Abstract
Mammalian sperm require to spend a limited period of time in the female reproductive tract to become competent to fertilize in a process called capacitation. It is well established that HCO3− is essential for capacitation because it activates the atypical soluble adenylate cyclase ADCY10 leading to cAMP production, and promotes alkalinization of cytoplasm and membrane hyperpolarization. However, how HCO3− is transported into the sperm is not well understood. There is evidence that CFTR activity is involved in the human sperm capacitation but how this channel is integrated in the complex signaling cascades associated with this process remains largely unknown. In the present work we have analyzed the extent to which CFTR regulates different events in human sperm capacitation. We observed that inhibition of CFTR affects HCO3− -entrance dependent events resulting in lower PKA activity. CFTR inhibition also affected cAMP/PKA-downstream events such as the increase in tyrosine phosphorylation, hyperactivated motility and acrosome reaction. In addition, we demonstrated for the first time, that CFTR and PKA activity are essential for the regulation of intracellular pH and membrane potential in human sperm. Addition of permeable cAMP partially recovered all the PKA-dependent events altered in the presence of inh-172 which is consistent with a role of CFTR upstream of PKA activation.
Keywords: Sperm, cystic fibrosis transmembrane conductance regulator (CFTR), protein kinase A (PKA), capacitation, pH
Introduction
At ejaculation, mature sperm are not able to fertilize an oocyte. They require to spend a limited period of time in the female reproductive tract and undergo several maturational changes, all grouped under the name of capacitation (Austin 1951; Chang 1951). Capacitation prepares sperm to acquire the ability to develop hyperactivated motility and to undergo acrosomal exocytosis (Buffone, et al. 2014; Stival et al. 2016). From a molecular point of view, one of the first events that occur during capacitation is a HCO3−-dependent activation of the atypical soluble adenylyl cyclase ADCY10 (aka sAC, SACY) that leads to cAMP synthesis and the subsequent activation of PKA (Buffone, et al. 2014 and references therein). Also, HCO3− triggers alkalinization of the cytoplasm and membrane hyperpolarization (Demarco et al. 2003; Zeng et al. 1995). However, the mechanisms by which HCO3− is transported into the sperm are not well established. Evidence from our group showed the presence of a Na+-dependent electrogenic HCO3− permeability in mouse sperm which was attributed to one of the members of the sodium bicarbonate cotransporter (NBC) (Demarco et al. 2003). Another molecule that was proposed to play a role in this transport is the cystic fibrosis transmembrane conductance regulator channel (CFTR) (Chávez et al. 2012; Hernández-González et al. 2007; Xu et al. 2007). CFTR is an ATP-gated PKA-regulated anion channel that conducts Cl− and HCO3− with a modest permeability ratio (Tang et al. 2009). Experiments in mouse sperm suggest that this channel works in association with other Cl−/HCO3− cotransporters such as SLC26A3, SLC26A6 and SLC26A8 and might provide the driving force for the sustained uptake of HCO3− (Chávez et al. 2012; Rode et al. 2012).
Electrophysiological evidence indicates that functional CFTR channels are present in mouse sperm (Figueiras-Fierro et al. 2013). CFTR-mutated heterozygous mice have sperm with reduced fertilizing capacity in vitro and in vivo, supporting an important role of CFTR in determining sperm-fertilizing ability (Xu et al. 2007). Consistently, inhibition of CFTR impaired the ability of sperm to undergo increases in intracellular pH (Xu et al. 2007), and membrane hyperpolarization (Chávez et al. 2012). In addition, Hernández-González and coworkers (2007) presented evidence that CFTR is involved in mouse sperm hyperpolarization by regulating Na+ channel activity (ENaC). In mouse sperm CFTR co-immunoprecipitate with SLC26A3 and SLC26A6, and it was demonstrated that these transporters are involved in pHi increase (Chávez et al. 2012). Also, a functional interaction between CFTR and SLC26A3 was reported in guinea pig sperm (Chen et al. 2009). Considering that the role of CFTR as a Cl− channel is well established, its involvement in sperm function is consistent with the requirement of Cl− in mouse and guinea pig sperm capacitation events (Chen et al. 2009; Wertheimer et al. 2008). In addition, it was also demonstrated that CFTR co-immunoprecipitates with SLC26A8 in mouse testis and that SLC26A8 is involved in cAMP/PKA activation (Rode et al. 2012). Moreover, missense mutations of SLC26A8 results in proteasomal degradation of both CFTR and SLC26A8 (Dirami et al. 2013).
In humans, mutations in the CFTR gene that reduce or impair CFTR activity cause a fatal illness called cystic fibrosis (CF). CF is the most common autosomal disease in Caucasian population (1:25 mutation frequency) (Bobadilla et al. 2002). Around 97% CF male patients are infertile due to congenital bilateral absence of the vas deferens. In addition, the higher incidence of CFTR mutations in a male infertile subpopulation may indicate its participation in other fertilization related events like sperm capacitation (Schulz et al. 2006). Supporting this hypothesis, it was reported that human sperm treated with a specific CFTR inhibitor reduces the percentage of sperm undergoing progesterone-induced acrosome reaction, sperm hyperactivation and sperm penetration of zona-free hamster eggs (Li et al. 2010). However, how CFTR is integrated in the complex signaling cascades associated with human sperm capacitation remains largely unknown. Taking into account that the reason for infertility in about ~30% of infertile men is unexplained (Esteves et al. 2012) and given the high frequency of mutations of this gene, the study of the molecular mechanisms involving the activity of CFTR during capacitation may be important to understand certain causes of male infertility. In the present work we found that CFTR plays an essential role in mediating HCO3− entrance during capacitation, and therefore regulating PKA activation and downstream events such as increase in tyrosine phosphorylation, hyperactivated motility, increase in pHi, changes on membrane potential and acrosome reaction.
Materials & Methods
Reagents
Chemicals were obtained from the following sources: Pisum Sativum agglutinin (PSA), Bovine Serum Albumin (BSA), calcium ionophore A23187 and 3-isobutyl-1-methylxanthine (IBMX), 2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate (dbcAMP), anti-β-Tubulin T4026, were purchased from Sigma (St. Louis, MO); anti-phosphotyrosine (pY) clone 4G10 from Millipore Corporation (Temecula, USA); anti-pPKAs clone 100G7E and horseradish peroxidase-conjugated (HRP) anti-rabbit IgG from Cell Signaling, (Danvers, USA), HRP IgG from Vector Laboratories (Burlingame, CA). 2′,7′-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein, Acetoxymethyl Ester (BCECF-AM), H89, GlyH-101 and inh-172 from Cayman Chemicals, (Ann Arbor, MI). Propidium iodide (PI) from Santa Cruz (Santa Cruz, USA), 3,3-dipropylthiadicarbocyanine iodide (DiSC3(5)) from Invitrogen (Carlsbad, USA) and Eosin-Y from Biopack (Buenos Aires, Argentina).
PSA was dissolved in PBS; calcium ionophore A23187, BCECF-AM, DiSC3(5), H89, GlyH-101, inh-172 and IBMX were dissolved in DMSO; dbcAMP and PI were dissolved in hexa-distilled water.
Ethical approval
The study protocol was approved by the Bioethics Committee of the Instituto de Biología y Medicina Experimental (IByME) and by the Bioethics Committee of the Instituto de Biotecnología-Universidad Nacional Autónoma de México. Human donors were provided with written information about the study prior to giving informed consent. Experiments involving animals were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the NIH.
Culture media
The non-capacitating medium used in this study was HEPES-buffered human tubal fluid (HTF) containing KCl 4.7 mM, KH2PO2 0.3 mM, NaCl 90.7 mM, MgSO 41.2 mM, Glucose 2.8 mM, CaCl2 1.6 mM, Sodium Piruvate 3.4 mM, Sodium Lactate 60 mM, Hepes 23.8 mM from Sigma (St. Louis, MO). To study the role of Cl− in capacitation NaCl, KCl, CaCl2 were respectively replaced by Sodium Gluconate, Potassium Gluconate and Calcium Lactate (Sigma, St. Louis, MO) at the same concentrations and this non-capacitating medium without Cl− was used for cells washes at this condition. For capacitating media with or without Cl−, 25 mM NaHCO3 and 0.5% w/v BSA was added (Sigma MO). In all cases, pH was adjusted to 7.4 with NaOH.
Human sperm capacitation
Semen samples were obtained by masturbation from 15 healthy donors after 3–5 days of abstinence and analyzed following WHO recommendations (World Health Organization. 2010). All samples fulfilled semen parameters (total fluid volume, sperm concentration, motility, viability and morphology) according to WHO normality criteria. Samples were allowed to liquefy for 1 h at room temperature, then, sperm ejaculates were allowed to swim-up in non-capacitating media at 37°C for 1 hour. Motile selected spermatozoa were washed 5 min 400 g and pre-incubated in 250 μl of non-capacitating media containing inhibitor or vehicle for 10 min. After pre-incubation, an equal volume (250 μl) of two-fold concentrated capacitating media were added to cell suspensions to a final cell concentration of 2–8×106 cells/ml and incubated for different time periods at 37°C in an atmosphere of 5% v/v CO2. Sperm were capacitated for 1, 3 or 5 hours to evaluate PKA substrate phosphorylation or Tyr phosphorylation and hyperactivation or pHi and Em, respectively. Viability was evaluated by Eosin-Y staining (World Health Organization. 2010).
Extraction of sperm proteins and Immunoblotting
Sperm were washed by centrifugation (5 min, 400 g) in 0.5 ml of non-capacitating media, resuspended in sample buffer (62.5 mM Tris–HCl pH 6.8, 2% w/v SDS, 10% v/v glycerol) and boiled for 5 min. After centrifugation for 5 min at 13,000 g, 5% v/v ß-mercaptoethanol and 0.0005% w/v bromophenol blue was added to the supernatants then boiled again for 5 min. Protein extracts equivalent to 2–4×106 sperm per lane were separated by SDS-PAGE in gels containing 10% w/v polyacrilamide according to the method of Laemmli (Laemmli 1970) and transferred onto nitrocellulose membranes (Towbin et al. 1979). Blots were blocked in 5% w/v nonfat dry milk in PBS containing 0.1% v/v Tween 20 (T-PBS) for 30 min at room temperature and incubated for 1 hour at room temperature with the first and then 1 hour at room temperature with the secondary antibody. Antibodies were diluted in 2% w/v non-fat dry milk in T-PBS: 1:5,000 for anti-pY, 1:3,000 for anti-pPKA and 1:5,000 for anti-ß-Tubulin. The corresponding secondary antibodies were diluted in 2% w/v nonfat dry milk in T-PBS: 1:3,000 for both, anti-rabbit and anti-mouse. In all cases the reactive bands were visualized using a chemiluminescence detection solution consisting of 100 mM Tris-HCl buffer pH 8, 205 μM coumaric acid, 1.3 mM luminol, 0.01% v/v H2O2 Sigma (St. Louis, MO) and were exposed for 10 s to CL-XPosure film (Pierce). When necessary, membranes were stripped for 10 min in 2% w/v SDS, 0.67% v/v ß-mercaptoethanol and 62.5 mM of buffer Tris pH 6.8. In all experiments, molecular masses were expressed in kilodaltons. Image J 1.48k (National Institute of Health, USA) was used for analysis of the Western blot images following the specifications of ImageJ User Guide, IJ 1.46r. The optical density of all bands including 150 kDa to 50 kDa were quantified and relativized first, to ß-Tubulin band and then, to the CAP condition of each membrane.
Determination of intracellular pH and membrane potential by Flow cytometry
Sperm plasma membrane potential (Em) and intracellular pH (pHi) changes were assessed using DiSC3(5) and BCECF-AM respectively according to (López-González et al. 2014). Briefly, after 5 h incubation on capacitating media, samples were centrifuged at 400 g for 5 min, resuspended in 500 μl of non-capacitating media and the concentration was adjusted to 2×106 cells/ml. Then, cells were loaded with 0.5 μM BCECF-AM for 10 min, washed again and resuspended in 500 μl of non-capacitating media. Two aliquots from the same tube were divided: one for the pHi assay and the other for the Em assay. For pHi estimation, 50 nM of PI was added 30 sec before collecting data to monitor viability. For the Em assay the cell suspension was loaded with 50 nM DiSC3(5), during 3 min. Data were recorded as individual cellular events using a FACSCanto II TM cytometer (Becton Dickinson). Forward scatter (FSC) and side scatter (SSC) fluorescence data were collected from 20,000 events per sample. Positive cells for BCECF-AM were collected using the filter for Fluorescein isothiocyanate (FITC; 530/30), and for PI, the filter for Peridinin chlorophyll PerCP (670LP) (Suppl. Fig. 3A). As it is shown in Suppl. Fig. 3B, BCECF-AM is only incorporated in PI negative cells, but negative IP cells are present in BCECF-AM negative population. Thus, BCECF-AM can be used as a viability marker but it is important to remark that cell viability is underestimated with this probe. Because compensation between PI and DiSC3(5) was difficult to perform and taking into account that BCECF-AM only is incorporated in living cells, positive cells for BCECF-AM were used to monitor viability for DiSC3(5) (Suppl. Fig. 3G). Positive cells for DiSC3(5) were detected using the filter for Allophycocyanine (APC) (660/20). Data were analyzed using FACS Diva and FlowJo software (Tree Star 7.6.2).
Computer-assisted sperm analysis (CASA)
Aliquots of 5 μL of the sperm suspension were placed into a Makler chamber pre-warmed at 37°C. CASA analysis was performed using a Hamilton-Thorne digital image analyzer (HTR-IVOS v.10.8s; Hamilton-Thorne Research, Beverly, MA). The settings used for the analysis were as follows: frames acquired, 30; frame rate, 60 Hz; minimum contrast, 85; minimum cell size, 4 pixels; straightness threshold, 80%; low path velocity (VAP) cutoff, 5 μm/second−1; medium VAP cutoff, 25 μm/second−1; head size, non-motile, 12 pixels; head intensity, non-motile, 130 pixels; static head size, 0.68–2.57 pixels; static head intensity, 0.31–1.25 pixels; and static elongation, 23–100 pixels. The playback function of the HTR was used to accurately identify motile and immotile sperm cells. The criterion for detecting hyperactivated sperm was: VCL >150 um/s, ALH >7.0 um, LIN <50% (Mortimer 1998).
Acrosome reaction
Human sperm were exposed to 10 μM of calcium ionophore A23187 diluted in non-capacitating media 30 min before the end of incubation for assessment of acrosomal status (Köhn et al. 1997). Briefly, sperm were fixed in 2% w/v paraformaldehyde in PBS, stained with 100 μg/ml of FITC-labeled Pisum Sativum agglutinin (PSA, Sigma) dissolved in PBS, and observed under a Nikon Optiphot microscope equipped with epifluorescence optics (1,250×). Sperm were scored as acrosome intact when a bright staining was observed in the acrosome, or as acrosome reacted when either fluorescent staining was restricted to the equatorial segment or no labeling was observed.
Calculations and statistical analysis
The percentages of western blot quantifications viability and hyperactivation were analyzed by one-way analysis of variance (ANOVA). FSC-A/SSC-A ratio and BCECF-AM and DiSC3(5) relative medians of flow cytometry histograms were analyzed by t-test. The AR and motility parameters were analyzed by two-way analysis of variance (ANOVA). Calculations were performed with Libre Office 4.3.2.2 spreadsheet and statistical analysis with GraphPad Prism version 4.00 for Windows, GraphPad Software (San Diego, CA, USA). Independent experiments were carried out using different donors. A probability (p) value p<0.05 was considered statistically significant.
Results
Cl− is necessary for activation of capacitation-associated cAMP/PKA pathway
When sperm are exposed to HCO3− after ejaculation, there is a fast increase in cAMP synthesis, which results in PKA activation (Okamura et al. 1985). Here, the activation of the cAMP/PKA pathway was evaluated by the increase in phosphorylation of PKA-substrates (pPKAs) and the phosphorylation of tyrosine residues (pY) which is up-regulated downstream PKA activation (Visconti, et al. 1995)
Previous studies showed that Cl− is essential for pY in guinea pig and mouse sperm (Chen et al. 2009; Wertheimer et al. 2008). To investigate whether Cl− is necessary for activation of capacitation-associated cAMP/PKA pathway in human sperm, we evaluated the effect of different Cl− concentrations in the phosphorylation of PKA substrates and tyrosine residues (pY). To maintain the total anion concentration in the capacitation media, Cl− was replaced by equivalent concentrations of gluconate as described in “Materials & Methods”. Overall, we observed that both pY and pPKA strongly depend on Cl− content in the incubation medium (Fig. 1A). To assess whether the requirement for Cl− is upstream the increase of cAMP, sperm were incubated in media with or without Cl− and in the presence or absence of dbcAMP/IBMX. Addition of dbcAMP/IBMX restored the levels of pPKA and pY in sperm incubated in Cl−-free media (Fig. 1B). The quantification and statistical analysis is shown in Supplementary Figure 2.
Figure 1. cAMP is downstream the effect of Cl− on capacitation-associated increase activation of cAMP/PKA pathway.
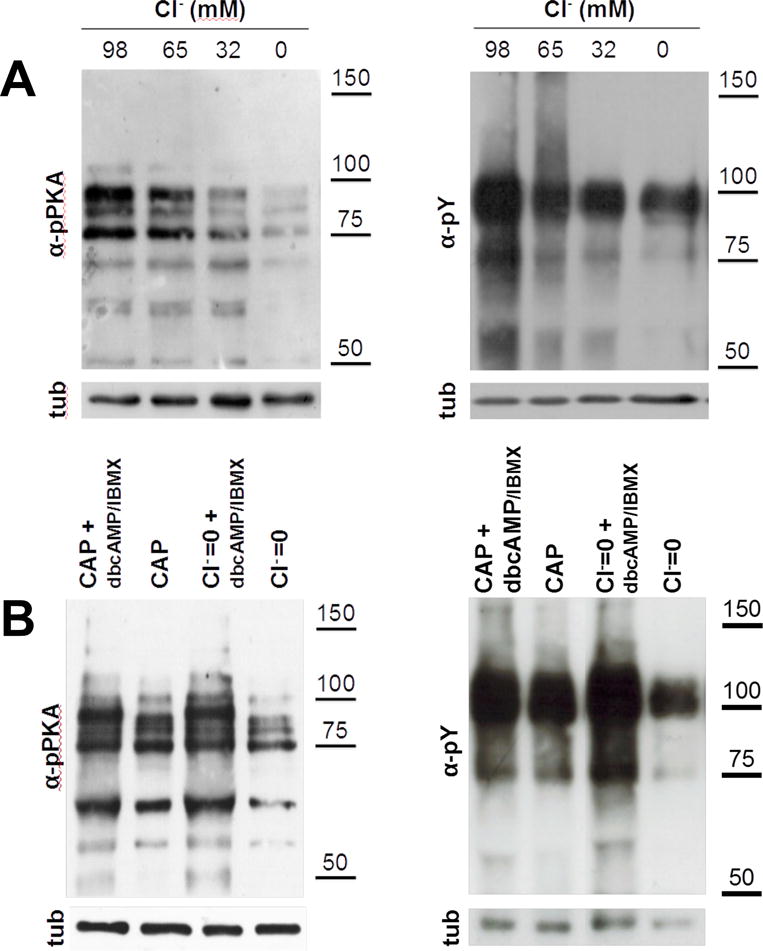
(A) As Cl− is one of the major anions transported by CFTR, sperm incubated in capacitating media for 1 (for pPKA) or 3 hours (for pY) with different Cl− concentrations (replaced by gluconate) to evaluate the effect of Cl− on the activation of cAMP/PKA pathway. Complete medium contains 98 mM of Cl−. Aliquots from each condition were processed for Western blotting with anti-pPKA antibodies (upper panel) and then membranes were reblotted with an anti-β-tubulin antibody for loading control (lower panel). The increase in the pY and pPKA is dependent on Cl− concentration and cAMP is downstream of the effect of Cl−. (B) Human sperm were incubated for 1 (for pPKA) or 3 hours (for pY) in media in the presence (CAP) or absence of Cl− (Cl−=0), IBMX (0.2 mM), and dbcAMP (1 mM). cAMP agonists rescued the inhibition of pY by the absence of Cl−. This experiment was repeated at least 4 times with similar results. For statistical analysis see Suppl. Fig. 2
Inhibition of CFTR reduced activation of the capacitation-associated cAMP/PKA pathway
The observation that Cl− was required for the increase in PKA-dependent tyrosine phosphorylation suggests that its transport in sperm plays an important role in capacitation. Previous observations reported electrophysiological evidence that functional CFTR channels are present in mouse sperm (Figueiras-Fierro et al. 2013). Because Cl− is the main ion transported by CFTR and this channel was proposed to play a role in HCO3− in many cells, we evaluated the effect of two different CFTR inhibitors (inh-172 and GlyH-101) on the activation of HCO3− dependent cAMP/PKA pathway. As shown in Figure 2A and Supplementary Figure 1A, there was a concentration-dependent decrease on the levels of pY in the presence of CFTR inhibitors. The concentration of 5 μM for inh-172 was chosen for all the experiments because not only the levels of pY were strongly decreased but also, because viability was similar to the control condition (Fig. 5A). Similarly, GlyH-101 also displayed a concentration-dependent inhibition of pY. In this case, the concentration of 10 μM was chosen because a clear inhibitory effect on pY was evident and the percentage of viable cells was similar to the control condition (Suppl. Fig. 1A). Inh-172 is widely considered the best inhibitor of CFTR channel. At low concentrations (<10 μM), inh-172 represents the best experimental condition to provide fully inhibition of the CFTR conductance, with minimum effects on other Cl− conductances, ATP-sensitive K+ channels, or other currents like CaCC (Ca2+-dependent Cl− conductance) and VSORC (volume-sensitive outwardly rectifying Cl− conductance) (Ma et al. 2002; Melis et al. 2014). Although GlyH101 is not as specific as inh-172, at concentrations used in our experiments (10 μM) the off-target effects were reported to be highly reduced (Muanprasat et al. 2004).
Figure 2. cAMP is downstream the effect of inh-172 on capacitation-associated activation of cAMP/PKA pathway.
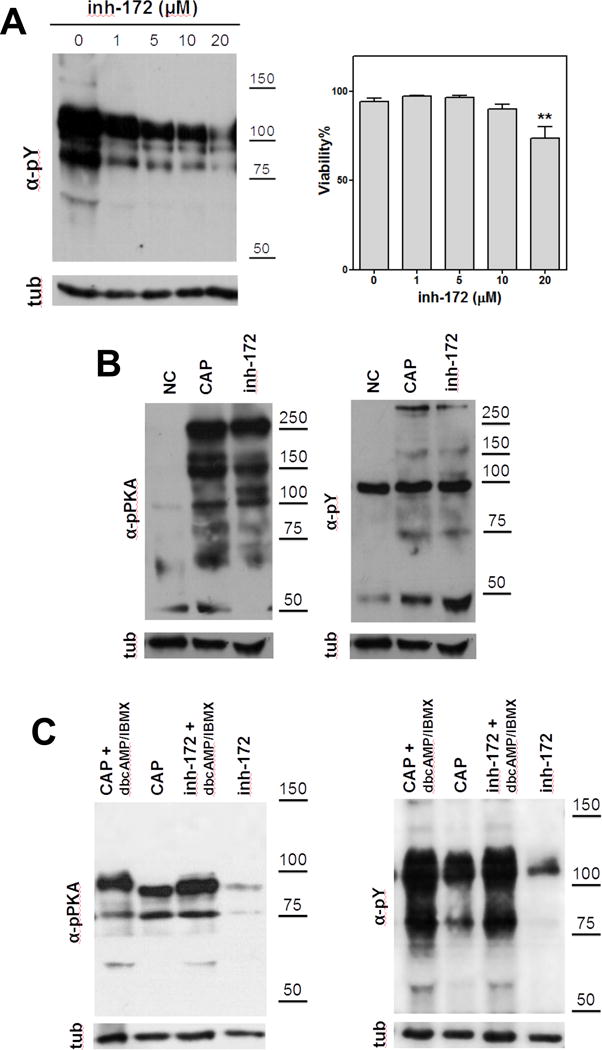
(A) Human sperm were incubated for 3 hours under capacitating conditions in the presence of different concentrations of inh-172. A concentration-dependent decrease on the levels of tyrosine phosphorylation (pY) was observed in the presence of inh-172. On the right, the percentage of viable cells using different concentrations of inh-172 was evaluated (*denotes p<0.05 vs 0) (B) Mouse sperm were incubated for 90 min in media that do not support (−BSA, − HCO3−; NC) or support capacitation (+BSA, + HCO3−) in the presence (inh-172) or absence (CAP) of inh-172 (10 μM). Subsequently, aliquots from each condition were processed for Western blotting with anti-pY (upper right panel) and anti-pPKA (upper left panel) antibodies and then membranes were reblotted with an anti-β-tubulin antibody for loading control (lower panel). Inh-172 did not inhibit capacitation-associated increase in tyrosine PKA substrate phosphorylation in mouse sperm. (C) On the contrary, in human sperm inh-172 (5 μM) blocked capacitation-associated activation of cAMP/PKA pathway. Human sperm were incubated for 1 (for pPKA) or 3 hours (for pY) in media that support capacitation in the presence (inh-172) or absence (CAP) of inh-172 (5 μM). cAMP agonists rescue the inhibition of capacitation-associated activation of cAMP/PKA pathway by inh-172 (5 μM). These experiments were repeated at least 3 times with similar results. For statistical analysis see Suppl. Fig. 2.
Figure 5. PKA inhibition by H-89 caused pHi acidification and Em depolarization.
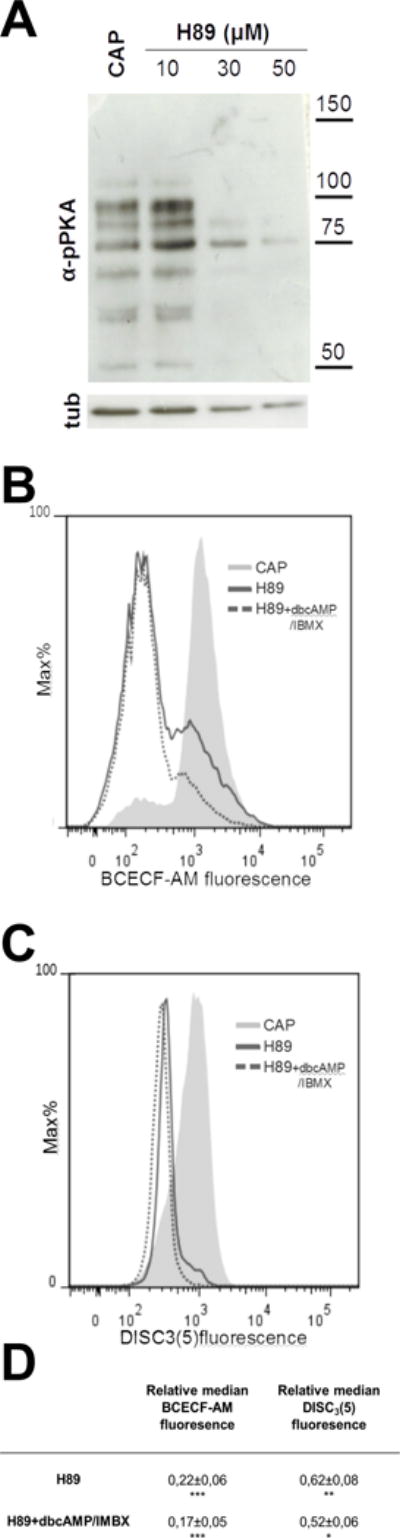
Human sperm were incubated for 1 hour in media that supports capacitation in the absence (CAP) or presence of different concentrations of H89 (10, 30 and 50 μM). Aliquots from each condition were processed for Western blotting with anti-pPKA antibodies and then membranes were reblotted with an anti-β-tubulin antibody for loading control. (A) PKA activity was inhibited at 30 μM of H89. At this concentration, intracellular acidification (B) and depolarization (C) was observed when compared to the control condition (CAP). cAMP agonists (dbcAMP, 1 mM + IBMX 0.2 mM, dotted line) did not rescue the acidification nor the depolarization produced by H89 (30 μM) as expected. Blot and histograms are representative of at least 4 experiments. (D) Relative median compared to the control condition (CAP) of BCECF and DiSC3(5) fluorescence of samples incubated with H89 in the presence or absence of 1 mM dbcAMP and 0.2 mM IBMX.
As previously reported (Wertheimer et al. 2008), we observed that CFTR inhibitor inh-172 (5 μM) has no effect on the capacitation-induced PKA activation or in the increase in pY in mouse sperm (Fig. 2B). In contrast, human sperm incubated in the presence of inh-172 displayed lower levels of both pPKAs and pY, suggesting that CFTR could play different physiological functions in these two species (Fig. 2C). Similar results were obtained with GlyH-101 (10 μM) (Suppl. Fig. 1A). Addition of permeable cAMP agonists such as IBMX and dbcAMP induced phosphorylation of PKA substrates and pY even in the presence of inh-172 (Fig. 1C). These results are consistent with a role of CFTR upstream of PKA activation. The quantification of these results and the corresponding statistical analysis are shown in the supplementary information (Suppl. Fig. 2).
CFTR inhibition reduced sperm hyperactivation but not the percentage of total motile cells
HCO3− and the consequent activation of cAMP-dependent pathways are critical for sperm motility and hyperactivation (Neill & Olds-Clarke 1987; Okamura et al. 1985). To investigate the effect of CFTR inhibition on sperm motility, we evaluated several sperm motility parameters using CASA. Although inh-172 did not significantly affect the percentage of motile sperm (Fig. 3A), the curvilinear velocity (VCL) was significantly lower compared to the control. This is consistent with a significantly reduced percentage of hyperactivated cells (Fig. 3B). The addition of dbcAMP/IBMX restored the percentage of hyperactivated sperm incubated with inh-172. These results suggest that CFTR activity regulate hyperactivation and that its role is mediated by cAMP-dependent pathways.
Figure 3. Sperm hyperactivation but not total motility was affected by inh-172.
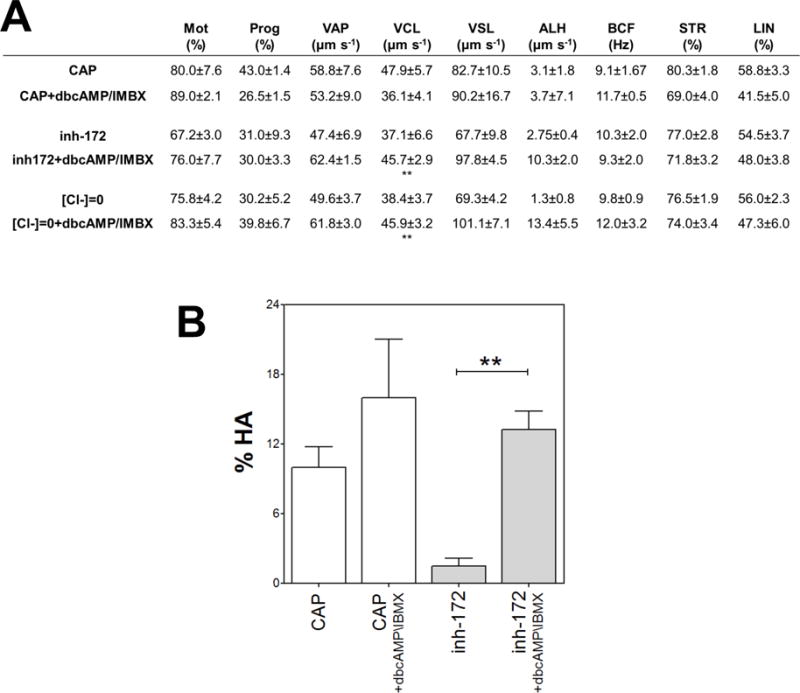
Human sperm were incubated for 5 hours in media that support capacitation in the presence or absence of inh-172 (5 μM), IBMX (0.2 mM) and dbcAMP (1 mM) and the motility parameters were analyzed by CASA. (A) Sperm motion parameter assessed by CASA. Mot: total motility; Prog: progressive motility; VAP: average path velocity; VCL: curvilinear velocity; VSL: straight-line velocity; ALH: amplitude of lateral head displacement; BCF: Beat frequency; STR: straightness; LIN: linearity. Values represent the mean ± SEM of 4 experiments ** vs inh-172 + dbcAMP/IMBX condition p<0.01. (B) Percentage of hyperactivated motility (%HA). Bars represent mean value ± SEM from at least 4 experiments. ** p<0.01.
CFTR inhibition blocked the capacitation-associated increase in pHi
Capacitation is associated with elevation of pHi (Nishigaki et al. 2014; Zeng et al. 1996) and in mouse sperm, this event was shown to depend on CFTR activity (Xu et al. 2007). For this reason, we evaluated whether CFTR inhibition altered the capacitation-associated intracellular pH increase. As described in “Materials & Methods”, sperm were incubated for 5 hours in capacitating medium and loaded with the pH sensor BCECF-AM and PI to monitor viability. BCECF-AM is a cell permeant probe with pKa of ~6.98 that is hydrolyzed by cytosolic esterases and accumulates only in live cells. Due to the pH-dependent fluorescence of the probe, a more alkalized sperm population would present a higher fluorescence. Flow cytometry analysis was performed as shown in Suppl. Fig. 3 A–D. We found that the presence of different concentrations of inh-172 (Fig. 4A, left panel) resulted in acidification of cytoplasm with respect to the control condition. This effect was not due to fluorescence quenching as shown in Suppl. Fig. 3K. Addition of dbcAMP/IBMX partially reverted the acidification caused by inh-172 (dotted line in Fig. 4B). These results were also observed in the presence of the other CFTR inhibitor GlyH-101 (Suppl. Fig. 1B).
Figure 4. cAMP agonists partially rescued the inhibitory effects on pHi and Em caused by inh-172.
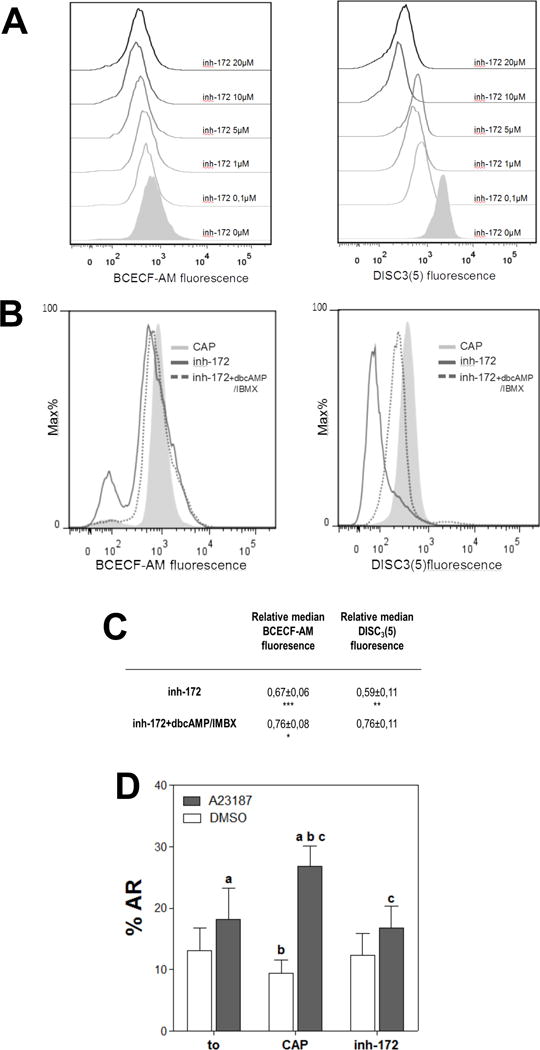
(A) The presence of inh-172 during sperm incubation in capacitating media acidified pHi and depolarized Em with respect to control conditions. Human sperm were incubated for 5 hours in media that support capacitation in the absence (CAP) or presence of different concentrations of CFTR inhibitor (inh-172; 0.1–20 μM). Subsequently, aliquots from each condition were processed by flow cytometry to evaluate pHi with BCECF-AM probe and Em with DiSC3(5). (B) Addition of dbcAMP 1mM and IBMX 0.2 mM (dotted line) partially rescued the acidification and depolarization produced by inh-172 (5 μM; solid line). Histograms of percentage of the maximum (% Max) versus BCECF and DiSC3(5) fluorescence of non-PI stained sperm are shown. (C) Relative median compared to the control condition (CAP) of BCECF and DiSC3(5) fluorescence of samples incubated with inh-172 in the presence or absence of 1 mM dbcAMP and 0.2 mM IBMX. Values represent the mean ± SEM of at least 4 experiments. *** p<0.001, ** p<0.01 * p<0.05 represents statistical significance vs CAP condition (D) Acrosome reaction (%AR) induced by 10 μM of ionophore A23187 or DMSO was evaluated before (t0) and after incubation for 6 hours in capacitating media in the absence (CAP) or presence of inh-172 5 μM (inh-172). For columns with the same letter, the difference between means is statistically significant (Student t-test at a, b and c p<0.001).
CFTR inhibition produced a membrane depolarization and inhibited acrosome reaction
Sperm from several mammalian species, including human, exhibit a capacitation-associated plasma membrane hyperpolarization (López-González et al. 2014; Zeng et al. 1995). Previously, it was described that inhibition of CFTR blocks hyperpolarization in mouse sperm (Hernández-González et al. 2007; Xu et al. 2007). To evaluate if inhibition of CFTR impact the human sperm Em, sperm were incubated for 5 hours in media that support capacitation in the absence (CAP) or presence of inh-172. As shown in “Materials & Methods”, cells were loaded with DiSC3(5) to estimate membrane potential by flow cytometry and analysis was performed as described in Suppl. Fig. 3E–H. Because DiSC3(5) is positively charged, a more hyperpolarized sperm population would present a higher fluorescence due to cationic dye influx into the cell. We observed that a population of cells incubated with different concentrations of inh-172 during capacitation were more depolarized than the capacitated control condition (Fig. 4A). This effect was not due to fluorescence quenching because addition of inh-172 did not result in a decrease in the fluorescence intensity (Suppl. Fig. 3L). These results were also observed in the presence of the other CFTR inhibitor GlyH-101 (Suppl. Fig. 1B).
Because it was described in mouse sperm that membrane hyperpolarization occurs downstream the cAMP-dependent pathway (Escoffier et al. 2015), human sperm were co-incubated with inh-172 and cAMP agonists. As shown in Figure 4B, dbcAMP/IBMX partially rescued the decrease in Em produced by inh-172.
We have previously presented evidence that in mouse sperm, the capacitation-induced hyperpolarization is necessary to prepare the sperm for the acrosome reaction (De La Vega-Beltran et al. 2012). Here, we evaluated exocytosis in sperm incubated in the presence of inh-172. The acrosome reaction induced by 10 μM ionophore A23187 was strongly inhibited in the presence of CFTR inhibitors inh-172 (Fig. 4D) or GlyH-101 (Suppl. Fig. 1D).
PKA inhibition caused intracellular acidification and membrane depolarization
CFTR activity depends on its phosphorylation by PKA (Berger et al. 1993; Gadsby et al. 2006; Sheppard & Welsh 1999; Sorum et al. 2015; Tabcharani et al. 1991). Thus, we decided to evaluate if PKA inhibitor H89 alters pH and Em in human sperm. For this purpose, cells were incubated with 30 μM H89 because at this concentration, phosphorylation of PKA substrates were abolished (Fig. 5A). Inhibition of PKA activity resulted in pHi acidification, and as expected, the cAMP and IBMX addition did not rescued this condition due to PKA blockage (Fig. 5B). On the other hand, addition of H89 caused a depolarization when compared to control condition (Fig. 5C). These results are in accordance with previous studies in mouse showing that capacitation-associated hyperpolarization is blocked by PKA inhibitors (Escoffier et al. 2015). Similarly, cAMP addition did not affect depolarization caused by H89 (Fig. 5C). The effect on BCECF and DiSC3(5) fluorescence was not due to quenching by H89 as shown in Suppl. Fig. 3 K–L.
Despite that H89 is frequently used as a PKA inhibitor in mammalian sperm (Visconti, et al. 1995; Wennemuth et al. 2003; Leclerc et al. 1996; Nolan et al. 2004), it has also been demonstrated that it may inhibit other kinases (Lochner & Moolman 2006). For this reason, we have also tested other PKA inhibitors such as Rp-cAMPS and KT5720 (Suppl. Fig 5). Both inhibitors displayed similar results in Em and pHi. The inhibitors KT5720 and H89 have similar mechanism of action, they inhibit PKA by competing with ATP (Lochner & Moolman 2006; Kase et al. 1987). This inhibition cannot be bypassed by cAMP analogs. In the case of Rp-cAMPS, it binds with high affinity to the cAMP binding site of PKA and as a result, direct competition with cAMP analogs do not allow to use this strategy to bypass the PKA inhibitory effect (Dostmann, 1995; Lochner & Moolman 2006).
Discussion
In this work, we investigated the hypothesis that CFTR influences the HCO3− entrance-dependent events in human sperm capacitation. Supporting this hypothesis, we show that inhibition of CFTR blocks PKA-dependent events. In contrast to what was observed in mouse sperm, we found that human sperm incubated in the presence of inh-172 displayed low levels of pPKA and pY. This difference between species could be expected because Cftr was subject to different selective pressures due to an advantage in humans of heterozygous mutants compared to non-mutated individuals (Gabriel et al. 1994). Since CFTR is differentially expressed in mouse and human tissues (Ellsworth et al. 2000), the function of this channel may also differ among these species. In fact, previous reports showed that human sperm incubated in the presence of inh-172 display low cAMP concentration (Diao et al. 2013; Li et al. 2010). Here, we report that direct stimulation of PKA by-passed the inhibitory effect of inh-172 on pPKA and pY (Hernández-González et al. 2007). From a mechanistic point of view, CFTR phosphorylation by PKA is required for its activation (Berger et al. 1993; Gadsby et al. 2006; Sorum et al. 2015; Tabcharani et al. 1991). Thus, an initial increase in PKA activity is necessary to activate the channel and allow a sustained increase in HCO3− and cAMP. In human sperm a fast and transient HCO3− influx may occur when sperm encounter high extracellular bicarbonate concentration in the seminal plasma at the time of ejaculation (Luconi et al. 2005; Okamura et al. 1985). In mouse, this fast increase is mainly mediated by the NBC but in humans, this influx is still unresolved. This transient raise in HCO3− concentration may stimulate ADCY10, resulting in cAMP production and an increase in the basal PKA activity. In addition, Ca2+ transported through CatSper channels is also necessary for the complete activation of ADCY10 (Navarrete et al. 2015). Then CFTR phosphorylation by PKA stimulates its activity resulting in a sustained increase in intracellular HCO3−. Based on our observations, this HCO3− uptake could be occurring indirectly through other Cl−/HCO3− cotransporters and not directly through CFTR. Supporting this hypothesis, in other systems under physiological conditions, HCO3− exits the cell through CFTR resulting in cytoplasmic acidification. Alkalinization due to CFTR function can only occurs after membrane depolarization and dbcAMP addition (Ishiguro et al. 2009). Thus, HCO3− entrance through CFTR would not be favored since sperm Em hyperpolarizes during capacitation. In addition, given that HCO3− transport through CFTR should be facilitated in the absence of Cl−, we tested the last hypothesis by replacing Cl− by gluconate in capacitating medium. We observed that pPKA and pY were dependent on the Cl− concentration, suggesting that HCO3− transported directly by CFTR would not be the main mediator of PKA activation. As expected, direct activation of PKA in Cl−-free medium restored pPKA and pY. Direct activation of ADCY10 by Cl− would not occur since earlier studies demonstrated that other anions, such as chloride, sulfate or phosphate do not stimulate its activity (Chen et al. 2000).
We and others (Li et al. 2010) observed that inhibition of CFTR in human sperm affects the percentage of sperm with hyperactivated motility with no significant difference in total motility. However, the role of CFTR in total motility cannot be excluded since its lower expression in the duct could also affect HCO3− secretion in the lumen and thus the motility of sperm (Chen et al. 2012). This result suggests that the function of this channel is critical in capacitation but not important for the maintenance of motility after leaving the epididymis. Direct activation of PKA could by-pass the inhibitory effect on hyperactivation revealing that the role of CFTR in this special type of motility is mediated by cAMP. Previous results in mouse sperm demonstrated that CFTR participates in the regulation of Em and pHi (Chávez et al. 2012; Xu et al. 2007). Given the heterogeneity of the human sperm population (Buffone et al. 2004; Buffone et al. 2009), flow cytometry was used to estimate pHi and Em changes because it is possible to discriminate between live and dead sperm and to discriminate subpopulations contributing to the average value (Escoffier et al. 2015; López-González et al. 2014). Sperm incubated in media treated with inh-172 showed a significant difference in the FSC-A parameter related with cell size (Suppl. Fig. 4), which is in accordance with previous observations in mouse sperm (Figueiras-Fierro et al. 2013). These results suggest that the participation of CFTR in volume regulation is related to chloride transport, as it was described for other cells (l’Hoste et al. 2010).
Regarding pHi, cytoplasmic alkalinization occurs during mammalian sperm capacitation. The main mechanisms responsible for this change could be divided into: 1) H+ efflux and 2) HCO3− entrance (Nishigaki et al. 2014). Regarding the first group, recently electrophysiological evidence of Hv1 activity in capacitated human sperm was reported (Lishko et al. 2010). The second group includes NBC, carbonic anhydrases (José et al. 2015) and CFTR among others. The latter modulates HCO3− entrance either directly or through SLC26 cotransporters couple to electroneutral Cl−/HCO3− exchangers (Chávez et al. 2012). In mouse sperm, it was demonstrated that CFTR inhibition during capacitation causes cytoplasm acidification when compared to control conditions. Here we show that CFTR inhibition causes cytoplasm acidification in human sperm suggesting Cl−/HCO3− exchangers could be mediating the entry of HCO3−. Our group, as well as others, has shown that there is a physical interaction between CFTR and the SLC26A3 and SLC26A6 exchangers in mouse (Chávez et al. 2012) and guinea pig sperm (Chen et al. 2009). The functional association between CFTR and the SLC26A3 modulates pHi (Chávez et al. 2012) and leads to sperm capacitation in guinea pig sperm as judged by the acrosome reaction (Chen et al. 2009). Interestingly, in humans, mutations in SLC26A3 also cause subfertility and oligoasthenoteratozoospermia. SLC26A6 is expressed in human efferent and epididymal ducts and colocalizes with CFTR (Kujala et al. 2007). SLC26A8 (TAT1) is specifically expressed in the male germ line. It was demonstrated that it physically interacts with CFTR in vitro and in vivo in mature sperm, it is a strong activator of CFTR and interestingly, is essential for cAMP/PKA pathway activation in mouse sperm (Rode et al. 2012). Additionally, missense mutations in SLC26A8 in human sperm are associated with asthenozoospermia (Dirami et al. 2013).
Considering that it is widely demonstrated in other systems that phosphorylation of CFTR by PKA is required for the activity of the channel (Berger et al. 1993; Gadsby et al. 2006; Sorum et al. 2015; Tabcharani et al. 1991; Sheppard & Welsh 1999), we evaluated whether the sustained activation of PKA could partially revert the acidification of the cytoplasm caused by the presence of inh-172. We found that addition of dbAMPc induced a partial reversal of the acidification caused by the inhibitor inh-172. These results suggest that the pHi changes observed may be regulated by other mechanisms besides the action of cAMP. In that sense, it was recently demonstrated by patch clamping human sperm that proton channel Hv1 is the dominant proton conductance (Lishko et al. 2010). A close connection between capacitation and Hv1 activity was provided by observing the increasing amplitude of Hv1 current in capacitated cells when compared to non-capacitated sperm. Considering that capacitation is affected by CFTR inhibition and to the best of our knowledge, the activity of Hv1 is not cAMP-dependent, we cannot exclude this effect could be related to the absence of Hv1 activity.
As mentioned above, another capacitation-related event is Em hyperpolarization (Escoffier et al. 2015). In mouse, this event is necessary and sufficient for the acrosome reaction to occur (De La Vega-Beltran et al. 2012). The molecular mechanisms involved in human sperm hyperpolarization are still not well established. Among the most likely events involved are: 1) the opening of K+ channels such as Slo1 and Slo3 (López-González et al. 2014) and 2) the closing of Na+ channels such as ENaCs, which are modulated through CFTR (Hernández-González et al. 2007). Regarding the first possibility, it was recently reported that although mouse sperm Em resting potential before capacitation is determined by K+, Cl− and Na+ permeabilities, the hyperpolarization observed during this process is mainly due to the activation of Slo3 channels (Chávez et al. 2013). In humans, as the hyperpolarization associated to capacitation is affected by high K+ concentrations, we proposed the participation of K+ channels in this process. The lack of specific inhibitors complicates the determination of the precise information about the contribution of Slo3 and/or Slo1 (López-González et al. 2014). Regarding the second possibility, in this work, we observed that sperm incubated with CFTR inhibitor during capacitation exhibited membrane depolarization when compared to control conditions and this effect could be partially recovered by direct stimulation of PKA. This result could be explained by two observations reported in mouse sperm: a) inhibition of CFTR could lead to ENaC activation and hence, membrane depolarization (Hernández-González et al. 2007) and b) CFTR inhibition would prevent PKA activation and at the same time, PKA activity would be upstream of the changes in Em observed during capacitation (Escoffier et al. 2015). Taking into account that PKA also affects CFTR activity, the connecting-loop between PKA and CFTR could explain the two observations made in mouse sperm: CFTR inhibition would affect ENaC and PKA, and PKA inhibition would modulate CFTR and ENaC activity. We cannot rule out that AMPc could regulate Em through additional targets besides CFTR. For example, in mouse sperm, cSrc is stimulated downstream PKA activation and its inhibition blocked the capacitation-induced hyperpolarization (Stival et al. 2015).
To explain many of the molecular mechanisms by which CFTR is involved in the capacitation process requires including PKA as a central player (Fig. 6). Thereby, if CFTR activity is required for activation of PKA and PKA for CFTR activity: does PKA inhibition cause the same effects on the capacitation-associated events as CFTR? Previous studies demonstrated that PKA activity is essential for capacitation-associated events in human sperm such as hyperactivation (Luconi et al0. 2005), pY (Leclerc et al. 1996) and AR (De Jonge et al. 1991). However, it is not yet known whether PKA modulates human sperm Em and/or pHi. To test this hypothesis, we estimated Em and pHi in the presence of H89. Indeed, PKA inhibition affected pHi and Em and addition of cAMP analogs did not revert this condition.
Figure 6. Proposed model for CFTR participation in the capacitation signaling pathways described in this work.
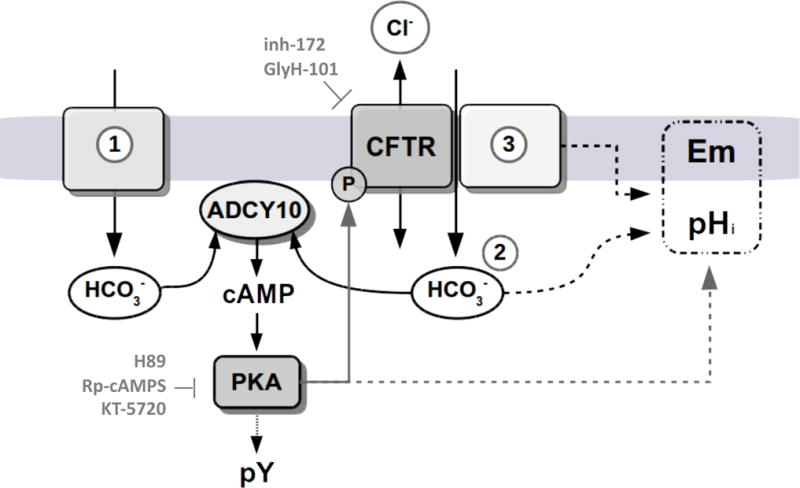
CFTR is essential for the occurrence of bicarbonate-dependent capacitation events. In human sperm a fast and transient HCO3− influx occurs when sperm encounter high extracellular bicarbonate concentration in the seminal plasma at ejaculation. This transient raise in HCO3− concentration may stimulate the ADCY10, resulting in cAMP production and in an increase in the basal PKA activity to phosphorylate and activate CFTR (1). The sustained HCO3− (2) responsible of PKA activation or pHi changes could be transported either directly by CFTR or indirectly, by providing a recycling pathway for Cl−/HCO3− cotransporters associated with this channel (3). CFTR modulation of Em could be explained by its influence on HCO3− transport, or by its association with other transporters like ENaC (3). Because pHi, Em and CFTR activity depend on PKA, we propose a connecting-loop between PKA and CFTR activity in human sperm.
In conclusion, we demonstrated that CFTR plays an essential role in human sperm capacitation because its functional state is required for HCO3− entrance-dependent events indirectly as a chloride channel, and therefore PKA activation and its related events such as: Em, hyperactivated motility, increase in pHi, tyrosine kinase activity and acrosome reaction. Further investigation is needed to answer questions about the involvement of Cl−/HCO3− transporter(s) in HCO− entrance in human sperm and the mechanistic relations between PKA activation and pHi and Em changes.
Supplementary Material
Supplementary figure 1. CFTR inhibitor GlyH-101 affects pY, pPKA, Em and pHi changes associated to capacitation and the acrosome reaction induced by ionophore A23187. Human sperm were incubated in media that support capacitation in the absence (CAP) or presence of GlyH-101. (A) On the left, a concentration-dependent decrease on the levels of tyrosine phosphorylation (pY) was observed in the presence of GlyH-101. In the middle, the percentage of viable cells using different concentrations of GlyH101 was evaluated (*denotes p<0.05 vs 0). On the right, a western blot with anti-pY and anti-PKA antibodies in sperm incubated with 10 μM of GlyH-101. Membranes were reblotted with an anti-β-tubulin antibody for loading control. (B) Histograms of percentage of the maximum (% Max) versus BCECF-AM fluorescence of non-PI stained sperm or (% Max) versus DiSC3(5) fluorescence of BCECF-AM stained sperm were shown. Histograms are representative of at least 4 experiments. (C) Median values of BCECF-AM and DiSC3(5) histograms from each experiment were relativized to CAP condition. Values represent the mean ± SEM of at least 4 experiments. ** p<0.01 * p<0.05 vs 1 (relative to median fluorescence of CAP). (D) Acrosome reaction (%AR) induced by 10 μM of ionophore A23187 or DMSO was evaluated after incubation for 6 hours in media that support capacitation in the absence (CAP) or presence of GlyH-101 (10 μM). Statistical analysis for 3 experiments was made between vehicle and induced %AR from each condition using two-way ANOVA. * p<0.05.
Supplementary figure 5. PKA inhibition by KT-5720 and RP-cAMPS caused pHi acidification and Em depolarization. Human sperm were incubated for 1 hour in media that support capacitation in the absence (CAP) or presence of different concentrations of KT-5720 and RP-cAMPS. Aliquots from each condition were processed for Western blotting with anti-pPKA antibodies and then membranes were reblotted with an anti-β-tubulin antibody for loading control. (A) On the left, PKA activity was inhibited at 30 μM of KT-5720. At this concentration, after 5 hours of capacitation intracellular acidification and depolarization was observed when compared to the control condition (CAP). cAMP agonists (dbcAMP, 1 mM + IBMX 0.2 mM, dotted line) did not rescue the acidification nor the depolarization produced by KT-5720 (30 μM) as expected. (B) On the left, a concentration-dependent decrease on the levels of pPKA was observed. PKA activity was inhibited at 100 μM of RP-cAMPS. At this concentration, intracellular acidification and depolarization was observed when compared to the control condition (CAP). Blot and histograms are representative of at least 3 experiments.
Supplementary figure 2. Western Blot quantification and statistical analysis. (A) Blots were quantified as described in “Materials & Methods”. Quantification of blot values represents the mean ± SEM of at least 3 experiments. Values represent the mean ± SEM of at least 4 experiments. *** p<0.001, ** p<0.01 * p<0.05.
Supplementary figure 3. Flow cytometry analyses of Em and pHi changes. Sperm Em and pHi changes were monitored using DiSC3(5) and BCECF-AM. Two aliquots from the same tube were divided to assess pHi and Em. (A) For pHi estimation, Forward scatter (FSC) and Side scatter (SSC) fluorescence data were collected from 20,000 events per sample. Threshold levels for FSC and SSC were set to exclude signals from cellular debris or abnormal morphology for all samples. (B) Cells loaded with 0.5 μM BCECF-AM and 50 nM of PI were collected using Fluorescein isothiocyanate FITC (530/30) and PI Peridinin chlorophyll PerCP (670LP) lasers to determine viability. BCECF-AM is only incorporated in living cells (Q3). (C and D) Non-PI stained sperm were analyzed for FITC to estimate pHi. (E) For membrane potential assay, Forward scatter (FSC) and side scatter (SSC) fluorescence data were collected from 20,000 events per sample. Because compensation between PI and DiSC3(5) was difficult to perform and taking into account that BCECF-AM only is incorporated in living cells, positive cells for BCECF-AM were used to monitor viability for DiSC3(5). (F) Cells loaded with 0.5 μM BCECF-AM and 50 nM DiSC3(5) were assessed using APC and FITC lasers. (G and H) BCECF stained sperm were analyzed for APC to estimate Em. (I) Histogram of percentage of the maximum (%Max) vs BCECF-AM fluorescence before and after the addition of NH4Cl (20 mM). Addition of NH4Cl in cells collected after swim up (t0=before capacitation) induced a mild intracellular alkalinization. (J) Histograms of percentage of the maximum (% Max) versus DiSC3(5) fluorescence before and after the addition of valinomycin (1μM). Addition of valinomycin in sperm collected after swim up (t0=before capacitation) induced hyperpolarization of the membrane potential. (K) Emission spectra of BCECF without cells at λexc=488nm before (−inh-172 and −H89) and after the addition of 5 μM inh-172 or 30 μM H89 (+inh-172 and +H89). Because BCECF-AM is not fluorescent, the supernatant of cells incubated with this probe for 10 min was collected for this assay. (L) Emission spectra of DiSC3(5) without cells at λexc=635nm before (−inh-172 and −H89) and after the addition of 5 μM inh-172 or 30 μM H89 (+inh-172 and +H89). Background fluorescence from blank solution was subtracted (ΔF).
Supplementary figure 4. Inh-172 modified the side- (SSC-A) and forward-scatter (FSC-A) properties of human sperm. (A) Two dimensional plots of side (SSC) and forward-scatter (FSC) properties of human sperm incubated under capacitating conditions in the absence (CAP) or in the presence of 5 μM of inh-172 (inh-172). Sperm treated with inh-172 showed a difference in the FSC-A parameter related with cell size. (B) Treatment with of inh-172 did not affect viability. (C) Median of FSC-A and SSC-A from samples treated with 5 μM inh-172 were relativized with respect to control condition showing a significant difference in FSC-A parameter compared to SSC-A. *** p<0.001 vs 1 (relative median of CAP).
Acknowledgments
We thank Gabriel Rabinovich, Juan Pablo Cerliani, Carlos Davio, Nicolás Gilio and Juan Jeremías Incicco for their advice and technical assistance. We would also like to thank Williams and Rene Baron Foundation.
Funding: This work was supported by National Institutes of Health (HD038082 and HD44044 to PEV), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2013-1175), the Alexander von Humboldt Foundation (to CT); CONACyT-Mexico Fronteras de la Ciencia 71 to AD and CT and DGAPA/UNAM (IN203116 to CT and IN205516 to AD).
Footnotes
The authors have no conflict of interest to declare
References
- Austin Cr. Observations on the penetration of the sperm into the mammalian egg. Australian Journal of Biological Sciences. 1951;4(4):581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- Berger HA, Travis SM, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl-channel by specific protein kinases and protein phosphatases. Journal of Biological Chemistry. 1993;268(3):2037–2047. [PubMed] [Google Scholar]
- Bobadilla JL, et al. Cystic fibrosis: a worldwide analysis of CFTR mutations—correlation with incidence data and application to screening. Human mutation. 2002;19(6):575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- Buffone MG, et al. Capacitation‐associated changes in membrane fluidity in asthenozoospermic human spermatozoa. International journal of andrology. 2009;32(4):360–375. doi: 10.1111/j.1365-2605.2008.00874.x. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Wertheimer EV, et al. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2014;1842(12):2610–2620. doi: 10.1016/j.bbadis.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, et al. Human sperm subpopulations: relationship between functional quality and protein tyrosine phosphorylation. Human reproduction. 2004;19(1):139–146. doi: 10.1093/humrep/deh040. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Hirohashi N, Gerton GL. Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biology of reproduction. 2014;90(5):112. doi: 10.1095/biolreprod.114.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. 1951 doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chávez JC, et al. Ion permeabilities in mouse sperm reveal an external trigger for SLO3-dependent hyperpolarization. PloS one. 2013;8(4):e60578. doi: 10.1371/journal.pone.0060578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez JC, et al. Participation of the Cl−/HCO3− exchangers SLC26A3 and SLC26A6, the Cl− channel CFTR, and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biology of reproduction. 2012;86(1):1–14. doi: 10.1095/biolreprod.111.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, et al. Regulation of male fertility by CFTR and implications in male infertility. Human reproduction update. 2012:dms027. doi: 10.1093/humupd/dms027. [DOI] [PubMed] [Google Scholar]
- Chen WY, et al. Cl− is required for HCO3− entry necessary for sperm capacitation in guinea pig: involvement of a Cl−/HCO3− exchanger (SLC26A3) and CFTR. Biology of reproduction. 2009;80(1):115–123. doi: 10.1095/biolreprod.108.068528. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289(5479):625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- De Jonge CJ, et al. Modulation of the human sperm acrosome reaction by effectors of the adenylate cyclase/cyclic AMP second‐messenger pathway. Journal of Experimental Zoology. 1991;258(1):113–125. doi: 10.1002/jez.1402580113. [DOI] [PubMed] [Google Scholar]
- De La Vega-Beltran JL, et al. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. Journal of Biological Chemistry. 2012;287(53):44384–44393. doi: 10.1074/jbc.M112.393488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarco IA, et al. Involvement of a Na+/HCO Cotransporter in Mouse Sperm Capacitation. Journal of Biological Chemistry. 2003;278(9):7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- Diao R, et al. Decreased expression of cystic fibrosis transmembrane conductance regulator impairs sperm quality in aged men. Reproduction. 2013;146(6):637–645. doi: 10.1530/REP-13-0146. [DOI] [PubMed] [Google Scholar]
- Dirami T, et al. Missense mutations in SLC26A8, encoding a sperm-specific activator of CFTR, are associated with human asthenozoospermia. The American Journal of Human Genetics. 2013;92(5):760–766. doi: 10.1016/j.ajhg.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostmann WR. (RP)-cAMPS inhibits the cAMP-dependent protein kinase by blocking the cAMP-induced conformational transition. FEBS Lett. 1995;375(3):231–234. doi: 10.1016/0014-5793(95)01201-o. 20. [DOI] [PubMed] [Google Scholar]
- Ellsworth RE, et al. Comparative genomic sequence analysis of the human and mouse cystic fibrosis transmembrane conductance regulator genes. Proceedings of the National Academy of Sciences. 2000;97(3):1172–1177. doi: 10.1073/pnas.97.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffier J, et al. Flow Cytometry Analysis Reveals That Only a Subpopulation of Mouse Sperm Undergoes Hyperpolarization During Capacitation. Biology of reproduction, p.biolreprod. 2015;114:127266. doi: 10.1095/biolreprod.114.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves SC, et al. Critical appraisal of World Health Organization’s new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79(1):16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Figueiras-Fierro D, et al. Electrophysiological evidence for the presence of cystic fibrosis transmembrane conductance regulator (CFTR) in mouse sperm. Journal of cellular physiology. 2013;228(3):590–601. doi: 10.1002/jcp.24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, et al. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994;266(5182):107–109. doi: 10.1126/science.7524148. [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Vergani P, Csanády L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440(7083):477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-González EO, et al. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. Journal of Biological Chemistry. 2007;282(33):24397–24406. doi: 10.1074/jbc.M701603200. [DOI] [PubMed] [Google Scholar]
- l’Hoste S, et al. CFTR mediates apoptotic volume decrease and cell death by controlling glutathione efflux and ROS production in cultured mice proximal tubules. American Journal of Physiology-Renal Physiology. 2010;298(2):F435–F453. doi: 10.1152/ajprenal.00286.2009. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, et al. CFTR functions as a bicarbonate channel in pancreatic duct cells. The Journal of general physiology. 2009;133(3):315–326. doi: 10.1085/jgp.200810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- José O, et al. Carbonic anhydrases and their functional differences in human and mouse sperm physiology. Biochemical and biophysical research communications. 2015;468(4):713–718. doi: 10.1016/j.bbrc.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Kase H, et al. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochemical and biophysical research communications. 1987;142(2):436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Köhn FM, et al. Detection of human sperm acrosome reaction: comparison between methods using double staining, Pisum sativum agglutinin, concanavalin A and transmission electron microscopy. Human reproduction. 1997;12(4):714–721. doi: 10.1093/humrep/12.4.714. [DOI] [PubMed] [Google Scholar]
- Kujala M, et al. Expression of ion transport-associated proteins in human efferent and epididymal ducts. Reproduction. 2007;133(4):775–784. doi: 10.1530/rep.1.00964. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′, 5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biology of reproduction. 1996;55(3):684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- Li CY, et al. CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Human reproduction. 2010;25(2):317–327. doi: 10.1093/humrep/dep406. [DOI] [PubMed] [Google Scholar]
- Lishko PV, et al. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140(3):327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- Lochner A, Moolman JA. The many faces of H89: a review. Cardiovascular drug reviews. 2006;24(3–4):261–274. doi: 10.1111/j.1527-3466.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- López-González I, et al. Membrane hyperpolarization during human sperm capacitation. Molecular human reproduction. 2014;20(7):619–629. doi: 10.1093/molehr/gau029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luconi M, et al. Tyrosine phosphorylation of the a kinase anchoring protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biology of reproduction. 2005;72(1):22–32. doi: 10.1095/biolreprod.104.032490. [DOI] [PubMed] [Google Scholar]
- Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin–induced intestinal fluid secretion. The Journal of clinical investigation. 2002;110(11):1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis N, et al. Revisiting CFTR inhibition: a comparative study of CFTRinh‐172 and GlyH‐101 inhibitors. British journal of pharmacology. 2014;171(15):3716–3727. doi: 10.1111/bph.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer ST. Minimum sperm trajectory length for reliable determination of the fractal dimension. Reproduction, Fertility and Development. 1998;10(6):465–470. doi: 10.1071/rd98123. [DOI] [PubMed] [Google Scholar]
- Muanprasat C, et al. Discovery of Glycine Hydrazide Pore-occluding CFTR Inhibitors Mechanism, Structure–Activity Analysis, and In Vivo Efficacy. The Journal of general physiology. 2004;124(2):125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, et al. Biphasic role of calcium in mouse sperm capacitation signaling pathways. Journal of cellular physiology. 2015;230(8):1758–1769. doi: 10.1002/jcp.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JM, Olds‐Clarke P. A computer‐assisted assay for mouse sperm hyperactivation demonstrates that bicarbonate but not bovine serum albumin is required. Gamete research. 1987;18(2):121–140. doi: 10.1002/mrd.1120180204. [DOI] [PubMed] [Google Scholar]
- Nishigaki T, et al. Intracellular pH in sperm physiology. Biochemical and biophysical research communications. 2014;450(3):1149–1158. doi: 10.1016/j.bbrc.2014.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MA, et al. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(37):13483–13488. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, et al. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. Journal of Biological Chemistry. 1985;260(17):9699–9705. [PubMed] [Google Scholar]
- Rode B, et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Human molecular genetics. 2012;21(6):1287–1298. doi: 10.1093/hmg/ddr558. [DOI] [PubMed] [Google Scholar]
- Schulz S, et al. Increased frequency of cystic fibrosis transmembrane conductance regulator gene mutations in infertile males. Fertility and sterility. 2006;85(1):135–138. doi: 10.1016/j.fertnstert.2005.07.1282. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiological reviews. 1999;79(1):S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Sorum B. Czégé, D. & Csanády, L., 2015. Timing of CFTR Pore Opening and Structure of Its Transition State. Cell. 163(3):724–733. doi: 10.1016/j.cell.2015.09.052. [DOI] [PubMed] [Google Scholar]
- Stival C, et al. Sperm Acrosome Biogenesis and Function During Fertilization. Springer; 2016. Sperm Capacitation and Acrosome Reaction in Mammalian Sperm; pp. 93–106. [DOI] [PubMed] [Google Scholar]
- Stival C, et al. Src Kinase Is the Connecting Player between Protein Kinase A (PKA) Activation and Hyperpolarization through SLO3 Potassium Channel Regulation in Mouse Sperm. Journal of Biological Chemistry. 2015;290(30):18855–18864. doi: 10.1074/jbc.M115.640326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani JA, et al. Phosphorylation-regulated CI− channel in CHO cells stably expressing the cystic fibrosis gene. 1991 doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Tang L, Fatehi M, Linsdell P. Mechanism of direct bicarbonate transport by the CFTR anion channel. Journal of Cystic Fibrosis. 2009;8(2):115–121. doi: 10.1016/j.jcf.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121(4):1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121(4):1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proceedings of the National Academy of Sciences. 2009;106(3):667–668. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennemuth G, et al. Bicarbonate actions on flagellar and Ca2+-channel responses: initial events in sperm activation. Development. 2003;130(7):1317–1326. doi: 10.1242/dev.00353. [DOI] [PubMed] [Google Scholar]
- Wertheimer EV, et al. Chloride Is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. The Journal of Biological Chemistry. 2008;283(51):35539–35550. doi: 10.1074/jbc.M804586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO laboratory manual for the Examination and processing of human semen. 5th. Switzerland: World Health Organization Press; 2010. [Google Scholar]
- Xu WM, et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proceedings of the National Academy of Sciences. 2007;104(23):9816–9821. doi: 10.1073/pnas.0609253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Clark EN, Florman HM. Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Developmental Biology. 1995;171(2):554–563. doi: 10.1006/dbio.1995.1304. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Oberdorf JA, Florman HM. pH Regulation in Mouse Sperm: Identification of Na+-, Cl−-, and [formula] Dependent and Arylaminobenzoate-Dependent Regulatory Mechanisms and Characterization of Their Roles in Sperm Capacitation. Developmental biology. 1996;173(2):510–520. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. CFTR inhibitor GlyH-101 affects pY, pPKA, Em and pHi changes associated to capacitation and the acrosome reaction induced by ionophore A23187. Human sperm were incubated in media that support capacitation in the absence (CAP) or presence of GlyH-101. (A) On the left, a concentration-dependent decrease on the levels of tyrosine phosphorylation (pY) was observed in the presence of GlyH-101. In the middle, the percentage of viable cells using different concentrations of GlyH101 was evaluated (*denotes p<0.05 vs 0). On the right, a western blot with anti-pY and anti-PKA antibodies in sperm incubated with 10 μM of GlyH-101. Membranes were reblotted with an anti-β-tubulin antibody for loading control. (B) Histograms of percentage of the maximum (% Max) versus BCECF-AM fluorescence of non-PI stained sperm or (% Max) versus DiSC3(5) fluorescence of BCECF-AM stained sperm were shown. Histograms are representative of at least 4 experiments. (C) Median values of BCECF-AM and DiSC3(5) histograms from each experiment were relativized to CAP condition. Values represent the mean ± SEM of at least 4 experiments. ** p<0.01 * p<0.05 vs 1 (relative to median fluorescence of CAP). (D) Acrosome reaction (%AR) induced by 10 μM of ionophore A23187 or DMSO was evaluated after incubation for 6 hours in media that support capacitation in the absence (CAP) or presence of GlyH-101 (10 μM). Statistical analysis for 3 experiments was made between vehicle and induced %AR from each condition using two-way ANOVA. * p<0.05.
Supplementary figure 5. PKA inhibition by KT-5720 and RP-cAMPS caused pHi acidification and Em depolarization. Human sperm were incubated for 1 hour in media that support capacitation in the absence (CAP) or presence of different concentrations of KT-5720 and RP-cAMPS. Aliquots from each condition were processed for Western blotting with anti-pPKA antibodies and then membranes were reblotted with an anti-β-tubulin antibody for loading control. (A) On the left, PKA activity was inhibited at 30 μM of KT-5720. At this concentration, after 5 hours of capacitation intracellular acidification and depolarization was observed when compared to the control condition (CAP). cAMP agonists (dbcAMP, 1 mM + IBMX 0.2 mM, dotted line) did not rescue the acidification nor the depolarization produced by KT-5720 (30 μM) as expected. (B) On the left, a concentration-dependent decrease on the levels of pPKA was observed. PKA activity was inhibited at 100 μM of RP-cAMPS. At this concentration, intracellular acidification and depolarization was observed when compared to the control condition (CAP). Blot and histograms are representative of at least 3 experiments.
Supplementary figure 2. Western Blot quantification and statistical analysis. (A) Blots were quantified as described in “Materials & Methods”. Quantification of blot values represents the mean ± SEM of at least 3 experiments. Values represent the mean ± SEM of at least 4 experiments. *** p<0.001, ** p<0.01 * p<0.05.
Supplementary figure 3. Flow cytometry analyses of Em and pHi changes. Sperm Em and pHi changes were monitored using DiSC3(5) and BCECF-AM. Two aliquots from the same tube were divided to assess pHi and Em. (A) For pHi estimation, Forward scatter (FSC) and Side scatter (SSC) fluorescence data were collected from 20,000 events per sample. Threshold levels for FSC and SSC were set to exclude signals from cellular debris or abnormal morphology for all samples. (B) Cells loaded with 0.5 μM BCECF-AM and 50 nM of PI were collected using Fluorescein isothiocyanate FITC (530/30) and PI Peridinin chlorophyll PerCP (670LP) lasers to determine viability. BCECF-AM is only incorporated in living cells (Q3). (C and D) Non-PI stained sperm were analyzed for FITC to estimate pHi. (E) For membrane potential assay, Forward scatter (FSC) and side scatter (SSC) fluorescence data were collected from 20,000 events per sample. Because compensation between PI and DiSC3(5) was difficult to perform and taking into account that BCECF-AM only is incorporated in living cells, positive cells for BCECF-AM were used to monitor viability for DiSC3(5). (F) Cells loaded with 0.5 μM BCECF-AM and 50 nM DiSC3(5) were assessed using APC and FITC lasers. (G and H) BCECF stained sperm were analyzed for APC to estimate Em. (I) Histogram of percentage of the maximum (%Max) vs BCECF-AM fluorescence before and after the addition of NH4Cl (20 mM). Addition of NH4Cl in cells collected after swim up (t0=before capacitation) induced a mild intracellular alkalinization. (J) Histograms of percentage of the maximum (% Max) versus DiSC3(5) fluorescence before and after the addition of valinomycin (1μM). Addition of valinomycin in sperm collected after swim up (t0=before capacitation) induced hyperpolarization of the membrane potential. (K) Emission spectra of BCECF without cells at λexc=488nm before (−inh-172 and −H89) and after the addition of 5 μM inh-172 or 30 μM H89 (+inh-172 and +H89). Because BCECF-AM is not fluorescent, the supernatant of cells incubated with this probe for 10 min was collected for this assay. (L) Emission spectra of DiSC3(5) without cells at λexc=635nm before (−inh-172 and −H89) and after the addition of 5 μM inh-172 or 30 μM H89 (+inh-172 and +H89). Background fluorescence from blank solution was subtracted (ΔF).
Supplementary figure 4. Inh-172 modified the side- (SSC-A) and forward-scatter (FSC-A) properties of human sperm. (A) Two dimensional plots of side (SSC) and forward-scatter (FSC) properties of human sperm incubated under capacitating conditions in the absence (CAP) or in the presence of 5 μM of inh-172 (inh-172). Sperm treated with inh-172 showed a difference in the FSC-A parameter related with cell size. (B) Treatment with of inh-172 did not affect viability. (C) Median of FSC-A and SSC-A from samples treated with 5 μM inh-172 were relativized with respect to control condition showing a significant difference in FSC-A parameter compared to SSC-A. *** p<0.001 vs 1 (relative median of CAP).


