting Ca2+ influx.153 Thus while MMPs may affect VSM contraction through modulation of surface membrane ion channels, further studies are needed to define the role of integrins and PARs as possible molecular mechanisms via which MMPs could inhibit VSM contraction.
We have shown that MMP-2 and MMP-9 cause inhibition of Ca2+ entry-dependent mechanisms of contraction not only in rat aorta,149 but also in rat IVC.154 Our studies support that MMPs are expressed in both the arterial and venous system and could have significant effects on the arterial and venous structure and function. However, the findings in certain arteries should not be generalized to other arteries in the systemic circulation or specialized arteries such as the coronary and cerebral arteries. Also, veins differ from arteries in their structure and function, and the effects of MMPs on the veins should not be generalized to the arteries. Veins have few layers of VSMCs compared to several layers in the arteries. Also, venous and arterial VSMCs originate from distinct embryonic locations and are exposed to different pressures and hemodynamic conditions in the circulation.155 Studies have shown that while cell migration and MMP-2 and MMP-9 levels could be similar in cultured saphenous vein VSMCs and internal mammary artery VSMCs, venous VSMCs exhibit more proliferative and invasive capabilities than arterial VSMCs.156 Other studies have shown that MMP-2 expression is greater in cultured human saphenous vein VSMCs than human coronary artery VSMCs. In contrast, the expression of MMP-3, MMP-10, MMP-20, and MMP-26 is greater in coronary artery than saphenous vein VSMCs.155 Similarly, TIMPs may show different expression levels in veins versus arteries. For instance, the levels of TIMP-1, TIMP-2, and TIMP-3 are greater in cultured human saphenous vein than coronary artery VSMCs.155 These observations highlight the importance of further studying the differences in the expression/activity of MMPs and TIMPs in veins versus arteries and in venous versus arterial disease.
7.2. MMP Inhibitors and Role of MMPs in Smooth Muscle Migration
MMPs play a role in VSMC migration. In rat aortic smooth muscle cells (RASMCs) cultured on collagen I gel to mimic ECM, exposure to interstitial flow enhanced cell motility. Upregulation of MMP-1 enhanced flow-induced cell motility, while the MMP inhibitor GM-6001 attenuated flow-induced cell migration. ERK1/2 phosphorylation and increased expression of activator protein-1 (AP-1) transcription factors c-Jun and c-Fos appear to be involved in MMP-mediated enhancement of flow-induced cell motility.157 Young human ASMCs produce active MMP-2 and show a greater migratory capability than aged cells. The activation of pro-MMP-2 in young cells is likely due to an increase in MT1-MMP. In contrast, aged cells produce only the inactive zymogen proMMP-2 form. Upregulation of TIMPs could also reduce MMP-2 activity in aged cells. Interestingly, treatment of young cells with TIMP-1 and TIMP-2 leads to a migratory behavior that mimics that of aged cells.158 MMP-2 activation may be involved in chemokine-induced chemotaxis in monolayers of human VSMCs.159 Also, MMP-2 knockout decreases VSMC migration and neointima formation in the mouse carotid ligation model (Table 2).160,161
Table 2.
Cardiovascular Effects of Gene Ablation of specific MMPs or TIMPs in Mice
| MMP/TIMP | Cardiovascular Phenotype | Reference |
|---|---|---|
|
| ||
| MMP-2 | Reduced neointima formation after vascular injury. | 161,321 |
| Protection from cardiac rupture post-myocardial infarction. | ||
|
| ||
| MMP-9 | Reduced neointima formation after vascular injury. | 161,224,322 |
| Protection from cardiac rupture post-myocardial infarction, vessel stiffness, increased pulse pressure. | ||
|
| ||
| MMP-11 | Accelerated neointima formation after vascular injury | 323 |
|
| ||
| MMP-14 | Defective angiogenesis | 5,324 |
|
| ||
| TIMP-1 | Accelerated neointima formation after vascular injury. | 325 |
| Spontaneous cardiac dilatation, increased cardiac dysfunction post-myocardial infarction. | ||
|
| ||
| TIMP-3 | Spontaneous dilated cardiomyopathy | 326 |
MMP-9 may also be involved in VSMC migration. Tanshinone IIA, a major constituent of Salvia miltiorrhiza bunge, inhibits tumor necrosis factor-α (TNF-α)-induced human ASMC migration, partly through inhibition of MMP-9 activity. Tanshinone IIA also inhibits TNF-α-induced ERK and c-jun phosphorylation, and NF-κB and AP-1 DNA-binding.162 Suppression of MMP-9 expression by downregulation of NF-κB may also mediate the inhibitory effects of curcumin on migration of human ASMCs.163 Also, MMP-9 knockout is associated with reduced VSMC migration and neointima formation in mouse models of filament loop injury164 and carotid artery occlusion (Table 2).165
Disruption of the basement membrane is required for VSMC migration.166 MMPs degrade the basement membrane and in turn facilitate ECM-integrin interactions, leading to activation of focal adhesion kinase (FAK) and increased cell migration. MMPs also cause fragmentation of membrane components such as type I collagen, thus creating new integrin-binding sites. Growth factor receptors, cadherins and integrins mediate signalling pathways that play a role in reorganization of the cytoskeleton in preparation for cell migration.167,168 MMPs cleave E-cadherin in epithelial cells, VE-cadherin in endothelial cells and N-cadherin in VSMCs,169,170 thus dissolve adherence junctions and free the cells to move and migrate.
MMPs not only facilitate migration by promoting proteolysis of ECM proteins, but could also directly enhance cell migration. MMP-1 promotes growth and invasion of cells by binding to and cleavage of PAR-1, which reveals a tethered ligand that initiates signaling via a GPCR and stimulates cell migration.171 This mechanism may allow the cells to sense a proteolytic environment and actively move towards an area of degraded matrix.
MMP inhibitors have been useful in demonstrating the effect of MMPs on VSMC migration. Gene transfer of TIMPs reduces VSMC migration in vitro and reduces neointma formation and intima thickening in in vivo models of vascular injury. TIMPs 1–4 delivered directly or by gene transfer inhibit migration of SMCs in vitro172,173 and reduce neointima formation in human saphenous vein organ culture.174 TIMP gene transfer also preserves the tunica media basement membrane and inhibits VSMC migration to the intima. Synthetic MMP inhibitors inhibit migration of VSMC in cultured baboon arterial explant,175 and early VSMC migration in the rat model of carotid balloon injury.176 Collectively, experimental evidence supports that MMPs enhance VSMC migration via their proteolytic degradation of ECM proteins as well as direct cellular effects, and MMP inhibitors could reverse or reduce VSMC migration.
7.3. MMP Inhibitors and Role of MMPs in Smooth Muscle Proliferation
In addition to their role in facilitating VSMC migration, MMPs may regulate VSMC proliferation. VSMC proliferation at sites of endothelial cell injury and subsequent lipid deposition play a role in atheroma formation, and MMPs appear to be involved in these processes. Pretreatment of human ASMCs with ethanol extract of Buddleja officinalis attenuates high-glucose-induced cell proliferation by suppressing MMP-9 activity.177 Also, MMP-9 knockout is associated with inhibition of VSMC proliferation in mouse model of filament loop arterial injury.164 Of note, MMP-9 knockout is not associated with decreased VSMC proliferation in mouse model of carotid artery occlusion,165 likely due to compensatory activation of other proteases.178
MMPs could regulate VSMC proliferation via several mechanisms. MMPs could promote permissive interactions between VSMCs and various components of ECM. Integrin-mediated pathways may be essential for stimulation of VSMC proliferation.179,180 MMPs may free growth factors from attachment to ECM components or cell surface so that they can act on their receptors. Heparin-binding growth factors such as fibroblast growth factor-1 (FGF-1) and FGF-2, are potent mitogens for VSMCs that are released through the action of MMPs on ECM proteoglycans.7 Together with ADAMs, MMPs could facilitate the release of cell surface heparin-bound epidermal growth factor (HB-EGF), which in turn stimulates VSMC proliferation.181,182 MMPs also activate transforming growth factor-h (TGF-h) by cleaving off the latency-associated peptide.183 MMPs can also liberate active insulin-like growth factor-1 (IGF-1) by degrading its binding proteins. Together with signals from FAK, these processes upregulate and/or stabilize key regulators of the cell cycle. Dismantling of cadherin-catenin complex occurs in balloon-injured rat carotid arteries leading to increased expression of the cell cycle gene cyclin D1 which stimulates VSMC proliferation.184 MMP-induced cadherin shedding promotes dissolution of adherens junctions and translocation of h-catenin to the nucleus where it acts as a transcription factor to further promote cell proliferation.168,170
MMP inhibitors have been useful to assess the role of MMPs in VSMC proliferation. Some studies have reported excess neointima formation in rat model of carotid arteries balloon injury after treatment with the MMP inhibitor GM-6001.185,186 Other studies have shown that synthetic MMP inhibitors inhibit VSMC proliferation in vitro.170,187 Also, inhibition of MMPs is associated with decreased N-cadherin shedding, increased cell membrane N-cadherin, decreased h-catenin nuclear translocation and decreased proliferation of cultured human VSMCs. Tetracycline-based MMP inhibitors reduce VSMC migration and neointima formation in rat model of carotid artery balloon injury.176,188 Collectively, experimental evidence largely points to a stimulatory effect of MMPs on VSMC proliferation, and reversal of this effect by MMP inhibitors.
7.4. MMP Inhibitors and Role of MMPs in Angiogenesis
Angiogenesis is the process of forming new blood vessels. Angiogenesis requires degradation of the vascular basement membrane and ECM remodeling in order to allow endothelial cells to migrate into the surrounding tissue. Angiogenesis plays a role in several biological processes and pathological conditions including the progression of atherosclerotic plaques and tumor growth.189,190 MMPs mediate the effects of several pro-angiogenic factors by virtue of their proteolytic activity. Angiogenic growth factors such as FGF, TGF-α, TGF-β, TNF-α, vascular endothelial growth factor (VEGF) and angiogenin are secreted by endothelial cells and other cells, and act in an autocrine or paracrine fashion to promote angiogenesis. The expression of MMP-1, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-13, and MMP-19 is up-regulated more than 1.5-fold in human umbilical vein endothelial cells (HUVECs) treated with VEGF. VEGF induces MMP-10 expression possibly via PI3K and MAPK pathways.191 MMPs take part in remodeling of the basement membrane and degradation of various components of ECM necessary for angiogenesis. MMPs also enhance angiogenesis by detaching pericytes from the vessels, releasing ECM-bound angiogenic factors, exposing cryptic pro-angiogenic integrin binding sites in ECM, generating promigratory ECM component fragments, and cleaving endothelial cell-cell adhesions.
MT1-MMP plays a specific role in angiogenesis.192,193 Semaphorin 4D is overexpressed in cancers and promotes neovascularization upon stimulation of its Plexin-B1 receptor on endothelial cells. MT1-MMP targets semaphorin 4D and releases it from its inactive membrane bound form to act in a paracrine manner on endothelial cells.194 MT1-MMP-dependent TGF-β signaling may also be involved in prostaglandin E2-induced endothelial cord formation in cultured HUVECs195.
Upregulation of MMPs has been positively linked to tumor size and the increased angiogenic and metastatic potential of tumors. Expression of MMP-2 and MMP-9 and VEGF is positively correlated to tumor size, depth of invasion, lymphatic and venous invasion, lymph node metastasis, and microvessel density of gastric carcinomas.196 MMP-2 mediates the angiogenic effect of pituitary tumor transforming gene expression in HEK293 cells.197. Downregulation of MMP-2 decreases tumor-induced angiogenesis in cultured human microvascular endothelial cells. MMP-2 inhibition causes apoptotic cell death in vitro, and suppresses tumor growth of pre-established U-251 intracranial xenografts in nude mice.198 Overexpression of MMP-9 in human breast cancer MCF-7 cells results in increased tumor angiogenesis, tumor growth, and VEGF/VEGFR-2 complex formation.199 MMP-9 may also be involved in FGF-2/FGFR-2 pathway in the mouse angiogenesis model,200 and downregulation of MMP-9 expression inhibits tumor growth in nude mice.201 Also, MMP-3 mediates matriptase/MT-SP1-induced tumor growth and angiogenesis by enhancing ECM degradation in tumor cell microenvironment.202
While angiogenic factors can induce MMP expression in endothelial and stromal cells, MMPs can in turn enhance the availability/bioactivity of angiogenic factors. Degradation of ECM releases ECM/basement membrane-sequestered angiogenic factors such as VEGF, bFGF and TGF-β. MMP-1 and MMP-3 degrade perlecan in endothelial cell basement membranes to release b-FGF. Connective tissue growth factor forms an inactive complex with VEGF165. and cleavage of connective tissue growth factor by MMP-1, MMP-3, MMP-7, or MMP-13 releases active VEGF165. MMP-2, -3, and -7 degrade the ECM proteoglycan decorin and release latent TGF-1, and MMP-2 and MMP-9 cleave the latency-associated peptide to activate TGF-β1.203.
In support of a role of MMPs in angiogenesis, dormant tumors may secrete TIMPs to prevent the tumor from switching to the angiogenic phenotype and thereby arrest tumor growth.204,205 We should note that MMPs may exert anti-angiogenic effects through the generation of endogenous angiogenesis inhibitors by proteolytic cleavage of certain collagen chains and plasminogen. MMP-9 mediates tamoxifen-induced increase in endostatin and thus decreases angiogenesis in hormone dependent ovarian cancer.206 MMP-7, MMP-9 and MMP-12 may block angiogenesis by converting plasminogen to angiostatin, a potent angiogenesis antagonist. MMP-14 cleaves endoglin, a TGF-β co-receptor, and thus inhibits its angiogenic effect.207 Thus MMPs are important regulators of angiogenesis with an overall tendency towards stimulation, and MMP inhibitors reverse these angiogenic effects.
7.5. MMP Inhibitors and Role of MMPs in Cell Apoptosis
Apoptosis is a form of cell death that involves activation of the intracellular cysteine proteases, caspases. Apoptosis of VSMCs plays a role in attenuating intimal thickening and destabilizing atherosclerotic plaques.208,209 Several factors promote apoptosis including death signals originating from outside the cell as well as intracellular factors such as DNA damage, cell cycle status and the levels of the tumor suppressor p53.209 MMP-7 is involved in the cleavage of N-cadherin and modulation of VSMC apoptosis. In contrast, survival signals maintain VSMC viability even in the face of a pro-apoptotic environment. Survival pathways are closely linked to those triggering proliferation and therefore could be influenced by MMPs. Survival factors such as platelet-derived growth factor (PDGF), HB-EGF and IGF-1 act via tyrosine kinase receptors to stimulate the PI3K/Akt pathway. MMP-2, MMP-7 and MMP-9 cleave cell surface pro-HB-EGF and liberate the soluble active growth factor which binds to EGF-R and promotes growth.181,210 In human coronary VSMCs, oxidized low density lipoprotein (oxLDL) and 4-hydroxynonenal activate PDGFR-β and the ERK1/2 pathway and in turn increase the production of MMP-1.211 MMP-1, MMP-2, MMP-8 and MMP-9 degrade members of the IGF binding protein family and thereby increase the bioavailability of IGF-1 and its anti-apoptotic effects.7
Cell–matrix contacts promote VSMC survival, and their disruption leads to apoptosis in a process originally termed anoikis.212 ECM–integrin interactions trigger FAK activation and induce the p53 survival signaling pathway.213,214 MMP production appears to favor FAK activation and hence survival signaling. On the other hand, excess MMPs could degrade ECM proteins or integrins and promote anoikis.215 MMPs may also modulate apoptosis by cleaving death ligands such as TNF-α and Fas ligand and their receptors. MMP-1, MMP-2, MMP-8, MMP-9 and MMP-13 and the MT-MMPs 14, 15, 16 and 17 can cleave pro-TNF-α.7,216 Similarly, MMP-7 sheds Fas-L from the cell surface.217,218 Caspase-mediated cleavage of the DNA repair enzyme poly(ADP-ribose) polymerase is an important step in apoptosis. MMP-2 has been localized in the nuclei of isolated cardiac myocytes and may be involved in cleaving nuclear poly(ADP-ribose) polymerase.219
TIMP-3, but not TIMP-1 or TIMP-2, stimulates apoptosis in many cell types including VSMCs.173,218 TIMP-4 also stimulates VSMC apoptosis.220 Thus, MMPs appear to regulate VSMC apoptosis and promote cell survival via several pathways, and MMP inhibitors could oppose the effects of MMPs on cell survival and promote apoptosis.
8. MMP INHIBITORS AS POTENTIAL TOOLS IN PATHOLOGICAL CONDITIONS
Altered MMP expression/activity and the resulting MMP/TIMP imbalance could cause unrestrained tissue remodeling in multiple pathological conditions including autoimmune and inflammatory disorders, osteoarthritis and cancer. MMP/TIMP imbalance has also been implicated in cardiovascular disorders such as hypertension. atherosclerosis and aneurysm.
8.1. MMP Inhibitors and Role of MMPs in Hypertension
Hypertension is a multifactorial disorder involving alterations in the renal, neuronal and vascular control mechanisms of blood pressure. Hypertension is often associated with vascular remodeling and rearrangement of various components of the vascular wall including ECM. The elevated plasma levels of some MMPs in hypertension have suggested that the underlying pathophysiology may involve excessive elastolysis or accumulation of collagen degradation products in the vascular wall.221 Several MMPs and TIMPs may be involved in the vascular remodeling associated with hypertension. Increased MMP activity could result in increased degradation of elastin relative to collagen leading to decreased elasticity. On the other hand, decreased TIMP-1 activity could lead to accumulation of poorly cross-linked immature and unstable fibrin degradation products, resulting in misdirected deposition of collagen.221 Some studies have shown a correlation between MMP levels and hypertension (Table 3). Other studies have shown low levels of MMPs and high levels of TIMPs levels in hypertension and suggested that decreased degradation of collagen type I could play a role in the development of hypertension (Table 3). Studies have compared the effects of early and late hypertension on ECM remodeling in Dahl rats of different age groups: young salt-resistant (control), young salt-sensitive (early hypertension), middle-age salt-resistant (aging), and middle-age salt-sensitive (late hypertension). In the early phase of hypertension, several MMPs decreased, TIMP-1 increased, and total collagen increased, consistent with increased fibrosis. MMP-8 activity decreased in young salt-sensitive rats. Also, MMP-14 correlated positively with changes in left ventricular mass in early hypertension. In contrast, late hypertension was associated with increased MMP-8 and MMP-14 and decreased total collagen levels. These findings suggest downregulation and upregulation of MMPs at early versus late stages of hypertension.222 We should note that ECM remodeling in response to pressure overload is a dynamic process involving both ECM accumulation and degradation, and, in addition to the stage of hypertension, antihypertensive treatment may further modulate collagen metabolism.
Table 3.
Representative clinical studies demonstrating the relationship between MMP or TIMP levels and hypertension
| Study Year Type |
Subjects | Design | Findings | Ref |
|---|---|---|---|---|
| 1998 Clinical Trial | 37 patients with essential hypertension, 23 control normotensive subjects | Measure serum levels of carboxy-terminal telopeptide of collagen type I (marker of collagen degradation), MMP-1, TIMP-1, and MMP-1/TIMP-1 ratio. Repeat measurements after 1 year treatment with the ACE inhibitor lisinopril | No difference in collagen type I levels. Decreased MMP-1 and increased TIMP-1 in hypertensive versus normotensive subjects. Hypertensive patients with left ventricular hypertrophy showed lower free MMP-1 and collagen type I and higher free TIMP-1 than hypertensive patients without left ventricular hypertrophy. Patients treated with lisinopril showed increased serum collagen type I and free MMP-1 and decreased free TIMP-1 | 327 |
| 2003 Clinical Trial | 42 hypertensive patients | 6 Months treatment with amlodipine | Normalized plasma levels of MMP-9, but not MMP-2 | 328 |
| 2006 Cross Sectional | 44 hypertensive patients, 44 controls | Measure plasma levels of MMP-2, MMP-9, and TIMP-1 | Higher plasma levels of MMP-2, MMP-9, and TIMP-1 in hypertensive versus control subjects | 329 |
| 2007 Cross Sectional | 202 hypertensive patients, 54 control | Measure carotid-femoral and carotid-radial pulse wave velocity to determine arterial elasticity. Measure serum levels of MMP-9 and TIMP-1 levels by ELISA | Higher serum levels of MMP-9 and TIMP-1 in hypertensive patients versus control subjects. Age, systolic blood pressure, heart rate and TIMP-1 levels were independent predictors of carotid-femoral pulse wave velocity in hypertensive subjects | 330 |
| 2009 Clinical Trial | 33 patients with stage 1 hypertension, 16 age-matched control | Assess serum levels of MMP-9 and TIMP-1 in the hypertensive group before and after 3-month-anti-hypertensive therapy | Pre-treatment serum MMP-9 levels were higher and TIMP-1 levels were lower in hypertensive group versus control. Anti-hypertensive treatment was associated with decreased serum MMP-9 levels and increased TIMP-1 levels | 221 |
| 2009 Randomized Clinical Trial | 595 Non-hypertensive Framingham Offspring Study, participants without prior heart failure or myocardial infarction, mean age 55 years, (360 women) | Measure plasma levels of MMP-9, TIMP-1, and procollagen III N-terminal peptide for 4 years | 81 Subjects (51 women) developed hypertension, and 198 (114 women) progressed to higher blood pressure. Subjects with detectable MMP-9 had 1.97-fold higher risk of blood pressure progression than those with undetectable MMP-9. A 1-SD increment of log-TIMP-1 was associated with 50% higher incidence of hypertension and 21% higher risk of blood pressure progression. Individuals in the top TIMP-1 tertile had a 2.15-fold increased risk of hypertension and 1.68-fold increased risk of blood pressure progression relative to the lowest tertile. Plasma procollagen III N-terminal peptide was not associated with hypertension or blood pressure progression. | 331 |
| 2010 Cross sectional | 64 Children (34 males, 30 females) | Measure circulating levels of MMP-2, MMP-9, TIMP-2, insulin-like growth factor-I and insulin growth factor binding protein-3 | Circulating levels of MMP-2 and MMP-9 correlate with systolic blood pressure and vascular function. MMP-2 was an independent predictor of systolic blood pressure. MMP-9 was an independent predictor of vascular dysfunction | 332 |
In addition to regulation of ECM turnover, MMPs could affect vascular remodeling in hypertension via other cellular mechanisms. MMPs may mediate EGFR transactivation induced by excessive stimulation of GPCRs such as α1-adrenergic receptors which in turn promote the synthesis of contractile proteins in VSMCs and thereby contribute to vasoconstriction and hypertension. Also, in fructose treated rat model of acquired systolic hypertension and insulin resistance, the insulin-resistant VSMCs showed increased expression/activity of MMP-2 and MMP-7, EGFR, the contractile proteins myosin light chain (MLC) kinase and MLC-II, and their transcriptional activators possibly through activation of ERK1/2. Disruption of MMP-EGFR signaling normalized the increased expression of contractile proteins and their transcriptional activators in insulin-resistant VSMCs and arteries and prevented the development of hypertension in fructose treated rats.223 Also, in a study comparing the effects of treatment with angiotensin II (AngII) for 10 days in wild-type and MMP-9 knockout mice, baseline blood pressure was equivalent in both phenotypes, but AngII treatment increased systolic blood pressure to a greater extent in MMP-9 knockout than wild-type mice. In response to AngII treatment, the carotid artery pressure-diameter relationship and arterial compliance were increased in wild-type, but reduced in MMP-9 knockout mice. Also, maximal carotid artery diameter was greater in wild-type versus MMP-9 knockout mice. AngII treatment induced MMP-2 and increased carotid media thickness equally in both phenotypes. On the other hand, AngII treatment induced MMP-9 and enhanced MMP-9 in situ gelatinase activity only in wild-type mice, and vessels from these mice produced more collagen I breakdown products than MMP-9 knockout mice. Conversely, staining for collagen IV was enhanced in vessels from AngII-treated MMP-9 knockout mice. These findings suggest that the onset of AngII-induced hypertension is accompanied by increased MMP-9 activity in conductance vessels, MMP-9 deficiency results in vessel stiffness and increased pulse pressure, and MMP-9 activation may have a beneficial role in early hypertension by preserving vessel compliance and alleviating the increase in blood pressure.224
MMP/TIMP imbalance in blood vessels, particularly in the intima and media, may account for the increased proteolytic activity and maladaptive vascular remodeling in hypertension. Increased levels of MMP-2, MMP-9, and MMP-14 and enhanced gelatinolytic activity were observed in the aortas of two kidney-one clip (2K-1C) rat model of hypertension. Doxycycline treatment for 8 weeks attenuated 2K-1C hypertension, prevented the increase in the aortic intima and media thicknesses, attenuated the increases in MMP-2, MMP-9, and MMP-14 in the intima and media, but did not change the levels of TIMPs 1–4.225 MMP-2 may contribute to arterial remodeling in early hypertension by decreasing the actin-binding protein calponin-1. The absence of calponin is associated with VSMC phenotype switch, and leads to VSMC migration and vascular remodeling. In a study of Sham-operated and 2K-1C rat model of hypertension, MMP-2 activity was increased in aortas from 2K-1C rats at 1 and 2 weeks of hypertension, followed by increased VSMC proliferation, and these effects were abolished by treating 2K-1C rats with doxycycline. Increased aortic media to lumen ratio started in 2K-1C rats at 1 week of hypertension, and was established by 2 weeks. MMP-2 and calponin-1 co-localized in the cytosol of VSMCs. Aortas from 2K-1C rats showed a decrease in calponin-1 protein levels but not calponin-1 mRNA expression, at 1 week of hypertension, and doxycycline treatment prevented the decrease in calponin-1 protein level. Conversely, calponin-1 was upregulated in 2K-1C rats at 2 weeks of hypertension. These findings suggest that MMP-2 may contribute to the post-translational decrease in calponin-1, and thereby contribute to hypertension-induced maladaptive arterial remodeling.226 In VSMCs from spontaneously hypertensive rats, TNF-α increased cell migration and MMP-9 expression. Upregulation of MMP-9 was transcriptionally regulated at the AP-1 and NF-κB sites in the MMP-9 promoter, suggesting a role for increased VSMC proliferative capacity, G1 to S-phase cell-cycle progress, and MMP-9 expression in the vascular remodeling in hypertension.227 Studies in Dahl salt-sensitive rats fed high-salt diet for 6 weeks have shown that intraperitoneal treatment with the MMP inhibitor GM6001 1.2 mg/kg body weight on alternate days for 4 weeks reduced blood pressure. MMP-9 expression and activity were reduced in cerebral vessels of GM6001-treated Dahl salt-sensitive rats. GM6001 treatment ameliorated oxidative/nitrosative stress and tight junction proteins in cerebral vessels of Dahl salt-sensitive rats, suggesting restoration of vascular integrity. These findings suggest that inhibition of MMP-9 attenuates high blood pressure and hypertension-associated cerebrovascular pathology in salt-sensitive hypertension.228 Also, in spontaneously hypertensive rats, induction of acute hypertension by AngII was associated with post-transcriptional activation of vascular MMP-7, transcription of myocardial ADAM-12, a major metalloproteinase implicated in cardiac hypertrophy, and overexpression of downstream hypertrophy marker genes. Knockdown of MMP-7 attenuated hypertension, inhibited ADAM-12 expression, and prevented cardiac hypertrophy.229 In addition to cardiac hypertrophy, MMPs may also play a role in other hypertensive complications such as intracranial hemorrhage.225,230,231
8.2. MMP Inhibitors and Role of MMPs in Atherosclerosis
Atherosclerosis is a multifactorial vascular disease. Dysfunctional endothelium recruits different inflammatory pathways leading to intimal differentiation, VSMC proliferation, ox-LDL deposition, platelet activation and aggregation, and the formation of an atheroma of fat, collagen and elastin with a thin fibrous cap. Dysregulated ECM metabolism may contribute to vascular remodeling during the development and complications of atherosclerotic lesions. Enhanced MMP expression has been detected in the atherosclerotic plaque, and activation of MMPs appears to facilitate atherogenesis, platelet aggregation and plaque destabilization.232,233 MMP-1, MMP-2, MMP-3, MMP-7, MMP-9 and MMP-12 are produced by SMCs and macrophages in the arterial wall, and are highly expressed in atherosclerotic lesions.234,235 Also, the plaques' shoulders and regions of foam cell accumulation display increased expression of MMP-1, MMP-3 and MMP-9. Activated gelatinases have been found in plaque extracts, and gelatinolytic and caseinolytic activities have been detected in atherosclerotic areas, but not in unaffected arterial tissue.236 Importantly, low-fat diet is associated with reduced plaque proteolysis and decline in MMP-1 levels and macrophage content.237 Patients on hemodialysis develop atherosclerosis rapidly and show evidence of fibrinolysis/proteolysis imbalance in their plasma, and MMP-2 may play a role in the development of atherosclerosis in these patients.238 Also, the urinary levels of MMP-9 and TIMP-1 are elevated in patients with coronary artery disease and acute coronary syndrome compared with healthy volunteers.239 Plasma levels of MMP-1, MMP-3, and MMP-7 are higher in patients with high compared with those with low intima-media thickness. MMP-7 is positively associated with carotid calcification,240 and plasma levels of MMP-8 are positively associated with the occurrence of carotid plaques.241 MMP-10 is induced by C-reactive protein in endothelial cells, and is overexpressed in atherosclerotic lesions. Also, high serum levels of MMP-10 are associated with increased inflammatory markers, increased carotid intima-media thickness and atherosclerotic plaques.242
Certain genetic variants of MMPs have been associated with the progression and complications of atherosclerosis. In a 3-year coronary atherosclerosis study, the 6A active variant of the MMP-3 promoter was correlated with progression of coronary arterial lumen narrowing243 and acute myocardial infarction.244 In a study on 139 patients with coronary artery disease and 119 healthy subjects, MMP-3 5A/6A genetic variant was associated with coronary artery disease, and the PON1 variant was associated with the number of diseased coronary vessels.245 In a subgroup of the Etude Cas-Temoin de l'Infarctus du Myocarde (ECTIM) study of acute myocardial infarction, the more active T allele of an MMP-9 functional promoter polymorphism (C1562T) was more common in patients with 3-coronary vessel disease, but did not predict myocardial infarction.246 In a study of 1127 patients, higher serum levels of MMP-9 were associated with the T allele, but did not predict cardiovascular death.247 Other studies have shown associations of MMP-9 genotypes with different stages of carotid artery atherosclerosis.248 Interestingly, MMP-9 ablation reduced the size of atherosclerotic lesion in ApoE−/− mice.249 Also, in the mouse carotid artery ligation model, the plaque burden was reduced in hypercholesterolemic MMP-9 knockout compared with wild-type mice.250
MMPs contribute to the pathophysiology of atherosclerosis through several processes and pathways. Vascular inflammation is an important factor in the atherogenic process that has been shown to promote MMP expression. In a study enrolling 18 patients with stable angina, 14 patients with unstable angina and non-ST-segment elevation myocardial infarction, 14 patients with ST-elevation myocardial infarction, and 16 healthy controls, the progression of coronary artery disease was paralleled with increased MMP-9/TIMP-1 ratio in circulating CD14+ monocytes and in the serum. Similar imbalance in the expression of MMP-9 and TIMP-1 was observed in monocyte-derived macrophages within the atherosclerotic plaques.251 Cholesterol lowering 3-HMGcoA reductase inhibitors decrease the expression of various MMPs in atheromatous plaques by reducing vascular inflammation.252 For example, rosuvastatin inhibits the expression of MMP-2 and MMP-9.253
VSMC migration and proliferation are also involved in atheroma formation. MMPs enhance VSMC migration to atherogenic areas where they proliferate and in turn increase the size of the lesion. In RASMCs, the herb Salvia miltorrhia extract inhibits VSMC migration in part through downregulation of both MMP-9 and TNF-α.254. In a study using mice with genetically modified collagen that resists digestion by MMP collagenases, in an atherogenic background, the lesion size was similar in collagenase-resistant and control mice, but collagen was more abundant and SMC number was decreased in the intimal lesions of collagenase-resistant mice. These findings suggest a role for MMP-mediated collagenolysis in regulating collagen turnover and SMC proliferation in the atheromatous plaque.255
MMP-1 may mediate ox-LDL induced activation of the PDGFR-β and ERK1/2 atherogenic pathways.211 Ox-LDL also activates MMP-2 through upregulation of MT1-MMP and increases in oxidative radicals generated by the xanthine/xanthine oxidase complex.256 AngII plays a role in the pathogenesis of atherosclerosis. AngII increases the expression of MMP-9 in VSMCs via angiotensin type 1 (AT1) receptor and NF-κB pathways.257 collectively, these studies have shown an association between MMPs levels, genetic variants of certain MMPs and the atherosclerotic process.
Because MMPs degrade ECM proteins and because increased MMP levels and activity has been detected in vulnerable atherosclerotic plaques, it has been proposed that MMPs reduce the strength of the fibrous cap and contribute to plaque rupture. High-mobility group box 1 is an intracellular gene regulator protein produced by activated VSMCs that causes the progression and increases the vulnerability of atherosclerotic lesions to rupture by increasing the expression of MMP-2, MMP-3 and MMP-9.258 Areas of atherosclerotic plaque rupture show a decrease in VSMCs and increased macrophage-derived foam cells. Studies have compared brachiocephalic artery plaque instability in apoE/MMP-3, apoE/MMP-7, apoE/MMP-9, and apoE/MMP-12 double knockout mice with their age-, strain-, and sex-matched apoE knockout controls, and concluded that MMP-12 supported lesion expansion and destabilization. MMP-7 had no effect on plaque growth or stability, although it was associated with decreased VSMCs in plaques, while MMP-3 and -9 appeared to play protective roles, limiting plaque growth and promoting a stable plaque phenotype.259 MMP-1, MMP-12 and MMP-13 derived from intimal macrophages have been suggested to play a role in both plaque initiation and progression.260 On the other hand, transgenic mice that specifically express MMP-1 in macrophages show smaller plaques and no evidence of plaque rupture when compared with control littermates.261 This is likely because these mice have altered MMP-1 from birth, which could reduce collagen accumulation. MMP-3 also appears to have a dual role. Mice lacking both MMP-3 and ApoE show extensive atheromas, but reduced aneurysm formation.262 MMP-3 deficiency is associated with increased collagen and fewer macrophages in plaques, which could contribute to greater stability of atheromatous plaques. Adenoviral gene transfer of TIMP-1 into ApoE−/− mice 6 weeks after commencing a high-fat diet reduced both lesion size and macrophage content, supporting the concept that MMPs adversely affect the stability of established plaques.263 Estrogen supplementation especially late after menopause may destabilize established plaques, in part due to estrogen’s ability to upregulate MT1-MMP without a corresponding increase in TIMP-2, and consequently increased activation of MMP-2.264
Although the MMP-induced degradation of ECM proteins could contribute to plaque instability, the ability of MMPs to promote VSMC migration and proliferation may contribute to atherosclerotic plaque cap growth and stability. MMPs such as MMP-2, MMP-9, MMP-13 and MMP-14 release growth factors such as TGF-h and VEGF that are stored in ECM.265 MMP-9 releases VEGF bound to proteoglycans in ECM, enhancing its bioavailability and thereby influencing plaque neovascularization. Collectively, evidence suggests dual role for MMPs in intimal thickening and athrogenesis as well as atherosclerotic plaque rupture.178 Further studies may allow targeting of individual MMPs with specific MMP inhibitors to limit the growth of the atherosclerotic lesions and promote their stability.
Atherosclerosis in the coronary arteries could lead to acute coronary syndrome including unstable anginas and myocardial infarction. Studies have shown an association between MMPs and the development of acute coronary syndrome.266 A case-control study on 261 patients who had suffered a myocardial infarction and 194 healthy controls, all Spanish male smokers, showed that MMP-1 promoter polymorphisms are associated with the risk of early myocardial infarction.267 MMP-2 and -9 were elevated following acute myocardial infraction in 91 patients compared to 172 control subjects with stable coronary artery disease. Higher early levels of MMP-9 were also associated with the extent of left ventricular remodeling and circulating white blood cell levels.268 Increased MMP expression is also observed after coronary angioplasty, suggesting a potential role of MMPs in coronary artery restenotic lesions.269
TIMPs may play a role in atherosclerosis. TIMP-4 is detected in cardiovascular tissue areas populated by inflammatory macrophages and CD3+ T cells. Human lymphocytes, monocytes, macrophages and mast cells produce TIMP-4. In advanced atherosclerotic lesions, TIMP-4 is detected around necrotic lipid cores, whereas TIMP-3 is detected within and around the core regions indicating different roles in inflammation-induced cell apoptosis and ECM turnover.270 In a study on 238 men, TIMP-1 was positively associated with carotid intima-media thickness and carotid-femoral pulse-wave velocity.271 In a study evaluating multiple carotid wall characteristics, including wall thickness, lumen area, calcium area, lipid core, and fibrous cap measures, for associations with plasma MMPs 1, 2, 3, 7, 8, and 9 and TIMP-1, the fibrous cap thickness was greater in individuals with elevated TIMP-1 levels. Also, TIMP-1 was positively associated with measures of lipid core.240 Of note, TIMP-1 deficiency produces macrophage-rich lesions with active proteinases and medial destruction in ApoE−/− mice.272 TIMP-1-deficient mice show 30% smaller atherosclerotic lesions but increased aneurysm formation compared with control mice.273 However, pharmacological MMP inhibitors did not appear to affect the lesion size in atheroma-prone mice.274,275
The levels of MMPs and TIMPs in early post-myocardial infarction period may provide an estimate of the extent of cardiac damage and remodeling. MMP-9 and TIMP-1 correlate with echocardiographic parameters of left ventricular dysfunction after acute myocardial infarction and may identify patients at risk of subsequent left ventricular remodeling and adverse prognosis.276 MMP inhibitors have not been used extensively in cardiovascular clinical trials partly because cancer trials showed side effects such as tendinitis (possibly due to inhibition of ADAMs), lack of efficacy, and possible adverse effects.142 In one clinical trial, 100 patients requiring carotid endarterectomy were randomized to receive 200 mg/d doxycycline or placebo for 2 to 8 weeks before surgery. Carotid plaques retrieved by endarterectomy showed that doxycycline penetrated the atherosclerotic plaques, achieved acceptable tissue levels, and reduced MMP-1 levels, but had no effect on atheroma progression.277 Also, most animal studies of post-angioplasty or in-stent stenosis have shown little or only short-term beneficial effects of MMP inhibitors.
8.3. MMP Inhibitors and Role of MMPs in Aneurysm
MMPs may play a role in the pathophysiology of thoracic aortic aneurysm (TAA) and abdominal aortic aneurysm (AAA). MMPs have been associated with aneurysm growth and rupture. Also, there is an association between certain haplotypes of MMP-1, MMP-3, MMP-7, MMP-12 and MMP-13 and the risk of coronary artery aneurysms in patients with Kawazaki disease.278
MMP/TIMP imbalance may promote TAA formation.279 High levels of MMP-2 and MMP-9 have been demonstrated in patients with TAA, with MMP-9 predominantly expressed in the faster-growing anterior wall of the aneurysm while MMP-2 is higher in the slower-growing posterior wall.280 Also, a study of 28 patients with degenerative TAA, 60 patients with thoracic aortic dissection, and 111 control subjects has shown an association between a genetic variant of MMP-9 (8202A/G), TAA and dissection.281 Interestingly, different sets of MMP/TIMP imbalances were detected within TAA of patients with bicuspid versus tricuspid aortic valves.282 Experimental studies have shown that the expression of MT1-MMP, which is important for macrophage-mediated elastolysis, increases progressively after induction of TAA in mice.283,284 Elevated levels of MMP-2, MMP-8, MMP-9 and MMP-12 are detected at various stages of TAA development in mice.285 Also, induction of TAA formation in rats is associated with increased levels of MMP-2 and MMP-9, and ADAM-10 and ADAM-17.286 In the mouse model of Marfan syndrome, TAA was prevented in mice treated with the MMP inhibitor doxycycline, while mild aneurysm was evident in mice treated with the β-blocker atenolol. Doxycycline improved elastic fiber integrity, normalized aortic stiffness, and prevented vessel weakening. Also, the impaired vascular contraction and endothelium-dependent relaxation observed in the nontreated and atenolol-treated mice was improved with doxycycline.287
MMPs could also play a role in the pathophysiology of AAA. Artic aneurysm shows several histopathological changes in tunica intima and media including accumulation of lipids in foam cells, extracellular free cholesterol crystals, calcifications, thrombosis, ulcerations and rupture of the vascular layers. There is also an adventitial inflammatory infiltrate. Degradation of tunica media is a major pathophysiological feature in AAA. Loss of elastin could be an initiating event in AAA formation, and loss of collagen causes continued expansion of the aneurysm wall. Medial neovascularization is characteristic of established AAA and involves proteolytic degradation of ECM by MMPs to facilitate endothelial cell proliferation and migration. Studies demonstrated upregulation of pro-angiogenic cytokines and increased medial neovascularization at the aneurysm rupture edge. Increased collagen turnover could be a major factor in growth and rupture of AAA.288
MMP-2 and MMP-9 appear to play a role in AAA formation.289,290 Patients with AAA show elevated plasma levels of MMP-2 and MMP-9 in the range of 0.06–0.6 µg/ml.289,291,292 MMP-9 is the most abundantly expressed MMP in AAA and is produced mainly by the aneurysm-infiltrating macrophages.293 The plasma level and aortic wall expression of MMPs are especially elevated in patients with imminent aneurysm rupture. In a study examining circulating levels of MMPs in non-ruptured and ruptured AAA immediately prior to open repair, MMP-1 and MMP-9 levels were elevated in the plasma of patients with ruptured AAA versus non-ruptured AAA. A 4-fold elevation in preoperative plasma levels of MMP-9 were associated with non-survival at 30 days from rupture surgery compared with patients surviving for greater than 30 days.294 Secretion of MMP-2 and MMP-9 by human ASMCs is enhanced in tissues of AAA in response to hypoxia.295 MMP-2 and MMP-9 appear to be necessary to induce experimental AAA formation in mice,296 and targeted gene disruption of MMP-9 in mice suppresses the development of AAA.297 MMP-8 may also have a role in AAA formation. MMP-8 levels are higher in infrarenal aortic biopsies taken from AAA compared with normal aorta. Immunohistochemistry localized MMP-8 to mesenchymal cells within the adventitia of the aortic wall. On the other hand, the levels of TIMP-1 and TIMP-2 were lower in AAA than in normal aortic samples. The high levels of MMP-8 and low levels of TIMP-1 and TIMP-2 in aortic aneurysms may represent a favorable environment for collagen degradation and aneurysm formation and expansion.298
MMPs may serve as potential biomarkers for estimation of aneurysmal area and proteolytic activity.299 Plasma MMP-9 levels are correlated with aneurysmal size and expansion.300 In a study measuring the levels of MMP-9 in peripheral venous blood from 25 patients with AAA, 15 patients with atherosclerotic occlusive disease, and 5 control subjects, plasma MMP-9 levels were directly correlated with the amount of MMP-9 produced within the aneurysm tissue. Elevated MMP-9 levels were observed in one half of patients with AAA and less than 10% of those with atherosclerotic occlusive disease. Importantly, plasma MMP-9 levels decreased substantially after aneurysm repair.291 A meta-analysis of data on 580 AAA cases and 258 controls concluded that an elevated MMP-9 has 48% sensitivity and 95% specificity as a diagnostic screening test for the presence of AAA.301 However, normal MMP-9 levels may not exclude the presence of AAA (negative predictive value, 52%). Also, some studies showed no significant correlation between serum levels of MMP-9 and AAA diameter,302–305 or between the plasma and aneurysm wall levels of any MMP or TIMP and AAA diameter,306 making it important to further explore the validity and accuracy of MMP-9 and other MMPs as investigational tools of AAA.
Studies have investigated whether genetic variants of MMPs are associated with AAA risk. A study in 51 patients with AAA and 48 controls showed that variations in MMP-2 gene do not contribute to the development of AAA.307 In contrast, a study enrolling 414 AAA patients and 203 control subjects showed an association between the T allele of the C-1562T functional promoter polymorphism of the MMP-9 gene and AAA formation.308 Another study enrolling 146 AAA patients and 156 healthy individuals showed no association between MMP-9 and AAA.309 A meta-analysis of 6 gene polymorphisms (ACE I/D, MTHFR+677C>T, MMP9-1562C>T, IL-1β/3953C>T, eNOS 4a/4b and TIMP-1/+434C>T) reported in multiple case control studies, showed that 3 of these polymorphisms, ACE RR 1.33 [95% CI 1.20–1.48], MTHFR RR 1.14 [1.08–1.21] and MMP-9 RR 1.09 [1.01–1.18], were associated with a significant risk of AAA.310
The mechanism of action of MMPs in aneurysm formation has largely been attributed to their proteolytic effects on ECM proteins and subsequent weakening of the aortic wall. MMP-2 has the greatest elastolytic activity and is produced mainly by VSMCs and fibroblasts.311 Additional inhibitory effects of MMP-2 and MMP-9 on Ca2+-dependent mechanism of aortic VSM contraction may play a role in the early development of aneurysm.149 MMP-9 is a more potent inhibitor of aortic contraction than MMP-2, consistent with the dominant MMP-9 expression in AAA wall.293 Aortic VSM contractile function may contribute to the structural integrity of the aortic wall and limit its tendency to dilate in response to pulsatile forces generated with each cardiac cycle. Atrophy of the tunica media and depletion of VSMCs are consistent histological findings in AAA.312 Also, disruption of structural integrity of the tunica media e.g. in chronic aortic dissection, often leads to late aneurysm formation. MMP-induced inhibition of VSM contraction may function synergistically with MMP-induced degradation of ECM, causing further weakening of the aortic wall and aneurysm formation.
Studies have investigated the effects of MMP inhibitors on AAA growth and rupture. Small randomized clinical trials suggested favorable effects of doxycycline on retarding AAA expansion.313 One study demonstrated that two weeks doxycycline treatment in patients with advanced AAA resulted in reduction of aortic wall neutrophil and cytotoxic T-cell content, and suppression of the inflammatory cytokines IL-6 and IL-8 and transcription factors AP-1, C/EBP and STAT3.314 In another study, patients undergoing endovascular AAA repair were randomized to doxycycline or placebo for 6 months following the procedure. Plasma MMP-9 decreased below baseline in doxycycline treated patients while there was an insignificant increase in the placebo group. In patients with endoleaks at 6 months, plasma MMP-9 increased in 83% of the placebo group, but in only 14% of doxycycline-treated group. Among endoleak-free patients with AneuRx or Excluder endografts, doxycycline caused greater decreases in maximum aortic diameter and the aortic neck dilatation than placebo.300 Thus, MMP inhibitors may provide an alternative therapeutic tool in AAA.
9. CONCLUDING REMARKS
MMPs play an important role in ECM metabolism and other biological processes, and increased MMP expression/activity has been associated with unrestrained tissue remodeling in autoimmune and vascular disease as well as cancer. MMPs, TIMPs and the MMP/TIMP ratio could provide an estimate of the extent of tissue remodeling in various biological processes and pathological conditions. One challenge is that studies often focus on measuring certain MMPs or TIMPs, and it is important to not generalize the findings to other MMPs and TIMPs. Tissue remodeling is a dynamic process, and an increase in one MMP in a certain region may be paralleled by a decrease of other MMPs in other regions. Also, because of the differences in the proteolytic activities of different MMPs towards different substrates, the activities of MMPs may vary during the course of cardiovascular disease. Therefore it is critical to examine different MMPs and TIMPs in various regions and at different stages of the disease.
MMP inhibitors have been used as investigational tools to determine the role of MMPs in biological processes and pathological conditions. Synthetic MMP inhibitors include ZBG, non-ZBG, and mechanism-based inhibitors. MMP inhibitors may represent potential therapeutic tools in the management of osteoarthritis, cancer and cardiovascular disease. However, most of the currently available MMP inhibitors show little specificity toward different MMPs. Also, most of the clinical trials of MMP inhibitors were not very successful, in part due to the lack of MMP inhibitor efficacy.315 The low efficacy of MMP inhibitors in clinical trials may be related to the design of the study where MMP inhibitors were often used as single agent therapy in patients with advanced disease, beyond the stage when these compounds could have been effective.142 Studies in animal models have suggested that MMP inhibitors could be effective in preventing the development and progression of early disease, but may have little effect in advanced stages of the disease. A promising preclinical proof-of-principle study tested whether early treatment with a selective MMP inhibitor between the time of diagnosis and definitive surgery, the so-called "window-of-opportunity," can inhibit breast cancer metastasis and thereby improve survival. The 4T1 mouse model of aggressive mammary carcinoma was treated with SD-7300 (SC-81490), an oral inhibitor of MMP-2, MMP-9, and MMP-13, starting after the initial detection of the primary tumor. Seven days later, the primary tumors were excised and analyzed for MMP activity, and the SD-7300 treatment was discontinued. After 4 weeks, the animals were sacrificed and their lungs were analyzed histologically for number of metastases and metastatic burden (metastases' area/lung section area). SD-7300 treatment inhibited 70% to 80% of tumor-associated MMP activity, reduced lung metastasis number and metastatic burden by 50% to 60%, and increased survival, relative to control vehicle. These findings suggest that treatment of early invasive breast cancer with selective MMP inhibitors can lower the risk of recurrence and increase long-term disease-free survival.316
Another limitation of MMP inhibitors is that they may cause musculoskeletal side effects including joint stiffness, inflammation, pain, and tendinitis.143 Classic MMP inhibitors may have poor selectivity to specific MMPs and could cause additional biological side effects.75 New approaches have attempted to address some of the drawbacks of classic MMP inhibitors. Specific antibodies could target the MMP active site in a more specific fashion and could identify sites on the MMP molecule that determine their extracellular location and substrate specificity. Specific MMP siRNA could inhibit the expression of specific MMP mRNA. Despite the marked advances in the design of MMP inhibitors, therapeutic inhibition of MMPs remains a challenge, and the tetracycline antibiotic doxycycline is the only FDA-approved MMP inhibitor.317 New MMP inhibitors with better selectivity could improve the specificity of MMP inhibitors, target specific MMPs in relevant pathological conditions, and mitigate some of the side effects. Targeted delivery of MMP inhibitors locally in the vicinity of the lesion could also minimize their systemic undesirable effects. Co-delivery of drug and gene is a new strategy in cancer therapy. Studies have utilized folate-targeted star-shaped cationic copolymer consisting of β-cyclodextrin and poly(L-lysine) dendron to co-deliver docetaxel chemotherapy and MMP-9 siRNA for treatment of nasopharyngeal carcinoma. Codelivery of docetaxel/MMP-9 siRNA induced more apoptosis and decreased invasive capacity of HEN-1 nasopharyngeal carcinoma cells. In vivo assays showed that codelivery of docetaxel/MMP-9 siRNA inhibited HNE-1 tumor growth and decreased expression of the cell proliferation marker proliferating-cell nuclear antigen (PCNA), suggesting that it could be a promising strategy for treatment of localized tumors such as nasopharyngeal carcinoma.318
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-111775). Dr. Jie Liu was a visiting scholar from the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital, Beijing, P.R. China, and a recipient of scholarship from the China Scholarship Council.
ABREVIATIONS
- AAA
abdominal aortic aneurysm
- ADAM
a disintegrin and metalloproteinase
- AngII
angiotensin II
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ERK
extracellular signal–regulated kinase
- FAK
focal adhesion kinase
- FGF
fibroblast growth factor
- GPCR
G-protein coupled receptor
- HUVECs
human umbilical vein endothelial cells
- IGF
insulin-like growth factor
- IVC
inferior vena cava
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- NO
nitric oxide
- oxLDL
oxidized low density lipoprotein
- PARs
protease-activated receptors
- PDGF
platelet-derived growth factor
- RASMCs
rat aortic smooth muscle cells
- TAA
thoracic aortic aneurysm
- TGF
transforming growth factor
- TIMP
tissue inhibitor of metalloproteinases
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VSM
vascular smooth muscle
- VSMCs
VSM cells
- Zn2+
zinc
- ZBG
zinc binding globulin
Footnotes
CONFLICT OF INTEREST
None
References
- 1.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42(3):113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 4.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272(4):2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 5.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 6.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 8.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108(5):1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 9.Eck SM, Blackburn JS, Schmucker AC, Burrage PS, Brinckerhoff CE. Matrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancer. J Autoimmun. 2009;33(3–4):214–221. doi: 10.1016/j.jaut.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 11.Kucukguven A, Khalil RA. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr Drug Targets. 2013;14(3):287–324. [PMC free article] [PubMed] [Google Scholar]
- 12.Bode W, Fernandez-Catalan C, Grams F, Gomis-Ruth FX, Nagase H, Tschesche H, Maskos K. Insights into MMP-TIMP interactions. Ann N Y Acad Sci. 1999;878:73–91. doi: 10.1111/j.1749-6632.1999.tb07675.x. [DOI] [PubMed] [Google Scholar]
- 13.Park HI, Jin Y, Hurst DR, Monroe CA, Lee S, Schwartz MA, Sang QX. The intermediate S1' pocket of the endometase/matrilysin-2 active site revealed by enzyme inhibition kinetic studies, protein sequence analyses, and homology modeling. J Biol Chem. 2003;278(51):51646–51653. doi: 10.1074/jbc.M310109200. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen JA, Major Jourden JL, Miller MT, Cohen SM. To bind zinc or not to bind zinc: an examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta. 2010;1803(1):72–94. doi: 10.1016/j.bbamcr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Pelmenschikov V, Siegbahn PE. Catalytic mechanism of matrix metalloproteinases: two-layered ONIOM study. Inorg Chem. 2002;41(22):5659–5666. doi: 10.1021/ic0255656. [DOI] [PubMed] [Google Scholar]
- 16.MacColl E, Khalil RA. Matrix Metalloproteinases as Regulators of Vein Structure and Function: Implications in Chronic Venous Disease. The Journal of pharmacology and experimental therapeutics. 2015;355(3):410–428. doi: 10.1124/jpet.115.227330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skiles JW, Gonnella NC, Jeng AY. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr Med Chem. 2001;8(4):425–474. doi: 10.2174/0929867013373417. [DOI] [PubMed] [Google Scholar]
- 18.Lovejoy B, Hassell AM, Luther MA, Weigl D, Jordan SR. Crystal structures of recombinant 19-kDa human fibroblast collagenase complexed to itself. Biochemistry. 1994;33(27):8207–8217. doi: 10.1021/bi00193a006. [DOI] [PubMed] [Google Scholar]
- 19.Manzetti S, McCulloch DR, Herington AC, van der Spoel D. Modeling of enzyme-substrate complexes for the metalloproteases MMP-3, ADAM-9 and ADAM-10. J Comput Aided Mol Des. 2003;17(9):551–565. doi: 10.1023/b:jcam.0000005765.13637.38. [DOI] [PubMed] [Google Scholar]
- 20.Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstet Gynecol. 2008;199(1):78 e71–76. doi: 10.1016/j.ajog.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, Zucker S. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340) Am J Respir Cell Mol Biol. 2001;25(6):717–724. doi: 10.1165/ajrcmb.25.6.4558f. [DOI] [PubMed] [Google Scholar]
- 22.Norgauer J, Hildenbrand T, Idzko M, Panther E, Bandemir E, Hartmann M, Vanscheidt W, Herouy Y. Elevated expression of extracellular matrix metalloproteinase inducer (CD147) and membrane-type matrix metalloproteinases in venous leg ulcers. Br J Dermatol. 2002;147(6):1180–1186. doi: 10.1046/j.1365-2133.2002.05025.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29(44):10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- 25.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension. 1996;28(3):505–506. [PubMed] [Google Scholar]
- 26.Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, Crabbe T, Clements J, d'Ortho MP, Murphy G. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273(2):871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275(34):26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, Aoki T, Seiki M. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20(17):4782–4793. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudo T, Takino T, Miyamori H, Thompson EW, Sato H. Substrate choice of membrane-type 1 matrix metalloproteinase is dictated by tissue inhibitor of metalloproteinase-2 levels. Cancer Sci. 2007;98(4):563–568. doi: 10.1111/j.1349-7006.2007.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Ven WJ, Voorberg J, Fontijn R, Pannekoek H, van den Ouweland AM, van Duijnhoven HL, Roebroek AJ, Siezen RJ. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990;14(4):265–275. doi: 10.1007/BF00429896. [DOI] [PubMed] [Google Scholar]
- 31.Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375(6528):244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- 32.Velasco G, Pendas AM, Fueyo A, Knauper V, Murphy G, Lopez-Otin C. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999;274(8):4570–4576. doi: 10.1074/jbc.274.8.4570. [DOI] [PubMed] [Google Scholar]
- 33.Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265(1–2):87–93. doi: 10.1016/s0378-1119(01)00360-2. [DOI] [PubMed] [Google Scholar]
- 34.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276(44):41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 35.Fu X, Kao JL, Bergt C, Kassim SY, Huq NP, d'Avignon A, Parks WC, Mecham RP, Heinecke JW. Oxidative cross-linking of tryptophan to glycine restrains matrix metalloproteinase activity: specific structural motifs control protein oxidation. J Biol Chem. 2004;279(8):6209–6212. doi: 10.1074/jbc.C300506200. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, Ichimori K, Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342(2):261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 37.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 38.Chen LC, Noelken ME, Nagase H. Disruption of the cysteine-75 and zinc ion coordination is not sufficient to activate the precursor of human matrix metalloproteinase 3 (stromelysin 1) Biochemistry. 1993;32(39):10289–10295. doi: 10.1021/bi00090a003. [DOI] [PubMed] [Google Scholar]
- 39.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267(6):3581–3584. [PubMed] [Google Scholar]
- 40.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006;69(3):625–635. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Murphy G, Houbrechts A, Cockett MI, Williamson RA, O'Shea M, Docherty AJ. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991;30(33):8097–8102. doi: 10.1021/bi00247a001. [DOI] [PubMed] [Google Scholar]
- 42.Williamson RA, Marston FA, Angal S, Koklitis P, Panico M, Morris HR, Carne AF, Smith BJ, Harris TJ, Freedman RB. Disulphide bond assignment in human tissue inhibitor of metalloproteinases (TIMP) Biochem J. 1990;268(2):267–274. doi: 10.1042/bj2680267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115(Pt 19):3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 44.Batra J, Robinson J, Soares AS, Fields AP, Radisky DC, Radisky ES. Matrix metalloproteinase-10 (MMP-10) interaction with tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2: binding studies and crystal structure. J Biol Chem. 2012;287(19):15935–15946. doi: 10.1074/jbc.M112.341156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng Q, Malinovskii V, Huang W, Hu Y, Chung L, Nagase H, Bode W, Maskos K, Brew K. Residue 2 of TIMP-1 is a major determinant of affinity and specificity for matrix metalloproteinases but effects of substitutions do not correlate with those of the corresponding P1' residue of substrate. J Biol Chem. 1999;274(15):10184–10189. doi: 10.1074/jbc.274.15.10184. [DOI] [PubMed] [Google Scholar]
- 46.Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102(5):647–655. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 47.Desrochers PE, Mookhtiar K, Van Wart HE, Hasty KA, Weiss SJ. Proteolytic inactivation of alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin by oxidatively activated human neutrophil metalloproteinases. J Biol Chem. 1992;267(7):5005–5012. [PubMed] [Google Scholar]
- 48.Kveiborg M, Jacobsen J, Lee MH, Nagase H, Wewer UM, Murphy G. Selective inhibition of ADAM12 catalytic activity through engineering of tissue inhibitor of metalloproteinase 2 (TIMP-2) Biochem J. 2010;430(1):79–86. doi: 10.1042/BJ20100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amour A, Knight CG, Webster A, Slocombe PM, Stephens PE, Knauper V, Docherty AJ, Murphy G. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473(3):275–279. doi: 10.1016/s0014-5793(00)01528-3. [DOI] [PubMed] [Google Scholar]
- 50.Jacobsen J, Visse R, Sorensen HP, Enghild JJ, Brew K, Wewer UM, Nagase H. Catalytic properties of ADAM12 and its domain deletion mutants. Biochemistry. 2008;47(2):537–547. doi: 10.1021/bi701629c. [DOI] [PubMed] [Google Scholar]
- 51.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276(16):12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293(1):501–508. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- 53.Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, Mak TW, Khokha R. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3) J Clin Invest. 2001;108(6):817–829. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fata JE, Leco KJ, Voura EB, Yu HY, Waterhouse P, Murphy G, Moorehead RA, Khokha R. Accelerated apoptosis in the Timp-3-deficient mammary gland. J Clin Invest. 2001;108(6):831–841. doi: 10.1172/JCI13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265(29):17401–17404. [PubMed] [Google Scholar]
- 56.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29(5):290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107(6):789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 58.Herman MP, Sukhova GK, Kisiel W, Foster D, Kehry MR, Libby P, Schonbeck U. Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest. 2001;107(9):1117–1126. doi: 10.1172/JCI10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goffin L, Fagagnini S, Vicari A, Mamie C, Melhem H, Weder B, Lutz C, Lang S, Scharl M, Rogler G, Chvatchko Y, Hausmann M. Anti-MMP-9 Antibody: A Promising Therapeutic Strategy for Treatment of Inflammatory Bowel Disease Complications with Fibrosis. Inflamm Bowel Dis. 2016;22(9):2041–2057. doi: 10.1097/MIB.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 60.Suenaga N, Mori H, Itoh Y, Seiki M. CD44 binding through the hemopexin-like domain is critical for its shedding by membrane-type 1 matrix metalloproteinase. Oncogene. 2005;24(5):859–868. doi: 10.1038/sj.onc.1208258. [DOI] [PubMed] [Google Scholar]
- 61.Ugarte-Berzal E, Vandooren J, Bailon E, Opdenakker G, Garcia-Pardo A. Inhibition of MMP-9-dependent Degradation of Gelatin, but Not Other MMP-9 Substrates, by the MMP-9 Hemopexin Domain Blades 1 and 4. The Journal of biological chemistry. 2016;291(22):11751–11760. doi: 10.1074/jbc.M115.708438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chetty C, Bhoopathi P, Joseph P, Chittivelu S, Rao JS, Lakka S. Adenovirus-mediated small interfering RNA against matrix metalloproteinase-2 suppresses tumor growth and lung metastasis in mice. Mol Cancer Ther. 2006;5(9):2289–2299. doi: 10.1158/1535-7163.MCT-06-0169. [DOI] [PubMed] [Google Scholar]
- 63.Marusak C, Bayles I, Ma J, Gooyit M, Gao M, Chang M, Bedogni B. The thiirane-based selective MT1-MMP/MMP2 inhibitor ND-322 reduces melanoma tumor growth and delays metastatic dissemination. Pharmacol Res. 2016;113(Pt A):515–520. doi: 10.1016/j.phrs.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Laan WH, Quax PH, Seemayer CA, Huisman LG, Pieterman EJ, Grimbergen JM, Verheijen JH, Breedveld FC, Gay RE, Gay S, Huizinga TW, Pap T. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 2003;10(3):234–242. doi: 10.1038/sj.gt.3301871. [DOI] [PubMed] [Google Scholar]
- 65.Mannello F, Medda V, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide inhibition of MMP-9 gelatinase secretion and activity: possible pharmacological role against collagen degradation in vascular chronic diseases. Curr Vasc Pharmacol. 2013;11(3):354–365. doi: 10.2174/1570161111311030010. [DOI] [PubMed] [Google Scholar]
- 66.Serra R, Gallelli L, Conti A, De Caridi G, Massara M, Spinelli F, Buffone G, Calio FG, Amato B, Ceglia S, Spaziano G, Scaramuzzino L, Ferrarese AG, Grande R, de Franciscis S. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Des Devel Ther. 2014;8:519–527. doi: 10.2147/DDDT.S61770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walter M, Simanovich E, Brod V, Lahat N, Bitterman H, Rahat MA. An epitope-specific novel anti-EMMPRIN polyclonal antibody inhibits tumor progression. Oncoimmunology. 2015;5(2):e1078056. doi: 10.1080/2162402X.2015.1078056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mix KS, Coon CI, Rosen ED, Suh N, Sporn MB, Brinckerhoff CE. Peroxisome proliferator-activated receptor-gamma-independent repression of collagenase gene expression by 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid and prostaglandin 15-deoxy-delta(12,14) J2: a role for Smad signaling. Mol Pharmacol. 2004;65(2):309–318. doi: 10.1124/mol.65.2.309. [DOI] [PubMed] [Google Scholar]
- 69.Dorecka M, Francuz T, Garczorz W, Siemianowicz K, Romaniuk W. The influence of elastin degradation products, glucose and atorvastatin on metalloproteinase-1, -2, -9 and tissue inhibitor of metalloproteinases-1, -2, -3 expression in human retinal pigment epithelial cells. Acta Biochim Pol. 2014;61(2):265–270. [PubMed] [Google Scholar]
- 70.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23(5):769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 71.Ichihara S, Noda A, Nagata K, Obata K, Xu J, Ichihara G, Oikawa S, Kawanishi S, Yamada Y, Yokota M. Pravastatin increases survival and suppresses an increase in myocardial matrix metalloproteinase activity in a rat model of heart failure. Cardiovasc Res. 2006;69(3):726–735. doi: 10.1016/j.cardiores.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Guo H, Lee JD, Uzui H, Toyoda K, Geshi T, Yue H, Ueda T. Effects of copper and zinc on the production of homocysteine-induced extracellular matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells. Acta Cardiol. 2005;60(4):353–359. doi: 10.2143/AC.60.4.2004982. [DOI] [PubMed] [Google Scholar]
- 73.Li PC, Pan CH, Sheu MJ, Wu CC, Ma WF, Wu CH. Deep sea water prevents balloon angioplasty-induced hyperplasia through MMP-2: an in vitro and in vivo study. PLoS One. 2014;9(5):e96927. doi: 10.1371/journal.pone.0096927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newsome AL, Johnson JP, Seipelt RL, Thompson MW. Apolactoferrin inhibits the catalytic domain of matrix metalloproteinase-2 by zinc chelation. Biochem Cell Biol. 2007;85(5):563–572. doi: 10.1139/o07-073. [DOI] [PubMed] [Google Scholar]
- 75.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 76.Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15(6):2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 77.Gall AL, Ruff M, Kannan R, Cuniasse P, Yiotakis A, Dive V, Rio MC, Basset P, Moras D. Crystal structure of the stromelysin-3 (MMP-11) catalytic domain complexed with a phosphinic inhibitor mimicking the transition-state. J Mol Biol. 2001;307(2):577–586. doi: 10.1006/jmbi.2001.4493. [DOI] [PubMed] [Google Scholar]
- 78.Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS (Experientia Supplementum) 2012;103:209–279. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao BG. Recent developments in the design of specific Matrix Metalloproteinase inhibitors aided by structural and computational studies. Curr Pharm Des. 2005;11(3):295–322. doi: 10.2174/1381612053382115. [DOI] [PubMed] [Google Scholar]
- 80.Brown S, Meroueh SO, Fridman R, Mobashery S. Quest for selectivity in inhibition of matrix metalloproteinases. Curr Top Med Chem. 2004;4(12):1227–1238. doi: 10.2174/1568026043387854. [DOI] [PubMed] [Google Scholar]
- 81.Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56(10):3326–3335. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- 82.Elaut G, Rogiers V, Vanhaecke T. The pharmaceutical potential of histone deacetylase inhibitors. Curr Pharm Des. 2007;13(25):2584–2620. doi: 10.2174/138161207781663064. [DOI] [PubMed] [Google Scholar]
- 83.Moss ML, Sklair-Tavron L, Nudelman R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008;4(6):300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 84.Cherney RJ, Mo R, Meyer DT, Hardman KD, Liu RQ, Covington MB, Qian M, Wasserman ZR, Christ DD, Trzaskos JM, Newton RC, Decicco CP. Sultam hydroxamates as novel matrix metalloproteinase inhibitors. J Med Chem. 2004;47(12):2981–2983. doi: 10.1021/jm049833g. [DOI] [PubMed] [Google Scholar]
- 85.Nakatani S, Ikura M, Yamamoto S, Nishita Y, Itadani S, Habashita H, Sugiura T, Ogawa K, Ohno H, Takahashi K, Nakai H, Toda M. Design and synthesis of novel metalloproteinase inhibitors. Bioorg Med Chem. 2006;14(15):5402–5422. doi: 10.1016/j.bmc.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 86.Rossello A, Nuti E, Catalani MP, Carelli P, Orlandini E, Rapposelli S, Tuccinardi T, Atkinson SJ, Murphy G, Balsamo A. A new development of matrix metalloproteinase inhibitors: twin hydroxamic acids as potent inhibitors of MMPs. Bioorg Med Chem Lett. 2005;15(9):2311–2314. doi: 10.1016/j.bmcl.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Subramaniam R, Haldar MK, Tobwala S, Ganguly B, Srivastava DK, Mallik S. Novel bis-(arylsulfonamide) hydroxamate-based selective MMP inhibitors. Bioorg Med Chem Lett. 2008;18(11):3333–3337. doi: 10.1016/j.bmcl.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitlock GA, Dack KN, Dickinson RP, Lewis ML. A novel series of highly selective inhibitors of MMP-3. Bioorg Med Chem Lett. 2007;17(24):6750–6753. doi: 10.1016/j.bmcl.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 89.Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5(3):203–220. doi: 10.2174/1568009053765799. [DOI] [PubMed] [Google Scholar]
- 90.Fingleton B. MMPs as therapeutic targets--still a viable option? Semin Cell Dev Biol. 2008;19(1):61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scozzafava A, Supuran CT. Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV N-hydroxysulfonamides inhibit both these zinc enzymes. J Med Chem. 2000;43(20):3677–3687. doi: 10.1021/jm000027t. [DOI] [PubMed] [Google Scholar]
- 92.Pochetti G, Gavuzzo E, Campestre C, Agamennone M, Tortorella P, Consalvi V, Gallina C, Hiller O, Tschesche H, Tucker PA, Mazza F. Structural insight into the stereoselective inhibition of MMP-8 by enantiomeric sulfonamide phosphonates. J Med Chem. 2006;49(3):923–931. doi: 10.1021/jm050787+. [DOI] [PubMed] [Google Scholar]
- 93.Wojtowicz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs. 1997;15(1):61–75. doi: 10.1023/a:1005722729132. [DOI] [PubMed] [Google Scholar]
- 94.Puerta DT, Lewis JA, Cohen SM. New beginnings for matrix metalloproteinase inhibitors: identification of high-affinity zinc-binding groups. J Am Chem Soc. 2004;126(27):8388–8389. doi: 10.1021/ja0485513. [DOI] [PubMed] [Google Scholar]
- 95.Auge F, Hornebeck W, Decarme M, Laronze JY. Improved gelatinase a selectivity by novel zinc binding groups containing galardin derivatives. Bioorg Med Chem Lett. 2003;13(10):1783–1786. doi: 10.1016/s0960-894x(03)00214-2. [DOI] [PubMed] [Google Scholar]
- 96.Ledour G, Moroy G, Rouffet M, Bourguet E, Guillaume D, Decarme M, Elmourabit H, Auge F, Alix AJ, Laronze JY, Bellon G, Hornebeck W, Sapi J. Introduction of the 4-(4-bromophenyl)benzenesulfonyl group to hydrazide analogs of Ilomastat leads to potent gelatinase B (MMP-9) inhibitors with improved selectivity. Bioorg Med Chem. 2008;16(18):8745–8759. doi: 10.1016/j.bmc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 97.Hurst DR, Schwartz MA, Jin Y, Ghaffari MA, Kozarekar P, Cao J, Sang QX. Inhibition of enzyme activity of and cell-mediated substrate cleavage by membrane type 1 matrix metalloproteinase by newly developed mercaptosulphide inhibitors. Biochem J. 2005;392(Pt 3):527–536. doi: 10.1042/BJ20050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffman A, Qadri B, Frant J, Katz Y, Bhusare SR, Breuer E, Hadar R, Reich R. Carbamoylphosphonate matrix metalloproteinase inhibitors 6: cis-2-aminocyclohexylcarbamoylphosphonic acid, a novel orally active antimetastatic matrix metalloproteinase-2 selective inhibitor--synthesis and pharmacodynamic and pharmacokinetic analysis. J Med Chem. 2008;51(5):1406–1414. doi: 10.1021/jm701087n. [DOI] [PubMed] [Google Scholar]
- 99.Breuer E, Salomon CJ, Katz Y, Chen W, Lu S, Roschenthaler GV, Hadar R, Reich R. Carbamoylphosphonates, a new class of in vivo active matrix metalloproteinase inhibitors. 1. Alkyl- and cycloalkylcarbamoylphosphonic acids. J Med Chem. 2004;47(11):2826–2832. doi: 10.1021/jm030386z. [DOI] [PubMed] [Google Scholar]
- 100.Li J, Rush TS, 3rd, Li W, DeVincentis D, Du X, Hu Y, Thomason JR, Xiang JS, Skotnicki JS, Tam S, Cunningham KM, Chockalingam PS, Morris EA, Levin JI. Synthesis and SAR of highly selective MMP-13 inhibitors. Bioorg Med Chem Lett. 2005;15(22):4961–4966. doi: 10.1016/j.bmcl.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 2003;167(8):1083–1089. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- 102.Jacobsen FE, Lewis JA, Cohen SM. A new role for old ligands: discerning chelators for zinc metalloproteinases. J Am Chem Soc. 2006;128(10):3156–3157. doi: 10.1021/ja057957s. [DOI] [PubMed] [Google Scholar]
- 103.Cook GR, Manivannan E, Underdahl T, Lukacova V, Zhang Y, Balaz S. Synthesis and evaluation of novel oxazoline MMP inhibitors. Bioorg Med Chem Lett. 2004;14(19):4935–4939. doi: 10.1016/j.bmcl.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 104.Grams F, Brandstetter H, D'Alo S, Geppert D, Krell HW, Leinert H, Livi V, Menta E, Oliva A, Zimmermann G, Gram F, Livi VE. Pyrimidine-2,4,6-Triones: a new effective and selective class of matrix metalloproteinase inhibitors. Biol Chem. 2001;382(8):1277–1285. doi: 10.1515/BC.2001.159. [DOI] [PubMed] [Google Scholar]
- 105.Foley LH, Palermo R, Dunten P, Wang P. Novel 5,5-disubstitutedpyrimidine-2,4,6-triones as selective MMP inhibitors. Bioorg Med Chem Lett. 2001;11(8):969–972. doi: 10.1016/s0960-894x(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 106.Kim SH, Pudzianowski AT, Leavitt KJ, Barbosa J, McDonnell PA, Metzler WJ, Rankin BM, Liu R, Vaccaro W, Pitts W. Structure-based design of potent and selective inhibitors of collagenase-3 (MMP-13) Bioorg Med Chem Lett. 2005;15(4):1101–1106. doi: 10.1016/j.bmcl.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 107.Blagg JA, Noe MC, Wolf-Gouveia LA, Reiter LA, Laird ER, Chang SP, Danley DE, Downs JT, Elliott NC, Eskra JD, Griffiths RJ, Hardink JR, Haugeto AI, Jones CS, Liras JL, Lopresti-Morrow LL, Mitchell PG, Pandit J, Robinson RP, Subramanyam C, Vaughn-Bowser ML, Yocum SA. Potent pyrimidinetrione-based inhibitors of MMP-13 with enhanced selectivity over MMP-14. Bioorg Med Chem Lett. 2005;15(7):1807–1810. doi: 10.1016/j.bmcl.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 108.Reiter LA, Freeman-Cook KD, Jones CS, Martinelli GJ, Antipas AS, Berliner MA, Datta K, Downs JT, Eskra JD, Forman MD, Greer EM, Guzman R, Hardink JR, Janat F, Keene NF, Laird ER, Liras JL, Lopresti-Morrow LL, Mitchell PG, Pandit J, Robertson D, Sperger D, Vaughn-Bowser ML, Waller DM, Yocum SA. Potent, selective pyrimidinetrione-based inhibitors of MMP-13. Bioorg Med Chem Lett. 2006;16(22):5822–5826. doi: 10.1016/j.bmcl.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 109.Freeman-Cook KD, Reiter LA, Noe MC, Antipas AS, Danley DE, Datta K, Downs JT, Eisenbeis S, Eskra JD, Garmene DJ, Greer EM, Griffiths RJ, Guzman R, Hardink JR, Janat F, Jones CS, Martinelli GJ, Mitchell PG, Laird ER, Liras JL, Lopresti-Morrow LL, Pandit J, Reilly UD, Robertson D, Vaughn-Bowser ML, Wolf-Gouviea LA, Yocum SA. Potent, selective spiropyrrolidine pyrimidinetrione inhibitors of MMP-13. Bioorg Med Chem Lett. 2007;17(23):6529–6534. doi: 10.1016/j.bmcl.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 110.Maquoi E, Sounni NE, Devy L, Olivier F, Frankenne F, Krell HW, Grams F, Foidart JM, Noel A. Anti-invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin Cancer Res. 2004;10(12 Pt 1):4038–4047. doi: 10.1158/1078-0432.CCR-04-0125. [DOI] [PubMed] [Google Scholar]
- 111.Puerta DT, Cohen SM. Examination of novel zinc-binding groups for use in matrix metalloproteinase inhibitors. Inorg Chem. 2003;42(11):3423–3430. doi: 10.1021/ic026029g. [DOI] [PubMed] [Google Scholar]
- 112.Jacobsen FE, Lewis JA, Cohen SM. The design of inhibitors for medicinally relevant metalloproteins. ChemMedChem. 2007;2(2):152–171. doi: 10.1002/cmdc.200600204. [DOI] [PubMed] [Google Scholar]
- 113.Puerta DT, Griffin MO, Lewis JA, Romero-Perez D, Garcia R, Villarreal FJ, Cohen SM. Heterocyclic zinc-binding groups for use in next-generation matrix metalloproteinase inhibitors: potency, toxicity, and reactivity. J Biol Inorg Chem. 2006;11(2):131–138. doi: 10.1007/s00775-005-0053-x. [DOI] [PubMed] [Google Scholar]
- 114.Puerta DT, Mongan J, Tran BL, McCammon JA, Cohen SM. Potent, selective pyrone-based inhibitors of stromelysin-1. J Am Chem Soc. 2005;127(41):14148–14149. doi: 10.1021/ja054558o. [DOI] [PubMed] [Google Scholar]
- 115.Agrawal A, Romero-Perez D, Jacobsen JA, Villarreal FJ, Cohen SM. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem. 2008;3(5):812–820. doi: 10.1002/cmdc.200700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang YM, Fan X, Chakaravarty D, Xiang B, Scannevin RH, Huang Z, Ma J, Burke SL, Karnachi P, Rhodes KJ, Jackson PF. 1-Hydroxy-2-pyridinone-based MMP inhibitors: synthesis and biological evaluation for the treatment of ischemic stroke. Bioorg Med Chem Lett. 2008;18(1):409–413. doi: 10.1016/j.bmcl.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 117.Yan YL, Cohen SM. Efficient synthesis of 5-amido-3-hydroxy-4-pyrones as inhibitors of matrix metalloproteinases. Org Lett. 2007;9(13):2517–2520. doi: 10.1021/ol0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Acharya MR, Venitz J, Figg WD, Sparreboom A. Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. Drug Resist Updat. 2004;7(3):195–208. doi: 10.1016/j.drup.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 119.Zakeri B, Wright GD. Chemical biology of tetracycline antibiotics. Biochem Cell Biol. 2008;86(2):124–136. doi: 10.1139/O08-002. [DOI] [PubMed] [Google Scholar]
- 120.Voils SA, Evans ME, Lane MT, Schosser RH, Rapp RP. Use of macrolides and tetracyclines for chronic inflammatory diseases. Ann Pharmacother. 2005;39(1):86–94. doi: 10.1345/aph.1E282. [DOI] [PubMed] [Google Scholar]
- 121.Li JJ, Nahra J, Johnson AR, Bunker A, O'Brien P, Yue WS, Ortwine DF, Man CF, Baragi V, Kilgore K, Dyer RD, Han HK. Quinazolinones and pyrido[3,4-d]pyrimidin-4-ones as orally active and specific matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis. J Med Chem. 2008;51(4):835–841. doi: 10.1021/jm701274v. [DOI] [PubMed] [Google Scholar]
- 122.Morales R, Perrier S, Florent JM, Beltra J, Dufour S, De Mendez I, Manceau P, Tertre A, Moreau F, Compere D, Dublanchet AC, O'Gara M. Crystal structures of novel non-peptidic, non-zinc chelating inhibitors bound to MMP-12. J Mol Biol. 2004;341(4):1063–1076. doi: 10.1016/j.jmb.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 123.Engel CK, Pirard B, Schimanski S, Kirsch R, Habermann J, Klingler O, Schlotte V, Weithmann KU, Wendt KU. Structural basis for the highly selective inhibition of MMP-13. Chem Biol. 2005;12(2):181–189. doi: 10.1016/j.chembiol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 124.Dublanchet AC, Ducrot P, Andrianjara C, O'Gara M, Morales R, Compere D, Denis A, Blais S, Cluzeau P, Courte K, Hamon J, Moreau F, Prunet ML, Tertre A. Structure-based design and synthesis of novel non-zinc chelating MMP-12 inhibitors. Bioorg Med Chem Lett. 2005;15(16):3787–3790. doi: 10.1016/j.bmcl.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 125.Gooljarsingh LT, Lakdawala A, Coppo F, Luo L, Fields GB, Tummino PJ, Gontarek RR. Characterization of an exosite binding inhibitor of matrix metalloproteinase 13. Protein Sci. 2008;17(1):66–71. doi: 10.1110/ps.073130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pochetti G, Montanari R, Gege C, Chevrier C, Taveras AG, Mazza F. Extra binding region induced by non-zinc chelating inhibitors into the S1' subsite of matrix metalloproteinase 8 (MMP-8) J Med Chem. 2009;52(4):1040–1049. doi: 10.1021/jm801166j. [DOI] [PubMed] [Google Scholar]
- 127.Jacobsen JA, Major Jourden JL, Miller MT, Cohen SM. To bind zinc or not to bind zinc: an examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta. 1803(1):72–94. doi: 10.1016/j.bbamcr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 128.Johnson AR, Pavlovsky AG, Ortwine DF, Prior F, Man CF, Bornemeier DA, Banotai CA, Mueller WT, McConnell P, Yan C, Baragi V, Lesch C, Roark WH, Wilson M, Datta K, Guzman R, Han HK, Dyer RD. Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 that reduces cartilage damage in vivo without joint fibroplasia side effects. J Biol Chem. 2007;282(38):27781–27791. doi: 10.1074/jbc.M703286200. [DOI] [PubMed] [Google Scholar]
- 129.Bernardo MM, Brown S, Li ZH, Fridman R, Mobashery S. Design, synthesis, and characterization of potent, slow-binding inhibitors that are selective for gelatinases. J Biol Chem. 2002;277(13):11201–11207. doi: 10.1074/jbc.M111021200. [DOI] [PubMed] [Google Scholar]
- 130.Morrison JF, Walsh CT. The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol Relat Areas Mol Biol. 1988;61:201–301. doi: 10.1002/9780470123072.ch5. [DOI] [PubMed] [Google Scholar]
- 131.Toth M, Bernardo MM, Gervasi DC, Soloway PD, Wang Z, Bigg HF, Overall CM, DeClerck YA, Tschesche H, Cher ML, Brown S, Mobashery S, Fridman R. Tissue inhibitor of metalloproteinase (TIMP)-2 acts synergistically with synthetic matrix metalloproteinase (MMP) inhibitors but not with TIMP-4 to enhance the (Membrane type 1)-MMP-dependent activation of pro-MMP-2. J Biol Chem. 2000;275(52):41415–41423. doi: 10.1074/jbc.M006871200. [DOI] [PubMed] [Google Scholar]
- 132.Kruger A, Arlt MJ, Gerg M, Kopitz C, Bernardo MM, Chang M, Mobashery S, Fridman R. Antimetastatic activity of a novel mechanism-based gelatinase inhibitor. Cancer Res. 2005;65(9):3523–3526. doi: 10.1158/0008-5472.CAN-04-3570. [DOI] [PubMed] [Google Scholar]
- 133.Bonfil RD, Sabbota A, Nabha S, Bernardo MM, Dong Z, Meng H, Yamamoto H, Chinni SR, Lim IT, Chang M, Filetti LC, Mobashery S, Cher ML, Fridman R. Inhibition of human prostate cancer growth, osteolysis and angiogenesis in a bone metastasis model by a novel mechanism-based selective gelatinase inhibitor. Int J Cancer. 2006;118(11):2721–2726. doi: 10.1002/ijc.21645. [DOI] [PubMed] [Google Scholar]
- 134.Bonfil RD, Dong Z, Trindade Filho JC, Sabbota A, Osenkowski P, Nabha S, Yamamoto H, Chinni SR, Zhao H, Mobashery S, Vessella RL, Fridman R, Cher ML. Prostate cancer-associated membrane type 1-matrix metalloproteinase: a pivotal role in bone response and intraosseous tumor growth. Am J Pathol. 2007;170(6):2100–2111. doi: 10.2353/ajpath.2007.060720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Banke IJ, Arlt MJ, Mueller MM, Sperl S, Stemberger A, Sturzebecher J, Amirkhosravi A, Moroder L, Kruger A. Effective inhibition of experimental metastasis and prolongation of survival in mice by a potent factor Xa-specific synthetic serine protease inhibitor with weak anticoagulant activity. Thromb Haemost. 2005;94(5):1084–1093. doi: 10.1160/TH05-04-0249. [DOI] [PubMed] [Google Scholar]
- 136.Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, Lipton SA. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25(27):6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee M, Villegas-Estrada A, Celenza G, Boggess B, Toth M, Kreitinger G, Forbes C, Fridman R, Mobashery S, Chang M. Metabolism of a highly selective gelatinase inhibitor generates active metabolite. Chem Biol Drug Des. 2007;70(5):371–382. doi: 10.1111/j.1747-0285.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 138.Celenza G, Villegas-Estrada A, Lee M, Boggess B, Forbes C, Wolter WR, Suckow MA, Mobashery S, Chang M. Metabolism of (4-phenoxyphenylsulfonyl) methylthiirane, a selective gelatinase inhibitor. Chem Biol Drug Des. 2008;71(3):187–196. doi: 10.1111/j.1747-0285.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 139.Lee M, Celenza G, Boggess B, Blase J, Shi Q, Toth M, Bernardo MM, Wolter WR, Suckow MA, Hesek D, Noll BC, Fridman R, Mobashery S, Chang M. A potent gelatinase inhibitor with anti-tumor-invasive activity and its metabolic disposition. Chem Biol Drug Des. 2009;73(2):189–202. doi: 10.1111/j.1747-0285.2008.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nuti E, Tuccinardi T, Rossello A. Matrix metalloproteinase inhibitors: new challenges in the era of post broad-spectrum inhibitors. Curr Pharm Des. 2007;13(20):2087–2100. doi: 10.2174/138161207781039706. [DOI] [PubMed] [Google Scholar]
- 141.Georgiadis D, Yiotakis A. Specific targeting of metzincin family members with small-molecule inhibitors: progress toward a multifarious challenge. Bioorg Med Chem. 2008;16(19):8781–8794. doi: 10.1016/j.bmc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 142.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 143.Renkiewicz R, Qiu L, Lesch C, Sun X, Devalaraja R, Cody T, Kaldjian E, Welgus H, Baragi V. Broad-spectrum matrix metalloproteinase inhibitor marimastat-induced musculoskeletal side effects in rats. Arthritis Rheum. 2003;48(6):1742–1749. doi: 10.1002/art.11030. [DOI] [PubMed] [Google Scholar]
- 144.Harvey MB, Leco KJ, Arcellana-Panlilio MY, Zhang X, Edwards DR, Schultz GA. Proteinase expression in early mouse embryos is regulated by leukaemia inhibitory factor and epidermal growth factor. Development. 1995;121(4):1005–1014. doi: 10.1242/dev.121.4.1005. [DOI] [PubMed] [Google Scholar]
- 145.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14(17):2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 146.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6(4):391–407. [PubMed] [Google Scholar]
- 148.Nagareddy PR, Chow FL, Hao L, Wang X, Nishimura T, MacLeod KM, McNeill JH, Fernandez-Patron C. Maintenance of adrenergic vascular tone by MMP transactivation of the EGFR requires PI3K and mitochondrial ATP synthesis. Cardiovasc Res. 2009;84(3):368–377. doi: 10.1093/cvr/cvp230. [DOI] [PubMed] [Google Scholar]
- 149.Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40(5):1001–1010. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 150.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45(2):373–380. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, Trzeciakowski JP, Davis MJ, Davis GE, Meininger GA. alpha(4)beta(1) Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90(4):473–480. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- 152.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53(2):245–282. [PubMed] [Google Scholar]
- 153.Hamilton JR, Nguyen PB, Cocks TM. Atypical protease-activated receptor mediates endothelium-dependent relaxation of human coronary arteries. Circ Res. 1998;82(12):1306–1311. doi: 10.1161/01.res.82.12.1306. [DOI] [PubMed] [Google Scholar]
- 154.Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res. 2010;159(2):755–764. doi: 10.1016/j.jss.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deng DX, Spin JM, Tsalenko A, Vailaya A, Ben-Dor A, Yakhini Z, Tsao P, Bruhn L, Quertermous T. Molecular signatures determining coronary artery and saphenous vein smooth muscle cell phenotypes: distinct responses to stimuli. Arterioscler Thromb Vasc Biol. 2006;26(5):1058–1065. doi: 10.1161/01.ATV.0000208185.16371.97. [DOI] [PubMed] [Google Scholar]
- 156.Turner NA, Ho S, Warburton P, O'Regan DJ, Porter KE. Smooth muscle cells cultured from human saphenous vein exhibit increased proliferation, invasion, and mitogen-activated protein kinase activation in vitro compared with paired internal mammary artery cells. J Vasc Surg. 2007;45(5):1022–1028. doi: 10.1016/j.jvs.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 157.Shi ZD, Ji XY, Berardi DE, Qazi H, Tarbell JM. Interstitial flow induces MMP-1 expression and vascular SMC migration in collagen I gels via an ERK1/2-dependent and c-Jun-mediated mechanism. American journal of physiology. Heart and circulatory physiology. 2010;298(1):H127–135. doi: 10.1152/ajpheart.00732.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vigetti D, Moretto P, Viola M, Genasetti A, Rizzi M, Karousou E, Clerici M, Bartolini B, Pallotti F, De Luca G, Passi A. Aortic smooth muscle cells migration and the role of metalloproteinases and hyaluronan. Connect Tissue Res. 2008;49(3):189–192. doi: 10.1080/03008200802143141. [DOI] [PubMed] [Google Scholar]
- 159.Haque NS, Fallon JT, Pan JJ, Taubman MB, Harpel PC. Chemokine receptor-8 (CCR8) mediates human vascular smooth muscle cell chemotaxis and metalloproteinase-2 secretion. Blood. 2004;103(4):1296–1304. doi: 10.1182/blood-2002-05-1480. [DOI] [PubMed] [Google Scholar]
- 160.Cheng XW, Kuzuya M, Sasaki T, Arakawa K, Kanda S, Sumi D, Koike T, Maeda K, Tamaya-Mori N, Shi GP, Saito N, Iguchi A. Increased expression of elastolytic cysteine proteases, cathepsins S and K, in the neointima of balloon-injured rat carotid arteries. Am J Pathol. 2004;164(1):243–251. doi: 10.1016/S0002-9440(10)63114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24(1):54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 162.Jin UH, Suh SJ, Chang HW, Son JK, Lee SH, Son KH, Chang YC, Kim CH. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J Cell Biochem. 2008;104(1):15–26. doi: 10.1002/jcb.21599. [DOI] [PubMed] [Google Scholar]
- 163.Yu YM, Lin HC. Curcumin prevents human aortic smooth muscle cells migration by inhibiting of MMP-9 expression. Nutr Metab Cardiovasc Dis. 2010;20(2):125–132. doi: 10.1016/j.numecd.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 164.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91(9):845–851. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 165.Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91(9):852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 166.Aguilera CM, George SJ, Johnson JL, Newby AC. Relationship between type IV collagen degradation, metalloproteinase activity and smooth muscle cell migration and proliferation in cultured human saphenous vein. Cardiovasc Res. 2003;58(3):679–688. doi: 10.1016/s0008-6363(03)00256-6. [DOI] [PubMed] [Google Scholar]
- 167.Carragher NO, Frame MC. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14(5):241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 168.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Savani RC, Wang C, Yang B, Zhang S, Kinsella MG, Wight TN, Stern R, Nance DM, Turley EA. Migration of bovine aortic smooth muscle cells after wounding injury. The role of hyaluronan and RHAMM. J Clin Invest. 1995;95(3):1158–1168. doi: 10.1172/JCI117764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Uglow EB, Slater S, Sala-Newby GB, Aguilera-Garcia CM, Angelini GD, Newby AC, George SJ. Dismantling of cadherin-mediated cell-cell contacts modulates smooth muscle cell proliferation. Circ Res. 2003;92(12):1314–1321. doi: 10.1161/01.RES.0000079027.44309.53. [DOI] [PubMed] [Google Scholar]
- 171.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 172.Forough R, Koyama N, Hasenstab D, Lea H, Clowes M, Nikkari ST, Clowes AW. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res. 1996;79(4):812–820. doi: 10.1161/01.res.79.4.812. [DOI] [PubMed] [Google Scholar]
- 173.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101(6):1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH. Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-3. Circulation. 2000;101(3):296–304. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]
- 175.Kenagy RD, Vergel S, Mattsson E, Bendeck M, Reidy MA, Clowes AW. The role of plasminogen, plasminogen activators, and matrix metalloproteinases in primate arterial smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 1996;16(11):1373–1382. doi: 10.1161/01.atv.16.11.1373. [DOI] [PubMed] [Google Scholar]
- 176.Islam MM, Franco CD, Courtman DW, Bendeck MP. A nonantibiotic chemically modified tetracycline (CMT-3) inhibits intimal thickening. Am J Pathol. 2003;163(4):1557–1566. doi: 10.1016/S0002-9440(10)63512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee YJ, Kim JS, Kang DG, Lee HS. Buddleja officinalis suppresses high glucose-induced vascular smooth muscle cell proliferation: role of mitogen-activated protein kinases, nuclear factor-kappaB and matrix metalloproteinases. Experimental biology and medicine. 2010;235(2):247–255. doi: 10.1258/ebm.2009.009222. [DOI] [PubMed] [Google Scholar]
- 178.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85(1):1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 179.Walker HA, Whitelock JM, Garl PJ, Nemenoff RA, Stenmark KR, Weiser-Evans MC. Perlecan up-regulation of FRNK suppresses smooth muscle cell proliferation via inhibition of FAK signaling. Mol Biol Cell. 2003;14(5):1941–1952. doi: 10.1091/mbc.E02-08-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Morla AO, Mogford JE. Control of smooth muscle cell proliferation and phenotype by integrin signaling through focal adhesion kinase. Biochem Biophys Res Commun. 2000;272(1):298–302. doi: 10.1006/bbrc.2000.2769. [DOI] [PubMed] [Google Scholar]
- 181.Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110(23):3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- 182.Hollenbeck ST, Sakakibara K, Faries PL, Workhu B, Liu B, Kent KC. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004;120(2):288–294. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 183.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(Pt 2):217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 184.Slater SC, Koutsouki E, Jackson CL, Bush RC, Angelini GD, Newby AC, George SJ. R-cadherin:beta-catenin complex and its association with vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2004;24(7):1204–1210. doi: 10.1161/01.ATV.0000130464.24599.e0. [DOI] [PubMed] [Google Scholar]
- 185.Bendeck MP, Irvin C, Reidy MA. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res. 1996;78(1):38–43. doi: 10.1161/01.res.78.1.38. [DOI] [PubMed] [Google Scholar]
- 186.Zempo N, Koyama N, Kenagy RD, Lea HJ, Clowes AW. Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler Thromb Vasc Biol. 1996;16(1):28–33. doi: 10.1161/01.atv.16.1.28. [DOI] [PubMed] [Google Scholar]
- 187.Lovdahl C, Thyberg J, Hultgardh-Nilsson A. The synthetic metalloproteinase inhibitor batimastat suppresses injury-induced phosphorylation of MAP kinase ERK1/ERK2 and phenotypic modification of arterial smooth muscle cells in vitro. J Vasc Res. 2000;37(5):345–354. doi: 10.1159/000025750. [DOI] [PubMed] [Google Scholar]
- 188.Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160(3):1089–1095. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109(4):520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 190.Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312(5):594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 191.Heo SH, Choi YJ, Ryoo HM, Cho JY. Expression profiling of ETS and MMP factors in VEGF-activated endothelial cells: role of MMP-10 in VEGF-induced angiogenesis. Journal of cellular physiology. 2010;224(3):734–742. doi: 10.1002/jcp.22175. [DOI] [PubMed] [Google Scholar]
- 192.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21(7):1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 193.Mimura T, Han KY, Onguchi T, Chang JH, Kim TI, Kojima T, Zhou Z, Azar DT. MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009;46(6):541–550. doi: 10.1159/000226222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282(9):6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- 195.Alfranca A, Lopez-Oliva JM, Genis L, Lopez-Maderuelo D, Mirones I, Salvado D, Quesada AJ, Arroyo AG, Redondo JM. PGE2 induces angiogenesis via MT1-MMP-mediated activation of the TGFbeta/Alk5 signaling pathway. Blood. 2008;112(4):1120–1128. doi: 10.1182/blood-2007-09-112268. [DOI] [PubMed] [Google Scholar]
- 196.Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26(5A):3579–3583. [PubMed] [Google Scholar]
- 197.Malik MT, Kakar SS. Regulation of angiogenesis and invasion by human Pituitary tumor transforming gene (PTTG) through increased expression and secretion of matrix metalloproteinase-2 (MMP-2) Mol Cancer. 2006;5:61. doi: 10.1186/1476-4598-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kargiotis O, Chetty C, Gondi CS, Tsung AJ, Dinh DH, Gujrati M, Lakka SS, Kyritsis AP, Rao JS. Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene. 2008;27(35):4830–4840. doi: 10.1038/onc.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 199.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284(38):25854–25866. doi: 10.1074/jbc.M109.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ezhilarasan R, Jadhav U, Mohanam I, Rao JS, Gujrati M, Mohanam S. The hemopexin domain of MMP-9 inhibits angiogenesis and retards the growth of intracranial glioblastoma xenograft in nude mice. Int J Cancer. 2009;124(2):306–315. doi: 10.1002/ijc.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jin X, Yagi M, Akiyama N, Hirosaki T, Higashi S, Lin CY, Dickson RB, Kitamura H, Miyazaki K. Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci. 2006;97(12):1327–1334. doi: 10.1111/j.1349-7006.2006.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chung AS, Kao WJ. Fibroblasts regulate monocyte response to ECM-derived matrix: the effects on monocyte adhesion and the production of inflammatory, matrix remodeling, and growth factor proteins. J Biomed Mater Res A. 2009;89(4):841–853. doi: 10.1002/jbm.a.32431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115(6):849–860. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 205.Moller MN, Werther K, Nalla A, Stangerup SE, Thomsen J, Bog-Hansen TC, Nielsen HJ, Caye-Thomasen P. Angiogenesis in vestibular schwannomas: expression of extracellular matrix factors MMP-2, MMP-9, and TIMP-1. Laryngoscope. 120(4):657–662. doi: 10.1002/lary.20834. [DOI] [PubMed] [Google Scholar]
- 206.Bendrik C, Karlsson L, Dabrosin C. Increased endostatin generation and decreased angiogenesis via MMP-9 by tamoxifen in hormone dependent ovarian cancer. Cancer Lett. 2010;292(1):32–40. doi: 10.1016/j.canlet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 207.Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E, Sier CF, ten Dijke P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010;70(10):4141–4150. doi: 10.1158/0008-5472.CAN-09-4466. [DOI] [PubMed] [Google Scholar]
- 208.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22(9):1370–1380. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- 209.Stoneman VE, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond) 2004;107(4):343–354. doi: 10.1042/CS20040086. [DOI] [PubMed] [Google Scholar]
- 210.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94(1):68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 211.Akiba S, Kumazawa S, Yamaguchi H, Hontani N, Matsumoto T, Ikeda T, Oka M, Sato T. Acceleration of matrix metalloproteinase-1 production and activation of platelet-derived growth factor receptor beta in human coronary smooth muscle cells by oxidized LDL and 4-hydroxynonenal. Biochim Biophys Acta. 2006;1763(8):797–804. doi: 10.1016/j.bbamcr.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 212.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 213.Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149(3):741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143(2):547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Levkau B, Kenagy RD, Karsan A, Weitkamp B, Clowes AW, Ross R, Raines EW. Activation of metalloproteinases and their association with integrins: an auxiliary apoptotic pathway in human endothelial cells. Cell Death Differ. 2002;9(12):1360–1367. doi: 10.1038/sj.cdd.4401106. [DOI] [PubMed] [Google Scholar]
- 216.Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4(6):216. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mannello F, Luchetti F, Falcieri E, Papa S. Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis. 2005;10(1):19–24. doi: 10.1007/s10495-005-6058-7. [DOI] [PubMed] [Google Scholar]
- 218.Bond M, Murphy G, Bennett MR, Amour A, Knauper V, Newby AC, Baker AH. Localization of the death domain of tissue inhibitor of metalloproteinase-3 to the N terminus. Metalloproteinase inhibition is associated with proapoptotic activity. J Biol Chem. 2000;275(52):41358–41363. doi: 10.1074/jbc.M007929200. [DOI] [PubMed] [Google Scholar]
- 219.Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18(6):690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 220.Guo YH, Gao W, Li Q, Li PF, Yao PY, Chen K. Tissue inhibitor of metalloproteinases-4 suppresses vascular smooth muscle cell migration and induces cell apoptosis. Life Sci. 2004;75(20):2483–2493. doi: 10.1016/j.lfs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 221.Onal IK, Altun B, Onal ED, Kirkpantur A, Gul Oz S, Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med. 2009;20(4):369–372. doi: 10.1016/j.ejim.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 222.Lin J, Davis HB, Dai Q, Chou YM, Craig T, Hinojosa-Laborde C, Lindsey ML. Effects of early and late chronic pressure overload on extracellular matrix remodeling. Hypertens Res. 2008;31(6):1225–1231. doi: 10.1291/hypres.31.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nagareddy PR, MacLeod KM, McNeill JH. GPCR agonist-induced transactivation of the EGFR upregulates MLC II expression and promotes hypertension in insulin-resistant rats. Cardiovascular research. 2010;87(1):177–186. doi: 10.1093/cvr/cvq030. [DOI] [PubMed] [Google Scholar]
- 224.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50(1):212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 225.Castro MM, Rizzi E, Prado CM, Rossi MA, Tanus-Santos JE, Gerlach RF. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010;29(3):194–201. doi: 10.1016/j.matbio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 226.Belo VA, Parente JM, Tanus-Santos JE, Castro MM. Matrix metalloproteinase (MMP)-2 decreases calponin-1 levels and contributes to arterial remodeling in early hypertension. Biochemical pharmacology. 2016;118:50–58. doi: 10.1016/j.bcp.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 227.Lee YH, Kim TY, Hong YM. Metalloproteinase-3 genotype as a predictor of cardiovascular risk in hypertensive adolescents. Korean Circ J. 2009;39(8):328–334. doi: 10.4070/kcj.2009.39.8.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kalani A, Pushpakumar SB, Vacek JC, Tyagi SC, Tyagi N. Inhibition of MMP-9 attenuates hypertensive cerebrovascular dysfunction in Dahl salt-sensitive rats. Mol Cell Biochem. 2016;413(1–2):25–35. doi: 10.1007/s11010-015-2623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang X, Chow FL, Oka T, Hao L, Lopez-Campistrous A, Kelly S, Cooper S, Odenbach J, Finegan BA, Schulz R, Kassiri Z, Lopaschuk GD, Fernandez-Patron C. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119(18):2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]
- 230.Franz M, Berndt A, Altendorf-Hofmann A, Fiedler N, Richter P, Schumm J, Fritzenwanger M, Figulla HR, Brehm BR. Serum levels of large tenascin-C variants, matrix metalloproteinase-9, and tissue inhibitors of matrix metalloproteinases in concentric versus eccentric left ventricular hypertrophy. Eur J Heart Fail. 2009;11(11):1057–1062. doi: 10.1093/eurjhf/hfp128. [DOI] [PubMed] [Google Scholar]
- 231.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30(1):56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Beaudeux JL, Giral P, Bruckert E, Foglietti MJ, Chapman MJ. Matrix metalloproteinases, inflammation and atherosclerosis: therapeutic perspectives. Clin Chem Lab Med. 2004;42(2):121–131. doi: 10.1515/CCLM.2004.024. [DOI] [PubMed] [Google Scholar]
- 233.Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology. 2005;56(2):173–189. doi: 10.1177/000331970505600208. [DOI] [PubMed] [Google Scholar]
- 234.Uzui H, Harpf A, Liu M, Doherty TM, Shukla A, Chai NN, Tripathi PV, Jovinge S, Wilkin DJ, Asotra K, Shah PK, Rajavashisth TB. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: role of activated macrophages and inflammatory cytokines. Circulation. 2002;106(24):3024–3030. doi: 10.1161/01.cir.0000041433.94868.12. [DOI] [PubMed] [Google Scholar]
- 235.Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther. 2007;5(2):265–282. doi: 10.1586/14779072.5.2.265. [DOI] [PubMed] [Google Scholar]
- 236.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aikawa M, Rabkin E, Voglic SJ, Shing H, Nagai R, Schoen FJ, Libby P. Lipid lowering promotes accumulation of mature smooth muscle cells expressing smooth muscle myosin heavy chain isoforms in rabbit atheroma. Circ Res. 1998;83(10):1015–1026. doi: 10.1161/01.res.83.10.1015. [DOI] [PubMed] [Google Scholar]
- 238.Pawlak K, Pawlak D, Mysliwiec M. Urokinase-type plasminogen activator and metalloproteinase-2 are independently related to the carotid atherosclerosis in haemodialysis patients. Thromb Res. 2008;121(4):543–548. doi: 10.1016/j.thromres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 239.Fitzsimmons PJ, Forough R, Lawrence ME, Gantt DS, Rajab MH, Kim H, Weylie B, Spiekerman AM, Dehmer GJ. Urinary levels of matrix metalloproteinase 9 and 2 and tissue inhibitor of matrix metalloproteinase in patients with coronary artery disease. Atherosclerosis. 2007;194(1):196–203. doi: 10.1016/j.atherosclerosis.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 240.Gaubatz JW, Ballantyne CM, Wasserman BA, He M, Chambless LE, Boerwinkle E, Hoogeveen RC. Association of circulating matrix metalloproteinases with carotid artery characteristics: the Atherosclerosis Risk in Communities Carotid MRI Study. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(5):1034–1042. doi: 10.1161/ATVBAHA.109.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Djuric T, Zivkovic M, Stankovic A, Kolakovic A, Jekic D, Selakovic V, Alavantic D. Plasma levels of matrix metalloproteinase-8 in patients with carotid atherosclerosis. J Clin Lab Anal. 2010;24(4):246–251. doi: 10.1002/jcla.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rodriguez JA, Orbe J, Martinez de Lizarrondo S, Calvayrac O, Rodriguez C, Martinez-Gonzalez J, Paramo JA. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front Biosci. 2008;13:2916–2921. doi: 10.2741/2896. [DOI] [PubMed] [Google Scholar]
- 243.Ye S, Watts GF, Mandalia S, Humphries SE, Henney AM. Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J. 1995;73(3):209–215. doi: 10.1136/hrt.73.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Terashima M, Akita H, Kanazawa K, Inoue N, Yamada S, Ito K, Matsuda Y, Takai E, Iwai C, Kurogane H, Yoshida Y, Yokoyama M. Stromelysin promoter 5A/6A polymorphism is associated with acute myocardial infarction. Circulation. 1999;99(21):2717–2719. doi: 10.1161/01.cir.99.21.2717. [DOI] [PubMed] [Google Scholar]
- 245.Ozkok E, Aydin M, Babalik E, Ozbek Z, Ince N, Kara I. Combined impact of matrix metalloproteinase-3 and paraoxonase 1 55/192 gene variants on coronary artery disease in Turkish patients. Med Sci Monit. 2008;14(10):CR536–542. [PubMed] [Google Scholar]
- 246.Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5(5):397–403. doi: 10.3748/wjg.v5.i5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107(12):1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 248.Rauch I, Iglseder B, Paulweber B, Ladurner G, Strasser P. MMP-9 haplotypes and carotid artery atherosclerosis: an association study introducing a novel multicolour multiplex RealTime PCR protocol. Eur J Clin Invest. 2008;38(1):24–33. doi: 10.1111/j.1365-2362.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- 249.Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, Moons L. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004;109(11):1408–1414. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- 250.Choi ET, Collins ET, Marine LA, Uberti MG, Uchida H, Leidenfrost JE, Khan MF, Boc KP, Abendschein DR, Parks WC. Matrix metalloproteinase-9 modulation by resident arterial cells is responsible for injury-induced accelerated atherosclerotic plaque development in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25(5):1020–1025. doi: 10.1161/01.ATV.0000161275.82687.f6. [DOI] [PubMed] [Google Scholar]
- 251.Brunner S, Kim JO, Methe H. Relation of matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio in peripheral circulating CD14+ monocytes to progression of coronary artery disease. Am J Cardiol. 2010;105(4):429–434. doi: 10.1016/j.amjcard.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 252.Cevik C, Otahbachi M, Nugent K, Warangkana C, Meyerrose G. Effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition on serum matrix metalloproteinase-13 and tissue inhibitor matrix metalloproteinase-1 levels as a sign of plaque stabilization. J Cardiovasc Med (Hagerstown) 2008;9(12):1274–1278. doi: 10.2459/JCM.0b013e328316912f. [DOI] [PubMed] [Google Scholar]
- 253.Guo Z, Sun X, He Z, Jiang Y, Zhang X. Role of matrix metalloproteinase-9 in apoptosis of hippocampal neurons in rats during early brain injury after subarachnoid hemorrhage. Neurol Sci. 2010;31(2):143–149. doi: 10.1007/s10072-009-0192-x. [DOI] [PubMed] [Google Scholar]
- 254.Jin UH, Kang SK, Suh SJ, Hong SY, Park SD, Kim DW, Chang HW, Son JK, Lee SH, Son KH, Kim CH. Inhibitory effect of Salvia miltiorrhia BGE on matrix metalloproteinase-9 activity and migration of TNF-alpha-induced human aortic smooth muscle cells. Vascul Pharmacol. 2006;44(5):345–353. doi: 10.1016/j.vph.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 255.Fukumoto Y, Deguchi JO, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FJ, Krane SM, Aikawa M. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation. 2004;110(14):1953–1959. doi: 10.1161/01.CIR.0000143174.41810.10. [DOI] [PubMed] [Google Scholar]
- 256.Valentin F, Bueb JL, Kieffer P, Tschirhart E, Atkinson J. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam Clin Pharmacol. 2005;19(6):661–667. doi: 10.1111/j.1472-8206.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 257.Guo RW, Yang LX, Wang H, Liu B, Wang L. Angiotensin II induces matrix metalloproteinase-9 expression via a nuclear factor-kappaB-dependent pathway in vascular smooth muscle cells. Regul Pept. 2008;147(1–3):37–44. doi: 10.1016/j.regpep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 258.Inoue S, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy M. Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: a role for sphingosine-1-phosphate receptors. J Vasc Surg. 2007;46(4):756–763. doi: 10.1016/j.jvs.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 259.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102(43):15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Koike Y, Shima F, Nakamizo A, Miyagi Y. Direct localization of subthalamic nucleus supplemented by single-track electrophysiological guidance in deep brain stimulation lead implantation: techniques and clinical results. Stereotact Funct Neurosurg. 2008;86(3):173–178. doi: 10.1159/000120430. [DOI] [PubMed] [Google Scholar]
- 261.Lemaitre V, O'Byrne TK, Borczuk AC, Okada Y, Tall AR, D'Armiento J. ApoE knockout mice expressing human matrix metalloproteinase-1 in macrophages have less advanced atherosclerosis. J Clin Invest. 2001;107(10):1227–1234. doi: 10.1172/JCI9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Silence J, Lupu F, Collen D, Lijnen HR. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol. 2001;21(9):1440–1445. doi: 10.1161/hq0901.097004. [DOI] [PubMed] [Google Scholar]
- 263.Rouis M, Adamy C, Duverger N, Lesnik P, Horellou P, Moreau M, Emmanuel F, Caillaud JM, Laplaud PM, Dachet C, Chapman MJ. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein E-deficient mice. Circulation. 1999;100(5):533–540. doi: 10.1161/01.cir.100.5.533. [DOI] [PubMed] [Google Scholar]
- 264.Grandas OH, Mountain DH, Kirkpatrick SS, Cassada DC, Stevens SL, Freeman MB, Goldman MH. Regulation of vascular smooth muscle cell expression and function of matrix metalloproteinases is mediated by estrogen and progesterone exposure. J Vasc Surg. 2009;49(1):185–191. doi: 10.1016/j.jvs.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 265.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16(5):558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59(4):812–823. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 267.Roman-Garcia P, Coto E, Reguero JR, Cannata-Andia JB, Lozano I, Avanzas P, Moris C, Rodriguez I. Matrix metalloproteinase 1 promoter polymorphisms and risk of myocardial infarction: a case-control study in a Spanish population. Coron Artery Dis. 2009;20(6):383–386. doi: 10.1097/MCA.0b013e32832fa9cf. [DOI] [PubMed] [Google Scholar]
- 268.Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, Squire IB. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J. 2007;28(6):711–718. doi: 10.1093/eurheartj/ehm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ikeda U, Shimada K. Matrix metalloproteinases and coronary artery diseases. Clin Cardiol. 2003;26(2):55–59. doi: 10.1002/clc.4960260203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Koskivirta I, Rahkonen O, Mayranpaa M, Pakkanen S, Husheem M, Sainio A, Hakovirta H, Laine J, Jokinen E, Vuorio E, Kovanen P, Jarvelainen H. Tissue inhibitor of metalloproteinases 4 (TIMP4) is involved in inflammatory processes of human cardiovascular pathology. Histochem Cell Biol. 2006;126(3):335–342. doi: 10.1007/s00418-006-0163-8. [DOI] [PubMed] [Google Scholar]
- 271.Zureik M, Beaudeux JL, Courbon D, Benetos A, Ducimetiere P. Serum tissue inhibitors of metalloproteinases 1 (TIMP-1) and carotid atherosclerosis and aortic arterial stiffness. J Hypertens. 2005;23(12):2263–2268. [PubMed] [Google Scholar]
- 272.Lemaitre V, Soloway PD, D'Armiento J. Increased medial degradation with pseudo-aneurysm formation in apolipoprotein E-knockout mice deficient in tissue inhibitor of metalloproteinases-1. Circulation. 2003;107(2):333–338. doi: 10.1161/01.cir.0000044915.37074.5c. [DOI] [PubMed] [Google Scholar]
- 273.Silence J, Collen D, Lijnen HR. Reduced atherosclerotic plaque but enhanced aneurysm formation in mice with inactivation of the tissue inhibitor of metalloproteinase-1 (TIMP-1) gene. Circ Res. 2002;90(8):897–903. doi: 10.1161/01.res.0000016501.56641.83. [DOI] [PubMed] [Google Scholar]
- 274.Prescott MF, Sawyer WK, Von Linden-Reed J, Jeune M, Chou M, Caplan SL, Jeng AY. Effect of matrix metalloproteinase inhibition on progression of atherosclerosis and aneurysm in LDL receptor-deficient mice overexpressing MMP-3, MMP-12, and MMP-13 and on restenosis in rats after balloon injury. Ann N Y Acad Sci. 1999;878:179–190. doi: 10.1111/j.1749-6632.1999.tb07683.x. [DOI] [PubMed] [Google Scholar]
- 275.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23(3):483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 276.Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, Squire IB. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008;29(17):2116–2124. doi: 10.1093/eurheartj/ehn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, Thompson MM. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke. 2002;33(12):2858–2864. doi: 10.1161/01.str.0000038098.04291.f6. [DOI] [PubMed] [Google Scholar]
- 278.Shimizu C, Matsubara T, Onouchi Y, Jain S, Sun S, Nievergelt CM, Shike H, Brophy VH, Takegawa T, Furukawa S, Akagi T, Newburger JW, Baker AL, Burgner D, Hibberd ML, Davila S, Levin M, Mamtani M, He W, Ahuja SK, Burns JC. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J Hum Genet. 2010;55(12):779–784. doi: 10.1038/jhg.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139(2):292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 280.Sinha I, Bethi S, Cronin P, Williams DM, Roelofs K, Ailawadi G, Henke PK, Eagleton MJ, Deeb GM, Patel HJ, Berguer R, Stanley JC, Upchurch GR., Jr A biologic basis for asymmetric growth in descending thoracic aortic aneurysms: a role for matrix metalloproteinase 9 and 2. J Vasc Surg. 2006;43(2):342–348. doi: 10.1016/j.jvs.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 281.Chen L, Wang X, Carter SA, Shen YH, Bartsch HR, Thompson RW, Coselli JS, Wilcken DL, Wang XL, LeMaire SA. A single nucleotide polymorphism in the matrix metalloproteinase 9 gene (−8202A/G) is associated with thoracic aortic aneurysms and thoracic aortic dissection. J Thorac Cardiovasc Surg. 2006;131(5):1045–1052. doi: 10.1016/j.jtcvs.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, 3rd, Spinale FG, Gorman RC. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133(4):1028–1036. doi: 10.1016/j.jtcvs.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 283.Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg. 2008;47(1):166–172. doi: 10.1016/j.jvs.2007.09.016. discussion 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Jones JA, Ruddy JM, Bouges S, Zavadzkas JA, Brinsa TA, Stroud RE, Mukherjee R, Spinale FG, Ikonomidis JS. Alterations in membrane type-1 matrix metalloproteinase abundance after the induction of thoracic aortic aneurysm in a murine model. American journal of physiology. Heart and circulatory physiology. 2010;299(1):H114–124. doi: 10.1152/ajpheart.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Barbour JR, Stroud RE, Lowry AS, Clark LL, Leone AM, Jones JA, Spinale FG, Ikonomidis JS. Temporal disparity in the induction of matrix metalloproteinases and tissue inhibitors of metalloproteinases after thoracic aortic aneurysm formation. J Thorac Cardiovasc Surg. 2006;132(4):788–795. doi: 10.1016/j.jtcvs.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 286.Geng L, Wang W, Chen Y, Cao J, Lu L, Chen Q, He R, Shen W. Elevation of ADAM10, ADAM17, MMP-2 and MMP-9 expression with media degeneration features CaCl2-induced thoracic aortic aneurysm in a rat model. Exp Mol Pathol. 2010;89(1):72–81. doi: 10.1016/j.yexmp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 287.Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res. 2008;102(8):e73–85. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- 288.Choke E, Cockerill GW, Dawson J, Wilson RW, Jones A, Loftus IM, Thompson MM. Increased angiogenesis at the site of abdominal aortic aneurysm rupture. Ann N Y Acad Sci. 2006;1085:315–319. doi: 10.1196/annals.1383.007. [DOI] [PubMed] [Google Scholar]
- 289.Goodall S, Crowther M, Hemingway DM, Bell PR, Thompson MM. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001;104(3):304–309. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- 290.Petersen E, Gineitis A, Wagberg F, Angquist KA. Activity of matrix metalloproteinase-2 and -9 in abdominal aortic aneurysms. Relation to size and rupture. Eur J Vasc Endovasc Surg. 2000;20(5):457–461. doi: 10.1053/ejvs.2000.1211. [DOI] [PubMed] [Google Scholar]
- 291.Hovsepian DM, Ziporin SJ, Sakurai MK, Lee JK, Curci JA, Thompson RW. Elevated plasma levels of matrix metalloproteinase-9 in patients with abdominal aortic aneurysms: a circulating marker of degenerative aneurysm disease. J Vasc Interv Radiol. 2000;11(10):1345–1352. doi: 10.1016/s1051-0443(07)61315-3. [DOI] [PubMed] [Google Scholar]
- 292.Nagashima H, Aoka Y, Sakomura Y, Sakuta A, Aomi S, Ishizuka N, Hagiwara N, Kawana M, Kasanuki H. A 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, cerivastatin, suppresses production of matrix metalloproteinase-9 in human abdominal aortic aneurysm wall. J Vasc Surg. 2002;36(1):158–163. doi: 10.1067/mva.2002.123680. [DOI] [PubMed] [Google Scholar]
- 293.Sakalihasan N, Delvenne P, Nusgens BV, Limet R, Lapiere CM. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J Vasc Surg. 1996;24(1):127–133. doi: 10.1016/s0741-5214(96)70153-2. [DOI] [PubMed] [Google Scholar]
- 294.Wilson WR, Anderton M, Choke EC, Dawson J, Loftus IM, Thompson MM. Elevated plasma MMP1 and MMP9 are associated with abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2008;35(5):580–584. doi: 10.1016/j.ejvs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 295.Erdozain OJ, Pegrum S, Winrow VR, Horrocks M, Stevens CR. Hypoxia in abdominal aortic aneurysm supports a role for HIF-1alpha and Ets-1 as drivers of matrix metalloproteinase upregulation in human aortic smooth muscle cells. Journal of vascular research. 2010;48(2):163–170. doi: 10.1159/000318806. [DOI] [PubMed] [Google Scholar]
- 296.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110(5):625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wilson WR, Schwalbe EC, Jones JL, Bell PR, Thompson MM. Matrix metalloproteinase 8 (neutrophil collagenase) in the pathogenesis of abdominal aortic aneurysm. Br J Surg. 2005;92(7):828–833. doi: 10.1002/bjs.4993. [DOI] [PubMed] [Google Scholar]
- 299.Razavian M, Zhang J, Nie L, Tavakoli S, Razavian N, Dobrucki LW, Sinusas AJ, Edwards DS, Azure M, Sadeghi MM. Molecular imaging of matrix metalloproteinase activation to predict murine aneurysm expansion in vivo. J Nucl Med. 2010;51(7):1107–1115. doi: 10.2967/jnumed.110.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hackmann AE, Rubin BG, Sanchez LA, Geraghty PA, Thompson RW, Curci JA. A randomized, placebo-controlled trial of doxycycline after endoluminal aneurysm repair. J Vasc Surg. 2008;48(3):519–526. doi: 10.1016/j.jvs.2008.03.064. discussion 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sangiorgi G, D'Averio R, Mauriello A, Bondio M, Pontillo M, Castelvecchio S, Trimarchi S, Tolva V, Nano G, Rampoldi V, Spagnoli LG, Inglese L. Plasma levels of metalloproteinases-3 and -9 as markers of successful abdominal aortic aneurysm exclusion after endovascular graft treatment. Circulation. 2001;104(12 Suppl 1):I288–295. doi: 10.1161/hc37t1.094596. [DOI] [PubMed] [Google Scholar]
- 302.Cui Y, Takamatsu H, Kakiuchi T, Ohba H, Kataoka Y, Yokoyama C, Onoe H, Watanabe Y, Hosoya T, Suzuki M, Noyori R, Tsukada H. Neuroprotection by a central nervous system-type prostacyclin receptor ligand demonstrated in monkeys subjected to middle cerebral artery occlusion and reperfusion: a positron emission tomography study. Stroke. 2006;37(11):2830–2836. doi: 10.1161/01.STR.0000245088.60282.22. [DOI] [PubMed] [Google Scholar]
- 303.Eugster T, Huber A, Obeid T, Schwegler I, Gurke L, Stierli P. Aminoterminal propeptide of type III procollagen and matrix metalloproteinases-2 and -9 failed to serve as serum markers for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2005;29(4):378–382. doi: 10.1016/j.ejvs.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 304.van Laake LW, Vainas T, Dammers R, Kitslaar PJ, Hoeks AP, Schurink GW. Systemic dilation diathesis in patients with abdominal aortic aneurysms: a role for matrix metalloproteinase-9? Eur J Vasc Endovasc Surg. 2005;29(4):371–377. doi: 10.1016/j.ejvs.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 305.Wilson WR, Anderton M, Schwalbe EC, Jones JL, Furness PN, Bell PR, Thompson MM. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation. 2006;113(3):438–445. doi: 10.1161/CIRCULATIONAHA.105.551572. [DOI] [PubMed] [Google Scholar]
- 306.Wilson WR, Choke EC, Dawson J, Loftus IM, Thompson MM. Plasma matrix metalloproteinase levels do not predict tissue levels in abdominal aortic aneurysms suitable for elective repair. Vascular. 2008;16(5):248–252. doi: 10.2310/6670.2008.00043. [DOI] [PubMed] [Google Scholar]
- 307.Hinterseher I, Bergert H, Kuhlisch E, Bloomenthal A, Pilarsky C, Ockert D, Schellong S, Saeger HD, Krex D. Matrix metalloproteinase 2 polymorphisms in a caucasian population with abdominal aortic aneurysm. J Surg Res. 2006;133(2):121–128. doi: 10.1016/j.jss.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 308.Jones GT, Phillips VL, Harris EL, Rossaak JI, van Rij AM. Functional matrix metalloproteinase-9 polymorphism (C-1562T) associated with abdominal aortic aneurysm. J Vasc Surg. 2003;38(6):1363–1367. doi: 10.1016/s0741-5214(03)01027-9. [DOI] [PubMed] [Google Scholar]
- 309.Armani C, Curcio M, Barsotti MC, Santoni T, Di Stefano R, Dell'omodarme M, Brandi ML, Ferrari M, Scatena F, Carpi A, Balbarini A. Polymorphic analysis of the matrix metalloproteinase-9 gene and susceptibility to sporadic abdominal aortic aneurysm. Biomed Pharmacother. 2007;61(5):268–271. doi: 10.1016/j.biopha.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 310.Thompson AR, Drenos F, Hafez H, Humphries SE. Candidate gene association studies in abdominal aortic aneurysm disease: a review and meta-analysis. Eur J Vasc Endovasc Surg. 2008;35(1):19–30. doi: 10.1016/j.ejvs.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 311.Wall SJ, Sampson MJ, Levell N, Murphy G. Elevated matrix metalloproteinase-2 and -3 production from human diabetic dermal fibroblasts. Br J Dermatol. 2003;149(1):13–16. doi: 10.1046/j.1365-2133.2003.05262.x. [DOI] [PubMed] [Google Scholar]
- 312.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150(3):993–1007. [PMC free article] [PubMed] [Google Scholar]
- 313.Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, Saikku P, Juvonen T. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34(4):606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 314.Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation. 2009;119(16):2209–2216. doi: 10.1161/CIRCULATIONAHA.108.806505. [DOI] [PubMed] [Google Scholar]
- 315.Milner JM, Cawston TE. Matrix metalloproteinase knockout studies and the potential use of matrix metalloproteinase inhibitors in the rheumatic diseases. Curr Drug Targets Inflamm Allergy. 2005;4(3):363–375. doi: 10.2174/1568010054022141. [DOI] [PubMed] [Google Scholar]
- 316.Winer A, Janosky M, Harrison B, Zhong J, Moussai D, Siyah P, Schatz-Siemers N, Zeng J, Adams S, Mignatti P. Inhibition of Breast Cancer Metastasis by Presurgical Treatment with an Oral Matrix Metalloproteinase Inhibitor: A Preclinical Proof-of-Principle Study. Mol Cancer Ther. 2016;15(10):2370–2377. doi: 10.1158/1535-7163.MCT-16-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Liu T, Wu X, Wang Y, Zhang T, Wu T, Liu F, Wang W, Jiang G, Xie M. Folate-targeted star-shaped cationic copolymer co-delivering docetaxel and MMP-9 siRNA for nasopharyngeal carcinoma therapy. Oncotarget. 2016;7(27):42017–42030. doi: 10.18632/oncotarget.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31(31):7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- 320.Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL. T cell gelatinases mediate basement membrane transmigration in vitro. Journal of immunology. 1995;154(9):4379–4389. [PubMed] [Google Scholar]
- 321.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115(3):599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106(1):55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lijnen HR, Van Hoef B, Vanlinthout I, Verstreken M, Rio MC, Collen D. Accelerated neointima formation after vascular injury in mice with stromelysin-3 (MMP-11) gene inactivation. Arterioscler Thromb Vasc Biol. 1999;19(12):2863–2870. doi: 10.1161/01.atv.19.12.2863. [DOI] [PubMed] [Google Scholar]
- 324.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97(8):4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284(1):H364–371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 326.Fedak PW, Smookler DS, Kassiri Z, Ohno N, Leco KJ, Verma S, Mickle DA, Watson KL, Hojilla CV, Cruz W, Weisel RD, Li RK, Khokha R. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation. 2004;110(16):2401–2409. doi: 10.1161/01.CIR.0000134959.83967.2D. [DOI] [PubMed] [Google Scholar]
- 327.Laviades C, Varo N, Fernandez J, Mayor G, Gil MJ, Monreal I, Diez J. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98(6):535–540. doi: 10.1161/01.cir.98.6.535. [DOI] [PubMed] [Google Scholar]
- 328.Zervoudaki A, Economou E, Stefanadis C, Pitsavos C, Tsioufis K, Aggeli C, Vasiliadou K, Toutouza M, Toutouzas P. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17(2):119–124. doi: 10.1038/sj.jhh.1001518. [DOI] [PubMed] [Google Scholar]
- 329.Derosa G, D'Angelo A, Ciccarelli L, Piccinni MN, Pricolo F, Salvadeo S, Montagna L, Gravina A, Ferrari I, Galli S, Paniga S, Tinelli C, Cicero AF. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;13(3):227–231. doi: 10.1080/10623320600780942. [DOI] [PubMed] [Google Scholar]
- 330.Tan J, Hua Q, Xing X, Wen J, Liu R, Yang Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007;30(10):959–963. doi: 10.1291/hypres.30.959. [DOI] [PubMed] [Google Scholar]
- 331.Dhingra R, Pencina MJ, Schrader P, Wang TJ, Levy D, Pencina K, Siwik DA, Colucci WS, Benjamin EJ, Vasan RS. Relations of matrix remodeling biomarkers to blood pressure progression and incidence of hypertension in the community. Circulation. 2009;119(8):1101–1107. doi: 10.1161/CIRCULATIONAHA.108.821769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sesso R, Franco MC. Abnormalities in metalloproteinase pathways and IGF-I axis: a link between birth weight, hypertension, and vascular damage in childhood. American journal of hypertension. 2010;23(1):6–11. doi: 10.1038/ajh.2009.200. [DOI] [PubMed] [Google Scholar]