Abstract
Normal pregnancy is associated with marked hemodynamic and uterine changes that allow adequate uteroplacental blood flow and uterine expansion for the growing fetus. These pregnancy-associated changes involve significant uteroplacental and vascular remodeling. Matrix metalloproteinases (MMPs) are important regulators of vascular and uterine remodeling. Increases in MMP-2 and MMP-9 have been implicated in vasodilation, placentation and uterine expansion during normal pregnancy. The increases in MMPs could be induced by the increased production of estrogen and progesterone during pregnancy. MMP expression/activity may be altered during complications of pregnancy. Decreased vascular MMP-2 and MMP-9 may lead to decreased vasodilation, increased vasoconstriction, hypertensive pregnancy and preeclampsia. Abnormal expression of uteroplacental integrins, cytokines and MMPs may lead to decreased maternal tolerance, apoptosis of invasive trophoblast cells, inadequate remodeling of spiral arteries, and reduced uterine perfusion pressure (RUPP). RUPP may cause imbalance between the anti-angiogenic factors soluble fms-like tyrosine kinase-1 and soluble endoglin and the pro-angiogenic vascular endothelial growth factor and placental growth factor, or stimulate the release of inflammatory cytokines, hypoxia-inducible factor, reactive oxygen species, and angiotensin AT1 receptor agonistic autoantibodies. These circulating factors could target MMPs in the extracellular matrix as well as endothelial and vascular smooth muscle cells, causing generalized vascular dysfunction, increased vasoconstriction and hypertension in pregnancy. MMP activity can also be altered by endogenous tissue inhibitors of metalloproteinases (TIMPs) and changes in the MMP/TIMP ratio. In addition to their vascular effects, decreases in expression/activity of MMP-2 and MMP-9 in the uterus could impede uterine growth and expansion and lead to premature labor. Understanding the role of MMPs in uteroplacental and vascular remodeling and function could help design new approaches for prediction and management of preeclampsia and premature labor.
Keywords: Blood Vessels, Contraction, Cytokines, Estrogen, Growth Factors, Hypertension, Hypoxia, Placental Ischemia, Progesterone, Uterus
1. INTRODUCTION
Normal pregnancy is associated with several uteroplacental and hemodynamic changes in order to meet the growing and metabolic demands of the developing fetus. The pregnant uterus undergoes hypertrophy and distension in order to provide sufficient space for the growing fetus. Placental remodeling and cytotrophoblast invasion of spiral arteries maintain adequate blood supply to the developing fetus.1,2 Also, the increases in maternal blood volume and cardiac output are counterbalanced by systemic vasodilation and decreased vascular resistance, leading to only slight change in blood pressure.3,4 These uterine and vascular changes involve marked uteroplacental and vascular remodeling and redistribution of blood flow in different maternal tissues and organs.5,6
Matrix metalloproteinases (MMPs) are zinc-dependent proteases that play a role in tissue remodeling.7,8 MMPs include collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs with different tissue expression, distribution and substrate specificity.9 MMPs degrade various proteins in the extracellular matrix (ECM) including collagen and elastin.9 MMPs are produced as pro-MMPs which are cleaved by other MMPs or proteases into active MMPs.9,10 MMPs play a role in endometrial tissue remodeling during the estrous cycle and menstrual cycle, and may be involved in the uterine and vascular tissue remodeling during normal pregnancy.8
In 5 to 8% of pregnancies, women may have hypertension in pregnancy manifested in one of four forms: chronic hypertension that predates pregnancy, preeclampsia-eclampsia, chronic hypertension with superimposed preeclampsia, and nonproteinuric gestational hypertension.11 Preeclampsia is diagnosed after the 20th week of pregnancy by new onset hypertension (systolic pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg), often proteinuria and may be associated with edema and increased platelet aggregation.12 Preeclampsia may also be a part of hemolysis elevated liver enzymes low platelets (HELLP) syndrome. If not treated, preeclampsia may progress to eclampsia, characterized by severe hypertension and convulsions, which could culminate into coma and death, causing an estimated 14% of pregnancy-related maternal deaths.13
Premature labor is another major complication of pregnancy that could lead to prematurity at birth and neonatal death. Preterm delivery complicates 10% to 15% of all pregnancies, and is a leading cause of perinatal morbidity and death.14 Preeclampsia may be associated with intrauterine growth restriction (IUGR), a condition that could cause premature birth.15 Of the spontaneous births, 3.2% of preterm births and 2.2% of very preterm births are associated with preeclampsia.16 These complications of pregnancy are particularly important in developing countries where the incidence of preeclampsia is greater and the rates of maternal mortality and preterm births are higher than those in developed countries.17
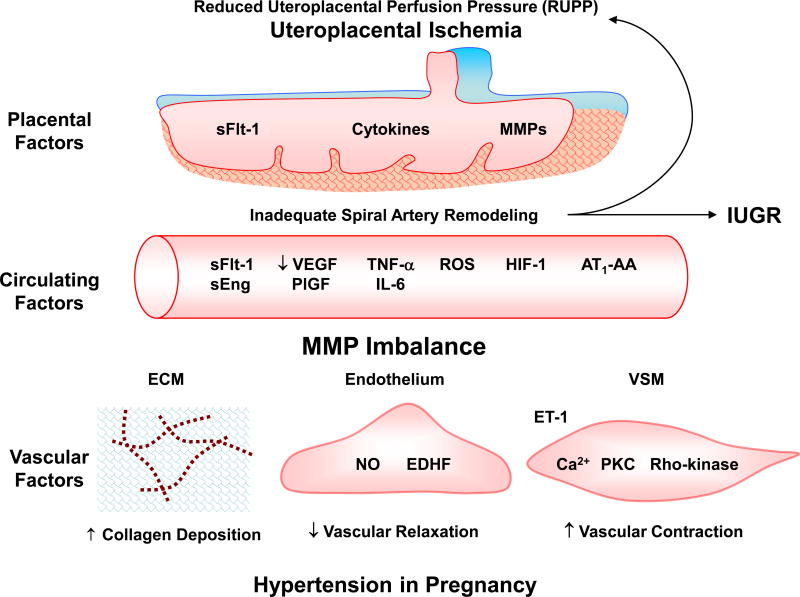
Although preeclampsia and premature labor are major causes of maternal and fetal morbidity and constitute a significant burden on the healthcare system, their etiology and pathophysiology are not fully understood. Certain genetic and environmental risk factors may trigger reduction in uteroplacental perfusion pressure (RUPP) and the resulting placental ischemia/hypoxia could cause the release of bioactive factors that could target the blood vessels and the uterus (Fig. 1). Because of the difficulty to perform mechanistic studies in pregnant women, and in order to further understand the pathophysiological mechanisms of preeclampsia, animal models of hypertension in pregnancy have been developed. Studies in animal models support that placental ischemia could be an initiating event, and RUPP in pregnant rats shows some of the characteristics of preeclampsia including hypertension in pregnancy and IUGR.18–20 Studies have also shown changes in the local and circulating levels of pro-angiogenic vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), the anti-angiogenic factors soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng), cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), hypoxia-inducible factor (HIF), reactive oxygen species (ROS) and angiotensin II (AngII) type 1 receptor (AT1R) agonistic autoantibodies (AT1-AA). These bioactive factors could target systemic vessels causing generalized vascular dysfunction and hypertension, renal glomeruli causing glomerular endotheliosis and increased glomerular permeability and proteinuria, cerebral vessels causing cerebral edema and seizures,11,21 and possibly the uterus leading to premature labor.
Fig. 1.
Role of MMPs in hypertension in pregnancy and IUGR. Initial reduction of uteroplacental perfusion pressure (RUPP) and uteroplacental ischemia causes the release of cytoacive and circulating factors, which target MMPs in uteroplacental tissues leading to intrauterine growth restriction (IUGR) and in blood vessels leading to increased collagen deposition in extracellular matrix (ECM), decreased endothelium-dependent vascular relaxation pathways, and increased enothelin-1 (ET-1) and mechanisms of VSM contraction, resulting in increased vascular resistance and hypertension in Pregnancy.
AT1-AA, AngII AT1R agonistic autoantibodies; EDHF, endothelium-derived hyperpolarizing factor; HIF, hypoxia-inducible factor; IL-6, interleukin-6; NO, nitric oxide; PKC, protein kinase C; PlGF, placental growth factor; ROS, reactive oxygen species; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; VSM, vascular smooth muscle
Because normal pregnancy involves significant uterine and vascular remodeling, and because MMPs are major regulators of tissue remodeling. MMPs may be involved in the uteroplacental and vascular remodeling during normal pregnancy. Changes in MMP expression/activity could also cause uterine and vascular dysfunction and in turn contribute to the pathogenesis of preeclampsia and premature birth. These observations have raised interest in the hormonal and growth factors that could affect MMP expression/activity during normal pregnancy, and the bioactive factors that could target uteroplacental and vascular MMPs in the setting of preeclampsia and premature labor.
In this chapter, we will discuss MMP expression/activity in blood vessels during pregnancy and how changes in vascular MMP could lead to severe vasoconstriction and increased blood pressure in human preeclampsia and animal models of hypertension in pregnancy. We will describe the potential upstream mechanisms that could cause changes in MMPs including the genetic and demographic risk factors, RUPP and placental ischemia, and discuss how the release of bioactive factors could target MMPs in the systemic vessels. We will also describe MMP expression/activity in the uterus during normal pregnancy and how changes in uterine MMP could lead to excessive uterine contraction and premature labor. Because of the ambiguity of the etiology and pathogenesis of preeclampsia and premature labor, the available remedies are currently limited. We will discuss how the advances in our knowledge of the role of MMPs and the potential predisposing factors and circulating bioactive factors could help design new biomarkers for diagnosis, and novel approaches for management of preeclampsia and premature labor.
2. VASCULAR AND PLACENTAL MMPs DURING PREGNANCY
Normal pregnancy is associated with significant hemodynamic, vascular and uteroplacental changes to ensure adequate placentation of the embryo, and sufficient blood and nutrient supply to the developing fetus. Normal pregnancy is associated with marked vasodilation of the maternal uterine, renal and systemic vessels,22 and reduction in the mechanisms of vascular contraction.23,24 The pregnancy-associated vasodilation and reduction in vascular contraction could be related to increased plasma levels of the female sex hormones estrogen and progesterone.25 For instance, estrogen causes relaxation of vascular smooth muscle (VSM) of the rat aorta and uterine artery.26,27 Also, progesterone inhibits contraction of rat blood vessels.26 Adequate placentation is also critical during normal pregnancy. Extravillous trophoblasts invade the maternal decidua and remodels spiral arteries to achieve maximal vasodilation and adequate nutrient supply to the embryo. Trophoblast invasion into the decidual stroma my require degradation of ECM proteins by proteolytic enzymes such as MMPs. MMP-2 (gelatinase A) and MMP-9 (gelatinase B) play a role in endometrial tissue remodeling during the menstrual cycle and pregnancy28–30. MMP-2 and MMP-9 are abundantly expressed in invading extravillous trophoblast cells, and the expression of these two gelatinases is highly related to trophoblast cell invasiveness.31–34 Also, factors that promote trophoblast invasion appear to have regulatory effects on MMP-2 and/or MMP-9 activity. For instance, epidermal growth factor (EGF)–mediated induction of trophoblast invasion is associated with increased expression/activity of MMP-2 and MMP-9.35,36 MMP-2 is the main MMP in the umbilical cord11, and serum MMP-9 level is elevated in normal pregnant women.7 The pregnancy-associated increase in expression/activity of vascular MMPs may be partly induced by the female sex hormones estrogen and progesterone. We have shown that the expression and activity of MMP-2 and -9 are increased in the aorta during pregnancy in rats.37 Also, consistent with the report that estrogen enhances the release of MMP-2 from human VSM cells,38 we found that estrogen+progesterone enhanced MMP-2 and MMP-9 expression/activity in the aorta of virgin rats. The pregnancy-associated increases in vascular MMPs could play a role in vascular remodeling, angiogenesis, and the systemic changes in blood vessels.39 In addition to their proteolytic effects, MMPs may affect membrane receptors and cell signaling. For instance, MMP-2 and MMP-9 cause relaxation of precontracted rat aorta40 and inferior vena cava.41,42 The increased expression/activity of MMPs in the aorta together with reported effects of MMPs effects on vascular remodeling and contraction mechanisms are consistent with a role of MMPs in the reduced vascular contraction and enhanced systemic vasodilation during pregnancy.
Another factor that could affect MMP expression/activity is extracellular MMP inducer (EMMPRIN, CD147, Basigin, BSG). EMMPRIN is a widely expressed membrane protein of the immunoglobulin superfamily,43 that has been implicated in tissue remodeling44 and various pathological conditions including cancer,43 rheumatoid arthritis45, heart failure,46 and atherosclerosis.47 EMMPRIN stimulates the production of MMP-1, MMP-2, MMP-3, and MMP-9,48 and may regulate MMPs in endothelial cells and tumors.49 We have shown that EMMPRIN expression is increased in the aorta of late-pregnant compared with virgin and mid-pregnant rats as well as in the aorta of virgin rats pretreated with estrogen+progesterone. The sex hormone-induced increases in aortic MMP-2 and MMP-9 expression/activity were blocked by EMMPRIN neutralizing antibody, supporting a role of EMMPRIN in the increases in vascular MMPs.37 These observations are consistent with the contention that the pregnancy-associated increase in expression/activity of vascular MMP-2 and MMP-9 are mediated by EMMPRIN and induced by the female sex hormones estrogen and progesterone.
In contrast with the pregnancy-associated increases in MMPs in the aorta, MMP-2 and MMP-9 expression did not increase in the placenta of late pregnant rats. In effect, a decrease in the expression/activity of placental MMP-2 and MMP-9 was observed in late compared with mid-pregnancy in rats.37 Some studies have shown an increase in MMP-2 and MMP-9 in the placenta of diabetic rats at mid-gestation,50 but did not follow the changes in MMPs during late-gestation. Other studies have suggested that MMPs are involved in placental remodeling during pregnancy.50 Serum MMP-2 and MMP-9 activity are increased in pregnant bitches.51 Also, the expression of MMP-2, MMP-14 and EMMPRIN is increased in the bovine placenta during late gestation.52 The differences in the expression of MMPs in the rat versus canine or bovine placenta could be related to species differences in the MMP regulation mechanisms or differences in the role of MMPs in early, mid, and late pregnancy. It is possible that the placenta as a potential source of MMPs may have finite capacity particularly during the late stages of pregnancy. Also, most of the placental remodeling takes place during the peri-implantation period and during fetal and organ development in early and mid-gestation, and further placental remodeling may not be needed during late pregnancy. Interestingly, we observed that the pregnancy-associated decrease in placental MMPs expression was associated with a decrease in the expression of placental EMMPRIN in late-pregnant compared with mid-pregnant rats, supporting a role of EMMPRIN as a critical inducer of MMPs during pregnancy.37 The decreased MMP-2 and MMP-9 and reduced EMMPRIN expression in the placenta of late-pregnant rats suggest reduced role of these MMPs in the feto-placental circulation during late pregnancy. While the observed changes in MMP-2 and MMP-9 highlight their role in vascular remodeling during pregnancy, that should not minimize possible involvement of other members of the MMP family.
3. UTEROPLACENTAL AND VASCULAR CHANGES IN PREECLAMPSIA
Preeclampsia is a major complication of pregnancy characterized by hypertension in pregnancy and often proteinuria,11,21 and is a major cause maternal and fetal morbidity and mortality, IUGR, fetal programming of cardiovascular and metabolic disease, and predisposition to adulthood hypertension and diabetes.53,54 Observational studies in preeclamptic women and mechanistic studies in animal models of hypertension in pregnancy have helped to uncover some of the pathogenic mechanisms.19,55–58 RUPP during late pregnancy in sheep, dog, rabbit and rat has been shown to induce a hypertensive state that closely resembles preeclampsia.19,56 Also, certain risk factors have been associated with placental ischemia as a potential initiating pathogenic event.18,23 Placental ischemia has also been associated with increased release of bioactive factors,20,59–62 which could target various vascular mediators and MMPs in the vascular ECM, endothelial cells and VSM leading to increased vasoconstriction and hypertension in pregnancy (Fig. 1).
4. RISK FACTORS IN PREECLAMPSIA
Predisposing genetic, demographic and environmental factors could affect placental development. Mutations in placental genes have been associated with preeclampsia, and 31 out of 36 placental genes are downregulated in preeclampsia.63 Mutations in placental mitochondrial genes could interfere with oxygen reduction leading to accumulation of ROS and oxidative stress in the uteroplacental circulation.64 Susceptibility genes include ACVR2A gene on chromosome 2q22 and STOX1 gene on chromosome 10q22. STOX1 Y153H polymorphism has been linked to inadequate trophoblast invasion and IUGR, and was detected in families with several generations of women who developed early and severe preeclampsia.65 Also, wild-type female mice crossed with transgenic male mice overexpressing human STOX1 show preeclamptic features including hypertension and proteinuria.66 FOXP3 is another gene that plays a role in the activation of regulatory T cells (Tregs) and thereby controls the immune response and maternal tolerance during normal pregnancy. Downregulation or polymorphism in the FOXP3 gene could alter the maternal immune response, reduce maternal tolerance and predispose to preeclampsia.67,68 The role of paternal genes in preeclampsia has been the subject of debate. Although some studies showed a 2.7% risk of preeclampsia associated with men whose mothers developed preeclampsia compared with men whose mothers had normal pregnancy,69 other studies showed a limited association between paternal genes and preeclampsia.70
Ethnic background, age, maternal lifestyle, pre-pregnancy weight, previous and family history of preeclampsia, primiparity, and multiple pregnancy could be risk factors for preeclampsia.6 The rate of preeclampsia is higher among African-American (5.2%) than Asian women (3.5%).71 Very young <16 years or older women >40 years are more prone to preeclampsia, and studies in Finland and India have supported that older women are at higher risk of developing preeclampsia than young women.72,73 The incidence of preeclampsia is ~3% in women with normal body mass index (BMI, 18.5–24.9), but increases to 7% in overweight women with BMI 30–34.9 and to 13% in obese women with BMI around 50.74 Preexisting medical condition such as heart disease, chronic respiratory conditions, diabetes, renal disorders, systemic lupus erythematosus, mental stress, reproductive tract surgery and history of antepartum hemorrhage may also increase the risk for preeclampsia.6 Importantly, cardiovascular and pulmonary disorders are associated with changes in tissue expression/activity of MMPs, which could contribute to the inadequate uteroplacental and vascular remodeling in preeclampsia.
5. ABNORMAL PLACENTATION AND PLACENTAL ISCHEMIA IN PREECLAMPSIA
During early pregnancy, the placenta is developed as a maternal-fetal interface through several processes including vasculogenesis, angiogenesis, trophoblast invasion and vascular remodeling. Vasculogenesis is the development of de novo vessels from pluripotent mesenchymal stem cells and occurs ~18–35 days after conception in humans. Angiogenesis is the sprouting of new blood vessels from preexisting vessels and is regulated by the coordinated actions of pro-angiogenic growth factors and the invasive capability of trophoblast cells.75 Healthy pregnancy requires sufficient placental vascularization. During the first trimester, the placental extravillous trophoblasts invade deep into the maternal decidua up to one-third of the myometrium, progressively invading the spiral arteries, replacing endothelial cells and VSM, and substituting the elastic tissue with fibrinoid material.76 This causes gradual dilation and transformation of the spiral arteries from low-capacity high-resistance to high-capacity low-resistance vessels, thus ensuring sufficient blood and nutrient supply to the developing fetus (Fig. 2).
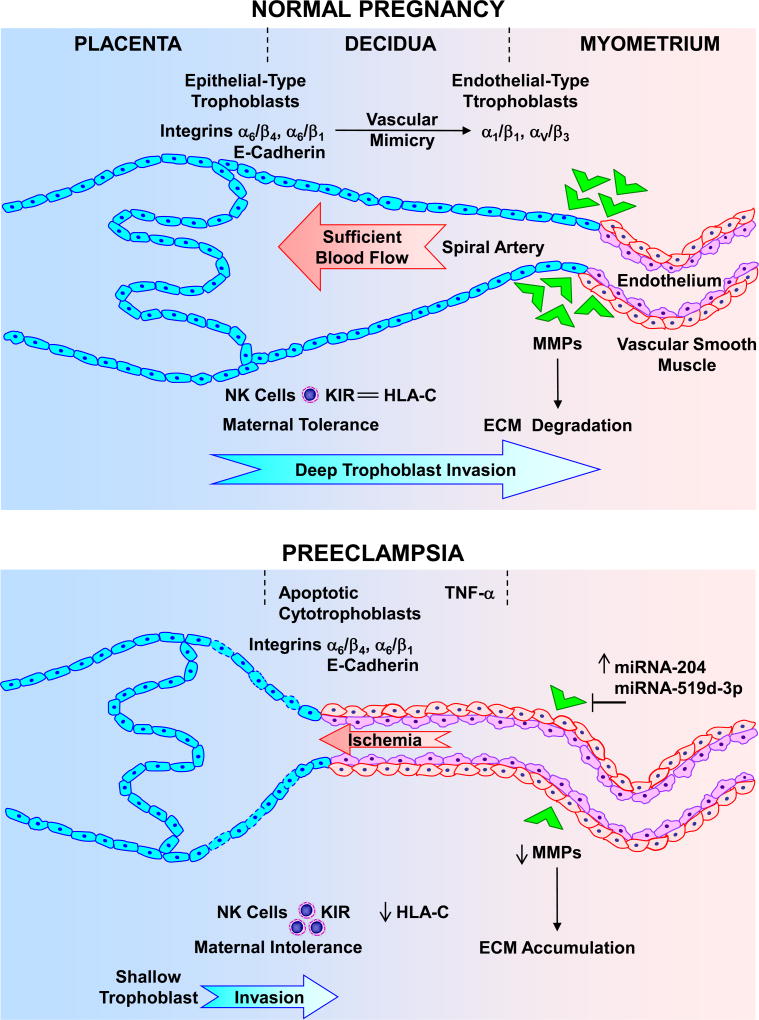
Fig. 2.
Deficient placentation in preeclampsia. During normal pregnancy, cytotrophoblasts initially express epithelial-type adhesion molecules such as integrins α6/β4 and α6/β1, and E-cadherin. As cytotrophoblasts become invasive, they express endothelial-type integrins α1/β1 and αV/β3 (“vascular mimicry”). Matrix metalloproteinases (MMPs) also cause degradation of extracellular matrix (ECM) and allow vascular remodeling. Cytotrophoblasts overexpress HLA-C that interact with the inhibitory KIR receptor and decrease natural killer (NK) cells, thus contributing to maternal tolerance. As a result, cytotrophoblasts invade the decidua to one-third of the myometrium, causing extensive remodeling of the spiral arteries from small-caliber resistance vessels to high-caliber capacitance vessels, and providing sufficent placental blood flow. In preeclampsia, increased immune response causes the release of cytokines such as TNF-α, apoptosis of cytotrophoblasts and maintained expression of epithelial-type integrins α6/β4 and α6/β1, and E-cadherin. Increased miRNA-519d-3p and −204 also decrease MMPs leading to decreased ECM degradation and vascular remodeling. Decreased HLA-C interaction with the inhibitory KIR receptor increases NK cells and decreases maternal tolerance. The decrease in trophoblast invasion of spiral arteries leads to shallow placentation to only superficial layers of the decidua, resulting in decreased blood flow and placental ischemia.
The symptoms of preeclampsia remit after delivery of the baby and the placenta, implicating the placenta as a central culprit in the disorder. Abnormal placentation, RUPP and placental ischemia/hypoxia are important initiating events in preeclampsia.18,20,56 Inadequate placentation could be caused by abnormal inflammatory and immune responses and accumulation of natural killer (NK) cells and macrophages, apoptosis of trophosblast cells and decreased invasion of spiral arteries, and abnormal expression of integrins and MMPs leading to decreased ECM remodeling, shallow trophoblast invasion and poor spiral arteries remodeling (Fig. 2).
5.1. Immune Responses and Inadequate Placentation in Preeclampsia
Pregnancy is a physiological process that poses a challenge to maternal tolerance and the immune response. For healthy pregnancy, the maternal systems must tolerate the semi-allogenic fetus, and likewise, the fetus needs to be protected from rejection by excessive maternal immune response.77 Preeclampsia is associated with augmented immune and inflammatory responses and increased production of the pro-inflammatory cytokines TNF-α and IL-6. In support of altered immune response in preeclampsia, HIV-positive women, who often have suppressed immune response, show lower incidence rates of hypertensive disorders and preeclampsia.78
During normal pregnancy, cytotrophoblasts overexpress the major histocompatibility complex molecules HLA-C, HLA-E and HLA-G which interact with their respective inhibitory receptors KIR, CD 94/NKGs and ILT-2 on NK cells. These interactions reduce the activity of NK cells and prevent them from attacking normal placental and fetal tissues.79 A decrease in the HLA-C/KIR interaction would lead to increased activity of NK cells, which would in turn attack placental and fetal tissues and lead to preeclampsia.80
Healthy pregnancy is associated with moderate activation of the complement system. Increased complement activation products Bb, C3a and C5a have been associated with preeclampsia.81 Also, small subcutaneous vessels from preeclamptic women show more neutrophils adherent to the endothelium than vessels from normal pregnant women, which may contribute to the endothelial dysfunction in preeclampsia.82 Interestingly, inhibition of complement activation or depletion of neutrophils decreases blood pressure in the RUPP rat model of placental ischemia, supporting a role of complement activation and innate immune response in hypertension in pregnancy.81,83
MMPs are released by inflammatory cells and MMP expression/activity is increased in various inflammatory and autoimmune disorders. An increase in the inflammatory and immune responses is expected to alter uteroplacental and vascular MMP expression/activity and consequently uteroplacental and vascular remodeling in preeclampsia.
5.2. Integrins and Reduced Trophoblast Invasion and Spiral Artery Remodeling
Trophoblast invasion and remodeling of the spiral arteries is in part regulated by integrins and other adhesion molecules. Cytotrophoblasts initially express epithelial cell-type adhesion molecules such as integrins α6/β4 and α6/β1, and E-cadherin. During normal pregnancy cytotrophoblasts become more invasive, and the epithelial cell-type adhesion molecules are replaced by the endothelial-type integrins α1/β1 and αV/β3; a process known as vascular mimicry or pseudovasculogenesis (Fig. 2).84 These phenotypic changes in integrins may be impaired during placental hypoxia and preeclampsia. Hypoxia alters the placental expression of integrins and fibronectin, causing increased expression of integrin α5 and fibronectin and decreased expression of integrin α1.85 Also, during preeclampsia, abnormal expression of epithelial cell-type adhesion molecules and apoptosis of cytotrophoblasts cause limited invasion of spiral arteries, placental ischemia and RUPP.84,86,87 Ezrin is one of the integrins involved in cell adhesion, organization and migration. Ezerin is downregulated in syncytiotrophoblast microvesicles from preeclamptic women, resulting in reduced invasiveness of cytotrophoblats, shallow placentation and defective vascularization of the placenta.88 The decreased trophoblast invasion and replacement of vascular cells also leads to retention of VSM cells in the spiral arteries, which promote vasoconstriction,89 and further decrease uteroplacental blood flow and aggravate placental ischemia (Fig. 2).
Endothelial cell adhesion molecules such as soluble intracellular adhesion molecule-1 (ICAM-1) and soluble vascular cell adhesion molecule-1 (VCAM-1) are downregulated during normal pregnancy, thus minimizing leukocyte adhesion to endothelial cells, and maintaining patency and blood flow in the spiral arteries. The plasma levels of ICAM-1 and VCAM-1 are increased in preeclampsia, leading to increased leukocyte adhesion to endothelial cells and restricted blood flow in the spiral arteries.90
Preeclampsia is also associated with increased placental expression of microRNA miRNA-125b-1-3p which could reduce the expression of S1PR1, a G-protein coupled receptor that facilitates invasion of human trophoblasts.91 Preeclampsia is also associated with increased expression of placental miRNA-517a/b and miRNA-517c, which have been shown to be expressed and to decrease trophoblast invasion in extravillous trophoblasts under hypoxic conditions92.
5.3. MMPs, Abnormal Placentation, and Placental Ischemia
MMPs such as MMP-2 and MMP-9 may be involved in ECM remodeling and trophoblast invasion of the spiral arteries during pregnancy.31–34 The amount and activity of MMP-2 and MMP-9 are increased in the aorta of normal pregnant rats, suggesting a role of MMPs in the pregnancy-associated vascular remodeling.37,93 In support of a role of MMPs in uteroplacental remodeling, in first trimester trophoblasts suppression of MMP-9 expression inhibits the invasive capability of trophoblasts.94 Also, MMP-9 ablation in MMP-9 knockout mice shows a phenotype that mimics preeclampsia possibly due to impaired trophoblast differentiation and invasion.95 Genetic polymorphisms in MMP-2 and MMP-9 transcription have been described in preeclampsia.96 Decreased levels of MMP-9 have also been observed in preeclamptic compared with normal placenta.97,98 In preeclampsia, increased expression of miRNA-519d-3p and miRNA-204,99 could target MMP-2 and MMP-9 and decrease trophoblast invasion of spiral arteries.94 Collectively, these observation suggest a relationship between decreased MMP-2 and MMP-9 and impaired trophoblast invasion in preeclampsia. On the other hand, measurements of the levels of MMPs have not been consistent in preeclampsia, with some studies showing an increase in serum levels of MMP-2 and MMP-9,100 while other studies showing a decrease in circulating MMP-9 (Table 1).7 Further measurements of the plasma levels of MMPs and their correlation with MMP levels in the placenta and other maternal tissues are needed in both human preeclampsia and animal models of hypertension in pregnancy.
Table 1.
MMP and TIMP levels in human normal pregnancy and preeclampsia
| MMP/TIMP | Specimen (Units) | Normal pregnancy | Preeclampsia | Reference |
|---|---|---|---|---|
|
| ||||
| MMP-1 | Umbilical cord serum (pg/mL) | 294.33±11.53 | 177.67±12.63 | 101 |
|
| ||||
| MMP-2 | Serum (ng/mL) | 669 (560–760) | 834 (656–1002) | 7,100 |
| Plasma (ng/mL) | 241.1±35.3 SD | 290.5±48.4 SD | ||
|
| ||||
| MMP-9 | Serum (ng/mL) | 390 (277–569) | 290 (280–470) | 7,100 |
| Plasma (ng/mL) | 240.0±197.7 SD | 262.4±153.8 SD | ||
|
| ||||
| TIMP-1 | Serum (ng/mL) | 148 (121–188) | 213 (212–220) | 7,100,101 |
| Plasma (ng/mL) | 142.8±39.2 SD | 187.1±35.4 SD | ||
| Umbilical cord serum (pg/mL) | 1304.20±69.66 | 1363.00±71.50 | ||
|
| ||||
| TIMP-2 | Serum (ng/mL) | 228 (207–267) | 232 (225–245) | 7 |
| Plasma (ng/mL) | 158.3±32.3 SD | 194.3±49.3 SD | 100 | |
Values represent means±standard error of the mean.
indicates standard deviation. Numbers in parenthesis indicates range.
Because collagen is a major substrate of MMPs, a decrease in MMP-2 and MMP-9 is expected to cause excessive collagen deposition, and in turn decrease uteroplacental vascularization and spiral arteries remodeling.8 However, gelatinases mainly degrade collagen IV, and partially degrade collagen I, suggesting that other MMPs are involved. The collagenase MMP-1 is expressed in cytotrophoblasts and syncytiotrophoblasts of the placenta and decidua and may play a role in trophoblast invasion. Some studies have shown low levels of MMP-1 in umbilical cord blood, placenta and decidua of preeclamptic versus normal pregnant women, and the low MMP-1 levels are correlated with the severity preeclampsia.101 Other studies suggest a role of MMP-1 in the pathogenesis of preeclampsia.102 Also, the matrilysin MMP-7 could play a role in endometrial tissue remodeling during the menstrual cycle and pregnancy.103 Studies have also shown that cytotrophoblasts and VSM release MMP-12, which could mediate elastolysis and remodeling of the uterine spiral arteries during pregnancy.104 Whether the uteroplacental and vascular MMP balance is altered in hypertension in pregnancy is a subject of topical interest. Also, the upstream mechanisms influencing MMPs activity and the downstream substrates and pathways via which MMP imbalance could affect uteroplacental remodeling and vascular function are emerging new areas for research.
We have examined whether alteration of MMP expression/activity is a potential pathogenic mechanism in the uteroplacental and vascular remodeling and placental ischemia in hypertension in pregnancy. We measured the hemodynamic and uteroplacental changes in normal pregnant rats and the RUPP rat model of placental ischemia. Blood pressure was increased, and the litter size and individual pup weight were decreased in RUPP versus normal pregnant rats.105,106 We further examined the specific changes in three important tissues during pregnancy; the uterus which undergoes remodeling to accommodate the growing fetus, the placenta which provides nutrient supply to the developing fetus, and the aorta for the vascular changes in the maternal circulation. The uterus, placenta, and aortic tissue weight was reduced in RUPP rats. Also, histological morphometry showed reduction in uterine, placental and aortic cross-sectional area in RUPP versus normal pregnant rats, supporting growth-restrictive remodeling in the uterus, placenta and vasculature of RUPP rats. Also, the fetal litter size and individual pup weight are decreased in RUPP compared with normal pregnant rats.8
In search for the mechanisms involved in the changes in uteroplacental and vascular remodeling, Western blots, gelatin zymography and immunohistochemical analysis revealed that MMP-2 and MMP-9 were abundantly expressed in tissues of normal pregnant rats, supporting a role of MMPs in the uteroplacental and vascular remodeling during normal pregnancy.37 MMPs immunostaining was particularly apparent in the aortic media, consistent with reports that VSMCs are a major source of MMPs.107,108 The levels of MMP-2 and MMP-9 appear to be decreased in the uterus, placenta and aorta of RUPP compared with normal pregnant rats (Table 2). Western blots and gelatin zymography revealed decreases in protein amount and gelatinase activity of MMP-2 and MMP-9 in tissues of RUPP rats. MMP-2 and MMP-9 immunostaining was also reduced in uterine and placenta tissue sections and less intense in the aortic media of RUPP versus normal pregnant rats. The decreases in MMPs expression/activity, in parallel with the decreases in uterine, placental, and aortic tissue weight and cross-sectional area suggest a role for reduced MMPs expression/activity in growth-restrictive remodeling in tissues of RUPP rats.8
Table 2.
MMP levels in normal pregnant and RUPP rats
| MMP | Specimen (Units) | Normal Pregnant | RUPP | Reference |
|---|---|---|---|---|
|
| ||||
| MMP-2 | Uterus (OD) | ~1.0 | ~0.7 | 8 |
| Placenta (OD) | ~0.9 | ~0.5 | ||
| Aorta (OD) | ~0.9 | ~0.6 | ||
|
| ||||
| MMP-9 | Uterus (OD) | ~0.4 | ~0.2 | 8 |
| Placenta (OD) | ~0.3 | ~0.2 | ||
| Aorta (OD) | ~0.4 | ~0.2 | ||
OD: optical densitometry of Western blot bands normalized to β-actin
While the changes in MMPs in the aorta of RUPP rats suggest a role in hypertension in pregnancy, the mechanisms linking localized RUPP to the systemic changes in MMPs and the potential bioactive factors involved need to be examined.
6. CIRCULATING BIOACTIVE FACTORS and MMPs IN PREECLAMPSIA
Placental hypoxia/ischemia is believed to trigger the release of several bioactive factors including the antiangiogenic factors sFlt-1 and sEng, pro-inflammatory cytokines such as TNF-α and IL-6, HIF, ROS and AT1-AA (see Fig. 1).6,57–59,62,109–111 These factors could target uteroplacental and vascular MMPs and cause further vasoconstriction of spiral arteries and placental ischemia, as well as generalized vasoconstriction and increase in blood pressure in preeclamptic women and animal models of hypertension in pregnancy.21,112
6.1. Pro-angiogenic and Anti-angiogenic Factors in Preeclampsia
6.1.1 Vascular Endothelial Growth Factor (VEGF)
The VEGF gene has a gene locus on chromosome 6p21.3, which consists of 8 exons involved in the expression of a family of growth factors including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF.112 VEGF-A, VEGF-B and PlGF bind to tyrosine kinase receptor Flt-1 (VEGFR-1). VEGF-A binds to VEGFR-2 (Flk-1 or KDR) to promote the development of placental blood vessels.112 VEGF regulates endothelial cell proliferation, angiogenesis and vascular permeability.21,112 In endothelial cells, VEGF increases [Ca2+]i, Ca2+/calmodulin, endothelial nitric oxide synthase (eNOS) activity, and prostacyclin (PGI2).113–115 VEGF could also promote Ca2+-independent generation of NO by promoting Akt activation and eNOS Ser1177 phosphorylation in human umbilical vein endothelial cells (HUVECs).115
Some studies show an increase in circulating VEGF in preeclampsia.116–118 Also, villous explants from preeclampsia produce greater amounts of VEGF than those form normal pregnant women.119 It is likely that the severe vasoconstriction in preeclampsia would increase vascular shear-stress, and in turn increase circulating VEGF.6 Other studies have shown a decrease or unchanged serum levels of VEGF in preeclampsia.120,121 Women with the T allele of VEGF 936C/T have lower levels of VEGF and a higher risk of preeclampsia than women with VEGF 936C/C.122 Plasma VEGF levels are decreased in RUPP rat model of hypertension in pregnancy,20 although as with the findings in human villous explants, placenta from RUPP rats show greater production of VEGF.123 The differences in the results may be related to differences in the methods of VEGF measurement.120 Also, in preeclampsia, an increase in circulating anti-angiogenic factors may bind to VEGF. Thus, total (bound and unbound) VEGF measured using radioimmunoassay or competitive enzyme immunoassay could be higher while free VEGF measured using enzyme-linked immunosorbent assay (ELISA) may be lower in preeclamptic than normal pregnant women.124
A decrease in VEGF may also play a role in the glomerular endotheliosis and proteinuria in preeclampsia. VEGF is synthesized constitutively by podocytes in the glomerulus where it maintains endothelial cell health and induces the formation of fenestrae. Endotheliosis and loss of fenestrae has been detected in genetic glomerular VEGF deficiency.125 Also, in clinical cancer trials the use of VEGF-neutralizing antibodies is associated with proteinuria.126 In mice, infusion of VEGF antibodies leads to glomerular endotheliosis and proteinuria.127 Also, mice lacking one VEGF allele in renal podocytes develop a renal pathology similar to that in preeclampsia. Thus, a decrease in VEGF could cause proteinuria and other renal pathology in preeclampsia. Importantly, infusion of VEGF ameliorates the renal lesions, glomerulonephritis and thrombotic microangiopathy in RUPP rats, suggesting potential benefits of pro-angiogenic factors in the glomerular endotheliosis associated with hypertension in pregnancy.128,129
MMPs may induce the release of growth factors by cleaving the growth factor-binding proteins or matrix molecules, and these effects may contribute to the altered tissue remodeling in preeclampsia. MMPs may also be regulated by growth factors.130 Platelet derived growth factor-BB increases MMP-2 expression in rat VSMCs, possibly via Rho-associated protein kinase, extracellular signal-regulated kinases, and p38 mitogen-activated protein kinase (MAPK).131 Also, in carotid plaques, EGF enhances MMP-9 activity and increases MMP-1 and MMP-9 mRNA transcripts in VSMCs.132 Angiogenic growth factors such as VEGF and TGF-β are secreted by endothelial cells and other cells and act in an autocrine or paracrine fashion to accelerate angiogenesis. MMPs may mediate the angiogenic effects of VEGF by virtue of their proteolytic activity and other mechanisms including helping to detach pericytes from the vessels undergoing angiogenesis, releasing ECM-bound angiogenic growth factors, exposing cryptic pro-angiogenic integrin binding sites in ECM, generating pro-migratory ECM component fragments, and cleaving endothelial cell-cell adhesions.39 Interestingly, VEGF increases the expression of MMP-1, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-13, and MMP-19 in HUVECs, and induces MMP-10 expression via PI3K and MAPK pathways.133 The interaction between MMPs and VEGF in the setting of uteroplacental and vascular remodeling in normal pregnancy and preeclampsia should be further examined.
6.1.2 Placental Growth Factor (PlGF)
PlGF is a pro-angiogenic factor that binds to VEGFR-1 and enhances the angiogenic effects of VEGF.134 PlGF has only 1/10th the affinity of VEGF for VEGFR-1, but its levels are ~40 times higher than those of VEGF during normal pregnancy. PIGF promotes endothelial cell growth, placental vasculogenesis and development, and vasodilation of uterine vessels.21
Plasma PlGF levels are low in non-pregnant women (~44 pg/mL), and markedly increase during normal pregnancy.134 PlGF levels are ~353 pg/mL during gestational weeks 21 and 22, rising steadily to ~574 pg/mL after gestational weeks 29 and 30.135 Circulating PlGF levels decrease in preeclampsia116,136–138 and the decrease is more apparent in early than late preeclampsia.139 PlGF has four alternatively spliced mRNA forms (PIGF 1–4), and its predominant isoform PIGF-1 is downregulated in preeclampsia.124 Circulating levels of PlGF are also decreased in RUPP and deoxycorticosterone acetate (DOCA)-salt hypertensive rats.20,140
In addition to its growth promoting effects, PlGF promotes vasodilation via VEGFR-1 and endothelium-derived hyperpolarizing factor (EDHF)-mediated activation of small conductance Ca2+-activated K+ channels (SKCa).141,142 In small mesenteric arteries of pregnant rats treated with L-NAME and indomethacin, a second exposure to PlGF produces greater vasodilation and greater reduction in VSM [Ca2+]i than the first PlGF application. VEGF and PlGF may promote VEGFR-1 dimerization, and the initial exposure to PlGF may facilitate the formation of receptor homodimers and their submembrane signaling, leading to augmented vasodilator responses to repeated PlGF stimulation.142 A decrease in the levels of PlGF may be partly responsible for the decreased vasodilator responses in preeclampsia.
6.1.3 Soluble fms-like Tyrosine Kinase-1 (sFlt-1)
sFlt-1 (sVEGFR-1) is an anti-angiogenic factor expressed as an alternatively spliced variant of VEGFR-1 that lacks both the transmembrane and cytoplasmic domains. sFlt-1 binds VEGF and PlGF and blocks their angiogenic effects on VEGFR. sFlt-1 may also form a heterodimer with the surface membrane VEGFR-1 and inhibit its post-receptor signaling actions.143 Trophoblast cells express sFlt-1 mRNA, and the sFlt-1 level increases to ~1.5 ng/mL in normal pregnant women compared to ~0.15 ng/mL in non-pregnant women.21 sFlt-1 levels are largely stable in normal pregnant women, and show an increase after gestational week 36. Throughout the third trimester, an increase in sFlt-1 is associated with some reduction in VEGF and PlGF levels. Preeclamptic women show imbalance between sFlt-1, VEGF and PlGF.116,120,137,139,144–149 The sFlt-1 gene has a gene locus on chromosome 13q12. In women with trisomy 13, an extra copy of the sFlt-1 gene is associated with increased circulating levels of sFlt-1, reduced PlGF and increased risk of preeclampsia.150 Several reports have shown higher circulating levels of sFlt-1 in early and late preeclampsia.116,136–139,149 Serum sFlt-1 is also higher in women with previous preeclampsia (~0.5 ng/mL) than in women with previous normal pregnancy (~0.3 ng/mL), and the increases can be detected even 6 months or more after delivery.149 sFlt-1 levels are also greater in villous explants from preeclamptic compared with normal pregnant women.119
Placental ischemia/hypoxia may trigger the production of sFlt-1. During placental hypoxia, HIF-1 may bind to the promoter region of flt-1 gene leading to up-regulation of sFlt-1.119,120 In extravillous trophoblasts, overexpression of miR-517a/b and miR-517c increase the expression of TNFSF15, a cytokine that promotes Flt-1 splicing, and increases the production of sFlt-1.92 sFlt-1 e15a, a splice variant of sFlt-1 and the most abundant form released by the placenta, binds VEGF and in turn decreases endothelial cell migration, invasion, and tube formation. sFlt-1 e15a is expressed in syncytiotrophoblasts and its serum levels are 10-fold higher in preeclamptic versus normal pregnant women.151
Because of the increased levels of sFlt-1, a 53% decrease in VEGF/sFlt-1 ratio and a 70% decrease in PlGF/sFlt-1 ratio have been observed in preeclamptic placenta.119 The circulating sFlt-1/PlGF ratio is higher in preeclamptic than normal pregnant women from second trimester onwards and could be a potential predictor of the onset of preeclampsia,138,139 However, some studies suggest that the circulating sFlt-1/PlGF ratio could be lower in late versus early preeclampsia.139,152 Circulating sFlt-1 levels and sFlt-1/PlGF ratio are higher in twin than singleton pregnancies, and the difference is likely related to the greater placental mass in twin pregnancies.153,154 The proportionate increases in sFlt-1 and sFlt-1/PlGF ratio in twin versus singleton pregnancies support the concept that the placenta is a major source of these factors. Angiogenic imbalance has also been implicated in the changes in endothelin-1 (ET-1) levels. Preeclamptic women with sFlt-1/PlGF ratio >85 have higher levels of ET-1 than women with sFlt-1/PlGF ratio <85.155 Importantly, extracorporeal removal of circulating sFlt-1 from preeclamptic patients decreases sFlt-1/PlGF ratio, improves symptoms and prolongs pregnancy,156 further supporting a role of sFlt-1 in preeclampsia.
RUPP rats show increases in plasma and placental levels of sFlt-1 and plasma sFlt-1/PlGF ratio.20,123,157 Other animal models of hypertension in pregnancy show either increased or little change in circulating levels of sFlt-1.140,158–162 Importantly, Infusion of exogenous sFlt-1 or adenoviral overexpression of sFlt-1 in pregnant rats causes increases in blood pressure, decreased plasma VEGF, proteinuria, and glomerular endotheliosis with occlusion of renal capillaries and focal fibrin deposition in glomerular cells.120,163–166 Also, mice treated with sFlt-1 show increased vascular response to ET-1.167 Treatment of endothelial cells with plasma of preeclamptic patients decreases angiogenesis, and removal of sFlt-1 or treatment with VEGF or a sFlt-1 antibody reverses the anti-angiogenic effects of sFlt-1 and restores endothelial cell angiogenesis.119
Of note, VEGF through an action on VEGFR-2 stimulates the production of sFlt-1 in human placental explants.168 This feedback modulation of VEGF by sFlt-1 may represent a local protective mechanism at the maternal-fetal interface to control the levels of VEGF and prevent any damage to the placenta or fetus caused by excess VEGF during normal pregnancy.168 Dysregulation of this VEGF-sFlt-1 feedback mechanism may be involved in the pathogenesis of preeclampsia.
It has been suggested that sFlt-1 could cause generalized endotheliosis in blood vessels leading to hypertension, in the glomeruli leading to proteinuria, and in the brain vessels leading to seizures and eclampsia.166 Whether sFlt-1 could target tissue MMPs particularly during pregnancy is not clear. Some studies suggest that sFlt-1-induced inhibition of VEGFR-2 could decrease endothelial VEGF production and MMP-2 and MMP-9 expression/activity.169 Also, in mouse model of abdominal aortic aneurysm treatment with sFlt-1 reduces aneurysm size and attenuates MMP-2 and MMP-9 activity in peri-aortic tissue.170 Our recent studies have supported a role of sFlt-1 as a potential upstream mechanism linking placental ischemia to the decrease in MMPs in hypertension in pregnancy.8 We found that sFlt-1 reduced MMPs expression/activity in uterine, placental and vascular tissues of normal pregnant rats. VEGF reversed the sFlt-1 induced decreases in MMPs in tissues of normal pregnant rats. Also, VEGF increased MMPs levels in tissues of RUPP rats to levels similar to those in normal pregnant rats. These observations are consistent with the reports that infusion of VEGF reduces blood pressure in RUPP rats.171,172
6.1.4 Soluble Endoglin (sEng)
Transforming growth factor-β1 (TGF-β1) is known to bind to TGF receptors and to induce proliferation and migration of endothelial cells.112 Endoglin (Eng) is a co-receptor for TGF-β1 and TGF-β3 that is highly expressed on cell membrane of endothelial cells and syncytiotrophoblasts, where it mediates proliferation of angiogenic endothelial cells and possibly trophoblasts.173 Mutations in Eng gene are associated with loss of capillaries, arteriovenous malformations, and hereditary hemorrhagic telangiectasia.174 In comparison with Eng, sEng is an anti-angiogenic protein that binds TGF-β1 and prevents it from binding to its natural angiogenic receptor, and thereby inhibits TGF-β1-induced eNOS activation and vasodilation.112 Hypoxia induces the release of sEng. In placental extracts, exposure to hypoxia increases the expression of sEng.175
Serum levels of sEng are barely detectable in non-pregnant women and are much lower in normal pregnant women.176 The levels of sEng are 3-, 5- and 10-fold higher in women with mild preeclampsia, severe preeclampsia and HELLP syndrome, respectively, compared with gestational age–matched control pregnant women.176 Serum levels of sEng may be increased in both early and late preeclampsia.136,177 However, one study showed an increase in sEng levels at gestational weeks 10–17 in women who developed early preeclampsia, but not in those who developed late preeclampsia.152
In RUPP rat model of hypertension in pregnancy, the levels of sEng are increased in the serum and placenta, and the serum levels of TGF-β are decreased.62,178 However, sEng levels did not show detectable change in DOCA-salt or L-NAME treated rat models of hypertension in pregnancy.140,158 It is likely that sEng acts in concert with the antiangiogenic factor sFlt-1 to promote vascular permeability, proteinuria, IUGR and severe hypertension.176 In support, pregnant rats infused with both sEng and sFlt-1 show HELLP syndrome-like characteristics.179 In cultured HUVECs, sEng impairs endothelial formation.176 Whether sEng targets MMPs and affects uteroplacental and vascular remodeling in hypertension in pregnancy is unclear. Of note, MMP-14 cleaves Eng, the TGF-β co-receptor, and thereby inhibits its angiogenic effects,180 and whether these effects play a role in preeclampsia need to be examined.
6.2. Cytokines, TNF-α, and Interleukins
Placental ischemia/hypoxia causes the release of pro-inflammatory cytokines.11,21,181 During placental reperfusion injury, reestablished blood flow causes the releases of TNF-α and interleukins (ILs).77 The circulating levels of TNF-α are greater in preeclamptic than normal pregnant women,182–184 although the placental levels of TNF-α may not be different in preeclampsia versus normal pregnancy.185 LIGHT, or TNF superfamily member 14, is also increased in preeclampsia and may contribute to placental ischemia.186 The plasma levels and CD4+T cell production of TNF-α are increased in RUPP versus normal pregnant rats.109,110,178,181,187 Infusion of TNFα causes hypertension and proteinuria in late pregnant mice, rats, and baboons.161,162,188 Similarly, infusion of the TNF superfamily member LIGHT in pregnant mice causes increases in blood pressure, proteinuria, and the expression of ET-1 and sFlt-1.186 TNF-α may work in concert with other inflammatory cytokines such as IL-6 to increase ET-1 levels and cause hypertension in RUPP rats.181 TNF-α may also function in synergy with sFlt-1 to promote a pro-inflammatory and antiangiogenic state. Treatment of HUVECs with both TNF-α and sFlt-1 causes an increase in the adhesion molecules ICAM and VCAM and promotes the release of markers of endothelial dysfunction such as ET-1 and von Willebrand factor.115 In support of a role of TNF-α in hypertension in pregnancy, blockade of TNF-α with the TNF-α decoy receptor etanercept reduces blood pressure in RUPP rats. Also, treatment of HUVECs with serum from RUPP rats treated with the TNF-α blocker etanercept produces less ET-1 than serum from nontreated RUPP rats.181
TNF-α is an important modulator of the immune response. TNF-α increases vascular permeability, fibroblast proliferation and lymphocyte activation, and promotes the production of IL-6 and IL-8. TNF-α downregulates eNOS and mitochondrial biogenesis, leading to mitochondrial dysfunction, oxidative stress and increased production of ROS.189 TNF-α can also alter the expression of adhesion molecules in placental vessels181 and the production of MMPs in preeclampsia.96
Another pro-inflammatory cytokine that could be elevated in preeclampsia is IL-6.136,182 RUPP rats show increased plasma levels and higher CD4+T cell production of IL-6.178,187 Chronic infusion of pregnant rats with IL-6 causes hypertension, proteinuria,190 enhanced vascular contraction and reduced endothelium-dependent NO-cGMP relaxation pathway.58 IL-6 may promote dimerization of the surface receptor GP-130 on endothelial cells leading to abnormal cell signaling and vascular dysfunction. IL-6 may also increase vascular permeability by disrupting the tight junctions in endothelial cells.191
IL-1β is another pro-inflammatory cytokine that could promote the inflammatory response and disrupt endothelial function in preeclampsia. The production of IL-1β is greater by monocytes from preeclamptic women compared with those from normal pregnant women.192
IL-10 is an anti-inflammatory cytokine whose levels are reduced in the plasma and placenta of preeclamptic women183,185 and in the plasma of RUPP rats.178 Also, in placental trophoblasts, exposure to hypoxia is associated with increased pro-inflammatory cytokines and decreased IL-10.193
The source of pro-inflammatory cytokine in preeclampsia is mostly in the maternal circulation. Monocytes and macrophages are the main reservoirs of pro-inflammatory cytokines and are the first cells to be activated in nonspecific immune response.194 Monocytes produce more TNF-α and IL-6 when treated with plasma from preeclamptic than normal pregnant women.194 IL-10 may have regulatory effects on monocytes and the inflammatory response during normal pregnancy by controlling TNF-α and IL-1β gene expression,192 and these IL-10-mediated regulatory effects appear to be lost in preeclampsia. Interestingly, uric acid stimulates monocytes to release cytokines, and hyperuricemia is often observed in preeclamptic patients. Also, monocytes from preeclamptic patients with high levels of uric acid produce more TNF-α and IL-1β than monocytes from normal pregnant women.192 In addition to their proteolytic activities, MMPs may increase the release of cytokines in preeclampsia.60
6.3. Hypoxia-Inducible Factor (HIF)
HIF is a transcriptional factor that plays a role in the physiologic responses to hypoxia. HIF-1 is a heterodimer consisting of an oxygen-regulated HIF-1α and HIF-2α subunits and a constitutively expressed HIF-1β subunit. While hypoxia is an important inducer of HIF, de novo synthesis of HIF-1α may occur in response to non-hypoxic stimuli such as pro-inflammatory factors. For example, TNF-α upregulates HIF-1α mRNA expression.162 Also, a large number of genes are regulated by HIF-1 including VEGF, leptin, TGF-β3, and NOS. Of note, DNA microarray analysis in arterial endothelial cells have shown that more than 2% of human genes are regulated directly or indirectly by HIF-1.11
HIF expression may increase during pregnancy, and the changes may be related to the pregnancy-related increase in production of estrogen and progesterone. Estrogen stimulates uterine HIF-2α, and progesterone primarily up-regulates uterine HIF-1α expression.195 HIF shows further increase in preeclampsia.196 The circulating levels of HIF-1α are increased in preeclamptic compared with normal pregnant women.197 HIF-1α may participate in the pathogenesis of preeclampsia by upregulating the antiangiogenic factors sFlt-1 and sEng, binding to ET-1 gene and regulating ET-1 mRNA expression, reducing the trophoblast invasion capability, and inducing AngII-converting enzyme (ACE) expression in the lungs and kidney and AngII production.11,198 In support, HIF-1α increases the production of sFlt-1 in human villous trophoblasts.199
The placental levels of HIF-1α are elevated in the RUPP rat model of hypertension in pregnancy.62 Also, downregulation of HIF-1α mRNA using siRNA reverses the increases in blood pressure, proteinuria, renal damage and serum levels of sFlt-1 in mice models of hypertension in pregnancy.199
In addition to the role of HIF in oxygen homeostasis and its regulation by oxygen,200 cytokines, hormones, metallic ions and mechanical stretch may induce HIF expression.200,201 Prolonged mechanical stretch increases HIF-1α and HIF-2α mRNA expression and protein levels in skeletal muscle fibers.202,203 Also, upregulation of HIF-1α has been observed in rat cardiac myocytes, aortic VSM cells and fibroblasts exposed to mechanical stretch.204–206 Studies have shown upregulation of HIF-1α mRNA in VSM cells subjected to cyclic stretch for 4 hours.204 Similar increases in HIF-1α protein have been shown in fibroblasts subjected to cyclic stretch for 24 hours.206 The mechanisms via which mechanical stretch upregulate HIF are unclear, but may involve PI3K and MAPK.201,202,204 Studies have suggested that the expression/activity of MMP-2 and MMP-9 are regulated by HIF.207,208 We have also shown that mechanical stretch is associated with increased HIF-1α expression, and that HIF could increase MMP expression in rat inferior vena cava.209 Whether HIF functions as a transducing signaling mechanism between vascular mechanical stretch and the expression of MMPs during pregnancy needs to be examined.
6.4. Reactive Oxygen Species (ROS)
ROS such as superoxide anion (O2•−), H2O2 and hydroxyl ion (OH−) contain highly reactive O2. Normal pregnancy may represent a state of oxidative stress caused by increased maternal metabolism and metabolic activity of the placenta. The generation of ROS is increased during pregnancy;210 however, the placental production of ROS is normally counterbalanced by antioxidants.11 In preeclampsia, defective trophoblast invasion and decreased uteroplacental blood flow result in periods of ischemia/reperfusion and a hypoxic environment that favors oxidative stress, inflammation, and vascular dysfunction.189 In preeclampsia, the levels of antioxidants may be too low to counterbalance the increased ROS production.211 The expression of antioxidants such as hemeoxygenase-1, hemeoxygenase-2, copper/zinc superoxide dismutase, glutathione peroxidase and catalase is decreased in preeclampsia. Also, the total antioxidant capacity is lower in serum from preeclamptic compared with normal pregnant women.212 The ROS/antioxidants imbalance would then lead to lipid peroxidation, increased TXA2 and loss of glutathione peroxidase activity in the placenta.59 Antioxidant levels were reduced in women who were later diagnosed with early preeclampsia,213 suggesting a role of oxidative stress in the pathogenesis of preeclampsia. Interestingly, in preeclampsia women, brachial artery flow-mediated dilation is reduced and is associated with decreased plasma levels of the antioxidant ascorbate, and administration of ascorbic acid improved flow-mediated dilation, supporting a relation between oxidative stress and endothelial dysfunction in preeclampsia.214 Also, the placental levels of hemeoxygenase-1 are reduced in RUPP compared with normal pregnant rats,62 supporting a link between ROS and hypertension in pregnancy.
Neutrophils and monocytes are major sources of ROS in preeclampsia. Monocytes from preeclamptic women produce more H2O2 and O2•− and cause more endothelial cell damage compared to monocytes from normal pregnant women.215,216 We should note that neutrophils also produce NO, which can protect cells from the damaging effects of O2•− during normal pregnancy. However, in preeclampsia, excess O2•− scavenge the NO produced by neutrophils to form peroxynitrite (ONOO−), thus reducing NO bioavailability and causing endothelial cell damage.216. NADPH oxidase is a membrane-bound enzyme that catalyzes the one-electron reduction of oxygen to O2•− via NADPH. NADPH oxidase isoform NOX1 is overexpressed in the placenta of preeclamptic women.217 In HUVECs, treatment with serum from preeclamptic women increases the mRNA expression of the NADPH oxidase subunit gp91phox, and augments O2•− production.218 Treatment of HUVECs with preeclamptic serum also causes overexpression of iNOS,218 which could produce excessive amounts of NO and in turn increase the production of ROS and promote endothelial cell injury. Interestingly, in the RUPP rat model of hypertension in pregnancy treatment with specific inhibitors of iNOS is associated with a decrease in blood pressure, aortic levels of ROS and NADPH-dependent production of ROS.219 Biopterin (BH4) promotes eNOS dimerization and activity. Hypoxia reduces BH4 and in turn causes eNOS uncoupling, increased ROS production, and decreased bioavailability of NO.220 In DOCA-salt hypertensive rats, supplementation with a BH4 such as sepiapterin decreases the production of ONOO− and O2•− and increases the production of NO.220
Other markers of lipid peroxidation and oxidative stress such as malondialdehyde and prostaglandin F2α are increased in serum of preeclamptic women at gestational weeks 10–14. This may cause gradual oxidative damage in the placenta, even before overt symptoms of preeclampsia.221 The plasma levels of the oxidative stress marker 8-isoprostane, and the total aortic and placental levels of ROS are higher in RUPP compared with normal pregnant rats.178,219 In first-trimester villous trophoblasts, excessive oxidative stress affects the expression of several miRNAs including those involved in angiogenesis, apoptosis, immune response and inflammation, and this could be a potential mechanism in the pathogenesis of preeclampsia.222 MMPs may also contribute to the increases in ROS in preeclampsia.60
6.5. AngII and AT1 Receptor Agonistic Autoantibodies (AT1-AA)
AngII is an important circulating hormone in the control of water and electrolyte homeostasis and blood pressure. AngII activation of vascular AT1R promotes vascular growth, inflammation, and vasoconstriction by increasing [Ca2+]i and Rho-kinase activity in VSM. AngII activation of endothelial AT2R is coupled to increases in eNOS activity and NO production, release of PGI2, and vasodilation, and thereby counteracts AngII-induced vasoconstriction. Therefore, while normal pregnancy is associated with increased plasma levels of renin and AngII, the pressor response to AngII is decreased due to decreased expression of AT1R or increased expression of AT2R. On the other hand, the dose of AngII required to elicit a 20 mmHg pressor response in the diastolic blood pressure in women at gestational weeks 23–26 is lower in women who subsequently developed preeclampsia compared with normal pregnant women who remained normotensive,223 suggesting that the increased pressor response to AngII occurs long before overt clinical manifestations of preeclampsia.
AngII levels and AT1R mRNA expression are increased in chorionic villi and placenta of preeclamptic versus normal pregnant women.224,225 Studies have shown that plasma hemopexin activity increases during normal gestation from week 10 onward, and that active hemopexin downregulates AT1R in human monocytes and endothelial cells, and decreases functional AT1R and AngII-induced contraction in rat aortic rings. These observations have suggested that during preeclampsia inhibition of hemopexin activity may result in enhanced AT1R expression and increased vasoconstriction.226
AT1-AA is a bioactive factor with vasoconstrictor and growth promoting properties via AT1R. The serum levels of AT1-AA are elevated in preeclamptic compared with normal pregnant women,160,227 and are further elevated in severe preeclampsia and in early versus late preeclampsia.228 The circulating levels of AT1-AA are also increased in RUPP compared with normal pregnant rats.178,229–231 Also, infusion of AT1-AA in pregnant mice causes some of the manifestations of preeclampsia including increased blood pressure, proteinuria and increased plasma sFlt-1 levels.160 Also, infusion of AT1-AA in pregnant rats is associated with increases in ET-1 levels approximately 4-fold in the placenta and 11-fold in the renal cortex.232 Interestingly, endothelium-dependent acetycholine (ACh)-induced vasodilation is reduced in the renal interlobar arteries of pregnant rats infused with AT1-AA, suggesting a link between AT1-AA and renal endothelial dysfunction in the pathogenesis of hypertension in pregnancy. Of note, the impaired ACh-induced vasodilation in AT1-AA infused pregnant rats was abolished by concomitant treatment with an ETAR antagonist, suggesting an interplay between the AngII and endothelin system in the setting of endothelial dysfunction and hypertension in pregnancy.233 AT1-AA has been linked to increased blood pressure, reduced trophoblast invasion, increased sFlt-1, ROS and cellular Ca2+, activation of coagulation tissue factor and thrombosis, vascular damage in the adrenal glands, and reduced aldosterone secretion in preeclampsia.160,234 AT1-AA also promotes collagen-induced platelet aggregation, which may contribute to the hypercoagulability observed in preeclampsia.227 In cultured trophoblasts, stimulation of AT1R with IgG isolated from preeclamptic women causes increases in sFlt-1 levels.235 In HUVECs, treatment with AT1-AA isolated from preeclamptic women induces the release of the cell death and necrosis marker lactate dehydrogenase,236 suggesting that AT1-AA may cause endothelial cell damage and necrosis. Also in HUVECs, AT1-AA induces the activity of caspase-3 and caspase-8, suggesting that it may promote endothelial cell apoptosis.236 While the mechanisms causing the release of AT1-AA in preeclampsia are not clearly understood, the plasma levels of AT1-AA are increased in pregnant rats infused with TNF-α, suggesting the involvement of cytokine-induced pathways.231
7. MMPs AND VASCULAR DYSFUNCTION IN HYPERTENSIVE PREGNANCY and PREECLAMPSIA
7.1 MMPs and Extracellular Matrix Abnormalities in Preeclampsia
MMPs degrade different substrates including collagen, gelatin, and other proteins.9,10,237 We have investigated the changes in MMPs substrates in the RUPP rat model of hypertension in pregnancy. Picro-Sirius Red staining revealed an increase in collagen content in uterus, placenta and aorta of RUPP compared with normal pregnant rats.8 Because MMPs facilitate cell growth and migration by promoting proteolysis of ECM, the decreased MMPs and increased collagen deposition in RUPP tissues could impede smooth muscle cell growth, proliferation and migration, and thus interfere with uteroplacental tissue invasion and decrease uterine and placental growth. Also, while the aortic collagen content increased, there was a decrease in aortic tissue weight and thickness in RUPP rats, likely because the decreased MMPs and increased collagen content would interfere with VSMC growth and migration. In support, MMP-2 knockout (KO) is associated with decreased VSMC migration and neointima formation in the mouse carotid ligation model.238,239 Also, MMP-9 KO is associated with reduced VSMC migration and neointima formation in mouse carotid occlusion model,240 and reduced VSMC migration and proliferation and neointima formation in mouse model of carotid artery injury.241 The decreased MMP activity and increased vascular collagen content could also increase vessel rigidity and decrease its plasticity and thus contribute to increased vascular resistance and hypertension. This is consistent with reports that MMP-1, MMP-2, and MMP-9 gelatinolytic activity is decreased and collagen deposition is increased in internal mammary artery from hypertensive compared with normotensive patients undergoing coronary artery bypass surgery.242 AngII infusion and high salt diet in mice are also associated with hypertension and increased MMP-9 activity in carotid artery, and MMP-9 KO is associated with vessel stiffness and increased pulse pressure, suggesting a beneficial role of MMP-9 in preserving vessel compliance and alleviating the increase in blood pressure in early hypertension.243 Also, in adult spontaneously hypertensive rat, a decrease in MMP-1, MMP-2, and MMP-3 may contribute to remodeling of resistance arteries and the setting of hypertension.244 It is important to note that collagen has 18 types and different subtypes.245 MMP-2 can degrade collagen I, II, III, IV, V, VII, X, and XI while MMP-9 can degrade collagen IV, V, VII, X, XIV.9,10,237 Studies should measure the changes in various collagen types and subtypes in hypertension in pregnancy. RT-PCR experiments should also determine whether any increases in a specific collagen subtype are solely due to decreased degradation or could also involve decreased de novo collagen mRNA expression and protein biosynthesis.
In contrast with collagen, elastin staining of uterus and placenta was sparse and diffuse and not different in RUPP versus normal pregnant rats, suggesting that elastin is less likely to be involved in the observed changes in tissue growth and remodeling. Although prominent and well-defined elastin bands were observed in the aorta, no significant changes were noted between RUPP and normal pregnant rats, suggesting little role of elastin in the observed changes in aortic growth and remodeling.8
We should note that MMPs is a large family of at least 28 proteolytic enzymes.9,10,237 While changes in MMP-2 and MMP-9 have been observed in RUPP rats, other MMPs have been detected in the uterus, placenta and aorta, and the changes in these MMPs in hypertension in pregnancy need to be investigated. Studies have shown that upregulation of MMP-1 enhances flow-induced VSMC motility in cell culture, and MMP-1 inhibition attenuates flow-induced migration.246 Importantly, MMPs activity could be influenced by other MMP activators and inhibitors. For example, some MMPs may cleave other pro-MMPs, and MT1-MMP is a key activator of proMMP-2.9,10,237,247 On the other hand, MMP expression/activiry could be influenced by TIMPs and other modulators of MMPs activity.9,10,237,247 For instance, TIMP-2 or specific MMP-2 blocking antibody inhibits cytotrophoblast invasion in vitro,34 and the role of modulators of MMP expression/activity in hypertension in pregnancy and preeclampsia needs to be further examined. Also, the time course and the progressive changes in MMPs during the course of pregnancy and their reversal in the postpartum period need to be examined. Plasma levels of MMPs may also vary at different stages and with the severity of preeclampsia. Furthermore, plasma MMPs represent the global changes in different maternal tissues, and the changes in MMPs in specific uteroplacental and vascular tissues need to measured.
While MMPs are largely known for their proteolytic effects ECM, we and others have identified novel MMP-induced downstream pathways that could affect uteroplacental remodeling and vascular function.40,41,93,102 Identifying the changes in the MMP-induced downstream pathways in hypertensive pregnancy would help define the molecular mechanisms of the increased vasoconstriction and blood pressure. In addition to their proteolytic effects on ECM proteins, MMPs may affect vascular function and the mechanisms of VSM contraction. We have shown that MMP-2 and MMP-9 cause relaxation of precontracted rat aorta40 and inferior vena cava.41,42 Also, changes in endothelial cell and VSM function have been observed in RUPP rats.19 Changes in vascular MMPs expression/activity could play a role in the endothelial dysfunction and increased vascular contraction in hypertension in pregnancy.
7.2 MMPs and Endothelial Dysfunction In Preeclampsia
The presence of functional endothelium is important for healthy gestation, and ensures a favorable prognosis for the mother and fetus.248 During normal pregnancy, there is an increase in brachial artery diameter and flow-mediated dilation as gestation progresses.249 Also, endothelium-dependent bradykinin-induced relaxation is increased in human small subcutaneous arteries from pregnant compared with non-pregnant women.250 Experimental studies corroborated endothelial cell adaptations during pregnancy. ATP causes periodic bursts in intracellular free Ca2+ concentration ([Ca2+]i) that are more frequent in uterine artery endothelial cells from pregnant compared with non-pregnant ewes,251 which could influence the vasodilator response and in turn attenuate the uterine artery myogenic tone and ensure adequate uterine blood flow during pregnancy.252
In addition to their effects on the uterus, gonadal hormones may contribute to the hemodynamic and vascular changes during pregnancy. Estrogen promotes endothelium-dependent vascular relaxation by increasing the release of NO, prostacyclin and EDHF.253 Estrogen also causes relaxation of endothelium-denuded vessels by inhibiting the mechanisms of VSM contraction including [Ca2+]i and protein kinase C.26,254–256 Estrogen may have additional effects on the vascular cytoskeleton, ECM, lipid profile and inflammatory response.253 Progesterone also causes vasodilation by mechanisms similar to estrogen.253,256,257 Some of the vascular effect of estrogen could involve MMPs. In cultured human coronary artery and umbilical artery VSM cells, estrogen causes dose-dependent increases in MMP-2 levels in culture media.38
In contrast with normal pregnancy, women with preeclampsia show systemic endothelial cell dysfunction and hypertension, glomerular endotheliosis causing renal injury and proteinuria, and cerebral endotheliosis which could lead to cerebral edema and seizures.11 Brachial artery flow-mediated dilation is less in preeclamptic than normal pregnant women.248,258 Preeclamptic women also show less vasodilation in the radial artery (~7.9%) when compared to normal pregnant women (~17.4%).259 Bradykinin-induced relaxation is decreased in small subcutaneous arteries of preeclamptic compared with normal pregnant women.250 Circulating endothelial cells and other markers of endothelial activation/injury such as soluble VCAM-1, E-selectin and endocan are increased in preeclamptic compared with normal pregnant women.149,184,260,261 Other studies have shown a decrease in circulating endothelial progenitor cells as a marker of endothelial damage in preeclamptic women.262
The RUPP rat has shown some of the characteristics of preeclampsia including high blood pressure, proteinuria, decreased glomerular filtration rate and renal plasma flow, and IUGR, and therefore has been used to study the vascular mechanisms of preeclampsia.56,172,263 ACh is less potent in inducing relaxation in the aorta and mesenteric microvessels of RUPP than normal pregnant rats, suggesting endothelial damage in RUPP rats.19,172 Endothelial cells release various vasodilator substances including nitric oxide (NO), prostacyclin (PGI2) and endothelium-derived hyperpolarizing factor (EDHF) as well as contracting factors as endothelin-1 (ET-1) and thromboxane A2 (TXA2). Endothelial dysfunction is associated with an imbalance in the release of endothelium-derived vasodilator versus vasoconstrictor factors.
7.2.1 Changes in Nitric Oxide (NO) in Preeclampsia
Nitric oxide (NO) is a potent vasodilator and relaxant of VSM. NO diffuses into VSM and increases cyclic guanosine monophosphate (cGMP), which promotes Ca2+ efflux, decreases VSM [Ca2+]i and causes VSM relaxation. Nitrites are important metabolites of NO that are increased in serum of normal pregnant compared with non-pregnant women.264 The plasma concentration and urinary excretion of cGMP, a second messenger of NO, are also increased in normal pregnancy. NOS expression and activity are also increased in human uterine artery and in the placenta with gestational age,265,266 supporting increased NO production during normal pregnancy. Also, the urinary levels of nitrites, the mRNA expression of eNOS, iNOS and nNOS, and the protein level of activated phospho-eNOS are increased in normal pregnant compared with virgin rats.267
Polymorphisms of eNOS gene could be a risk factor for preeclampsia. The VNTRa and 894T alleles of eNOS gene are associated with early and late severe preeclampsia, respectively. For the eNOS VNTRb/a polymorphism, plasma NO metabolites are lower in subjects homozygous for the “a” allele. Also, the eNOS 894T allele is subject to selective proteolytic cleavage in endothelial cells and vascular tissues, and this could account for the reduced vascular NO production in homozygous subjects for this variant.268 The T786C allele is also increased in preeclamptic compared with normal pregnant women.269,270 Genetic polymorphisms of eNOS could affect NO production. For instance, normal pregnant women with the TT phenotype for the T-786C allele have lower plasma nitrite levels than those with the CC phenotype271, and the TT phenotype has been proposed as a risk factor for preeclampsia in Tunisian women.269
Endothelial dysfunction is often associated with decreased NO bioavailability due to decreased synthesis or increased degradation.272 Clinical studies have shown increased273 or decreased274–277 plasma nitrite levels in preeclamptic compared with normal pregnant women. Also, urinary nitrite levels may not differ in preeclamptic versus normal pregnant women.274 The discrepancies in nitrite measurement could be related to the difficulty in controlling nitrate intake in diet. However, a study that carefully controlled dietary nitrate/nitrite intake did not show decreased NO production in preeclamptic women.278
The lack of change in whole-body NO despite the increase in blood pressure and the renal damage in preeclampsia suggest tissue-specific changes in NOS expression and NO bioavailability such that whole-body NO may not accurately reflect NO activity in the vasculature or the kidneys.11 Studies have shown a decrease in nitrites in placentae from preeclamptic women.277 Also, eNOS expression is decreased in umbilical cord of preeclamptic compared with normal pregnant women,279 and the decrease is greater in women with severe preeclampsia.280,281 However, some studies showed an increase in eNOS mRNA expression in placenta of preeclamptic women.282 Also, while the levels of cGMP are increased during normal pregnancy, the plasma and urinary cGMP levels are not different in preeclamptic versus normal pregnant women.274
The role of NO has also been examined in animal models of hypertension in pregnancy. In mid- to late pregnant rats, NOS blockade with Nω-nitro-L-arginine methyl ester (L-NAME) causes some of the changes observed in preeclampsia including increases in blood pressure and renal vasoconstriction, proteinuria, thrombocytopenia and IUGR,23,158,283 supporting that NO production is increased during gestation. Similar to the observation in humans, measurements of NO production in hypertensive pregnant animals have not been consistent. Studies showed no difference in nitrite levels in L-NAME treated versus nontreated normal pregnant rats.158 Also, while plasma nitrite levels were lower in RUPP than normal pregnant rats,263 no differences were observed in urinary nitrite levels.56,284 Also, consistent with the studies in human, no changes were observed in the NOS substrate L-arginine in the circulation of RUPP versus normal pregnant rats.285 Vascular function studies have shown increased aortic vascular reactivity to phenylephrine in L-NAME treated pregnant rats.23 Also, ACh-induced relaxation, eNOS expression, and NO production are reduced in mesenteric artery and aorta of RUPP versus normal pregnant rats, supporting specific reduction in NO synthesis in the vasculature.19,172,263 In rat model of hypertension in pregnancy produced by infusion of deoxycorticosterone acetate and replacement of drinking water with a 0.9% saline (DOCA-salt), NO-dependent relaxation was reduced in mesenteric vessels despite elevation of eNOS mRNA expression.220 MMPs may affect NO production by endothelial cells, and whether changes in MMPs during preeclampsia affect NO production should be examined.
7.2.2 MMPs and Endothelium-Derived Hyperpolarizing Factor (EDHF)
EDHF is a relaxing factor with specialized function in the control of small resistance vessels, local organ blood flow, peripheral vascular resistance and blood pressure. Although the nature of EDHF is unclear, it often presents as K+ efflux from endothelial cells through intermediate and small conductance Ca2+-activated K+ channels (IKCa and SKCa, respectively) causing hyperpolarization of endothelial cells. Endothelial cell hyperpolarization then spreads via myoendothelial gap junctions (MEGJs) and connexins to cause VSM hyperpolarization, reduction of Ca2+ influx via voltage-gated Ca2+ channels and suppression of the activity of phospolipase C, an enzyme involved in transduction pathways in VSM. The opening of endothelial cell IKCa and SKCa could also cause some accumulation of K+ ion in the myoendothelial interface which could induce VSM hyperpolarization by activating the inwardly rectifying K+ (KIR) channels and the Na+/K+-ATPase.286 EDHF relaxation may also be caused by diffusible factors released from endothelial cells. EDHF may be a product of cytochrome P450 (CYP450), such as epoxyeicosatrienoic acid (EET), which activate large conductance KCa (BKCa) and cause hyperpolarization of VSM. In some vessels, hydrogen peroxide (H2O2) may mimic EDHF-mediated responses by mechanisms involving KCa activation287. Thus multiple EDHFs may exist and the identity of EDHF could vary depending on the vascular bed and animal species studied.288
In small subcutaneous and myometrial arteries of normal pregnant women, EDHF is responsible for ~50% of bradykinin-induced relaxation, acting together with NO to maintain proper vascular tonus.289,290 The gap junction proteins connexins 37, 40 and 43 are partly involved in EDHF-mediated vascular response during normal pregnancy.291 An increase in endothelial cell [Ca2+]i may activate IKCa and SKCa and promote EDHF-mediated dilation in uterine radial arteries of pregnant rats.292 The delayed rectifier type of voltage-sensitive K+ channels (Kv) may also play a role in EDHF-mediated dilation in uterine artery of pregnant rats.293.
Studies in subcutaneous arteries from normal pregnant women have shown that MEGJs alone may be the main pathway of EDHF-mediated relaxation, while in women with preeclampsia MEGJs alone or in combination with H2O2 or CYP450 epoxygenase metabolites of arachidonic acid could mediate EDHF-induced vasodilation. The changes in the role of MEGJs may be caused by morphological changes within the vascular wall during preeclampsia.294 Studies in mice have shown pregnancy-associated adaptations in the form of decreased sensitivity to phenylephrine and enhanced bradykinin-induced vasodilation in normal pregnant wild-type mice but not in knockout mice lacking pregnane × receptor, a nuclear receptor that induces the expression of CYP450. Also, treatment with CYP450 inhibitor changed the vasodilatory response to bradykinin in wild-type but not the knockout mice, supporting that metabolites of CYP450 such as EET may play a role in the vascular adaptations during pregnancy.295 As EET is one of the possible factors involved in EDHF-mediated relaxation, it is plausible to suggest that alterations in EDHF may lead to impaired vascular function and hypertension in pregnancy. Although studies in mesenteric microvessels have suggested that the EDHF relaxation may not be compromised in RUPP versus normal pregnant rats,172 decreased EDHF-mediated relaxation is thought to contribute to the vasoconstriction observed in hypertension and diabetes, and its role in preeclampsia needs to be further examined.
Our data suggest that prolonged increases in intravascular pressure and wall tension cause increases in MMP-2 and MMP-9 expression40–42. Also, MMP-2 and MMP-9 cause relaxation of phenylephrine precontracted rat aorta. Thus during normal pregnancy, plasma volume expansion could lead to increased vascular MMP-2 and MMP-9, vasodilation and decreased blood pressure. The decrease in vascular MMP-2 and MMP-9 is expected to decrease relaxation in RUPP rats. Our data show that ACh-induced relaxation is reduced in blood vessels of RUPP versus pregnant rats. To test if the decreased vascular relaxation in RUPP rats reflects reduction in MMP-mediated NO-cGMP or PGI2-cAMP pathway, the effects of MMPs on NO and PGI2 production needs to be measured. To test if MMP-mediated EDHF is reduced in RUPP rats, MMP induced changes in membrane potential needs to be determined. We have shown that MMP-2 induces vascular relaxation partly via hyperpolarization and activation of K+ channels.41,42 Thus, the decrease in MMP-2 and MMP-9 mediated relaxation may contribute to the enhanced vascular contraction and increased blood pressure in RUPP versus normal pregnant rats.19,172,296
7.2.3 MMPs and Endothelin-1 (ET-1) in Preeclampsia
ET-1 is a major endothelium-derived vasoconstrictor that could play a role in preeclampsia.297 ET-1 synthesis is initiated by the production of the long 203 amino acid preproET, which is cleaved by furin-like protease to biologically inactive 37 to 41 amino acid big-ET. Big-ET is cleaved by endothelin converting enzymes, members of the metalloprotease family, to produce active 21 amino acid ET-1. Some of the circulating factors in preeclampsia including cytokines, hypoxia and AT1-AA may stimulate endothelial cells to produce ET-1,297 This is supported by reports that serum from preeclamptic women causes HUVECs to produce greater amounts of ET-1 than normal pregnant serum.298 Some studies suggest that plasma ET-1 levels are elevated in preeclampsia.299 ET-1 levels are higher during later stages of preeclampsia and return to normal levels within 48 hours after delivery,300 suggesting that ET-1 may be involved in the progression rather than the initiation of preeclampsia. However, in most studies serum ET-1 levels do not differ in preeclamptic versus normal pregnant women, and higher levels of ET-1 are observed mainly in patients with HELLP syndrome.117,301,302 Of note, ET-1 is released in a paracrine fashion from endothelial cells directly toward VSMCs, and the increases in ET-1 levels in preeclampsia may be localized in tissues. Studies have shown a 4- to 8-fold increase in ET-1 levels in umbilical cord cells303 and in renal tissues during later stages of preeclampsia.300 In perfused placentas under hypoxia, both the maternal and fetal side produced increased levels of ET-1.304 In RUPP rats, preproET levels show a 45% increase in renal cortex and 22% increase in renal medulla.305 Thus, measurements of circulating ET-1 may not always reflect ET-1 levels locally in tissues. It is possible that in severe PE and in HELLP syndrome the production of ET-1 is so augmented that ET-1 release “loses” its paracrine directionality and consequently leads to an increase in circulating ET-1 levels. This is supported by reports that rat models that mimic severe PE and HELLP syndrome have increased plasma levels of ET-1.179,306
ET-1 may play a role in the pathogenesis of preeclampsia by inducing apoptosis of trophoblast cells and increasing oxidant and anti-angiogenic substances.297,307 ET-1 activates endothelin receptor type A (ETAR) and endothelin receptor type B (ETBR).308–315 ET-1 activation of VSM ETAR stimulates Ca2+ release from the intracellular stores and Ca2+ entry through Ca2+ channels, and causes protein kinase C-dependent inhibition of K+ channels leading to increased [Ca2+]i and VSM contraction.308 ET-1-induced vasoconstriction is reduced in mesenteric vessels of normal pregnant compared with non-pregnant rats,316 and VSM ETAR are reduced in aortic media and VSMCs of late-pregnant rat.317 Also, treatment with ETAR antagonist reduces blood pressure in RUPP rat and other animal models of hypertension in pregnancy.305,318,319 Interestingly, among Brazilian women with preeclampsia, 52% of the patients diagnosed with severe preeclampsia exhibited increases in ETAR agonistic autoantibodies (ETA-AA) which could target ETAR and increase vasoconstriction.320
ET-1 also activates ETBR in endothelial cells and stimulates the release of NO, PGI2, and EDHF which in turn reduce myogenic vascular tone, promote vasodilation of renal arteries and hyperfiltration in pregnant rats.308,321 Downregulation of ETBR may impair trophoblast invasion in preeclampsia. Downregulation of ETBR decreases microvascular vasodilator activity in pregnant rats. ETBR expression is also reduced in endothelial cells and renal cells of RUPP rats. Also, ETBR-mediated NO production is less in the aorta and mesenteric artery of RUPP versus normal pregnant rats, supporting that downregulation of endothelial ETBR could play a role in hypertension in pregnancy.172
MMPs break big-ET-1 and into different endothelin forms that have different affinities for ETAR and ETBR and in turn differentially affect vasoconstriction in normal pregnancy and preeclampsia. MMPs could degrade big-ET into ET-1, which largely stimulates ETAR and promotes vasoconstriction.322 Studies have suggested a role of ET-1 and ETAR in some forms of hypertension including hypertension in pregnancy.309,318,323–328 In omental vessels of normal pregnant women, MMP-1 causes vasoconstriction and enhances reactivity to AngII via an endothelium-dependent protease-activated receptor (PAR) and ET-1 pathway.102 ET-1 in turn can stimulate VSM contraction mechanisms309,315,329 including [Ca2+]i,330–332 protein kinase C,333–339 and Rho-kinase.340–342
MMP-2 and MMP-9 could degrade big-ET to ET1–32 which preferentially stimulates endothelial ETBR and promotes relaxation. We have shown increases in MMP-2 and MMP-937,93 and ETBR in normal pregnant rats.316 Also, in line with our observed decrease in MMP-2 and MMP-9, ETBR is downregulated in RUPP rats, and infusion of the ETBR antagonist BQ788 increased blood pressure in pregnant rats.172
7.3 MMPs and Smooth Muscle Dysfunction in Preeclampsia
7.3.1 VSM Ca2+ in Preeclampsia
Ca2+ is a major determinant of VSM contraction and growth. Ca2+ release from the intracellular stores and Ca2+ entry from the extracellular space increase [Ca2+]i in VSM. Ca2+ binds calmodulin to form Ca2+-calmodulin complex which activates myosin light chain kinase, causes myosin phosphorylation, initiates actin-myosin interaction and produces VSM contraction. Decreased Ca2+ in VSM activates myosin phosphatase to dephosphorylate myosin light chain and dissociate the Ca2+-calmodulin complex. Endothelium-derived relaxing factors act on VSM to decrease [Ca2+]i. In hypoxia, decreased relaxing factors reduce VSM Ca2+ extrusion, while increased contracting factors increase [Ca2+]i and VSM contraction. In normal pregnancy, increased KCa channel activity decreases uterine artery tonicity and increases uteroplacental blood supply. In preeclampsia, KCa channel activity is suppressed leading to increased uterine artery [Ca2+]i, vasoconstriction and reduced fetal blood supply.343 Myometrial vessels may show similar vasoconstriction response to high KCI, phenylephrine and AngII in normal pregnancy and preeclampsia.344 However, basal and agonist-stimulated [Ca2+]i are reduced in renal arterial VSM of normal pregnant rats, and increased in pregnant rats treated with L-NAME.345 AT1R activation increases [Ca2+]i in the platelets, erythrocytes, and lymphocytes of preeclamptic women, and these effects subside 6 weeks after delivery.234 AngII- and caffeine-induced contraction and [Ca2+]i in Ca2+-free solution are similar in VSM of normal pregnant and RUPP rats, while KCl-induced maintained [Ca2+]i in a Ca2+-containing medium is greater in VSM of RUPP than normal pregnant rats, suggesting that it is not the release of intracellular Ca2+ but the increase in Ca2+ entry from extracellular space that increases vasoconstriction in hypertension in pregnancy.346
MMP-2 and MMP-9 may cause vascular relaxation by decreasing Ca2+ influx into VSM,40 and a decrease in these MMPs could lead to increased Ca2+ influx, vasoconstriction and hypertension in pregnancy.
7.3.2 Protein Kinase C (PKC), MAPK, and Rho-kinase in Preeclampsia
PKC is an important mediator of VSM contraction. PKC phosphorylates CPI-17 which inhibits myosin phosphatase and in turn increases myosin light chain phosphorylation and VSM contraction. PKC also phosphorylates calponin, an actin binding protein that inhibits myosin ATPase, leading to more actin-myosin interaction and VSM contraction. Phorbol esters activate PKC to cause VSM contraction with no detectable change in [Ca2+]i, suggesting that PKC increases Ca2+ sensitivity of the contractile proteins. PKC activity and contraction are reduced in the uterine artery of late pregnant ewes and gilts and the aorta of late pregnant rats.347–349 Also, the expression and subcellular redistribution of Ca2+-dependent α-PKC and Ca2+-independent δ- and ζ-PKC are reduced in aortic VSM of late pregnant rats, but are increased in L-NAME treated pregnant rat.348,350 PKC may increase the production of AT1-AA which stimulates AT1R. In cultured rat cardiomyocytes treatment with IgG obtained from preeclamptic women enhances AT1R-mediated response which is ameliorated with the PKC inhibitor calphostin C.351 Increased BKCa channel activity inhibits PKC-mediated contraction in ovine uterine arteries during pregnancy, and gestational hypoxia may upregulate PKC and inhibit BKCa.343 PKC inhibitors decrease TXA2 mediated contraction in uterine and mesenteric arteries of non-pregnant rats and in mesenteric artery of pregnant rats, supporting a role of PKC in mediating VSM contraction during pregnancy.352 Blocking PKC can prevent PKC-mediated vasoconstriction in preeclampsia. Cicletanine is an anti-hypertensive compound that prevents the increase in PKC and lower blood pressure in hypertensive pregnant rats. MMP-2 may reduce vascular contraction by degrading the actin-binding protein calponin.353 A decrease in MMP-2 would spare calponin and affect VSM contraction in hypertension in pregnancy.
Mitogen-activated protein kinase (MAPK) is a serine/threonine protein kinase that regulates cellular activities such as gene expression, mitosis, differentiation, and VSM contraction. During VSM contraction, PKC may phosphorylate MAPK kinase which in turn phosphorylates and activates MAPK. Activated MAPK phosphorylates the actin-binding protein caldesmon thus preventing its inhibition of ATPase and increases actin-myosin interaction and VSM contraction. Changes in PKC activity in VSM during normal pregnancy and hypertension in pregnancy could affect MAPK/caldesmon phosphorylation and VSM contraction.354 This is supported by reports that inhibition of p38 MAPK reduces TXA2-induced contraction in uterine and mesenteric arteries of virgin and pregnant rats.352
Rho is a family of small GTP-binding proteins that are involved in cell migration, cytoskeletal reorganization and VSM contraction. Rho-kinase is activated by GTP binding and inactivated by hydrolyzing GTP to GDP. There are two isoforms of Rho-kinase, rock I (ROKβ) and rock II (ROKα), which may be important in the formation of microvilli structures during pregnancy. Human placental villi show abnormal expression of rock II and apoptosis of the syncytium in preeclampsia.355 Rho-kinase increases Ca2+ sensitivity of the contractile proteins in subcutaneous resistance arteries of preeclamptic women.356 Also, AngII via AT1R induces RhoA/Rho-kinase activity in L-NAME treated hypertensive rats.357 Rho-kinase may stimulate IL-17 to phosphorylate the inhibitory eNOS Thr495 residue thus decreasing NO production in preeclampsia.358 Inhibition of Rho-kinase reduces TXA2-induced contraction in uterine vessels of non-pregnant rats.352 However, some studies show decreased expression of Rho-kinase in umbilical arteries of preeclamptic women.359
Specific MMP subtypes may increase the production of ET-1, which could activate ETAR in VSM, leading to activation of PKC, MAPK or Rho-kinase dependent pathway, and in turn increase the myofilament force sensitivity to Ca2+, and enhance vasoconstriction in the setting of hypertension in pregnancy.
8. MMPs AND UTERINE CONTRACTION DURING PREGNANCY AND LABOR
The uterus responds to different mechanical and chemical stimuli, and its response varies in the non-pregnant compared to the pregnant state, and during parturition. The non-pregnant uterus contracts in response to mechanical stretch. On the other hand, during pregnancy several mechanisms are activated in order to reduce the excitability of the myometrium, maintain uterine quiescence, and allow sufficient time for the development of the fetus before it is ready to be delivered.360 The uterine smooth muscle cells undergo sequential steps of hyperplasia, hypertrophy, and ECM elaboration in order to adapt to the physiological demands of pregnancy. These processes are regulated by both mechanical and endocrine factors and are accompanied by extensive remodeling of the uterine ECM.361–363 During pregnancy, the uterus dramatically expands and its volume markedly increases. Both hypertrophy and distension of the pregnant uterus allow sufficient space for the growing fetus. Also, the balance between uterine contraction and relaxation is tightly-controlled in order to maintain healthy and full-term pregnancy. Any disturbance in the mechanisms that maintain the uterus in a quiescent relaxed state could cause preterm uterine contraction and premature birth. At full-term, other uterine mechanisms are activated in order to terminate uterine quiescence, increase electrical excitability of the myometrium, coordinate uterine smooth muscle contraction, and ensure uncomplicated parturition. Uterine stretch, and steroid and neurohypophysial hormones could affect uterine quiescence and excitability, partly through MMPs.
8.1. Uterine stretch, sex hormones and MMPs during pregnancy
MMPs could play a role in the endometrial tissue remodeling during estrous and menstrual cycles and during pregnancy.28–30,103,364 MMP-2 and MMP-9 are expressed in the bovine uterus,365,366 and MMP-2, MMP-7 and MMP-9 are expressed in the rat uterus.37,93 Alterations in MMP-2, MMP-7 and MMP-9 expression/activity could be involved in the endometrial changes associated with menstrual disorders and endometriosis367,368 The plasma levels of MMP-2 and MMP-9 increase during normal pregnancy.369 MMP-2 and MMP-9 have been detected in uterine natural killer cells during early pregnancy in humans.370 Increases in MMP-2 expression/activity have also been observed in the final ripening stages and collagen denaturation of the cervix during late pregnancy.371 MMP-2 and MMP-9 have also been detected in the bovine endometrium and myometrium during pregnancy.28,103,366 We have shown an upregulation of MMP-2 and MMP-9 in the myometrium of late pregnant rats, and suggested a role of MMPs in pregnancy-related uterine remodeling.93 MMP-2 and MMP-9 degrade ECM proteins and could promote remodeling of the uterus and cervix during pregnancy.372 However, the mechanisms causing the changes in uterine MMPs during pregnancy are not clearly understood.
MMP expression/activity is regulated by multiple biophysical and biological factors including mechanical stretch and sex hormones. Mechanical stretch of skeletal muscle fibers is associated with increased MMP-2 expression.203 We have shown that protracted vein wall stretch and increases in vein wall tension are associated with increased MMP-2 and MMP-9 expression and decreased contraction of rat inferior vena cava.41,42,373 We have also tested if increased uterine stretch during pregnancy is associated with upregulation of MMPs, which could in turn cause inhibition of uterine contraction and promote uterine relaxation. We measured contraction in response to oxytocin, a nonapeptide produced by the hypothalamus-pituitary and other tissues such as adrenal medulla,374 corpus luteum,375,376 and placenta,377 in isolated uterine strips from virgin, mid-pregnant (day 12), and late-pregnant rats (day 19), and assessed uterine MMP expression and activity using RT-PCR, Western blots, and gelatin zymography. Consistent with the concept of uterine quiescence during the course of pregnancy, we found that oxytocin contraction of myometrium strips was reduced in mid- and late-pregnant rats compared with virgin rats. Some studies have shown that oxytocin contraction of mid-term and late-pregnant uterus is greater than that in non-pregnant uterus,378 which is opposite from our findings. This is likely because during late pregnancy the uterus is bigger and often thicker, and studies often measure uterine contraction in absolute grams. In effect, when we measured the uterine contraction in grams, the contraction in late-pregnant uterus was greater than that in mid-pregnant uterus, but less than that in virgin uterus. On the other hand, when the uterine contraction was normalized to the uterine tissue weight, the contraction was less in late-pregnant than mid-pregnant uterus and far less than that in the virgin uterus, supporting that uterine contraction is reduced during pregnancy.
We tested whether the pregnancy-associated reduction in uterine contraction reflect changes in MMP expression/activity. We observed that the reduced contraction in the mid-pregnant and late-pregnant rat uterus was reversed by the MMP inhibitors SB-3CT, BB-94 and Ro-28-2653, supporting a potential role of MMPs in the pregnancy-related reduction in uterine contraction. Also, the mRNA expression and protein levels of MMP-2 and MMP-9 were enhanced in mid- and late-pregnant compared with virgin rat uterus. Furthermore, MMP-2 and MMP-9 activity, as measured by gelatin zymography, was enhanced in mid- and late-pregnant compared with virgin rat uterus. Interestingly, SB-3CT, but not BB-94 or Ro-28-2653, enhanced oxytocin contraction in mid-pregnant uterus, while the three MMP inhibitors enhanced contraction in late-pregnant uterus. This is likely due to differences in uterine MMP activity during the course of pregnancy. In mid-pregnant uterus, moderate increases in MMPs occur, and therefore the most potent inhibitor SB-3CT potentiates the contraction. On the other hand, in late pregnant uterus, further increases in MMP expression/activity, and therefore MMP inhibitors with different potencies increase uterine contraction.93
We also simulated the changes associated with uterine expansion during pregnancy and tested the effects of induction of mechanical stretch in virgin uterus on uterine contraction and MMP expression/activity. Prolonged stretch of myometrium strips of virgin rats under 8 g basal tension for 18 hour was associated with reduced contraction and enhanced expression/activity of MMP-2 and MMP-9, that were reversed by MMP inhibitors. Also, treatment with MMP-2 and MMP-9 reduced oxytoxcin-induced contraction in myometrium of virgin rat. These observations support that prolonged increases in uterine wall tension (stretch) during pregnancy are associated with increased MMP-2 and MMP-9 and decreased contraction, leading to uterine expansion and better accommodation of the growing fetus.8,93
We examined whether the pregnancy-associated changes in MMPs are specific to MMP-2 and MMP-9, or involve other MMPs. MMP-7 (matrilysin-1) is expressed in the uterus.9 Studies have shown changes in MMP-7 during gestation, but not labor.379 We found that uterine MMP-7 expression did not increase during pregnancy in rats. In effect, MMP-7 showed a pregnancy-related decrease in expression, suggesting that MMP-7 may function via a mechanism different from that regulating MMP-2 and MMP-9.
Steroid and neurohypophysial hormones undergo marked changes during the menstrual cycle, pregnancy and parturition.380 Steroid and neurohypophysial hormones could influence the uterine structure and architecture and markedly affect its mechanical response. The plasma levels of estradiol are increased during the proliferative phase of the menstrual cycle. A decrease in plasma estrogen levels initiates endometrial shedding, and the plasma levels of progesterone are increased during the luteal phase of the menstrual cycle.381 Endometrial cellular concentrations of both estrogen and progesterone are positively correlated with the plasma levels of estrogen during the proliferative phase of the menstrual cycle.381 Uterine tissue remodeling and endometrium shedding during menstruation could involve estrogen-induced changes in MMPs activity.364,382,383 Progesterone may also control the initiation, maintenance and progression of endometrial lesions partly by affecting MMP expression/activity.384 During pregnancy the uterus is exposed to not only mechanical stretch caused by progressive fetal growth, but also hormonal changes that could influence the myometrium structure and mechanical properties. The plasma levels of estrogen and progesterone markedly increase during pregnancy,25,385 and may play a role in the pregnancy-related uterine relaxation and expansion.93,386–388 A large concentration of progesterone receptors is detected in the endometrium during early pregnancy endometrium.381 Studies have shown that estrogen causes relaxation of VSM of rat aorta and uterine artery,26,27 as well as the uterus.386 Also, progesterone inhibits contraction of rat blood vessels and human uterus.26,387 We have also shown that estrogen and progesterone decrease contraction of uterine strips of virgin rats, and further reduce contraction in uterine strips exposed to prolonged stretch.93
Uterine contraction could also be affected by changes in the levels of the neurohypophysial hormone oxytocin. The plasma levels of oxytocin are relatively low in the non-pregnant state, and do not detectably change during the menstrual cycle. The plasma oxytocin levels increase at gestational week 12 and progressively increase during the course of pregnancy.389 Oxytocin promotes cervical dilation before birth and stimulates uterine contraction during the second and third stages of labor. Steroid and neurohypophysial hormones target uterine receptors and other local enzymes and proteins that modulate uterine structure and function, including MMPs.
The uterine changes during pregnancy may involve effects of gonadal hormones on MMP expression/activity. The activity of MMP-2 and MMP-9 is higher in pregnant than non-pregnant bitches, and serum MMP activity correlates with the serum levels of estrogen,51 suggesting a relationship between sex hormones and MMP expression/activity. MMP-9 modulates uterine biology during various reproductive processes. In mouse uterus, gelatin zymography revealed that estrogen alone or in combination with progesterone increased MMP-9 activity, whereas Northern blot analysis showed that estrogen decreased MMP-9 mRNA expression. In contrast, uterine MMP-2 expression/activity was not affected by steroidal treatment, suggesting that estrogen and progesterone regulate uterine MMPs expression/activity and in turn uterine tissue remodeling via a complex mechanism.364 We should note that estrogen and progesterone are important regulators of myometrial growth and contractility, via both genomic and non-genomic mechanisms. It is generally thought that estrogen augments myometrial contractility and excitability, while progesterone sustains the pregnant state and promotes myometrial relaxation.390 While estrogen may have a stimulatory effects on uterine contractility at the time of parturition, these effects appear to be neutralized during the course of pregnancy, partly due to decreased uterine expression of estrogen receptors,390 or estrogen-induced expression of other factors that decrease myometrial contractility such as MMPs.93 For instance, the last phase of pregnancy in rats shows a correlation between the plasma level of estrogen, the increase in uterine contractility, and the density of α1-adrenergic receptors.385 On the other hand, uterine contraction is reduced in day-12 and day-19 of gestation in rats.93 Also, estrogen causes rapid non-genomic relaxation of spontaneous and depolarization-induced contraction of uterine strips of non-pregnant rat.386 We have also shown that prolonged treatment of virgin rat uterus with estrogen is associated with decreased uterine contraction.93 With regard to progesterone, in addition to its relaxing effects during pregnancy, it also decreases uterine contractility and promotes relaxation during the luteal phase, a phenomenon crucial for maximizing uterine receptivity to embryo implantation during in vitro fertilization cycles.391 For example, in women undergoing in vitro fertilization, administration of vaginal progesterone gel starting 2 days before embryo transfer reduces the frequency of uterine contraction at the time of embryo transfer, increases uterine relaxation, propitiates embryo permanence in the endometrial cavity, and thereby promotes implantation.387 Other studies have examined the long-term effects of estrogen and progesterone replacement on uterine contractility in women aged 25 to 41. Following 2 weeks of transdermal estrogen to duplicate the pattern of estrogen production normally seen in the late follicular phase, vaginal administration of sustained release progesterone gel every two days from cycle day 15 caused time-dependent decrease in uterine contractility over the course of 5 days (Cycle days 15–20).392
MMPs play a major role in uterine tissue remodeling, and some of the effects of sex hormones on the uterus may involve MMPs. Uterine tissue remodeling and endometrium shedding during menstruation may involve estrogen-induced changes in MMPs activity.364,382,383 Progesterone may also regulate important factors in the formation and maintenance of endometrial lesions partly by affecting MMPs expression.384 In the pregnant bitches, significant correlations are observed between the elevated serum MMP-2 and MMP-9 activity and the elevated serum levels of estrogen and progesterone.51. In mouse uterus, estrogen alone or in combination with progesterone increases MMP-9 activity.364 We have found that treatment of virgin uterus with estrogen+progesterone increases MMP-2 and MMP-9 expression/activity. These findings are consistent with reports that estrogen increases MMP-2 expression in the immature rat uterus,382 and suggest that estrogen and progesterone regulate uterine MMPs expression/activity during uterine tissue remodeling.
We have found that concomitant treatment of stretched myometrium of virgin rats with 17β-estradiol, progesterone, or estrogen+progesterone was associated with further reduction in contraction and increase in MMP expression/activity. The reduced contraction in sex hormone-treated uterus was reversed by MMP inhibitors. Also, estrogen and progesterone treatment of stretched virgin uterus was associated with greater increases in MMP-2 and MMP-9 mRNA expression, protein level and enzymatic activity. These observations suggest a synergistic relationship between mechanical stretch and sex hormones in the reduced uterine contraction and enhanced MMP expression/activity. The mechanisms via which estrogen enhances uterine MMP expression/activity are unclear, but may involve estrogen receptor-mediated MAPK pathway.393 Also, while some studies suggest anti-inflammatory effects of estrogen,394–396 estrogen may increase inflammatory cytokines such as TNF-α and IL-6, which could in turn increase MMP expression/activity.364,393,397–399
8.2. Uterine MMP Inducers and EMMPRIN during Pregnancy
Another factor that could affect uterine MMP expression during pregnancy is EMMPRIN, a known inducer of MMPs.29,52,366,400 We have examined whether the changes in uteroplacental MMPs expression/activity during pregnancy and in response to sex hormone involve changes in EMMPRIN.37 Using the uterus, placenta and aorta from virgin and pregnant rats we found that MMP-2 and MMP-9 expression/activity were upregulated in the uterus and aorta during mid and late pregnancy. Treatment of the uterus and aorta of virgin rats with estrogen and progesterone caused increases in MMP-2 and MMP-9 expression/activity, that were blocked by EMMPRIN antibody. EMMPRIN was upregulated in the uterus and aorta of pregnant rats and in uterus and aorta of virgin rats treated with sex hormones.37 These findings are consistent with reports that endometrial expression of EMMPRIN and MMPs is regulated by ovarian sex hormones in cycling baboons and that their expression patterns are dysregulated in endometriotic baboons.401 These observations are also in agreement with reports that EMMPRIN expression increases in the bovine endometrium during estrous cycle and early gestation,29 and support a role of EMMPRIN in inducing the changes in uterine MMPs expression during pregnancy. On the other hand, MMP-2 and MMP-9 expression/activity and EMMPRIN expression were downregulated in the placenta of late-pregnant compared with mid-pregnant rats,37 suggesting different regulatory mechanisms of MMPs and EMMPRIN in the placenta compared with the uterus and blood vessels.
8.3 MMPs, Uterine Extracellular Matrix, and Uterine Relaxation Mechanisms
The effects of MMPs are generally thought to involve degradation of ECM components and extensive tissue remodeling. However, MMP inhibitors cause relatively rapid enhancement of contraction in uterine strips of pregnant rats, suggesting other effects in addition to uterine tissue remodeling. We have shown that MMP-2 and MMP-9 inhibit VSM contraction in the absence of detectable tissue degradation.40,42 Also, MMP-2 may cause membrane hyperpolarization and reduction in Ca2+ influx in rat inferior vena cava.41,42 Treatment of uterine strips with MMP-2 or MMP-9 caused relaxation of oxytocin-induced contraction.93 These observations are consistent with our reports in vascular tissues.40–42 and point to inhibitory effects of MMPs on the mechanisms of uterine smooth muscle contraction (Fig. 3). Whether MMPs affect the plasma membrane channels or other mechanisms of uterine smooth muscle contraction remains to be examined.
Fig. 3.
Role of MMPs in normal and premature labor. During normal pregnancy increases in progesterone levels cause increases in uteroplacental MMP-2 and MMP-9 expression/activity, leading to uterine smooth muscle hyperpolarization and relaxation, uterine expansion to accommodate the growing fetus, and therefore full-term birth. Preeclampsia and other risk factors during pregnancy are associated with decreases in progesterone, leading to decreased MMP-2 and MMP-9 expression/activity, increased uterine smooth muscle depolarization, uterine contraction and premature birth.
AT1-AA, AngII AT1R agonistic autoantibodies; IL-6, interleukin-6; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; TNF-α, tumor necrosis factor-α
Consistent with other reports in the rat uterus,378,402 we have shown that oxytocin causes a steady increase in contraction and an additional spontaneous phasic contractile response.93 [Ca2+]I is a key regulator of uterine contraction. Membrane depolarization mainly stimulates Ca2+ influx into uterine smooth muscle cells. Uterine smooth muscle [Ca2+]I can also be modulated by different physiological and pharmacological agonists that affect the frequency, amplitude, and duration of uterine contraction.403,404 Studies in human myometrial cells have shown that oxytocin causes an initial [Ca2+]i transient followed by uniform relatively low frequency [Ca2+]i oscillations.405–407 The oxytocin-induced [Ca2+]i oscillations in myometrial cells are attenuated by caffeine and the voltage-dependent Ca2+ channel antagonist verapamil, and blocked by the inorganic Ca2+ antagonist La3+ and the Ca2+-ATPase inhibitor 2.5-di-tert-butylhydroquinone.408 Similarly, in rat uterine segments, oxytocin induces simultaneous [Ca2+]i oscillations and phasic contractions that are inhibited by the Ca2+ channel blocker nifedipine.402 Collectively, these studies support oxytocin-induced [Ca2+]i oscillations that are mediated by intracellular Ca2+ release from inositol 1,4,5-trisphosphate (IP3)-sensitive Ca2+ stores combined with voltage-dependent and capacitative Ca2+ influx. Interestingly, we have shown that MMP-2 causes hyperpolarization and reduction in Ca2+ influx in rat inferior vena cava,41,42 a smooth muscle preparation that also shows phasic contraction. These observations make it important to investigate the pregnancy-associated changes and the effects of MMPs on the magnitude and frequency of phasic uterine contractions, [Ca2+]i and Ca2+ regulatory mechanisms.
We should note that we measured uterine contraction and MMP expression at gestational day 12 and day 19.37 Other studies have shown that the contractility of rat uterus and the mRNA expression of the contractile agonists oxytocin, prostaglandin 2α, and ET-1 and their receptors are induced after gestational day 20,378 and that the last phase of pregnancy in rats shows an increase in uterine contractility and the density of α1-adrenergic receptors,385 further highlighting the importance of measuring MMPs and EMMPRIN during the different stages of pregnancy. Likewise, our uterine stretch experiments were limited to 8 g basal tension for 18 hours, and the progressive effects of different levels of basal tension for extended time periods would be more analogous to the progressive uterine stretch imposed by the growing fetus over the course of pregnancy. Also, while our studies suggest a role of MMP-2 and MMP-9 in the reduced uterine contraction during pregnancy and in response to uterine tissue stretch, that should not minimize the possibility of involvement of other MMPs.
9. DYSREGULATION OF UTERINE MMPs DURING PRETERM LABOR
Myometrium activity is tightly regulated during pregnancy. At the first and mid-trimester, myometrium relaxation is needed to accommodate fetal growth. As fetal growth nears its completion during late pregnancy the uterine activity is first stabilized then starts to increase in preparation for delivery. We have demonstrated a relationship between uterine stretch, MMPs expression and uterine relaxation during gestation. We have also shown a role of sex hormones in promoting the effects of uterine stretch on MMPs expression and uterine relaxation. MMP-1, MMP-2, MMP-3, MMP-7 and MMP-9 are found in the amniotic fluid and fetal membranes during normal pregnancy. MMP-2 and MMP-3 are expressed constitutively while MMP-9 is barely detectable until labor. At labor, MMP-9 is the major MMP responsible for gelatinolytic activity in the membranes, while MMP-2 is dominant in the decidua. These findings may have clinical relevance as a disturbance in the balance of MMPs or TIMPs could disturb uterine activity and lead to premature labor. The MMP/TIMP imbalance may be further aggravated by changes in the sex hormone levels or their uterine receptors.
Preterm labor complicates 10% to 15% of all pregnancies, and is a leading cause of perinatal morbidity and death;14 however, the mechanisms involved are not fully understood. MMP-2 and MMP-9 exhibit cell-specific expression in the human placenta. Studies have suggested that an increase in MMP-9 expression may contribute to degradation of ECM in the fetal membranes and placenta, thereby facilitating fetal membrane rupture and placental detachment from the maternal uterus at labor, both term and preterm.409 Also, studies on samples of amniochorion and amniotic fluid collected from women undergoing cesarean delivery before term, with either premature rupture of membranes or with preterm labor with no rupture of membranes, demonstrated an increased mRNA expression of MMP-2, MMP-9, and MT1-MMP and a decreased expression of TIMP-2 in prematurely ruptured membranes compared with preterm labor membranes. Enzyme-linked immunosorbent assay (ELISA) showed increases in the amniotic fluid concentrations of immunoreactive and bioactive MMP-2 and MMP-9 and immunoreactive MMP-3 and a decreased TIMP-2 concentration in fluids obtained from the premature rupture of membranes group compared with the preterm labor group.14 In contrast, in a study of 25 patients at preterm or term, MMP-2 protein and MMP-2 and MMP-9 pro-enzyme activities in the amnion markedly increased with labor at term, and were much higher than at preterm labor. There were no changes in chorion MMPs under any condition. These observations support a role of MMP-2 and MMP-9 in regulation of membrane rupture and other labor-associated mechanisms at term versus preterm parturition.410 Cervicovaginal and/or intrauterine infection may be associated with preterm premature rupture of membranes (PPROM) and spontaneous preterm birth, likely due to the triggered inflammatory response. Microbial invasion of the amniotic cavity is associated with marked reduction in the levels of active MMP-2.409,411 A decrease in uterine MMP-2 and MMP-9 in preeclampsia, intrauterine infection and other pregnancy-related risk conditions is expected to hinder uterine expansion and cause IUGR and premature birth (Fig. 3).8,93 Other MMPs may also be involved in the regulation of membrane rupture and uterine contraction in term and preterm labor. Studies have suggested that inflammation could induce myometrial activator protein-1 (AP-1) which could in turn drive the production of stromelysins MMP-3 and MMP-10 and result in preterm labor in mice.412 Also, a cross-sectional study in 275 women examined whether parturition (either term or preterm), premature rupture of the membranes, and microbial invasion of the amniotic cavity are associated with changes in the levels of matrilysin MMP-7 in the amniotic fluid. MMP-7 was detected in 97.4% (268/275) of the samples, and showed an increase with advancing gestational age. Parturition at term and premature rupture of membranes without microbial invasion of the amniotic cavity (either term or preterm) was not associated with a change in MMP-7. On the other hand, preterm parturition in the absence of microbial invasion of the amniotic cavity and intra-amniotic infection in both patients with preterm labor and patients with preterm premature rupture of membranes were associated with marked increase in MMP-7. It was concluded that MMP-7 is a physiologic constituent of amniotic fluid, and its levels increase with advancing gestational age, and markedly increase during microbial invasion of the amniotic cavity in preterm gestations, and the changes in MMP-7 may represent a maternal regulatory mechanism during infection and preterm labor.413 Studies have also shown that plasma progesterone levels are lower in some preterm delivery patients compared to normal term pregnancies. For example, progesterone concentration was ~30% lower at 28 to 34 weeks gestation in women who delivered prematurely than in women who delivered at term.414 Studies have suggested that progestin supplementation may prevent initiation of preterm labor or treat it once it is already established.415 Whether changes in MMPs and sex hormones could interfere with uterine relaxation and trigger uterus contraction and pre-term delivery needs to be further examined. Also, whether the potential beneficial effects of progesterone in preterm labor are mediated via modulation of MMP expression/activity warrant further investigation.
10. MMPs in PREDICTION AND MANAGEMENT OF COMPLICATIONS OF PREGNANCY
Because preeclampsia has a relatively long preclinical phase before clinically manifesting in late gestation, the identification of women at risk, early diagnosis using various biomarkers, and prompt management could improve the maternal and perinatal outcome.11 A decrease in brachial artery flow-mediated vasodilation could be an early indicator of compromised endothelial cell function between the 24th and 28th gestational weeks and before the clinical diagnosis of preeclampsia. The sensitivity of flow-mediated vasodilation is 87.5% and 95.5% for the prediction of early and late preeclampsia, respectively.248 Angiogenic/anti-angiogenic imbalance is an important feature in preeclampsia. Measurements of plasma VEGF, PlGF, sFlt-1 and sEng may help early detection in asymptomatic pregnant women, especially those at high risk for preeclampsia.11 In preeclampsia, circulating levels of sFlt-1 are increased more than one month before the onset of clinical symptoms, and PlGF is decreased in women who subsequently develop preeclampsia from the end of the first trimester.416 The sFlt-1/PlGF ratio is increased in preeclamptic versus normal pregnant women and in both early and late preeclampsia.139 A meta-analysis of 20 different studies revealed that the overall diagnostic accuracy of sFlt-1/PlGF ratio for preeclampsia is relatively high, and its diagnostic efficiency is higher in early than late preeclampsia.417
Assessment of the immune system could be useful in predicting preeclampsia in early pregnancy. An abnormal maternal immunological response could be presented as a change in monocytes and NK cells, increased inflammatory factors, altered release of cytokines, increased AT1-AA, and activation of pro-inflammatory AT1R.11 TNF-α levels could be an early predictor of preeclampsia. Plasma obtained at gestational weeks 11–13 showed higher TNF-α levels in women who later developed preeclampsia.418 Studies have suggested that elevated plasma TNF-α levels in association with changes in uterine artery Doppler at 11–13th gestational weeks have a 100% sensitivity in predicting preeclampsia.419 Other reports suggest that plasma TNF-α levels may be useful in predicting preeclampsia in the early third trimester, but not the first or second trimesters.420 Some studies suggest that at gestational week 28, miRNA-206, which interacts with several genes directly involved in the pathology of preeclampsia, is elevated in plasma and placenta from women who subsequently develop preeclampsia,421 although other studies show little predictive value of miRNAs.422 Measurements of the levels of MMPs have not been consistent in preeclampsia, with some studies showing an increase in MMP-2 and MMP-9,100 while other studies showing a decrease in MMP-9.7 The discrepancy in the results may be due to the fact that plasma MMPs represents global changes in MMPs in different tissues, and localized changes in MMPs in uteroplacental tissues and fluids may carry more predictive value. Because of the existence of several biomarkers, their predictive value should be further assessed in order to identify the best marker combinations for predicting preeclampsia.11
Currently, inducing labor is the most effective treatment for preeclampsia. International guidelines recommend the use of one of the antihypertensive agents methyldopa, labetalol, other beta-blockers (acebutolol, metoprolol, pindolol, and propranolol), or Ca2+ channel blockers (nifedipine).423 ACE inhibitors should not be used due to their teratogenic effects, and atenolol and prazosin are not recommended prior to delivery. If preeclampsia evolves to eclampsia, magnesium sulfate is often used to prevent eclamptic seizures.424
Sildenafil has been proposed for women with severe early-onset IUGR, as it has been shown to increase fetal growth with no maternal side effects.425 Sildenafil citrate vasodilates myometrial arteries isolated from women with IUGR-complicated pregnancies. Also, treatment with sildenafil citrate restored endothelial cell integrity in the placental vessels of L-NAME treated mouse model of hypertension in pregnancy.426 Eculizumab, an anti-C5 antibody, normalized laboratory values and prolonged pregnancy by 17 days in a woman with preeclampsia/HELLP syndrome, suggesting the benefits of manipulating the complement system during preeclampsia. However, complement inhibitors could increase susceptibility to infection and their long-term use requires close monitoring.427
Studies suggest potential benefits of correcting the angiogenic/anti-angiogenic imbalance in preeclampsia. In trophoblast cells and HUVECs treated with cobalt chloride to simulate hypoxic conditions, the free radical scavenger edaravone inhibit sFlt-1 expression in trophoblast cells and protects against the decrease in vascular development and tube formation in HUVECs.428 VEGF could improve the angiogenic/anti-angiogenic imbalance, but may impair bradykinin-induced vascular relaxation and enhance basal tone and vascular permeability in preeclampsia.76 Modulators of PlGF could be more promising in preeclampsia, and low molecular weight heparin therapy increases circulating PlGF levels during third trimester pregnancy.429
TNF-α antagonists such as etanercept decrease blood pressure, increase eNOS expression, decrease ET-1 levels and prevent cardiac changes in RUPP rats.181,430 Also, IL-17 soluble receptor C inhibits IL-17, prevents the recruitment of host defense cells, suppresses the inflammatory response, decreases AT1-AA and ROS, and ameliorates hypertension and improves pup and placental weight in RUPP rats.229
It is possible that RUPP causes the release of cytoactive factors which in turn disrupt MMP balance and lead to inadequate uteroplacental remodeling, vascular dysfunction and hypertension in pregnancy. Thus one way to manage hypertension in pregnancy is to target the cytoactive factors acting upstream of MMPs. If downregulation of MMP-2 and MMP-9 is a target of anti-angiogenic sFlt-1, then counteracting sFlt-1 by PlGF should reverse the reduction in MMP expression, improve MMP-mediated vascular relaxation and reduce vasoconstriction and blood pressure. In support, infusion of pro-angiogenic PlGF or VEGF reduces blood pressure in RUPP rats,171,431 and infusion of anti-inflammatory IL-10 decreases blood pressure in DOCA/salt hypertensive pregnant rats.432 An alternative approach is to aim at the common target affected by cytoactive factors. If MMPs are a central target in hypertension in pregnancy, then correcting MMP imbalance should promote vasodilation and reduce blood pressure. Doxycycline is a non-specific MMP inhibitor that was proposed to alleviate hypertension and vascular dysfunction in preeclampsia, but was found to decrease placenta weight and cause IUGR in both normal pregnant and hypertensive pregnant rats, and to reduce trophoblast invasion and placental perfusion in hypertensive pregnant rats.96 Thus, novel approaches to indirectly or directly correct MMP imbalance should provide new strategies in the management of hypertension in pregnancy and preeclampsia.
11. CONCLUDING REMARKS
Normal pregnancy is associated with uteroplacental and vascular remodeling in order to adapt for the growing fetus and the hemodynamic changes in the maternal circulation. Normal pregnancy is also associated with increased MMPs expression/activity. It is likely that myometrium stretch and the increase in the sex hormone progesterone during pregnancy alter the expression/activity of specific MMPs, and in turn inhibit uterine contraction and promote uterine relaxation. The pregnancy-associated upregulation of uterine MMPs is paralleled by increased expression/activity of vascular MMPs, and both appear to be mediated by EMMPRIN and induced by estrogen and progesterone, suggesting similar role of MMPs in uterine and vascular tissue remodeling and function during pregnancy. The decrease in MMP expression/activity and EMMPRIN expression in the placenta of late-pregnant rats suggest reduced role of MMPs in the feto-placental circulation during late pregnancy.
Preeclampsia is a complication of pregnancy manifested as maternal hypertension and often IUGR. Genetic and environmental factors may cause localized abnormal placentation, altered maternal immune response, and abnormal expression of integrins, inflammatory cytokines and MMPs, leading to increased apoptosis of trophoblast cells, shallow trophoblastic invasion and inadequate spiral artery remodeling, and causing RUPP and placental ischemia/hypoxia. Ischemic/hypoxic placenta causes the release of bioactive factors such as sFlt-1, sEng, TNF-α, IL-6, HIF, ROS and AT1-AA which could target MMPs in ECM and alter uteroplacental and vascular remodeling. Bioactive factors could also affect endothelial cells, cause endothelial dysfunction, decrease vasodilators or increase ET-1 and other vasoconstrictors and lead to hypertension in pregnancy.
Animal models of hypertension in pregnancy such as the RUPP rat have helped in understanding the mechanisms of preeclampsia. Maternal blood pressure is higher, and the litter size and pup weight are reduced in RUPP versus normal pregnant rats. MMP-2 and MMP-9 expression/activity is reduced and collagen deposition is increased in uterus, placenta and aorta of RUPP versus normal pregnant rats, suggesting a role for MMPs in growth-restrictive remodeling in hypertension in pregnancy. Anti-angiogenic factors such as sFlt-1 decrease MMPs expression/activity in uterus, placenta and aorta of normal pregnant rats, and the angiogenic factor VEGF reverses the effects of sFlt-1 in tissues of normal pregnant rats and the decreases in MMPs expression/activity in tissues of RUPP rats. Thus placental ischemia and anti-angiogenic sFlt-1 decrease uterine, placental and vascular MMP-2 and MMP-9, leading to increased uteroplacental and vascular collagen, and growth-restrictive remodeling in hypertension in pregnancy. Angiogenic factors and MMP inducers/activators may reverse the decrease in MMPs and enhance growth-permissive remodeling in preeclampsia.
While marked changes have been detected in uterine, placental and aortic MMPs in hypertensive pregnant rats, that should not minimize the importance of measuring the structural changes and MMPs expression in other tissues particularly the small resistance vessels which affect blood pressure. Also, RUPP during pregnancy may cause the release of not only anti-angiogenic factors, but also cytokines, ROS and HIF, which could in turn lead to uteroplacental and vascular dysfunction during hypertension in pregnancy.20,57–59,62,109,110 Interestingly, cytokines, ROS and HIF have been shown to affect MMPs expression/activity,209,398 and studying their differential effects as well as the effects of their inhibitors on MMPs in normal pregnancy and hypertension in pregnancy should be examined.
Further understanding of the interaction between bioactive factors, MMPs, and vascular mediators and cellular mechanisms should help design more specific and efficient measures for prevention, early detection, and management of preeclampsia. Targeting MMPs could provide a new approach for the detection and management of hypertension in pregnancy, preeclampsia, and premature labor.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-111775). Dr. J. Chen was a visiting scholar from the Department of Obstetrics & Gynecology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, P.R. China.
ABBREVIATIONS
- ACh
acetylcholine
- AngII
angiotensin II
- AT1R
AngII type 1 receptor
- AT1-AA
AngII AT1R agonistic autoantibodies
- [Ca2+]i
intracellular free Ca2+ concentration
- cGMP
cyclic guanosine monophosphate
- DOCA
deoxycorticosterone acetate
- ECM
extracellular matrix
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- EMMPRIN
extracellular MMP inducer
- EGF
epidermal growth factor
- ELISA
enzyme-linked immunosorbent assay
- ET-1
endothelin-1
- H2O2
hydrogen peroxide
- HIF
hypoxia-inducible factor
- HELLP
hemolysis elevated liver enzymes low platelets
- HUVECs
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule-1
- IL
interleukin
- IUGR
intrauterine growth restriction
- L-NAME
Nω-nitro-L-arginine methyl ester
- MAPK
mitogen-activated protein kinase
- MEGJ
myoendothelial gap junction
- MMP
matrix metalloproteinase
- NO
nitric oxide
- O2•−
superoxide anion
- PGI2
prostacyclin
- PlGF
placental growth factor
- PKC
protein kinase C
- ROS
reactive oxygen species
- RUPP
reduced uterine perfusion pressure
- sEng
soluble endoglin
- sFlt-1
soluble fms-like tyrosine kinase-1
- TGF-β
transforming growth factor-β
- TIMP
tissue inhibitor of metalloproteinases
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VCAM-1
vascular cell adhesion molecule-1
- VSM
vascular smooth muscle
Footnotes
CONFLICT OF INTEREST
None
References
- 1.Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30(3):317–329. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev. 2012;64(3):540–582. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacology & therapeutics. 1995;65(2):215–239. doi: 10.1016/0163-7258(94)00064-a. [DOI] [PubMed] [Google Scholar]
- 4.Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Seminars in perinatology. 2000;24(1):11–14. doi: 10.1016/s0146-0005(00)80047-6. [DOI] [PubMed] [Google Scholar]
- 5.Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reproductive biology and endocrinology : RB&E. 2009;7:79. doi: 10.1186/1477-7827-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanbe AF, Khalil RA. Circulating and Vascular Bioactive Factors during Hypertension in Pregnancy. Current bioactive compounds. 2010;6(1):60–75. doi: 10.2174/157340710790711737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montagnana M, Lippi G, Albiero A, Scevarolli S, Salvagno GL, Franchi M, Guidi GC. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J Clin Lab Anal. 2009;23(2):88–92. doi: 10.1002/jcla.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Mata KM, Mazzuca MQ, Khalil RA. Altered matrix metalloproteinase-2 and-9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochemical pharmacology. 2014;89(3):370–385. doi: 10.1016/j.bcp.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 11.Ali SM, Khalil RA. Genetic, immune and vasoactive factors in the vascular dysfunction associated with hypertension in pregnancy. Expert opinion on therapeutic targets. 2015;19(11):1495–1515. doi: 10.1517/14728222.2015.1067684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan LJ, Morton JS, Davidge ST. Vascular dysfunction in preeclampsia. Microcirculation. 2014;21(1):4–14. doi: 10.1111/micc.12079. [DOI] [PubMed] [Google Scholar]
- 13.Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. The Lancet. Global health. 2014;2(6):e323–333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 14.Fortunato SJ, Menon R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol. 2001;184(7):1399–1405. doi: 10.1067/mob.2001.115122. discussion 1405–1396. [DOI] [PubMed] [Google Scholar]
- 15.McBride CA, Bernstein IM, Badger GJ, Horbar JD, Soll RF. The effect of maternal hypertension on mortality in infants 22, 29weeks gestation. Pregnancy hypertension. 2015;5(4):362–366. doi: 10.1016/j.preghy.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Davies EL, Bell JS, Bhattacharya S. Preeclampsia and preterm delivery: A population-based case-control study. Hypertension in pregnancy. 2016;35(4):510–519. doi: 10.1080/10641955.2016.1190846. [DOI] [PubMed] [Google Scholar]
- 17.Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. Jama. 2002;287(24):3183–3186. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- 18.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. American journal of physiology. Regulatory, integrative and comparative physiology. 2002;283(1):R29–45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 19.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35(1 Pt 2):367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50(6):1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 21.Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochemical pharmacology. 2015;95(4):211–226. doi: 10.1016/j.bcp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R267–275. doi: 10.1152/ajpregu.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension. 1998;31(5):1065–1069. doi: 10.1161/01.hyp.31.5.1065. [DOI] [PubMed] [Google Scholar]
- 24.Crews JK, Novak J, Granger JP, Khalil RA. Stimulated mechanisms of Ca2+ entry into vascular smooth muscle during NO synthesis inhibition in pregnant rats. Am J Physiol. 1999;276(2 Pt 2):R530–538. doi: 10.1152/ajpregu.1999.276.2.R530. [DOI] [PubMed] [Google Scholar]
- 25.Risberg A, Olsson K, Lyrenas S, Sjoquist M. Plasma vasopressin, oxytocin, estradiol, and progesterone related to water and sodium excretion in normal pregnancy and gestational hypertension. Acta Obstet Gynecol Scand. 2009;88(6):639–646. doi: 10.1080/00016340902919002. [DOI] [PubMed] [Google Scholar]
- 26.Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol. 1999;26(9):707–715. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- 27.Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293(6):H3713–3719. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- 28.Ulbrich SE, Meyer SU, Zitta K, Hiendleder S, Sinowatz F, Bauersachs S, Buttner M, Frohlich T, Arnold GJ, Reichenbach HD, Wolf E, Meyer HH. Bovine endometrial metallopeptidases MMP14 and MMP2 and the metallopeptidase inhibitor TIMP2 participate in maternal preparation of pregnancy. Mol Cell Endocrinol. 2011;332(1–2):48–57. doi: 10.1016/j.mce.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Mishra B, Kizaki K, Koshi K, Ushizawa K, Takahashi T, Hosoe M, Sato T, Ito A, Hashizume K. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN) and its related extracellular matrix degrading enzymes in the endometrium during estrous cycle and early gestation in cattle. Reprod Biol Endocrinol. 2010;8:60. doi: 10.1186/1477-7827-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Qi C, Lin J. Enhanced expressions of matrix metalloproteinase (MMP)-2 and-9 and vascular endothelial growth factors (VEGF) and increased microvascular density in the endometrial hyperplasia of women with anovulatory dysfunctional uterine bleeding. Fertil Steril. 2010;93(7):2362–2367. doi: 10.1016/j.fertnstert.2008.12.142. [DOI] [PubMed] [Google Scholar]
- 31.Shimonovitz S, Hurwitz A, Dushnik M, Anteby E, Geva-Eldar T, Yagel S. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: a possible mechanism for control of trophoblast invasion. American journal of obstetrics and gynecology. 1994;171(3):832–838. doi: 10.1016/0002-9378(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 32.Suman P, Gupta SK. Comparative analysis of the invasion-associated genes expression pattern in first trimester trophoblastic (HTR-8/SVneo) and JEG-3 choriocarcinoma cells. Placenta. 2012;33(10):874–877. doi: 10.1016/j.placenta.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Su MT, Tsai PY, Tsai HL, Chen YC, Kuo PL. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors. 2017;43(2):210–219. doi: 10.1002/biof.1325. [DOI] [PubMed] [Google Scholar]
- 34.Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF, Takayama M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24(1):53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 35.Biadasiewicz K, Sonderegger S, Haslinger P, Haider S, Saleh L, Fiala C, Pollheimer J, Knofler M. Transcription factor AP-2alpha promotes EGF-dependent invasion of human trophoblast. Endocrinology. 2011;152(4):1458–1469. doi: 10.1210/en.2010-0936. [DOI] [PubMed] [Google Scholar]
- 36.Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128(3):355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- 37.Dang Y, Li W, Tran V, Khalil RA. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochemical pharmacology. 2013;86(6):734–747. doi: 10.1016/j.bcp.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wingrove CS, Garr E, Godsland IF, Stevenson JC. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. 1998;1406(2):169–174. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 39.Pepper MS. Extracellular proteolysis and angiogenesis. Thromb Haemost. 2001;86(1):346–355. [PubMed] [Google Scholar]
- 40.Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. Journal of vascular surgery. 2004;40(5):1001–1010. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 41.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45(2):373–380. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res. 2010;159(2):755–764. doi: 10.1016/j.jss.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55(2):434–439. [PubMed] [Google Scholar]
- 44.Huet E, Vallee B, Szul D, Verrecchia F, Mourah S, Jester JV, Hoang-Xuan T, Menashi S, Gabison EE. Extracellular matrix metalloproteinase inducer/CD147 promotes myofibroblast differentiation by inducing alpha-smooth muscle actin expression and collagen gel contraction: implications in tissue remodeling. FASEB J. 2008;22(4):1144–1154. doi: 10.1096/fj.07-8748com. [DOI] [PubMed] [Google Scholar]
- 45.Konttinen YT, Li TF, Mandelin J, Liljestrom M, Sorsa T, Santavirta S, Virtanen I. Increased expression of extracellular matrix metalloproteinase inducer in rheumatoid synovium. Arthritis Rheum. 2000;43(2):275–280. doi: 10.1002/1529-0131(200002)43:2<275::AID-ANR6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102(16):1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 47.Major TC, Liang L, Lu X, Rosebury W, Bocan TM. Extracellular matrix metalloproteinase inducer (EMMPRIN) is induced upon monocyte differentiation and is expressed in human atheroma. Arterioscler Thromb Vasc Biol. 2002;22(7):1200–1207. doi: 10.1161/01.atv.0000021411.53577.1c. [DOI] [PubMed] [Google Scholar]
- 48.Huet E, Gabison EE, Mourah S, Menashi S. Role of emmprin/CD147 in tissue remodeling. Connect Tissue Res. 2008;49(3):175–179. doi: 10.1080/03008200802151722. [DOI] [PubMed] [Google Scholar]
- 49.Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, Zucker S. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340) Am J Respir Cell Mol Biol. 2001;25(6):717–724. doi: 10.1165/ajrcmb.25.6.4558f. [DOI] [PubMed] [Google Scholar]
- 50.Pustovrh MC, Jawerbaum A, Capobianco E, White V, Lopez-Costa JJ, Gonzalez E. Increased matrix metalloproteinases 2 and 9 in placenta of diabetic rats at midgestation. Placenta. 2005;26(4):339–348. doi: 10.1016/j.placenta.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Schafer-Somi S, Ali Aksoy O, Patzl M, Findik M, Erunal-Maral N, Beceriklisoy HB, Polat B, Aslan S. The activity of matrix metalloproteinase-2 and-9 in serum of pregnant and non-pregnant bitches. Reprod Domest Anim. 2005;40(1):46–50. doi: 10.1111/j.1439-0531.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 52.Mishra B, Kizaki K, Koshi K, Ushizawa K, Takahashi T, Hosoe M, Sato T, Ito A, Hashizume K. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN) and its expected roles in the bovine endometrium during gestation. Domest Anim Endocrinol. 2012;42(2):63–73. doi: 10.1016/j.domaniend.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 54.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–474. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Losonczy G, Brown G, Venuto RC. Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits. Am J Med Sci. 1992;303(4):233–240. doi: 10.1097/00000441-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37(4):1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 57.Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):R390–399. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- 58.Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension. 2004;43(2):434–444. doi: 10.1161/01.HYP.0000113044.46326.98. [DOI] [PubMed] [Google Scholar]
- 59.Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem. 2010;8(4):204–226. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 2013;208(3):224–233. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53(2):399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30(1):15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trifonova EA, Gabidulina TV, Ershov NI, Serebrova VN, Vorozhishcheva AY, Stepanov VA. Analysis of the placental tissue transcriptome of normal and preeclampsia complicated pregnancies. Acta naturae. 2014;6(2):71–83. [PMC free article] [PubMed] [Google Scholar]
- 65.van Dijk M, Oudejans CB. STOX1: Key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. Journal of pregnancy. 2011;2011:521826. doi: 10.1155/2011/521826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doridot L, Passet B, Mehats C, Rigourd V, Barbaux S, Ducat A, Mondon F, Vilotte M, Castille J, Breuiller-Fouche M, Daniel N, le Provost F, Bauchet AL, Baudrie V, Hertig A, Buffat C, Simeoni U, Germain G, Vilotte JL, Vaiman D. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension. 2013;61(3):662–668. doi: 10.1161/HYPERTENSIONAHA.111.202994. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J, Saito S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clinical and experimental immunology. 2007;149(1):139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol. 2016;12(2):209–227. doi: 10.1586/1744666X.2016.1105740. [DOI] [PubMed] [Google Scholar]
- 69.Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J, Varner MW. Paternal and maternal components of the predisposition to preeclampsia. The New England journal of medicine. 2001;344(12):867–872. doi: 10.1056/NEJM200103223441201. [DOI] [PubMed] [Google Scholar]
- 70.Boyd HA, Tahir H, Wohlfahrt J, Melbye M. Associations of personal and family preeclampsia history with the risk of early-, intermediate- and late-onset preeclampsia. Am J Epidemiol. 2013;178(11):1611–1619. doi: 10.1093/aje/kwt189. [DOI] [PubMed] [Google Scholar]
- 71.Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health. 2005;95(9):1545–1551. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997–2008. BMC Pregnancy Childbirth. 2012;12:47. doi: 10.1186/1471-2393-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanagal DV, Rajesh A, Rao K, Devi UH, Shetty H, Kumari S, Shetty PK. Levels of Serum Calcium and Magnesium in Pre-eclamptic and Normal Pregnancy: A Study from Coastal India. J Clin Diagn Res. 2014;8(7):OC01–04. doi: 10.7860/JCDR/2014/8872.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spradley FT, Palei AC, Granger JP. Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms. American journal of physiology. Regulatory, integrative and comparative physiology. 2015;309(11):R1326–1343. doi: 10.1152/ajpregu.00178.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pereira RD, De Long NE, Wang RC, Yazdi FT, Holloway AC, Raha S. Angiogenesis in the placenta: the role of reactive oxygen species signaling. BioMed research international. 2015;2015:814543. doi: 10.1155/2015/814543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.VanWijk MJ, Kublickiene K, Boer K, VanBavel E. Vascular function in preeclampsia. Cardiovascular research. 2000;47(1):38–48. doi: 10.1016/s0008-6363(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 77.Zarate A, Saucedo R, Valencia J, Manuel L, Hernandez M. Early disturbed placental ischemia and hypoxia creates immune alteration and vascular disorder causing preeclampsia. Archives of medical research. 2014;45(7):519–524. doi: 10.1016/j.arcmed.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Hall D, Gebhardt S, Theron G, Grove D. Pre-eclampsia and gestational hypertension are less common in HIV infected women. Pregnancy hypertension. 2014;4(1):91–96. doi: 10.1016/j.preghy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Trowsdale J, Moffett A. NK receptor interactions with MHC class I molecules in pregnancy. Semin Immunol. 2008;20(6):317–320. doi: 10.1016/j.smim.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200(8):957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol. 2013;56(1–2):91–97. doi: 10.1016/j.molimm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44(1):72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 83.Regal JF, Lillegard KE, Bauer AJ, Elmquist BJ, Loeks-Johnson AC, Gilbert JS. Neutrophil Depletion Attenuates Placental Ischemia-Induced Hypertension in the Rat. PloS one. 2015;10(7):e0132063. doi: 10.1371/journal.pone.0132063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Seminars in nephrology. 2004;24(6):540–547. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- 85.Iwaki T, Yamamoto K, Matsuura T, Sugimura M, Kobayashi T, Kanayama N. Alteration of integrins under hypoxic stress in early placenta and choriocarcinoma cell line BeWo. Gynecol Obstet Invest. 2004;57(4):196–203. doi: 10.1159/000076688. [DOI] [PubMed] [Google Scholar]
- 86.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy hypertension. 2012;2(2):72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Dijk M, Oudejans C. (Epi)genetics of pregnancy-associated diseases. Front Genet. 2013;4:180. doi: 10.3389/fgene.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baig S, Kothandaraman N, Manikandan J, Rong L, Ee KH, Hill J, Lai CW, Tan WY, Yeoh F, Kale A, Su LL, Biswas A, Vasoo S, Choolani M. Proteomic analysis of human placental syncytiotrophoblast microvesicles in preeclampsia. Clin Proteomics. 2014;11(1):40. doi: 10.1186/1559-0275-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Sepulveda A, Torres MJ, Khoury M, Illanes SE. Innate immune system and preeclampsia. Front Immunol. 2014;5:244. doi: 10.3389/fimmu.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fei X, Hongxiang Z, Qi C, Daozhen C. Maternal plasma levels of endothelial dysfunction mediators including AM, CGRP, sICAM-1 and tHcy in pre-eclampsia. Adv Clin Exp Med. 2012;21(5):573–579. [PubMed] [Google Scholar]
- 91.Li Q, Pan Z, Wang X, Gao Z, Ren C, Yang W. miR-125b-1-3p inhibits trophoblast cell invasion by targeting sphingosine-1-phosphate receptor 1 in preeclampsia. Biochemical and biophysical research communications. 2014;453(1):57–63. doi: 10.1016/j.bbrc.2014.09.059. [DOI] [PubMed] [Google Scholar]
- 92.Anton L, Olarerin-George AO, Hogenesch JB, Elovitz MA. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PloS one. 2015;10(3):e0122707. doi: 10.1371/journal.pone.0122707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin Z, Sada AA, Reslan OM, Narula N, Khalil RA. Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones. Am J Physiol Endocrinol Metab. 2012;303(1):E55–70. doi: 10.1152/ajpendo.00553.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu Y, Wang L, Liu T, Guan H. MicroRNA-204 suppresses trophoblast-like cell invasion by targeting matrix metalloproteinase-9. Biochemical and biophysical research communications. 2015;463(3):285–291. doi: 10.1016/j.bbrc.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 95.Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, Werb Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci U S A. 2013;110(27):11109–11114. doi: 10.1073/pnas.1309561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palei AC, Granger JP, Tanus-Santos JE. Matrix metalloproteinases as drug targets in preeclampsia. Current drug targets. 2013;14(3):325–334. doi: 10.2174/1389450111314030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shokry M, Omran OM, Hassan HI, Elsedfy GO, Hussein MR. Expression of matrix metalloproteinases 2 and 9 in human trophoblasts of normal and preeclamptic placentas: preliminary findings. Exp Mol Pathol. 2009;87(3):219–225. doi: 10.1016/j.yexmp.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 98.Omran OM, Shokry M, Ismail H, Omar G, Rezk M. Expression of matrix metalloproteinases 2 and 9 in human trophoblasts of normal and preeclamptic placentas. Int J Health Sci (Qassim) 2011;5(2 Suppl 1):21–23. [PMC free article] [PubMed] [Google Scholar]
- 99.Choi SY, Yun J, Lee OJ, Han HS, Yeo MK, Lee MA, Suh KS. MicroRNA expression profiles in placenta with severe preeclampsia using a PNA-based microarray. Placenta. 2013;34(9):799–804. doi: 10.1016/j.placenta.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 100.Eleuterio NM, Palei AC, Rangel Machado JS, Tanus-Santos JE, Cavalli RC, Sandrim VC. Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy hypertension. 2015;5(2):205–208. doi: 10.1016/j.preghy.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 101.Deng CL, Ling ST, Liu XQ, Zhao YJ, Lv YF. Decreased expression of matrix metalloproteinase-1 in the maternal umbilical serum, trophoblasts and decidua leads to preeclampsia. Exp Ther Med. 2015;9(3):992–998. doi: 10.3892/etm.2015.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nugent WH, Mishra N, Strauss JF, 3rd, Walsh SW. Matrix Metalloproteinase 1 Causes Vasoconstriction and Enhances Vessel Reactivity to Angiotensin II via Protease-Activated Receptor 1. Reproductive sciences. 2016;23(4):542–548. doi: 10.1177/1933719115607998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roy SC, Ghosh J. Dynamic in vivo changes in the activities of gelatinases, matrix metalloproteinases (MMPs), and tissue inhibitor of metalloproteinases (TIMPs) in buffalo (Bubalus bubalis) uterine luminal fluid during estrous cycle and early pregnancy. Mol Reprod Dev. 2010;77(11):944–953. doi: 10.1002/mrd.21240. [DOI] [PubMed] [Google Scholar]
- 104.Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knofler M, Cartwright JE, Whitley GS, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol. 2010;177(4):2103–2115. doi: 10.2353/ajpath.2010.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2001;38(3 Pt 2):742–745. doi: 10.1161/01.hyp.38.3.742. [DOI] [PubMed] [Google Scholar]
- 106.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9(3):147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 107.Seo KW, Lee SJ, Kim YH, Bae JU, Park SY, Bae SS, Kim CD. Mechanical stretch increases MMP-2 production in vascular smooth muscle cells via activation of PDGFR-beta/Akt signaling pathway. PLoS One. 2013;8(8):e70437. doi: 10.1371/journal.pone.0070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li HX, Kong FJ, Bai SZ, He W, Xing WJ, Xi YH, Li GW, Guo J, Li HZ, Wu LY, Wang R, Yang GD, Tian Y, Xu CQ. Involvement of calcium-sensing receptor in oxLDL-induced MMP-2 production in vascular smooth muscle cells via PI3K/Akt pathway. Mol Cell Biochem. 2012;362(1–2):115–122. doi: 10.1007/s11010-011-1133-6. [DOI] [PubMed] [Google Scholar]
- 109.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46(4):1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 110.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep. 2007;9(6):480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 111.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jardim LL, Rios DR, Perucci LO, de Sousa LP, Gomes KB, Dusse LM. Is the imbalance between pro-angiogenic and anti-angiogenic factors associated with preeclampsia? Clinica chimica acta; international journal of clinical chemistry. 2015;447:34–38. doi: 10.1016/j.cca.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 113.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. The Journal of biological chemistry. 1999;274(35):25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 114.Shen BQ, Lee DY, Zioncheck TF. Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. The Journal of biological chemistry. 1999;274(46):33057–33063. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- 115.Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovascular research. 2011;89(3):671–679. doi: 10.1093/cvr/cvq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. The Journal of clinical endocrinology and metabolism. 2003;88(11):5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 117.Celik H, Avci B, Isik Y. Vascular endothelial growth factor and endothelin-1 levels in normal pregnant women and pregnant women with pre-eclampsia. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2013;33(4):355–358. doi: 10.3109/01443615.2013.769944. [DOI] [PubMed] [Google Scholar]
- 118.Hunter A, Aitkenhead M, Caldwell C, McCracken G, Wilson D, McClure N. Serum levels of vascular endothelial growth factor in preeclamptic and normotensive pregnancy. Hypertension. 2000;36(6):965–969. doi: 10.1161/01.hyp.36.6.965. [DOI] [PubMed] [Google Scholar]
- 119.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circulation research. 2004;95(9):884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 120.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Masoura S, Kalogiannidis I, Makedou K, Theodoridis T, Koiou K, Gerou S, Athanasiadis A, Agorastos T. Biomarkers of endothelial dysfunction in preeclampsia and neonatal morbidity: a case-control study. European journal of obstetrics, gynecology, and reproductive biology. 2014;175:119–123. doi: 10.1016/j.ejogrb.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 122.Papazoglou D, Galazios G, Koukourakis MI, Kontomanolis EN, Maltezos E. Association of −634G/C and 936C/T polymorphisms of the vascular endothelial growth factor with spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2004;83(5):461–465. doi: 10.1111/j.0001-6349.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 123.George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305(4):R397–403. doi: 10.1152/ajpregu.00216.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bates DO. An unexpected tail of VEGF and PlGF in pre-eclampsia. Biochem Soc Trans. 2011;39(6):1576–1582. doi: 10.1042/BST20110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. Journal of the American Society of Nephrology : JASN. 2007;18(8):2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 126.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 127.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. The New England journal of medicine. 2008;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, Ohashi R, Ishizaki M, Asano G, Sugisaki Y, Yamanaka N. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol. 2001;159(2):599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim YG, Suga SI, Kang DH, Jefferson JA, Mazzali M, Gordon KL, Matsui K, Breiteneder-Geleff S, Shankland SJ, Hughes J, Kerjaschki D, Schreiner GF, Johnson RJ. Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney international. 2000;58(6):2390–2399. doi: 10.1046/j.1523-1755.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 130.Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48(9):4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 131.Cui Y, Sun YW, Lin HS, Su WM, Fang Y, Zhao Y, Wei XQ, Qin YH, Kohama K, Gao Y. Platelet-derived growth factor-BB induces matrix metalloproteinase-2 expression and rat vascular smooth muscle cell migration via ROCK and ERK/p38 MAPK pathways. Mol Cell Biochem. 2014;393(1–2):255–263. doi: 10.1007/s11010-014-2068-5. [DOI] [PubMed] [Google Scholar]
- 132.Rao VH, Kansal V, Stoupa S, Agrawal DK. MMP-1 and MMP-9 regulate epidermal growth factor-dependent collagen loss in human carotid plaque smooth muscle cells. Physiol Rep. 2014;2(2):e00224. doi: 10.1002/phy2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heo SH, Choi YJ, Ryoo HM, Cho JY. Expression profiling of ETS and MMP factors in VEGF-activated endothelial cells: role of MMP-10 in VEGF-induced angiogenesis. J Cell Physiol. 2010;224(3):734–742. doi: 10.1002/jcp.22175. [DOI] [PubMed] [Google Scholar]
- 134.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(1):9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertension in pregnancy. 2004;23(1):101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 136.Ramma W, Buhimschi IA, Zhao G, Dulay AT, Nayeri UA, Buhimschi CS, Ahmed A. The elevation in circulating anti-angiogenic factors is independent of markers of neutrophil activation in preeclampsia. Angiogenesis. 2012;15(3):333–340. doi: 10.1007/s10456-012-9261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bian Z, Shixia C, Duan T. First-Trimester Maternal Serum Levels of sFLT1, PGF and ADMA Predict Preeclampsia. PloS one. 2015;10(4):e0124684. doi: 10.1371/journal.pone.0124684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Molvarec A, Czegle I, Szijarto J, Rigo J., Jr Increased circulating interleukin-17 levels in preeclampsia. J Reprod Immunol. 2015;112:53–57. doi: 10.1016/j.jri.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 139.March MI, Geahchan C, Wenger J, Raghuraman N, Berg A, Haddow H, McKeon BA, Narcisse R, David JL, Scott J, Thadhani R, Karumanchi SA, Rana S. Circulating Angiogenic Factors and the Risk of Adverse Outcomes among Haitian Women with Preeclampsia. PloS one. 2015;10(5):e0126815. doi: 10.1371/journal.pone.0126815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Agunanne EE, Uddin MN, Horvat D, Puschett JB. Contribution of angiogenic factors in a rat model of pre-eclampsia. Am J Nephrol. 2010;32(4):332–339. doi: 10.1159/000319463. [DOI] [PubMed] [Google Scholar]
- 141.Morton JS, Davidge ST. Arterial endothelium-derived hyperpolarization: potential role in pregnancy adaptations and complications. Journal of cardiovascular pharmacology. 2013;61(3):197–203. doi: 10.1097/FJC.0b013e31827b6367. [DOI] [PubMed] [Google Scholar]
- 142.Mandala M, Gokina N, Barron C, Osol G. Endothelial-derived hyperpolarization factor (EDHF) contributes to PlGF-induced dilation of mesenteric resistance arteries from pregnant rats. Journal of vascular research. 2012;49(1):43–49. doi: 10.1159/000329821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Charnock-Jones DS. Placental hypoxia, endoplasmic reticulum stress and maternal endothelial sensitisation by sFLT1 in pre-eclampsia. J Reprod Immunol. 2016;114:81–85. doi: 10.1016/j.jri.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the "chicken-and-egg" question. Endocrinology. 2004;145(11):4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 145.Wolf M, Shah A, Lam C, Martinez A, Smirnakis KV, Epstein FH, Taylor RN, Ecker JL, Karumanchi SA, Thadhani R. Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. Am J Obstet Gynecol. 2005;193(1):16–22. doi: 10.1016/j.ajog.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 146.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26(7):563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 147.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50(1):137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 148.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46(5):1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 149.Tuzcu ZB, Asicioglu E, Sunbul M, Ozben B, Arikan H, Koc M. Circulating endothelial cell number and markers of endothelial dysfunction in previously preeclamptic women. American journal of obstetrics and gynecology. 2015;213(4):533 e531–537. doi: 10.1016/j.ajog.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 150.Kakigano A, Mimura K, Kanagawa T, Nakayama M, Kanayama T, Fujita S, Kinugasa-Taniguchi Y, Endo M, Tomimatsu T, Kimura T. Imbalance of angiogenic factors and avascular edematous cystic villi in a trisomy 13 pregnancy: a case report. Placenta. 2013;34(7):628–630. doi: 10.1016/j.placenta.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 151.Palmer KR, Kaitu'u-Lino TJ, Hastie R, Hannan NJ, Ye L, Binder N, Cannon P, Tuohey L, Johns TG, Shub A, Tong S. Placental-Specific sFLT-1 e15a Protein Is Increased in Preeclampsia, Antagonizes Vascular Endothelial Growth Factor Signaling, and Has Antiangiogenic Activity. Hypertension. 2015;66(6):1251–1259. doi: 10.1161/HYPERTENSIONAHA.115.05883. [DOI] [PubMed] [Google Scholar]
- 152.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122(5):478–487. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 153.Bdolah Y, Lam C, Rajakumar A, Shivalingappa V, Mutter W, Sachs BP, Lim KH, Bdolah-Abram T, Epstein FH, Karumanchi SA. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? American journal of obstetrics and gynecology. 2008;198(4):428 e421–426. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- 154.Faupel-Badger JM, McElrath TF, Lauria M, Houghton LC, Lim KH, Parry S, Cantonwine D, Lai G, Karumanchi SA, Hoover RN, Troisi R. Maternal circulating angiogenic factors in twin and singleton pregnancies. American journal of obstetrics and gynecology. 2015;212(5):636 e631–638. doi: 10.1016/j.ajog.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Verdonk K, Saleh L, Lankhorst S, Smilde JE, van Ingen MM, Garrelds IM, Friesema EC, Russcher H, van den Meiracker AH, Visser W, Danser AH. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension. 2015;65(6):1316–1323. doi: 10.1161/HYPERTENSIONAHA.115.05267. [DOI] [PubMed] [Google Scholar]
- 156.Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T, Fridman A, Thome U, Kribs A, Danner M, Hamacher S, Mallmann P, Stepan H, Benzing T. Removal of Soluble Fms-Like Tyrosine Kinase-1 by Dextran Sulfate Apheresis in Preeclampsia. Journal of the American Society of Nephrology : JASN. 2016;27(3):903–913. doi: 10.1681/ASN.2015020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murphy SR, Cockrell K. Regulation of soluble fms-like tyrosine kinase-1 production in response to placental ischemia/hypoxia: role of angiotensin II. Physiol Rep. 2015;3(2):e12310. doi: 10.14814/phy2.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. European journal of obstetrics, gynecology, and reproductive biology. 2011;157(2):136–140. doi: 10.1016/j.ejogrb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 159.Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. American journal of physiology. Regulatory, integrative and comparative physiology. 2015;309(1):R62–70. doi: 10.1152/ajpregu.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Siddiqui AH, Irani RA, Zhang W, Wang W, Blackwell SC, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody-mediated soluble fms-like tyrosine kinase-1 induction contributes to impaired adrenal vasculature and decreased aldosterone production in preeclampsia. Hypertension. 2013;61(2):472–479. doi: 10.1161/HYPERTENSIONAHA.111.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas) Cytokine. 2011;56(2):192–199. doi: 10.1016/j.cyto.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 162.Bobek G, Surmon L, Mirabito KM, Makris A, Hennessy A. Placental Regulation of Inflammation and Hypoxia after TNF-alpha Infusion in Mice. American journal of reproductive immunology. 2015;74(5):407–418. doi: 10.1111/aji.12417. [DOI] [PubMed] [Google Scholar]
- 163.Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, Kang PM, van Goor H, McCurley A, Jaffe IZ, Karumanchi SA, Lely AT. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. Journal of the American Society of Nephrology : JASN. 2014;25(4):717–725. doi: 10.1681/ASN.2013030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jiang Z, Zou Y, Ge Z, Zuo Q, Huang SY, Sun L. A Role of sFlt-1 in Oxidative Stress and Apoptosis in Human and Mouse Pre-Eclamptic Trophoblasts. Biology of reproduction. 2015;93(3):73. doi: 10.1095/biolreprod.114.126227. [DOI] [PubMed] [Google Scholar]
- 165.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22(5):564–568. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O'Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50(4):686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 167.Amraoui F, Spijkers L, Hassani Lahsinoui H, Vogt L, van der Post J, Peters S, Afink G, Ris-Stalpers C, van den Born BJ. SFlt-1 elevates blood pressure by augmenting endothelin-1-mediated vasoconstriction in mice. PloS one. 2014;9(3):e91897. doi: 10.1371/journal.pone.0091897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, Gambhir SS, Ambati BK, Cross JC, Nayak NR. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. The Journal of clinical investigation. 2014;124(11):4941–4952. doi: 10.1172/JCI76864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78(2):356–365. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- 170.Kaneko H, Anzai T, Takahashi T, Kohno T, Shimoda M, Sasaki A, Shimizu H, Nagai T, Maekawa Y, Yoshimura K, Aoki H, Yoshikawa T, Okada Y, Yozu R, Ogawa S, Fukuda K. Role of vascular endothelial growth factor-A in development of abdominal aortic aneurysm. Cardiovasc Res. 2011;91(2):358–367. doi: 10.1093/cvr/cvr080. [DOI] [PubMed] [Google Scholar]
- 171.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55(2):380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mazzuca MQ, Li W, Reslan OM, Yu P, Mata KM, Khalil RA. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension. 2014;64(3):632–643. doi: 10.1161/HYPERTENSIONAHA.114.03315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luft FC. Soluble endoglin (sEng) joins the soluble fms-like tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant. 2006;21(11):3052–3054. doi: 10.1093/ndt/gfl439. [DOI] [PubMed] [Google Scholar]
- 174.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annual review of medicine. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 175.Yinon Y, Nevo O, Xu J, Many A, Rolfo A, Todros T, Post M, Caniggia I. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3. Am J Pathol. 2008;172(1):77–85. doi: 10.2353/ajpath.2008.070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 177.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. The New England journal of medicine. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 178.Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N, Scott J, Herse F, Haase N, Moseley J, Wallukat G, Dechend R, LaMarca B. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. American journal of physiology. Regulatory, integrative and comparative physiology. 2015;309(8):R884–891. doi: 10.1152/ajpregu.00154.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morris R, Spencer SK, Kyle PB, Williams JM, Harris A, Owens MY, Wallace K. Hypertension in an Animal Model of HELLP Syndrome is Associated With Activation of Endothelin 1. Reproductive sciences. 2015;23(1):42–50. doi: 10.1177/1933719115592707. [DOI] [PubMed] [Google Scholar]
- 180.Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E, Sier CF, ten Dijke P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010;70(10):4141–4150. doi: 10.1158/0008-5472.CAN-09-4466. [DOI] [PubMed] [Google Scholar]
- 181.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52(6):1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Moreno-Eutimio MA, Tovar-Rodriguez JM, Vargas-Avila K, Nieto-Velazquez NG, Frias-De-Leon MG, Sierra-Martinez M, Acosta-Altamirano G. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. BioMed research international. 2014;2014:413249. doi: 10.1155/2014/413249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pinheiro MB, Gomes KB, Ronda CR, Guimaraes GG, Freitas LG, Teixeira-Carvalho A, Martins-Filho OA, Dusse LM. Severe preeclampsia: association of genes polymorphisms and maternal cytokines production in Brazilian population. Cytokine. 2015;71(2):232–237. doi: 10.1016/j.cyto.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 184.Cakmak M, Yilmaz H, Baglar E, Darcin T, Inan O, Aktas A, Celik HT, Ozdemir O, Atalay CR, Akcay A. Serum levels of endocan correlate with the presence and severity of pre-eclampsia. Clinical and experimental hypertension. 2016;38(2):137–142. doi: 10.3109/10641963.2015.1060993. [DOI] [PubMed] [Google Scholar]
- 185.Peixoto AB, Araujo Junior E, Ribeiro JU, Rodrigues DB, Castro EC, Caldas TM, Rodrigues Junior V. Evaluation of inflammatory mediators in the deciduas of pregnant women with pre-eclampsia/eclampsia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016;29(1):75–79. doi: 10.3109/14767058.2014.987117. [DOI] [PubMed] [Google Scholar]
- 186.Wang W, Parchim NF, Iriyama T, Luo R, Zhao C, Liu C, Irani RA, Zhang W, Ning C, Zhang Y, Blackwell SC, Chen L, Tao L, Hicks MJ, Kellems RE, Xia Y. Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension. 2014;63(3):595–606. doi: 10.1161/HYPERTENSIONAHA.113.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN, Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57(5):949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. American journal of hypertension. 2002;15(2 Pt 1):170–175. doi: 10.1016/s0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- 189.Sanchez-Aranguren LC, Prada CE, Riano-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Frontiers in physiology. 2014;5:372. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lamarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate? International journal of interferon, cytokine and mediator research : IJIM. 2011;2011(3):59–64. doi: 10.2147/IJICMR.S16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lockwood CJ, Yen CF, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008;172(6):1571–1579. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Matias ML, Romao M, Weel IC, Ribeiro VR, Nunes PR, Borges VT, Araujo JP, Jr, Peracoli JC, de Oliveira L, Peracoli MT. Endogenous and Uric Acid-Induced Activation of NLRP3 Inflammasome in Pregnant Women with Preeclampsia. PloS one. 2015;10(6):e0129095. doi: 10.1371/journal.pone.0129095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Royle C, Lim S, Xu B, Tooher J, Ogle R, Hennessy A. Effect of hypoxia and exogenous IL-10 on the pro-inflammatory cytokine TNF-alpha and the anti-angiogenic molecule soluble Flt-1 in placental villous explants. Cytokine. 2009;47(1):56–60. doi: 10.1016/j.cyto.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 194.Rahardjo B, Widjajanto E, Sujuti H, Keman K. Different levels of IL-1alpha, IL-6, TNF-alpha, NF-kappaB and PPAR-gamma in monocyte cultures exposed by plasma preeclampsia and normotensive pregnancy. Pregnancy hypertension. 2014;4(3):187–193. doi: 10.1016/j.preghy.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 195.Daikoku T, Matsumoto H, Gupta RA, Das SK, Gassmann M, DuBois RN, Dey SK. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem. 2003;278(9):7683–7691. doi: 10.1074/jbc.M211390200. [DOI] [PubMed] [Google Scholar]
- 196.Patel J, Landers K, Mortimer RH, Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010;31(11):951–957. doi: 10.1016/j.placenta.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 197.Akhilesh M, Mahalingam V, Nalliah S, Ali RM, Ganesalingam M, Haleagrahara N. Hypoxia-inducible factor-1alpha as a predictive marker in pre-eclampsia. Biomed Rep. 2013;1(2):257–258. doi: 10.3892/br.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biology of reproduction. 2012;87(6):134. doi: 10.1095/biolreprod.112.102723. [DOI] [PubMed] [Google Scholar]
- 199.Iriyama T, Wang W, Parchim NF, Song A, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Hypoxia-independent upregulation of placental hypoxia inducible factor-1alpha gene expression contributes to the pathogenesis of preeclampsia. Hypertension. 2015;65(6):1307–1315. doi: 10.1161/HYPERTENSIONAHA.115.05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 201.Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis. 2005;64(7):971–980. doi: 10.1136/ard.2004.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Milkiewicz M, Doyle JL, Fudalewski T, Ispanovic E, Aghasi M, Haas TL. HIF-1alpha and HIF-2alpha play a central role in stretch-induced but not shear-stress-induced angiogenesis in rat skeletal muscle. J Physiol. 2007;583(Pt 2):753–766. doi: 10.1113/jphysiol.2007.136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Milkiewicz M, Haas TL. Effect of mechanical stretch on HIF-1{alpha} and MMP-2 expression in capillaries isolated from overloaded skeletal muscles: laser capture microdissection study. Am J Physiol Heart Circ Physiol. 2005;289(3):H1315–1320. doi: 10.1152/ajpheart.00284.2005. [DOI] [PubMed] [Google Scholar]
- 204.Chang H, Shyu KG, Wang BW, Kuan P. Regulation of hypoxia-inducible factor-1alpha by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin Sci (Lond) 2003;105(4):447–456. doi: 10.1042/CS20030088. [DOI] [PubMed] [Google Scholar]
- 205.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90(2):E25–33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 206.Petersen W, Varoga D, Zantop T, Hassenpflug J, Mentlein R, Pufe T. Cyclic strain influences the expression of the vascular endothelial growth factor (VEGF) and the hypoxia inducible factor 1 alpha (HIF-1alpha) in tendon fibroblasts. J Orthop Res. 2004;22(4):847–853. doi: 10.1016/j.orthres.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 207.Lee JD, Jeng SY, Lee TH. Increased expression of hypoxia-inducible factor-1alpha in the internal spermatic vein of patients with varicocele. J Urol. 2006;175(3 Pt 1):1045–1048. doi: 10.1016/S0022-5347(05)00417-9. discussion 1048. [DOI] [PubMed] [Google Scholar]
- 208.Fujiwara S, Nakagawa K, Harada H, Nagato S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S, Ohnishi T. Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol. 2007;30(4):793–802. [PubMed] [Google Scholar]
- 209.Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, Davies AH, Khalil RA. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg. 2011;53(3):764–773. doi: 10.1016/j.jvs.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Myatt L, Webster RP. Vascular biology of preeclampsia. Journal of thrombosis and haemostasis : JTH. 2009;7(3):375–384. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 211.Nakamura M, Sekizawa A, Purwosunu Y, Okazaki S, Farina A, Wibowo N, Shimizu H, Okai T. Cellular mRNA expressions of anti-oxidant factors in the blood of preeclamptic women. Prenat Diagn. 2009;29(7):691–696. doi: 10.1002/pd.2278. [DOI] [PubMed] [Google Scholar]
- 212.Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2015;15:189. doi: 10.1186/s12884-015-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cohen JM, Kramer MS, Platt RW, Basso O, Evans RW, Kahn SR. The association between maternal antioxidant levels in midpregnancy and preeclampsia. American journal of obstetrics and gynecology. 2015;213(5):695 e691–695 e613. doi: 10.1016/j.ajog.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 214.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. Jama. 2001;285(12):1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 215.Peracoli MT, Bannwart CF, Cristofalo R, Borges VT, Costa RA, Witkin SS, Peracoli JC. Increased reactive oxygen species and tumor necrosis factor-alpha production by monocytes are associated with elevated levels of uric acid in pre-eclamptic women. American journal of reproductive immunology. 2011;66(6):460–467. doi: 10.1111/j.1600-0897.2011.01016.x. [DOI] [PubMed] [Google Scholar]
- 216.Tsukimori K, Fukushima K, Tsushima A, Nakano H. Generation of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension. 2005;46(4):696–700. doi: 10.1161/01.HYP.0000184197.11226.71. [DOI] [PubMed] [Google Scholar]
- 217.Cui XL, Brockman D, Campos B, Myatt L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta. 2006;27(4–5):422–431. doi: 10.1016/j.placenta.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. The journal of obstetrics and gynaecology research. 2010;36(2):239–247. doi: 10.1111/j.1447-0756.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 219.Amaral LM, Pinheiro LC, Guimaraes DA, Palei AC, Sertorio JT, Portella RL, Tanus-Santos JE. Antihypertensive effects of inducible nitric oxide synthase inhibition in experimental pre-eclampsia. J Cell Mol Med. 2013;17(10):1300–1307. doi: 10.1111/jcmm.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mitchell BM, Cook LG, Danchuk S, Puschett JB. Uncoupled endothelial nitric oxide synthase and oxidative stress in a rat model of pregnancy-induced hypertension. American journal of hypertension. 2007;20(12):1297–1304. doi: 10.1016/j.amjhyper.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 221.Genc H, Uzun H, Benian A, Simsek G, Gelisgen R, Madazli R, Guralp O. Evaluation of oxidative stress markers in first trimester for assessment of preeclampsia risk. Archives of gynecology and obstetrics. 2011;284(6):1367–1373. doi: 10.1007/s00404-011-1865-2. [DOI] [PubMed] [Google Scholar]
- 222.Cross CE, Tolba MF, Rondelli CM, Xu M, Abdel-Rahman SZ. Oxidative Stress Alters miRNA and Gene Expression Profiles in Villous First Trimester Trophoblasts. BioMed research international. 2015;2015:257090. doi: 10.1155/2015/257090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. The Journal of clinical investigation. 1973;52(11):2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, Moorefield C, Gruver C, Ferrario CM, Brosnihan KB. Activation of local chorionic villi angiotensin II levels but not angiotensin (1–7) in preeclampsia. Hypertension. 2008;51(4):1066–1072. doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mistry HD, Kurlak LO, Broughton Pipkin F. The placental renin-angiotensin system and oxidative stress in pre-eclampsia. Placenta. 2013;34(2):182–186. doi: 10.1016/j.placenta.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 226.Bakker WW, Henning RH, van Son WJ, van Pampus MG, Aarnoudse JG, Niezen-Koning KE, Borghuis T, Jongman RM, van Goor H, Poelstra K, Navis G, Faas MM. Vascular contraction and preeclampsia: downregulation of the Angiotensin receptor 1 by hemopexin in vitro. Hypertension. 2009;53(6):959–964. doi: 10.1161/HYPERTENSIONAHA.108.127951. [DOI] [PubMed] [Google Scholar]
- 227.Bai K, Wang K, Li X, Wang J, Zhang J, Song L, Zhang S, Lau WB, Ma X, Liu H. Autoantibody against angiotensin AT1 receptor from preeclamptic patients enhances collagen-induced human platelet aggregation. Acta Biochim Biophys Sin (Shanghai) 2013;45(9):749–755. doi: 10.1093/abbs/gmt059. [DOI] [PubMed] [Google Scholar]
- 228.Yang J, Li L, Shang JY, Cai L, Song L, Zhang SL, Li H, Li X, Lau WB, Ma XL, Liu HR. Angiotensin II type 1 receptor autoantibody as a novel regulator of aldosterone independent of preeclampsia. Journal of hypertension. 2015;33(5):1046–1056. doi: 10.1097/HJH.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 229.Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, Wallukat G, Dechend R, LaMarca B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension. 2013;62(6):1068–1073. doi: 10.1161/HYPERTENSIONAHA.113.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN, Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;302(10):R1197–1201. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52(6):1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54(4):905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Parrish MR, Ryan MJ, Glover P, Brewer J, Ray L, Dechend R, Martin JN, Jr, Lamarca BB. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gender medicine. 2011;8(3):184–188. doi: 10.1016/j.genm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50(2):269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14(8):855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang X, Wang F, Lau WB, Zhang S, Liu H, Ma XL. Autoantibodies isolated from preeclamptic patients induce endothelial dysfunction via interaction with the angiotensin II AT1 receptor. Cardiovasc Toxicol. 2014;14(1):21–29. doi: 10.1007/s12012-013-9229-8. [DOI] [PubMed] [Google Scholar]
- 237.Kucukguven A, Khalil RA. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr Drug Targets. 2013;14(3):287–324. [PMC free article] [PubMed] [Google Scholar]
- 238.Cheng XW, Kuzuya M, Sasaki T, Arakawa K, Kanda S, Sumi D, Koike T, Maeda K, Tamaya-Mori N, Shi GP, Saito N, Iguchi A. Increased expression of elastolytic cysteine proteases, cathepsins S and K, in the neointima of balloon-injured rat carotid arteries. Am J Pathol. 2004;164(1):243–251. doi: 10.1016/S0002-9440(10)63114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Johnson C, Galis ZS. Matrix metalloproteinase-2 and-9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24(1):54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 240.Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91(9):852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 241.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91(9):845–851. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 242.Ergul A, Portik-Dobos V, Hutchinson J, Franco J, Anstadt MP. Downregulation of vascular matrix metalloproteinase inducer and activator proteins in hypertensive patients. Am J Hypertens. 2004;17(9):775–782. doi: 10.1016/j.amjhyper.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 243.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50(1):212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 244.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36(3):312–318. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- 245.Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 246.Shi ZD, Ji XY, Berardi DE, Qazi H, Tarbell JM. Interstitial flow induces MMP-1 expression and vascular SMC migration in collagen I gels via an ERK1/2-dependent and c-Jun-mediated mechanism. Am J Physiol Heart Circ Physiol. 2010;298(1):H127–135. doi: 10.1152/ajpheart.00732.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pascual G, Rodriguez M, Gomez-Gil V, Trejo C, Bujan J, Bellon JM. Active matrix metalloproteinase-2 upregulation in the abdominal skin of patients with direct inguinal hernia. Eur J Clin Invest. 2010;40(12):1113–1121. doi: 10.1111/j.1365-2362.2010.02364.x. [DOI] [PubMed] [Google Scholar]
- 248.Brandao AH, Felix LR, Patricio Edo C, Leite HV, Cabral AC. Difference of endothelial function during pregnancies as a method to predict preeclampsia. Archives of gynecology and obstetrics. 2014;290(3):471–477. doi: 10.1007/s00404-014-3243-3. [DOI] [PubMed] [Google Scholar]
- 249.Sierra-Laguado J, Garcia RG, Lopez-Jaramillo P. Flow-mediated dilatation of the brachial artery in pregnancy. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2006;93(1):60–61. doi: 10.1016/j.ijgo.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 250.Knock GA, Poston L. Bradykinin-mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. American journal of obstetrics and gynecology. 1996;175(6):1668–1674. doi: 10.1016/s0002-9378(96)70123-0. [DOI] [PubMed] [Google Scholar]
- 251.Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biology of reproduction. 2010;82(1):66–75. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance Ca(2+)-activated K(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58(6):1132–1139. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Reslan OM, Khalil RA. Vascular effects of estrogenic menopausal hormone therapy. Rev Recent Clin Trials. 2012;7(1):47–70. doi: 10.2174/157488712799363253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca(2+)](i) in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol. 2000;278(4):C834–844. doi: 10.1152/ajpcell.2000.278.4.C834. [DOI] [PubMed] [Google Scholar]
- 255.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280(1):C34–45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- 256.Murphy JG, Khalil RA. Decreased [Ca(2+)](i) during inhibition of coronary smooth muscle contraction by 17beta-estradiol, progesterone, and testosterone. J Pharmacol Exp Ther. 1999;291(1):44–52. [PubMed] [Google Scholar]
- 257.Crews JK, Khalil RA. Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol. 1999;19(4):1034–1040. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- 258.Guimaraes MF, Brandao AH, Rezende CA, Cabral AC, Brum AP, Leite HV, Capuruco CA. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Archives of gynecology and obstetrics. 2014;290(3):441–447. doi: 10.1007/s00404-014-3220-x. [DOI] [PubMed] [Google Scholar]
- 259.Yoshida A, Nakao S, Kobayashi M, Kobayashi H. Flow-mediated vasodilation and plasma fibronectin levels in preeclampsia. Hypertension. 2000;36(3):400–404. doi: 10.1161/01.hyp.36.3.400. [DOI] [PubMed] [Google Scholar]
- 260.Canbakan B, Keven K, Tutkak H, Danisman N, Ergun I, Nergizoglu G. Circulating endothelial cells in preeclampsia. Journal of human hypertension. 2007;21(7):558–563. doi: 10.1038/sj.jhh.1002199. [DOI] [PubMed] [Google Scholar]
- 261.Adekola H, Romero R, Chaemsaithong P, Korzeniewski SJ, Dong Z, Yeo L, Hassan SS, Chaiworapongsa T. Endocan, a putative endothelial cell marker, is elevated in preeclampsia, decreased in acute pyelonephritis, and unchanged in other obstetrical syndromes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28(14):1621–1632. doi: 10.3109/14767058.2014.964676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sakashita T, Higashi Y, Soga J, Miyoshi H, Kudo Y. Circulating endothelial progenitor cells and placental abruption in women with preeclampsia. Pregnancy hypertension. 2014;4(3):203–208. doi: 10.1016/j.preghy.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 263.Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN, Jr, LaMarca B. 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension. 2015;65(1):225–231. doi: 10.1161/HYPERTENSIONAHA.114.04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shaamash AH, Elsnosy ED, Makhlouf AM, Zakhari MM, Ibrahim OA, EL-dien HM. Maternal and fetal serum nitric oxide (NO) concentrations in normal pregnancy, pre-eclampsia and eclampsia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2000;68(3):207–214. doi: 10.1016/s0020-7292(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 265.Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circulation research. 2000;87(5):406–411. doi: 10.1161/01.res.87.5.406. [DOI] [PubMed] [Google Scholar]
- 266.Dotsch J, Hogen N, Nyul Z, Hanze J, Knerr I, Kirschbaum M, Rascher W. Increase of endothelial nitric oxide synthase and endothelin-1 mRNA expression in human placenta during gestation. European journal of obstetrics, gynecology, and reproductive biology. 2001;97(2):163–167. doi: 10.1016/s0301-2115(00)00532-7. [DOI] [PubMed] [Google Scholar]
- 267.Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension. 1999;33(1 Pt 2):435–439. doi: 10.1161/01.hyp.33.1.435. [DOI] [PubMed] [Google Scholar]
- 268.Alpoim PN, Gomes KB, Pinheiro Mde B, Godoi LC, Jardim LL, Muniz LG, Sandrim VC, Fernandes AP, Dusse LM. Polymorphisms in endothelial nitric oxide synthase gene in early and late severe preeclampsia. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2014;42:19–23. doi: 10.1016/j.niox.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 269.Ben Ali Gannoun M, Zitouni H, Raguema N, Maleh W, Gris JC, Almawi W, Mahjoub T. Association of common eNOS/NOS3 polymorphisms with preeclampsia in Tunisian Arabs. Gene. 2015;569(2):303–307. doi: 10.1016/j.gene.2015.05.072. [DOI] [PubMed] [Google Scholar]
- 270.Leonardo DP, Albuquerque DM, Lanaro C, Baptista LC, Cecatti JG, Surita FG, Parpinelli MA, Costa FF, Franco-Penteado CF, Fertrin KY, Costa ML. Association of Nitric Oxide Synthase and Matrix Metalloprotease Single Nucleotide Polymorphisms with Preeclampsia and Its Complications. PloS one. 2015;10(8):e0136693. doi: 10.1371/journal.pone.0136693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sandrim VC, Palei AC, Sertorio JT, Cavalli RC, Duarte G, Tanus-Santos JE. Effects of eNOS polymorphisms on nitric oxide formation in healthy pregnancy and in pre-eclampsia. Molecular human reproduction. 2010;16(7):506–510. doi: 10.1093/molehr/gaq030. [DOI] [PubMed] [Google Scholar]
- 272.Echeverri I, Ortega-Avila JG, Mosquera M, Castillo A, Jimenez E, Suarez-Ortegon MF, Mateus JC, Aguilar-de Plata C. Relationship between maternal and newborn endothelial function and oxidative stress. American journal of human biology : the official journal of the Human Biology Council. 2015;27(6):822–831. doi: 10.1002/ajhb.22733. [DOI] [PubMed] [Google Scholar]
- 273.Noorbakhsh M, Kianpour M, Nematbakhsh M. Serum levels of asymmetric dimethylarginine, vascular endothelial growth factor, and nitric oxide metabolite levels in preeclampsia patients. ISRN obstetrics and gynecology. 2013;2013:104213. doi: 10.1155/2013/104213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schiessl B, Strasburger C, Bidlingmaier M, Mylonas I, Jeschke U, Kainer F, Friese K. Plasma- and urine concentrations of nitrite/nitrate and cyclic Guanosinemonophosphate in intrauterine growth restricted and preeclamptic pregnancies. Archives of gynecology and obstetrics. 2006;274(3):150–154. doi: 10.1007/s00404-006-0149-8. [DOI] [PubMed] [Google Scholar]
- 275.Pimentel AM, Pereira NR, Costa CA, Mann GE, Cordeiro VS, de Moura RS, Brunini TM, Mendes-Ribeiro AC, Resende AC. L-arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsia. Hypertension research : official journal of the Japanese Society of Hypertension. 2013;36(9):783–788. doi: 10.1038/hr.2013.34. [DOI] [PubMed] [Google Scholar]
- 276.Eleuterio NM, Palei AC, Rangel Machado JS, Tanus-Santos JE, Cavalli RC, Sandrim VC. Relationship between adiponectin and nitrite in healthy and preeclampsia pregnancies. Clinica chimica acta; international journal of clinical chemistry. 2013;423:112–115. doi: 10.1016/j.cca.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 277.Ehsanipoor RM, Fortson W, Fitzmaurice LE, Liao WX, Wing DA, Chen DB, Chan K. Nitric oxide and carbon monoxide production and metabolism in preeclampsia. Reproductive sciences. 2013;20(5):542–548. doi: 10.1177/1933719112459231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NO(x) and cGMP during normal pregnancy and preeclampsia in women on a reduced NO(x) diet. The American journal of physiology. 1999;277(1 Pt 2):F48–57. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- 279.Bhavina K, Radhika J, Pandian SS. VEGF and eNOS expression in umbilical cord from pregnancy complicated by hypertensive disorder with different severity. BioMed research international. 2014;2014:982159. doi: 10.1155/2014/982159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wang L, Yang T, Ding Y, Zhong Y, Yu L, Peng M. Chemerin plays a protective role by regulating human umbilical vein endothelial cell-induced nitric oxide signaling in preeclampsia. Endocrine. 2015;48(1):299–308. doi: 10.1007/s12020-014-0286-y. [DOI] [PubMed] [Google Scholar]
- 281.Zawiejska A, Wender-Ozegowska E, Iciek R, Brazert J. Concentrations of endothelial nitric oxide synthase, angiotensin-converting enzyme, vascular endothelial growth factor and placental growth factor in maternal blood and maternal metabolic status in pregnancy complicated by hypertensive disorders. Journal of human hypertension. 2014;28(11):670–676. doi: 10.1038/jhh.2014.42. [DOI] [PubMed] [Google Scholar]
- 282.Smith-Jackson K, Hentschke MR, Poli-de-Figueiredo CE, Pinheiro da Costa BE, Kurlak LO, Broughton Pipkin F, Czajka A, Mistry HD. Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta. 2015;36(5):607–610. doi: 10.1016/j.placenta.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 283.Kemse NG, Kale AA, Joshi SR. A combined supplementation of omega-3 fatty acids and micronutrients (folic acid, vitamin B12) reduces oxidative stress markers in a rat model of pregnancy induced hypertension. PloS one. 2014;9(11):e111902. doi: 10.1371/journal.pone.0111902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Javadian P, Salmanian B, Javadi-Paydar M, Shamshirsaz AA, Ejtemaei Mehr S, Gharedaghi MH, Dehpour AR. Effect of morphine on the reduced uteroplacental perfusion model of pre-eclampsia in rats. European journal of obstetrics, gynecology, and reproductive biology. 2013;168(2):161–166. doi: 10.1016/j.ejogrb.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 285.Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension. 2004;43(4):832–836. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- 286.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clinical and experimental pharmacology & physiology. 2004;31(9):641–649. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 287.Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Archiv : European journal of physiology. 2010;459(6):915–922. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- 288.Feletou M, Vanhoutte PM. EDHF: an update. Clinical science. 2009;117(4):139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 289.Luksha L, Nisell H, Kublickiene K. The mechanism of EDHF-mediated responses in subcutaneous small arteries from healthy pregnant women. American journal of physiology. Regulatory, integrative and comparative physiology. 2004;286(6):R1102–1109. doi: 10.1152/ajpregu.00550.2003. [DOI] [PubMed] [Google Scholar]
- 290.Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. Differential mechanisms of endothelium-dependent vasodilator responses in human myometrial small arteries in normal pregnancy and pre-eclampsia. Clinical science. 2002;103(1):67–73. doi: 10.1042/cs1030067. [DOI] [PubMed] [Google Scholar]
- 291.Hammond S, Mathewson AM, Baker PN, Mayhew TM, Dunn WR. Gap junctions and hydrogen peroxide are involved in endothelium-derived hyperpolarising responses to bradykinin in omental arteries and veins isolated from pregnant women. European journal of pharmacology. 2011;668(1–2):225–232. doi: 10.1016/j.ejphar.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 292.Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. American journal of physiology. Heart and circulatory physiology. 2010;299(5):H1642–1652. doi: 10.1152/ajpheart.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Fulep EE, Vedernikov YP, Saade GR, Garfield RE. The role of endothelium-derived hyperpolarizing factor in the regulation of the uterine circulation in pregnant rats. American journal of obstetrics and gynecology. 2001;185(3):638–642. doi: 10.1067/mob.2001.117665. [DOI] [PubMed] [Google Scholar]
- 294.Luksha L, Nisell H, Luksha N, Kublickas M, Hultenby K, Kublickiene K. Endothelium-derived hyperpolarizing factor in preeclampsia: heterogeneous contribution, mechanisms, and morphological prerequisites. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;294(2):R510–519. doi: 10.1152/ajpregu.00458.2007. [DOI] [PubMed] [Google Scholar]
- 295.Hagedorn KA, Cooke CL, Falck JR, Mitchell BF, Davidge ST. Regulation of vascular tone during pregnancy: a novel role for the pregnane × receptor. Hypertension. 2007;49(2):328–333. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- 296.Zhu M, Ren Z, Possomato-Vieira JS, Khalil RA. Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. American journal of physiology. Regulatory, integrative and comparative physiology. 2016;311(3):R505–521. doi: 10.1152/ajpregu.00137.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. American journal of hypertension. 2011;24(9):964–969. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Scalera F, Schlembach D, Beinder E. Production of vasoactive substances by human umbilical vein endothelial cells after incubation with serum from preeclamptic patients. European journal of obstetrics, gynecology, and reproductive biology. 2001;99(2):172–178. doi: 10.1016/s0301-2115(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 299.Roberts JM. Endothelial dysfunction in preeclampsia. Seminars in reproductive endocrinology. 1998;16(1):5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 300.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. The Journal of clinical endocrinology and metabolism. 1990;71(6):1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 301.Naiker S, Naidoo S, Naicker T, Gathiram P, Nadar A, Moodley J. Immunolocalisation and endothelin-1 values In pre-eclampsia: an immunocytochemical study. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2001;21(1):39–45. doi: 10.1080/01443610020022104. [DOI] [PubMed] [Google Scholar]
- 302.Karakus S, Bozoklu Akkar O, Yildiz C, Sancakdar E, Cetin M, Cetin A. Serum levels of ET-1, M30, and angiopoietins-1 and -2 in HELLP syndrome and preeclampsia compared to controls. Archives of gynecology and obstetrics. 2016;293(2):351–359. doi: 10.1007/s00404-015-3803-1. [DOI] [PubMed] [Google Scholar]
- 303.Kourembanas S, McQuillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. The Journal of clinical investigation. 1993;92(1):99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jain A, Schneider H, Aliyev E, Soydemir F, Baumann M, Surbek D, Hediger M, Brownbill P, Albrecht C. Hypoxic treatment of human dual placental perfusion induces a preeclampsia-like inflammatory response. Lab Invest. 2014;94(8):873–880. doi: 10.1038/labinvest.2014.76. [DOI] [PubMed] [Google Scholar]
- 305.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37(2 Pt 2):485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 306.Johnson AC, Tremble SM, Chan SL, Moseley J, LaMarca B, Nagle KJ, Cipolla MJ. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PloS one. 2014;9(11):e113670. doi: 10.1371/journal.pone.0113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Fiore G, Florio P, Micheli L, Nencini C, Rossi M, Cerretani D, Ambrosini G, Giorgi G, Petraglia F. Endothelin-1 triggers placental oxidative stress pathways: putative role in preeclampsia. The Journal of clinical endocrinology and metabolism. 2005;90(7):4205–4210. doi: 10.1210/jc.2004-1632. [DOI] [PubMed] [Google Scholar]
- 308.Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochemical pharmacology. 2012;84(2):147–162. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hynynen MM, Khalil RA. The vascular endothelin system in hypertension--recent patents and discoveries. Recent Pat Cardiovasc Drug Discov. 2006;1(1):95–108. doi: 10.2174/157489006775244263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sumner MJ, Cannon TR, Mundin JW, White DG, Watts IS. Endothelin ETA and ETB receptors mediate vascular smooth muscle contraction. Br J Pharmacol. 1992;107(3):858–860. doi: 10.1111/j.1476-5381.1992.tb14537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.LaDouceur DM, Flynn MA, Keiser JA, Reynolds E, Haleen SJ. ETA and ETB receptors coexist on rabbit pulmonary artery vascular smooth muscle mediating contraction. Biochem Biophys Res Commun. 1993;196(1):209–215. doi: 10.1006/bbrc.1993.2236. [DOI] [PubMed] [Google Scholar]
- 312.Moreland S. Endothelin receptor antagonists: a brief review. Can J Physiol Pharmacol. 1994;72(11):1469–1471. doi: 10.1139/y94-212. [DOI] [PubMed] [Google Scholar]
- 313.Seo B, Oemar BS, Siebenmann R, von Segesser L, Luscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89(3):1203–1208. doi: 10.1161/01.cir.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 314.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J. 1995;9(12):1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- 315.Schiffrin EL, Touyz RM. Vascular biology of endothelin. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S2–13. [PubMed] [Google Scholar]
- 316.Mazzuca MQ, Dang Y, Khalil RA. Enhanced endothelin receptor type B-mediated vasodilation and underlying [Ca(2)(+)]i in mesenteric microvessels of pregnant rats. British journal of pharmacology. 2013;169(6):1335–1351. doi: 10.1111/bph.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ou M, Dang Y, Mazzuca MQ, Basile R, Khalil RA. Adaptive regulation of endothelin receptor type-A and type-B in vascular smooth muscle cells during pregnancy in rats. Journal of cellular physiology. 2014;229(4):489–501. doi: 10.1002/jcp.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46(1):82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 319.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55(2):394–398. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Velloso EP, Pimentel RL, Braga JF, Cabral AC, Reis ZS, Bader M, Santos RA, Wallukat G. Identification of a Novel Agonist-Like Autoantibody in Preeclamptic Patients. American journal of hypertension. 2016;29(3):405–412. doi: 10.1093/ajh/hpv099. [DOI] [PubMed] [Google Scholar]
- 321.Conrad KP, Gandley RE, Ogawa T, Nakanishi S, Danielson LA. Endothelin mediates renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. The American journal of physiology. 1999;276(5 Pt 2):F767–776. doi: 10.1152/ajprenal.1999.276.5.F767. [DOI] [PubMed] [Google Scholar]
- 322.Abdalvand A, Morton JS, Bourque SL, Quon AL, Davidge ST. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension. 2013;61(2):488–493. doi: 10.1161/HYPERTENSIONAHA.111.00055. [DOI] [PubMed] [Google Scholar]
- 323.Tam Tam KB, George E, Cockrell K, Arany M, Speed J, Martin JN, Jr, Lamarca B, Granger JP. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. American journal of obstetrics and gynecology. 2011;204(4):330 e331–334. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 325.Schiffrin EL. Endothelin and endothelin antagonists in hypertension. J Hypertens. 1998;16(12 Pt 2):1891–1895. doi: 10.1097/00004872-199816121-00007. [DOI] [PubMed] [Google Scholar]
- 326.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47(3):615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 327.LaMarca BD, Alexander BT, Gilbert JS, Ryan MJ, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med. 2008;5(Suppl A):S133–138. [Google Scholar]
- 328.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37(2 Part 2):485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 329.Cain AE, Tanner DM, Khalil RA. Endothelin-1--induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca(2+)](i) sensitization pathways. Hypertension. 2002;39(2 Pt 2):543–549. doi: 10.1161/hy0202.103129. [DOI] [PubMed] [Google Scholar]
- 330.Neylon CB. Vascular biology of endothelin signal transduction. Clin Exp Pharmacol Physiol. 1999;26(2):149–153. doi: 10.1046/j.1440-1681.1999.03013.x. [DOI] [PubMed] [Google Scholar]
- 331.Smith L, Payne JA, Sedeek MH, Granger JP, Khalil RA. Endothelin-induced increases in Ca2+ entry mechanisms of vascular contraction are enhanced during high-salt diet. Hypertension. 2003;41(3 Pt 2):787–793. doi: 10.1161/01.HYP.0000051643.05700.56. [DOI] [PubMed] [Google Scholar]
- 332.Chew DK, Orshal JM, Khalil RA. Elastase-induced suppression of endothelin-mediated Ca2+ entry mechanisms of vascular contraction. Hypertension. 2003;42(4):818–824. doi: 10.1161/01.HYP.0000086200.93184.8E. [DOI] [PubMed] [Google Scholar]
- 333.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 334.Kanashiro CA, Khalil RA. Signal transduction by protein kinase C in mammalian cells. Clin Exp Pharmacol Physiol. 1998;25(12):974–985. doi: 10.1111/j.1440-1681.1998.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 335.Khalil RA, Morgan KG. Phenylephrine-induced translocation of protein kinase C and shortening of two types of vascular cells of the ferret. J Physiol. 1992;455:585–599. doi: 10.1113/jphysiol.1992.sp019317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70(11):1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Khalil RA, Lajoie C, Resnick MS, Morgan KG. Ca(2+)-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am J Physiol. 1992;263(3 Pt 1):C714–719. doi: 10.1152/ajpcell.1992.263.3.C714. [DOI] [PubMed] [Google Scholar]
- 338.Khalil RA, Lajoie C, Morgan KG. In situ determination of [Ca2+]i threshold for translocation of the alpha-protein kinase C isoform. Am J Physiol. 1994;266(6 Pt 1):C1544–1551. doi: 10.1152/ajpcell.1994.266.6.C1544. [DOI] [PubMed] [Google Scholar]
- 339.Liou YM, Morgan KG. Redistribution of protein kinase C isoforms in association with vascular hypertrophy of rat aorta. Am J Physiol. 1994;267(4 Pt 1):C980–989. doi: 10.1152/ajpcell.1994.267.4.C980. [DOI] [PubMed] [Google Scholar]
- 340.Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25(8):613–615. doi: 10.1007/s10974-004-3146-1. [DOI] [PubMed] [Google Scholar]
- 341.Lee DL, Webb RC, Jin L. Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension. 2004;44(6):796–799. doi: 10.1161/01.HYP.0000148303.98066.ab. [DOI] [PubMed] [Google Scholar]
- 342.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25(9):1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 343.Xiao D, Zhu R, Zhang L. Gestational hypoxia up-regulates protein kinase C and inhibits calcium-activated potassium channels in ovine uterine arteries. Int J Med Sci. 2014;11(9):886–892. doi: 10.7150/ijms.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wimalasundera RC, Thom SA, Regan L, Hughes AD. Effects of vasoactive agents on intracellular calcium and force in myometrial and subcutaneous resistance arteries isolated from preeclamptic, pregnant, and nonpregnant woman. Am J Obstet Gynecol. 2005;192(2):625–632. doi: 10.1016/j.ajog.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 345.Murphy JG, Fleming JB, Cockrell KL, Granger JP, Khalil RA. [Ca(2+)](i) signaling in renal arterial smooth muscle cells of pregnant rat is enhanced during inhibition of NOS. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R87–99. doi: 10.1152/ajpregu.2001.280.1.R87. [DOI] [PubMed] [Google Scholar]
- 346.Murphy JG, Herrington JN, Granger JP, Khalil RA. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Heart Circ Physiol. 2003;284(1):H393–403. doi: 10.1152/ajpheart.00247.2002. [DOI] [PubMed] [Google Scholar]
- 347.Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol. 1991;260(3 Pt 1):E464–470. doi: 10.1152/ajpendo.1991.260.3.E464. [DOI] [PubMed] [Google Scholar]
- 348.Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R295–303. doi: 10.1152/ajpregu.2000.278.2.R295. [DOI] [PubMed] [Google Scholar]
- 349.Farley DB, Ford SP. Evidence for declining extracellular calcium uptake and protein kinase C activity in uterine arterial smooth muscle during gestation in gilts. Biol Reprod. 1992;46(3):315–321. doi: 10.1095/biolreprod46.3.315. [DOI] [PubMed] [Google Scholar]
- 350.Kanashiro CA, Alexander BT, Granger JP, Khalil RA. Ca(2+)-insensitive vascular protein kinase C during pregnancy and NOS inhibition. Hypertension. 1999;34(4 Pt 2):924–930. doi: 10.1161/01.hyp.34.4.924. [DOI] [PubMed] [Google Scholar]
- 351.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Goulopoulou S, Hannan JL, Matsumoto T, Webb RC. Pregnancy reduces RhoA/Rho kinase and protein kinase C signaling pathways downstream of thromboxane receptor activation in the rat uterine artery. Am J Physiol Heart Circ Physiol. 2012;302(12):H2477–2488. doi: 10.1152/ajpheart.00900.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Castro MM, Cena J, Cho WJ, Walsh MP, Schulz R. Matrix metalloproteinase-2 proteolysis of calponin-1 contributes to vascular hypocontractility in endotoxemic rats. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(3):662–668. doi: 10.1161/ATVBAHA.111.242685. [DOI] [PubMed] [Google Scholar]
- 354.Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Tamaki T. Atheroprotective effects of antioxidants through inhibition of mitogen-activated protein kinases. Acta Pharmacol Sin. 2004;25(8):977–985. [PubMed] [Google Scholar]
- 355.Ark M, Yilmaz N, Yazici G, Kubat H, Aktas S. Rho-associated protein kinase II (rock II) expression in normal and preeclamptic human placentas. Placenta. 2005;26(1):81–84. doi: 10.1016/j.placenta.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 356.VanWijk MJ, Boer K, van der Meulen ET, Bleker OP, Spaan JA, VanBavel E. Resistance artery smooth muscle function in pregnancy and preeclampsia. Am J Obstet Gynecol. 2002;186(1):148–154. doi: 10.1067/mob.2002.119184. [DOI] [PubMed] [Google Scholar]
- 357.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39(2):245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 358.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97(4):696–704. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Friel AM, Sexton DJ, O'Reilly MW, Smith TJ, Morrison JJ. Rho A/Rho kinase: human umbilical artery mRNA expression in normal and pre eclamptic pregnancies and functional role in isoprostane-induced vasoconstriction. Reproduction. 2006;132(1):169–176. doi: 10.1530/rep.1.01088. [DOI] [PubMed] [Google Scholar]
- 360.Parkington HC, Coleman HA. Ionic mechanisms underlying action potentials in myometrium. Clin Exp Pharmacol Physiol. 1988;15(9):657–665. doi: 10.1111/j.1440-1681.1988.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 361.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reproductive sciences. 2013;20(2):154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 362.Shynlova O, Tsui P, Dorogin A, Langille BL, Lye SJ. Insulin-like growth factors and their binding proteins define specific phases of myometrial differentiation during pregnancy in the rat. Biology of reproduction. 2007;76(4):571–578. doi: 10.1095/biolreprod.106.056929. [DOI] [PubMed] [Google Scholar]
- 363.Shynlova O, Mitchell JA, Tsampalieros A, Langille BL, Lye SJ. Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium. Biology of reproduction. 2004;70(4):986–992. doi: 10.1095/biolreprod.103.023648. [DOI] [PubMed] [Google Scholar]
- 364.Zhang X, Christenson LK, Nothnick WB. Regulation of MMP-9 expression and activity in the mouse uterus by estrogen. Mol Reprod Dev. 2007;74(3):321–331. doi: 10.1002/mrd.20582. [DOI] [PubMed] [Google Scholar]
- 365.Moreira L, de Carvalho EC, Caldas-Bussiere MC. Differential immunohistochemical expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in cow uteri with adenomyosis during follicular phase. Vet Res Commun. 2011;35(5):261–269. doi: 10.1007/s11259-011-9470-1. [DOI] [PubMed] [Google Scholar]
- 366.Kizaki K, Ushizawa K, Takahashi T, Yamada O, Todoroki J, Sato T, Ito A, Hashizume K. Gelatinase (MMP-2 and -9) expression profiles during gestation in the bovine endometrium. Reprod Biol Endocrinol. 2008;6:66. doi: 10.1186/1477-7827-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Shaco-Levy R, Sharabi S, Benharroch D, Piura B, Sion-Vardy N. Matrix metalloproteinases 2 and 9, E-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):226–232. doi: 10.1016/j.ejogrb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 368.Matsuzaki S, Maleysson E, Darcha C. Analysis of matrix metalloproteinase-7 expression in eutopic and ectopic endometrium samples from patients with different forms of endometriosis. Hum Reprod. 2010;25(3):742–750. doi: 10.1093/humrep/dep435. [DOI] [PubMed] [Google Scholar]
- 369.Merchant SJ, Davidge ST. The role of matrix metalloproteinases in vascular function: implications for normal pregnancy and pre-eclampsia. BJOG. 2004;111(9):931–939. doi: 10.1111/j.1471-0528.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 370.Naruse K, Lash GE, Innes BA, Otun HA, Searle RF, Robson SC, Bulmer JN. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum Reprod. 2009;24(3):553–561. doi: 10.1093/humrep/den408. [DOI] [PubMed] [Google Scholar]
- 371.van Engelen E, Breeveld-Dwarkasing VN, Taverne MA, Everts ME, van der Weijden GC, Rutten VP. MMP-2 expression precedes the final ripening process of the bovine cervix. Mol Reprod Dev. 2008;75(11):1669–1677. doi: 10.1002/mrd.20908. [DOI] [PubMed] [Google Scholar]
- 372.Lyons CA, Beharry KD, Nishihara KC, Akmal Y, Ren ZY, Chang E, Nageotte MP. Regulation of matrix metalloproteinases (type IV collagenases) and their inhibitors in the virgin, timed pregnant, and postpartum rat uterus and cervix by prostaglandin E(2)-cyclic adenosine monophosphate. Am J Obstet Gynecol. 2002;187(1):202–208. doi: 10.1067/mob.2002.123543. [DOI] [PubMed] [Google Scholar]
- 373.Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J Vasc Surg. 2008;48(2):447–456. doi: 10.1016/j.jvs.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ang VT, Jenkins JS. Neurohypophysial hormones in the adrenal medulla. J Clin Endocrinol Metab. 1984;58(4):688–691. doi: 10.1210/jcem-58-4-688. [DOI] [PubMed] [Google Scholar]
- 375.Wathes DC, Swann RW, Pickering BT, Porter DG, Hull MG, Drife JO. Neurohypophysial hormones in the human ovary. Lancet. 1982;2(8295):410–412. doi: 10.1016/s0140-6736(82)90441-x. [DOI] [PubMed] [Google Scholar]
- 376.Wathes DC, Swann RW. Is oxytocin an ovarian hormone? Nature. 1982;297(5863):225–227. doi: 10.1038/297225a0. [DOI] [PubMed] [Google Scholar]
- 377.Fields PA, Eldridge RK, Fuchs AR, Roberts RF, Fields MJ. Human placental and bovine corpora luteal oxytocin. Endocrinology. 1983;112(4):1544–1546. doi: 10.1210/endo-112-4-1544. [DOI] [PubMed] [Google Scholar]
- 378.Arthur P, Taggart MJ, Zielnik B, Wong S, Mitchell BF. Relationship between gene expression and function of uterotonic systems in the rat during gestation, uterine activation and both term and preterm labour. J Physiol. 2008;586(Pt 24):6063–6076. doi: 10.1113/jphysiol.2008.164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Weiss A, Goldman S, Shalev E. The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front Biosci. 2007;12:649–659. doi: 10.2741/2089. [DOI] [PubMed] [Google Scholar]
- 380.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21(5):514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 381.Levy C, Robel P, Gautray JP, De Brux J, Verma U, Descomps B, Baulieu EE. Estradiol and progesterone receptors in human endometrium: normal and abnormal menstrual cycles and early pregnancy. Am J Obstet Gynecol. 1980;136(5):646–651. doi: 10.1016/0002-9378(80)91018-2. [DOI] [PubMed] [Google Scholar]
- 382.Russo LA, Peano BJ, Trivedi SP, Cavalcanto TD, Olenchock BA, Caruso JA, Smolock AR, Vishnevsky O, Gardner RM. Regulated expression of matrix metalloproteinases, inflammatory mediators, and endometrial matrix remodeling by 17beta-estradiol in the immature rat uterus. Reprod Biol Endocrinol. 2009;7:124. doi: 10.1186/1477-7827-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Selvais C, Gaide Chevronnay HP, Lemoine P, Dedieu S, Henriet P, Courtoy PJ, Marbaix E, Emonard H. Metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 ectodomain decreases endocytic clearance of endometrial matrix metalloproteinase-2 and -9 at menstruation. Endocrinology. 2009;150(8):3792–3799. doi: 10.1210/en.2009-0015. [DOI] [PubMed] [Google Scholar]
- 384.Monckedieck V, Sannecke C, Husen B, Kumbartski M, Kimmig R, Totsch M, Winterhager E, Grummer R. Progestins inhibit expression of MMPs and of angiogenic factors in human ectopic endometrial lesions in a mouse model. Mol Hum Reprod. 2009;15(10):633–643. doi: 10.1093/molehr/gap063. [DOI] [PubMed] [Google Scholar]
- 385.Gaspar R, Foldesi I, Havass J, Marki A, Falkay G. Characterization of late-pregnant rat uterine contraction via the contractility ratio in vitro significance of alpha1-adrenoceptors. Life Sci. 2001;68(10):1119–1129. doi: 10.1016/s0024-3205(00)01014-6. [DOI] [PubMed] [Google Scholar]
- 386.Perusquia M, Navarrete E. Evidence that 17alpha-estradiol is biologically active in the uterine tissue: antiuterotonic and antiuterotrophic action. Reprod Biol Endocrinol. 2005;3:30. doi: 10.1186/1477-7827-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Fanchin R, Righini C, de Ziegler D, Olivennes F, Ledee N, Frydman R. Effects of vaginal progesterone administration on uterine contractility at the time of embryo transfer. Fertil Steril. 2001;75(6):1136–1140. doi: 10.1016/s0015-0282(01)01787-3. [DOI] [PubMed] [Google Scholar]
- 388.Mesiano S. Myometrial progesterone responsiveness. Semin Reprod Med. 2007;25(1):5–13. doi: 10.1055/s-2006-956771. [DOI] [PubMed] [Google Scholar]
- 389.Stock S, Bremme K, Uvnas-Moberg K. Plasma levels of oxytocin during the menstrual cycle, pregnancy and following treatment with HMG. Hum Reprod. 1991;6(8):1056–1062. doi: 10.1093/oxfordjournals.humrep.a137484. [DOI] [PubMed] [Google Scholar]
- 390.Mesiano S, Welsh TN. Steroid hormone control of myometrial contractility and parturition. Semin Cell Dev Biol. 2007;18(3):321–331. doi: 10.1016/j.semcdb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 391.Fanchin R, Righini C, Olivennes F, Taylor S, de Ziegler D, Frydman R. Uterine contractions at the time of embryo transfer alter pregnancy rates after in-vitro fertilization. Hum Reprod. 1998;13(7):1968–1974. doi: 10.1093/humrep/13.7.1968. [DOI] [PubMed] [Google Scholar]
- 392.Ayoubi JM, Fanchin R, Kaddouz D, Frydman R, de Ziegler D. Uterorelaxing effects of vaginal progesterone: comparison of two methodologies for assessing uterine contraction frequency on ultrasound scans. Fertil Steril. 2001;76(4):736–740. doi: 10.1016/s0015-0282(01)01998-7. [DOI] [PubMed] [Google Scholar]
- 393.He YY, Cai B, Yang YX, Liu XL, Wan XP. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci. 2009;100(6):1051–1061. doi: 10.1111/j.1349-7006.2009.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Sunday L, Osuna C, Krause DN, Duckles SP. Age alters cerebrovascular inflammation and effects of estrogen. Am J Physiol Heart Circ Physiol. 2007;292(5):H2333–2340. doi: 10.1152/ajpheart.01057.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Edwards KM, Mills PJ. Effects of estrogen versus estrogen and progesterone on cortisol and interleukin-6. Maturitas. 2008;61(4):330–333. doi: 10.1016/j.maturitas.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 397.Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest. 1994;93(1):17–25. doi: 10.1172/JCI116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, Oudit GY, Kassiri Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kgamma-dependent manner. Am J Physiol Cell Physiol. 2010;298(3):C679–692. doi: 10.1152/ajpcell.00351.2009. [DOI] [PubMed] [Google Scholar]
- 399.Lekontseva O, Jiang Y, Davidge ST. Estrogen replacement increases matrix metalloproteinase contribution to vasoconstriction in a rat model of menopause. J Hypertens. 2009;27(8):1602–1608. doi: 10.1097/HJH.0b013e32832c41b5. [DOI] [PubMed] [Google Scholar]
- 400.Mishra B, Kizaki K, Sato T, Ito A, Hashizume K. The role of extracellular matrix metalloproteinase inducer (EMMPRIN) in the regulation of bovine endometrial cell functions. Biol Reprod. 2012;87(6):149. doi: 10.1095/biolreprod.112.102152. [DOI] [PubMed] [Google Scholar]
- 401.Braundmeier AG, Fazleabas AT, Nowak RA. Extracellular matrix metalloproteinase inducer expression in the baboon endometrium: menstrual cycle and endometriosis. Reproduction. 2010;140(6):911–920. doi: 10.1530/REP-09-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Phillippe M, Basa A. Effects of sodium and calcium channel blockade on cytosolic calcium oscillations and phasic contractions of myometrial tissue. J Soc Gynecol Investig. 1997;4(2):72–77. [PubMed] [Google Scholar]
- 403.Wray S. Insights into the uterus. Exp Physiol. 2007;92(4):621–631. doi: 10.1113/expphysiol.2007.038125. [DOI] [PubMed] [Google Scholar]
- 404.Alotaibi M. The effect of cinnamon extract on isolated rat uterine strips. Reprod Biol. 2016;16(1):27–33. doi: 10.1016/j.repbio.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 405.Burghardt RC, Barhoumi R, Sanborn BM, Andersen J. Oxytocin-induced Ca2+ responses in human myometrial cells. Biol Reprod. 1999;60(4):777–782. doi: 10.1095/biolreprod60.4.777. [DOI] [PubMed] [Google Scholar]
- 406.Yue C, Ku CY, Liu M, Simon MI, Sanborn BM. Molecular mechanism of the inhibition of phospholipase C beta 3 by protein kinase C. J Biol Chem. 2000;275(39):30220–30225. doi: 10.1074/jbc.M004276200. [DOI] [PubMed] [Google Scholar]
- 407.Barhoumi R, Awooda I, Mouneimne Y, Safe S, Burghardt RC. Effects of benzo-a-pyrene on oxytocin-induced Ca2+ oscillations in myometrial cells. Toxicol Lett. 2006;165(2):133–141. doi: 10.1016/j.toxlet.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 408.Fu X, Liu YJ, Ciray N, Olovsson M, Ulmsten U, Gylfe E. Oxytocin-induced oscillations of cytoplasmic Ca2+ in human myometrial cells. Acta Obstet Gynecol Scand. 2000;79(3):174–179. [PubMed] [Google Scholar]
- 409.Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. The Journal of clinical endocrinology and metabolism. 2002;87(3):1353–1361. doi: 10.1210/jcem.87.3.8320. [DOI] [PubMed] [Google Scholar]
- 410.Yonemoto H, Young CB, Ross JT, Guilbert LL, Fairclough RJ, Olson DM. Changes in matrix metalloproteinase (MMP)-2 and MMP-9 in the fetal amnion and chorion during gestation and at term and preterm labor. Placenta. 2006;27(6–7):669–677. doi: 10.1016/j.placenta.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 411.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. American journal of obstetrics and gynecology. 2000;183(4):887–894. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 412.MacIntyre DA, Lee YS, Migale R, Herbert BR, Waddington SN, Peebles D, Hagberg H, Johnson MR, Bennett PR. Activator protein 1 is a key terminal mediator of inflammation-induced preterm labor in mice. FASEB J. 2014;28(5):2358–2368. doi: 10.1096/fj.13-247783. [DOI] [PubMed] [Google Scholar]
- 413.Maymon E, Romero R, Pacora P, Gervasi MT, Edwin SS, Gomez R, Seubert DE. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. American journal of obstetrics and gynecology. 2000;182(6):1545–1553. doi: 10.1067/mob.2000.107652. [DOI] [PubMed] [Google Scholar]
- 414.Stamatelou F, Deligeoroglou E, Farmakides G, Creatsas G. Abnormal progesterone and corticotropin releasing hormone levels are associated with preterm labour. Ann Acad Med Singapore. 2009;38(11):1011–1016. [PubMed] [Google Scholar]
- 415.Lucovnik M, Kuon RJ, Chambliss LR, Maner WL, Shi SQ, Shi L, Balducci J, Garfield RE. Progestin treatment for the prevention of preterm birth. Acta Obstet Gynecol Scand. 2011;90(10):1057–1069. doi: 10.1111/j.1600-0412.2011.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Herraiz I, Simon E, Gomez-Arriaga PI, Martinez-Moratalla JM, Garcia-Burguillo A, Jimenez EA, Galindo A. Angiogenesis-Related Biomarkers (sFlt-1/PLGF) in the Prediction and Diagnosis of Placental Dysfunction: An Approach for Clinical Integration. Int J Mol Sci. 2015;16(8):19009–19026. doi: 10.3390/ijms160819009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Liu Y, Zhao Y, Yu A, Zhao B, Gao Y, Niu H. Diagnostic accuracy of the soluble Fms-like tyrosine kinase-1/placental growth factor ratio for preeclampsia: a meta-analysis based on 20 studies. Archives of gynecology and obstetrics. 2015;292(3):507–518. doi: 10.1007/s00404-015-3671-8. [DOI] [PubMed] [Google Scholar]
- 418.Hamai Y, Fujii T, Yamashita T, Nishina H, Kozuma S, Mikami Y, Taketani Y. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. American journal of reproductive immunology. 1997;38(2):89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 419.Gomaa MF, Naguib AH, Swedan KH, Abdellatif SS. Serum tumor necrosis factor-alpha level and uterine artery Doppler indices at 11–13 weeks' gestation for preeclampsia screening in low-risk pregnancies: a prospective observational study. J Reprod Immunol. 2015;109:31–35. doi: 10.1016/j.jri.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 420.Serin IS, Ozcelik B, Basbug M, Kilic H, Okur D, Erez R. Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. European journal of obstetrics, gynecology, and reproductive biology. 2002;100(2):143–145. doi: 10.1016/s0301-2115(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 421.Akehurst C, Small HY, Sharafetdinova L, Forrest R, Beattie W, Brown CE, Robinson SW, McClure JD, Work LM, Carty DM, McBride MW, Freeman DJ, Delles C. Differential expression of microRNA-206 and its target genes in preeclampsia. Journal of hypertension. 2015;33(10):2068–2074. doi: 10.1097/HJH.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Luque A, Farwati A, Crovetto F, Crispi F, Figueras F, Gratacos E, Aran JM. Usefulness of circulating microRNAs for the prediction of early preeclampsia at first-trimester of pregnancy. Sci Rep. 2014;4:4882. doi: 10.1038/srep04882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy hypertension. 2014;4(2):105–145. doi: 10.1016/j.preghy.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 424.McDonald SD, Lutsiv O, Dzaja N, Duley L. A systematic review of maternal and infant outcomes following magnesium sulfate for pre-eclampsia/eclampsia in real-world use. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2012;118(2):90–96. doi: 10.1016/j.ijgo.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 425.von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, Lim KI, Liston RM, Miller SP, Rurak D, Sherlock RL, Skoll MA, Wareing MM, Baker PN. Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG. 2011;118(5):624–628. doi: 10.1111/j.1471-0528.2010.02879.x. [DOI] [PubMed] [Google Scholar]
- 426.Motta C, Grosso C, Zanuzzi C, Molinero D, Picco N, Bellingeri R, Alustiza F, Barbeito C, Vivas A, Romanini MC. Effect of Sildenafil on Pre-Eclampsia-Like Mouse Model Induced By L-Name. Reprod Domest Anim. 2015;50(4):611–616. doi: 10.1111/rda.12536. [DOI] [PubMed] [Google Scholar]
- 427.Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013;34(2):201–203. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 428.Zhao Y, Zheng YF, Luo QQ, Yan T, Liu XX, Han L, Zou L. Edaravone inhibits hypoxia-induced trophoblast-soluble Fms-like tyrosine kinase 1 expression: a possible therapeutic approach to preeclampsia. Placenta. 2014;35(7):476–482. doi: 10.1016/j.placenta.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 429.Yinon Y, Ben Meir E, Margolis L, Lipitz S, Schiff E, Mazaki-Tovi S, Simchen MJ. Low molecular weight heparin therapy during pregnancy is associated with elevated circulatory levels of placental growth factor. Placenta. 2015;36(2):121–124. doi: 10.1016/j.placenta.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 430.Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: effect of tumor necrosis factor blockade. Journal of hypertension. 2011;29(6):1203–1212. doi: 10.1097/HJH.0b013e3283468392. [DOI] [PubMed] [Google Scholar]
- 431.Spradley FT, Tan AY, Joo WS, Daniels G, Kussie P, Karumanchi SA, Granger JP. Placental Growth Factor Administration Abolishes Placental Ischemia-Induced Hypertension. Hypertension. 2016;67(4):740–747. doi: 10.1161/HYPERTENSIONAHA.115.06783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R713–719. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]