Abstract
Background
Early-life respiratory viral infection is a risk factor for asthma development. Rhinovirus (RV) infection of six-day old but not mature mice causes mucous metaplasia and airway hyperresponsiveness which is associated with expansion of lung type 2 innate lymphoid cells (ILC2s) and dependent on IL-13 and the innate cytokine IL-25. However, contributions of the other innate cytokines, IL-33 and thymic stromal lymphopoietin (TSLP), to the observed asthma-like phenotype have not been examined.
Objective
We reasoned that IL-33 and TSLP expression are also induced by RV infection in immature mice and required for maximum ILC2 expansion and mucous metaplasia.
Methods
We inoculated six day-old BALB/c (wild-type) and TSLP receptor knockout (TSLPR KO) mice with sham HeLa cell lysate or RV. Selected mice were treated with neutralizing antibodies to IL-33 or recombinant IL-33, IL-25 or TSLP. ILC2s were isolated from RV-infected immature mice and treated with innate cytokines ex vivo.
Results
RV infection of six-day old mice increased IL-33 and TSLP protein abundance. TSLP expression was localized to the airway epithelium, whereas IL-33 was expressed in both epithelial and subepithelial cells. RV-induced mucous metaplasia, ILC2 expansion, airway hyperresponsiveness and epithelial cell IL-25 expression were attenuated by anti-IL-33 treatment and in TSLPR KO mice. Administration of intranasal IL-33, but not TSLP, was sufficient for mucous metaplasia. Finally, TSLP was required for maximal ILC2 gene expression in response to IL-25 and IL-33.
Conclusion
The generation of mucous metaplasia in immature, RV-infected mice involves a complex interplay between the innate cytokines IL-25, IL-33 and TSLP.
Introduction
The epithelial-derived “innate cytokines” IL-25 (or IL-17E) (1, 2), IL-33 (3) and thymic stromal lymphoprotein (TSLP) (4–7), play a role in the maturation of Th2 cells via dendritic cell activation. IL-25 (8–12), IL-33 (8–10, 13–16) and TSLP (16) also induce activation and IL-13 production from innate immune cells including type 2 innate lymphoid cells (ILC2s).
The development of asthma is likely to be a result of genetic predisposition, immune dysfunction and environmental exposures in early infancy. Epidemiologic studies in high-risk infants indicate that early-life respiratory viral infection, particularly with rhinovirus (RV), is a major predisposing factor for asthma development (17–20). Because the airway epithelium is a major target of respiratory viral infection, the epithelium-derived cytokines IL-25, IL-33 and TSLP and their downstream cellular targets are uniquely positioned to play a role in viral-induced chronic airways disease. Previously, we have found that IL-25 plays pivotal role in asthma phenotype development in immature mice following RV infection (21). RV infection of six day-old but not mature mice induced mucous metaplasia and airways hyperresponsiveness which was accompanied by airway epithelial cell IL-25 production and expansion of IL-13-producing type 2 innate lymphoid cells (ILC2s). Neutralizing antibody against IL-25 abolished ILC2 expansion, mucous metaplasia and airways hyperresponsiveness (21). However, we did not examine the roles of IL-33 and TSLP in this model.
The role of IL-33 and TSLP in the pathogenesis of asthma has been recently highlighted. IL-33 is member of the IL-1 family of cytokines which signals through the IL-1 receptor homologue ST2L, is constitutively expressed as a nuclear precursor in alveolar epithelial cells (22) and released from producing cells upon cellular damage (23). In mice, IL-33 is expressed in airway epithelial and inflammatory cells after allergen challenge (24) and required for ovalbumin-, papain- and Alternaria-induced airway inflammation (16, 22, 25, 26). Allergic asthma, in particular childhood asthma and wheezing syndromes, has been associated with genetic variation of the genes encoding IL-33 and its receptor IL-1 receptor-like 1/ST2L (27–30). Clinically, expression of IL-33 is increased in the airway epithelium, endothelium and smooth muscle of adult patients with severe asthma (31) and the submucosal inflammatory cells of children with steroid-resistant asthma (32). In patients with asthma, experimental RV infection induces airway IL-33 production which is proportional to exacerbation severity and viral load (33).
TSLP is an IL-7-like cytokine which exerts its biological activity via a high-affinity TSLPR complex that is a heterodimer of the TSLPR chain and IL-7 receptor-α. In mice, transgenic mice that overexpress TSLP in the lungs show an augmented type 2 immune response, goblet cell hyperplasia and subepithelial fibrosis when challenged with ovalbumin, whereas TSLPR-deficient mice show attenuated responses(6). IL-25 and TSLP are secreted from airway epithelial cells upon house dust mite (HDM) challenge, leading to DC activation and an adaptive Th2 response (34). Genome-wide association studies have found that the TSLP gene locus is associated with asthma risk (35, 36). Several single-nucleotide polymorphisms of the TSLP gene locus are associated with asthma development (37–40). TSLP levels are increased in the airways of asthmatic patients and correlates with asthma severity (41, 42). Treatment of human asthmatic patients with anti-TSLP antibody blunts airway responses triggered by allergen inhalation (43). However, less is known about the role of TSLP in the development of asthma in childhood. RV infection increases nasal aspirate TSLP expression in young children (44), consistent with the notion that RV-induced TSLP production could play a role in the initiation of asthma. We therefore examined the effect of RV infection on IL-33 and TSLP expression in immature mice, and the requirement of IL-33 and TSLP for RV-induced mucus metaplasia and ILC2 expansion.
Material and Methods
Generation of RV and infection of immature mice
RV1B (ATCC, Manassas, VA) was partially purified from infected HeLa cell lysates by ultrafiltration using a 100 kD cut-off filter and titered by plaque assay (45, 46). Similarly concentrated and purified HeLa cell lysates were used for sham infection. BALB/c mice (Jackson Laboratories, Bar Harbor, ME), or TSLPR KO mice backcrossed to BALB/c for 7 generations (6) (graciously supplied by Dr. Steven Ziegler, Benaroya Research Institute, Seattle, WA) were inoculated through intranasal route under Forane anesthesia with 20 µl RV1B (1×108 PFU/ml) or sham HeLa cell lysates. Selected mice were treated with 0.5 µg of recombinant murine IL-33, IL-25 or TSLP (Pepro Tech, Rocky Hill, NJ) intranasal on day 0. Experiments were approved by the University of Michigan Institutional Animal Care and Use Committee.
Anti-IL-25 and anti-IL-33 neutralizing antibody treatment
Six-day-old mice were treated with either 100 µg of neutralizing antibody to IL-25 (clone 35B; BioLegend, San Diego, CA), neutralizing antibody to IL-33 (R&D Systems, Minneapolis, MN) or isotype control (rat, IgG1K, BioLegend) intraperitoneally 2 h prior to RV infection (day 0). Lungs were harvested 2 to 21 days after infection for analysis.
Histology and immunofluorescence microscopy
Lungs were perfused through the pulmonary artery with phosphate-buffered saline containing 5 mM EDTA. Next, lungs were fixed with 4% paraformaldehyde overnight. Five-micrometer-thick paraffin sections and processed for histology or fluorescence microscopy as described (47). Lung sections were stained with Periodic acid-Schiff (PAS) or AlexaFluor 488-conjugated anti-Muc5ac (Thermo Fisher Scientific, Rockford, IL) to visualize mucus (Sigma-Aldrich, St Louis, MO). Levels of Muc5ac staining in the airway epithelium were quantified by NIH ImageJ software (Bethesda, MD), as described (48). Muc5ac expression was represented as the fraction of Muc5ac + epithelium compared with the total basement membrane length. Other lung sections were incubated with Alexa Fluor 555-conjugated rabbit anti-mouse TSLP (Thermo Fisher Scientific), Alexa Fluor 555-conjugated goat anti-mouse IL-33 (R&D Systems), Alexa Fluor 633-conjugated rabbit anti-mouse IL-25/IL-17E (Millipore, Billerica, MA), Alexa Fluor 488-conjugated mouse anti-RV16 (clone R16–7, QED Bioscience, San Diego, CA), Alexa Fluor 555-conjugated antieosinophil major basic protein (Mayo Clinic, Rochester MN), Alexa Fluor 488-conjugated anti-Gr1 (R&D Systems), Alexa Fluor 647-conjugated anti-F4/80 (Biolegend) and Alexa Fluor 488-conjugated isotype control IgGs. The anti-RV16 antibody recognizes capsid protein VP2 of several RV serotypes including RV1B, as well as the VP2 precursors VP0 and P1.
Real-time quantitative PCR
Lung or ILC2 RNA was extracted with Trizol method (Invitrogen, Carlsbad, CA) with the combination of on-column digestion of genomic DNA (Qiagen, Valencia, CA). cDNA was synthesized from 1 µg of RNA and subjected to quantitative real-time PCR by using specific mRNA primers encoding for IL-13, Muc5ac, Gob5, IL-25, IL-33, TSLP, IL17RB and ST2L, as listed in Table S1. For each sample, the level of gene expression was normalized to its own GAPDH mRNA.
Measurement of IL-13, IL-25, IL-33, and TSLP protein levels
Lung cytokine levels were measured by ELISA (eBioscience catalog numbers 88–7137–22, 88–7002–22, 88–7333–22 and 887490–22, respectively). ELISA data were analyzed by BioTek Gen5 software (Winooski, VT).
Flow cytometric analysis
Lungs from sham- and RV-treated immature wild-type BALB/c or TSLPR KO mice were perfused with PBS containing EDTA, minced and digested in collagenase IV. Cells were filtered and washed with RBC lysis buffer, and dead cells were stained with Pac-Orange Live/Dead fixable dead staining dye (Invitrogen). To identify ILC2s, cells were then stained with fluorescent-tagged antibodies for lineage markers (CD3ε, TCRβ, B220/CD45R, Ter-119, Gr-1/Ly-6G/Ly-6C, CD11b, CD11c, F4/80 and FcεRIα, all from Biolegend), anti-CD25 (Biolegend), and anti-CD127 (eBioscience), as described (21). Cells were fixed, subjected to flow cytometry and analyzed on a LSR Fortessa (BD Biosciences, San Jose, CA). Data were collected using FACSDiva software (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Assessment of airway responsiveness
Airway cholinergic responsiveness was assessed by measuring changes in total respiratory system resistance in response to increasing doses of nebulized methacholine, as described previously (49). Mechanical ventilation was conducted and total respiratory system measured using a Buxco FinePointe operating system (Wilmington, NC). Airway responsiveness was assessed by measuring changes in resistance in response to increasing doses of nebulized methacholine.
ILC2 culture
To study lung ILC2s ex vivo, lung cells were stained with biotin-conjugated antibodies for lineage markers (CD3ε, TCRβ, B220/CD45R, Ter-119, Gr-1/Ly-6G/Ly-6C, CD11b, CD11c, F4/80 and FcεRIα), and mixed with anti-biotin microbeads (Miltenyi Biotech, Auburn, CA). The cell mixture was then subjected to a MACS Separator (Miltenyi Biotech). The flow through was collected for fluorescence-activated cell sorting. Lineage-negative CD25 and CD127 double-positive ILC2s were plated on round bottom 96-well plates at 5000 cells/well and cultured in RPMI1640 supplemented with 10% FBS, IL-2 and IL-7 (20 ng/ml each) (R&D Systems). Twenty-four h later, the cells were stimulated with different combinations of IL-2, IL-7, IL-25, IL-33 and TSLP (20 ng/ml each, all from R&D. After 48 h, plates were centrifuged and supernatant were tested for IL-13 with ELISA (eBioscience). Finally, cell pellet RNA was extracted for qPCR as described above.
For measurement of BrdU uptake, cells were co-treated with BrdU (10 µg/ml, Sigma-Aldrich) for 24 h. Forty-eight hours later, cytospin preparations were fixed and stained with Alexa Fluor 555-conjugated anti-BrdU antibody (Thermo Fisher Scientific). The cells were visualized under confocal microscopy.
Data analysis
Data are represented as mean ± standard error. Statistical significance was assessed by unpaired t-test or one-way ANOVA, as appropriate. Group differences were pinpointed by a Tukey multiple comparison test.
Results
RV induces expression of epithelial-derived innate cytokines
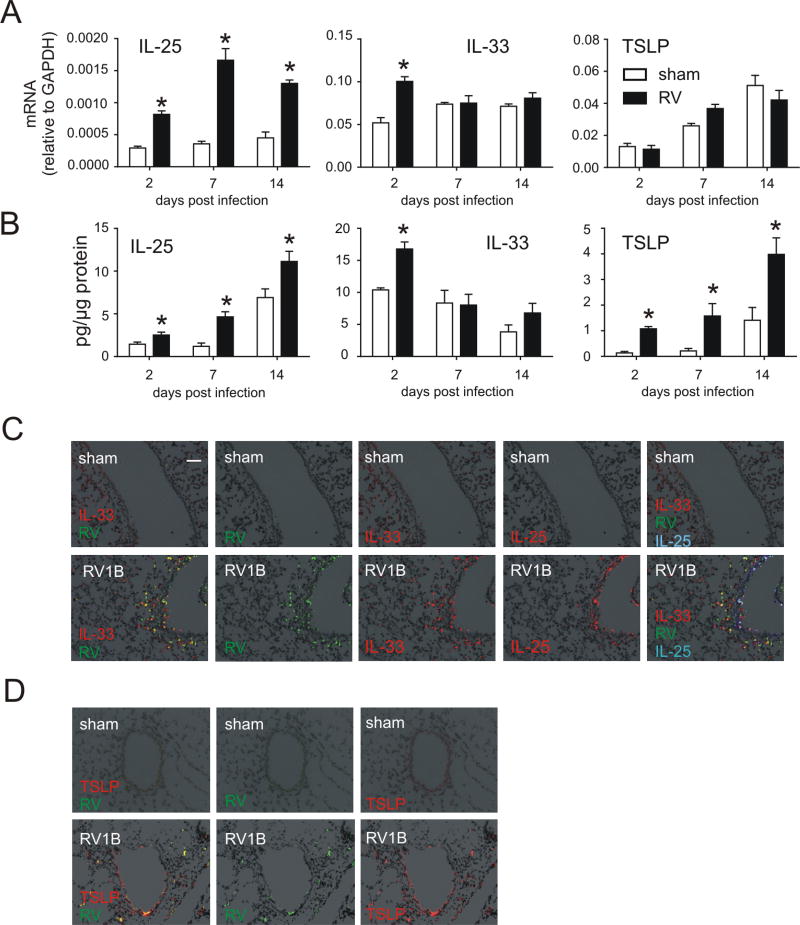
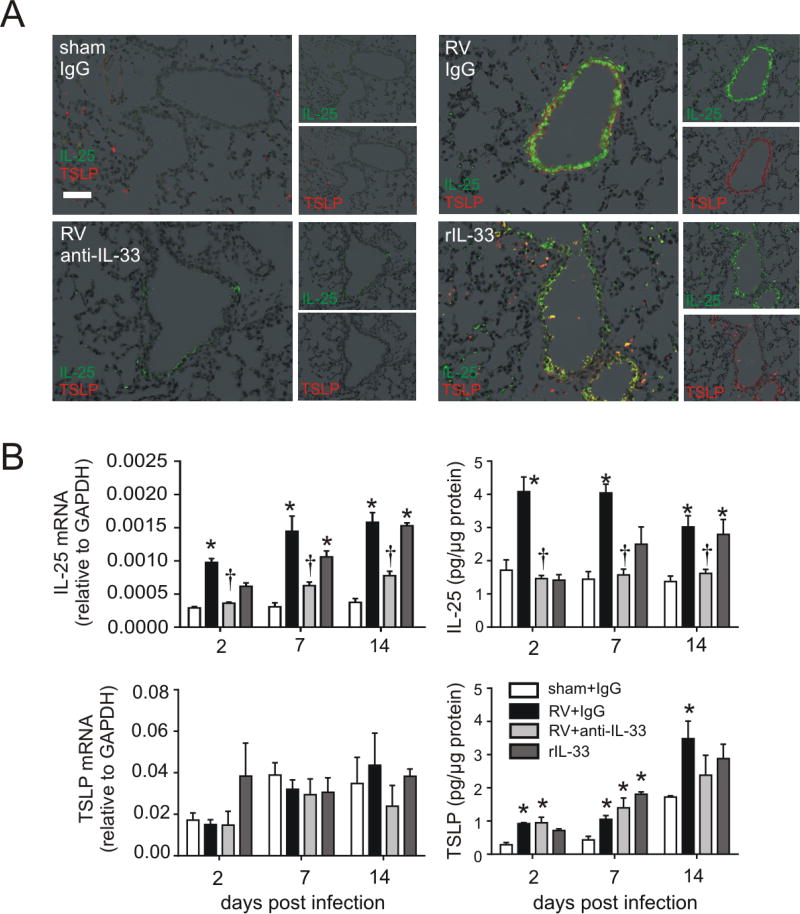
In our previous report, we found that epithelial IL-25 is increased in RV-infected immature mice and required for the development of mucus metaplasia and airways hyperresponsiveness (21). In the present study, we determined the effects of RV infection IL-33 and TSLP mRNA expression and protein abundance. We collected lungs from RV-infected 6 day-old mice at day 2, 7, and 14 postinfection and measured IL-25, IL-33, and TSLP mRNA and protein. Consistent with our previous report, IL-25 mRNA and protein expression was increased in RV-infected 6-day-old mice (Figure 1A, 1B). IL-33 mRNA and protein expression was increased at the early time points of infection. RV also increased protein but not mRNA level of TSLP.
FIG 1.
Innate cytokine expression after RV infection. Six-day-old BALB/c mice were inoculated with sham or RV. Lung mRNA (A) and protein (B) expression were measured 2, 7, or 14 days later. (N=3–5, mean±SEM, *different from sham, one-way ANOVA.). C and D, Two days after infection, lungs were stained for IL-33 (C, red), RV (green), IL-25 (shown separately in red, but merged on the right hand panel in blue) and nuclei (DAPI, black; bar, 50 µm). D, lungs were stained for TSLP (red), RV (green) and nuclei (DAPI, black; bar, 50 µm).
To better understand the expression pattern of epithelial-derived innate cytokines, we examined airway innate cytokine protein deposition by immunofluorescence. Infection with RV increased airway IL-33 expression, with the strongest signal found in the cytoplasm of RV-infected airway epithelial cells (Figure 1C). IL-33 was also expressed in subepithelial cells. RV infection also increased airway epithelial cell expression of IL-25. Infection also increased TSLP staining (Figure 1D). Both subepithelial and epithelial cells produced TSLP; the strongest signals were found in airway epithelial cells infected with RV.
IL-33 is required for mucous metaplasia in rhinovirus-infected immature mice
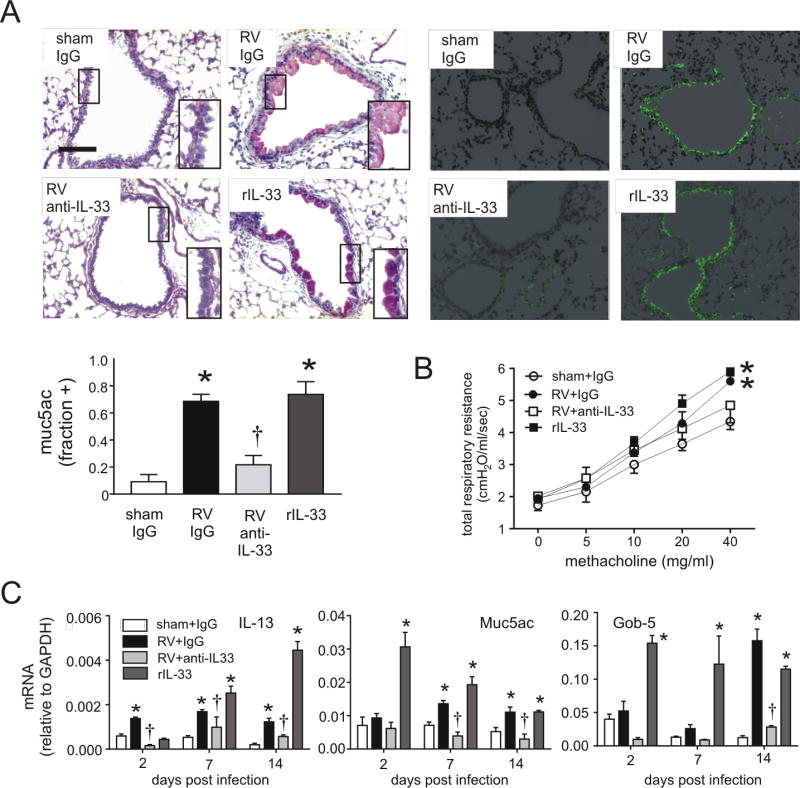
To test whether IL-33 is required for the development of an asthma-like phenotype, we treated rhinovirus-infected 6 day-old mice with a neutralizing antibody against IL-33. Since previous studies showed development of mucous metaplasia 2–4 weeks after RV infection (49), lung sections were analyzed three weeks after infection. Sham-infected animals showed no PAS staining in the non-cartilagenous airways. RV infection induced PAS staining of the airway epithelium (Figure 2A). Treatment with anti-IL-33 blocked PAS staining, while rIL-33 induced it. Like PAS staining, Muc5ac immunostaining in response to RV was reduced by anti-IL33 and increased by rIL-33. Similarly, anti-IL-33 blocked RV-increased airways hyperresponsiveness whereas rIL-33 induced increased airways responsiveness (Figure 2B). Expression of mRNAs encoding IL-13 and the mucus-related genes Muc5ac and Gob5 also decreased with anti-IL-33 treatment (Figure 2C). Taken together, these results show that IL-33 plays a key role in the development of mucous metaplasia in rhinovirus-infected immature mice.
FIG 2.
Mucous metaplasia and lung mRNA expression in anti-IL-33- and rIL-33 treated mice. Six-day-old BALB/c mice were inoculated with sham, RV or rIL-33. Anti-IL-33 was given 1 hour after infection to selected RV-treated mice. A, Mucous metaplasia was assessed by periodic acid-Schiff-staining and Muc5ac immunofluorescence. Lung sections prepared 3 weeks after treatment of six day-old mice with sham + IgG, RV + IgG, RV + anti-IL-33 or rIL-33. (Bar = 50 µM.) Muc5ac was quantified as the fraction of epithelium positively stained, as measured by NIH ImageJ software (N=3, mean±SEM, *different from sham + IgG, p<0.05; different from RV + IgG, †p<0.05, one-way ANOVA). B, Airways hyperresponsiveness of 4 week-old baby mice, 21 days after sham infection, RV infection, RV + anti-IL33 or recombinant (r) IL-33 (n=4, *different from sham, p<0.05, two-way ANOVA). C, Whole lung gene expression of the mucus-related genes Muc5ac, Gob5 and IL-13 was measured with quantitative PCR. (N=3–5, mean±SEM, *different from sham, p<0.05; †different from RV + IgG, p<0.05, one-way ANOVA).
IL-33 is required for expansion of ILC2s in RV-infected immature mice
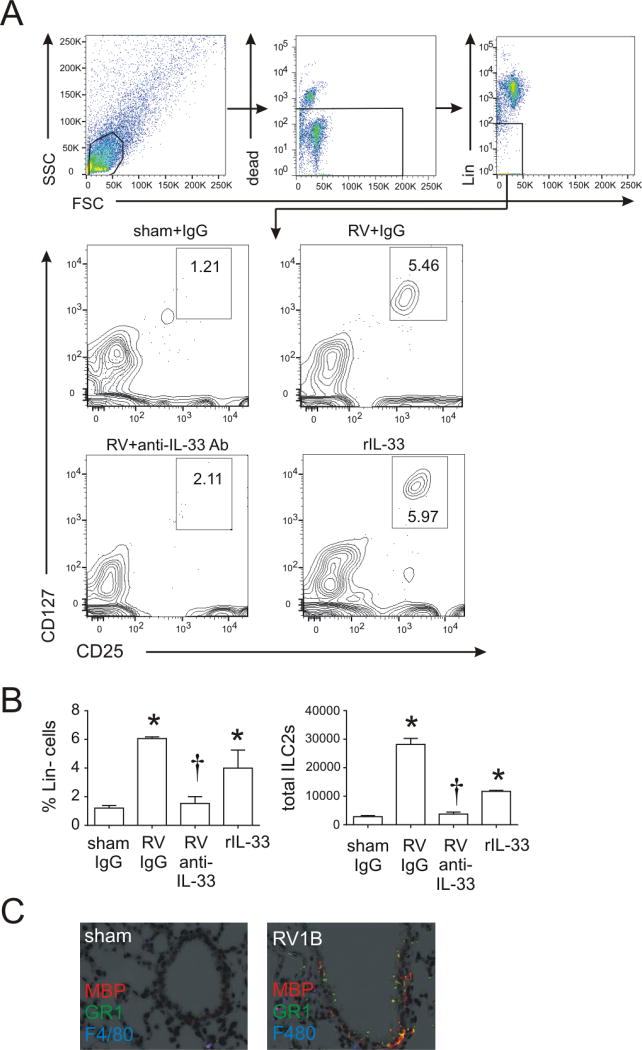
RV infection of six day-old mice expands the population of lung ILC2s, a major source of IL-13 after RV infection (21). The level of ILC2s peaks at 7 days and plateaus 7–21 days after infection. We asked whether anti-IL-33 suppresses expansion of ILC2s using flow cytometry, collecting lungs two weeks after sham or RV infection. Cells were incubated cells with a mixture of antibodies against hematopoietic lineage markers (CD3ε, TCRβ, B220, Ter-119, Gr-1, CD11b, CD11c, F4/80, FcεRIα), anti-CD25 and anti-CD127. After gating on small lineage-negative cells, a discrete population of CD25 and CD127 double-positive ILC2s was found. RV infection and recombinant IL-33 treatment significantly increased the number and percentage of lineage-negative CD25+ CD127+ cells (Figure 3A, 3B). Anti-IL33 treatment decreased the number and percentage of lineage-negative CD25+ CD127+ cells per lung. Finally, as shown previously (49), RV infection also increased airway granulocytes, including Gr1+ MBP+ eosinophils (Figure 3C).
FIG 3.
A, B, Lung lineage-, CD25+, CD127+ ILC2s in RV-infected BALB/c immature mice. Six day-old mice were treated with sham + IgG, RV + IgG, RV + anti-IL-33 and rIL-33. Live ILC2s were identified fourteen days later and analyzed as a percentage of lineage-negative and total cells. (N=3–5, mean±SEM, *different from sham; †different from RV+PBS, P<0.05, oneway ANOVA). C, Eosinophil major basic protein (red), Gr-1/Ly6G (green), and F4/80 (blue) were examined 14 days post-RV infection.
TSLP is required for RV-induced mucous metaplasia andILC2 expansion
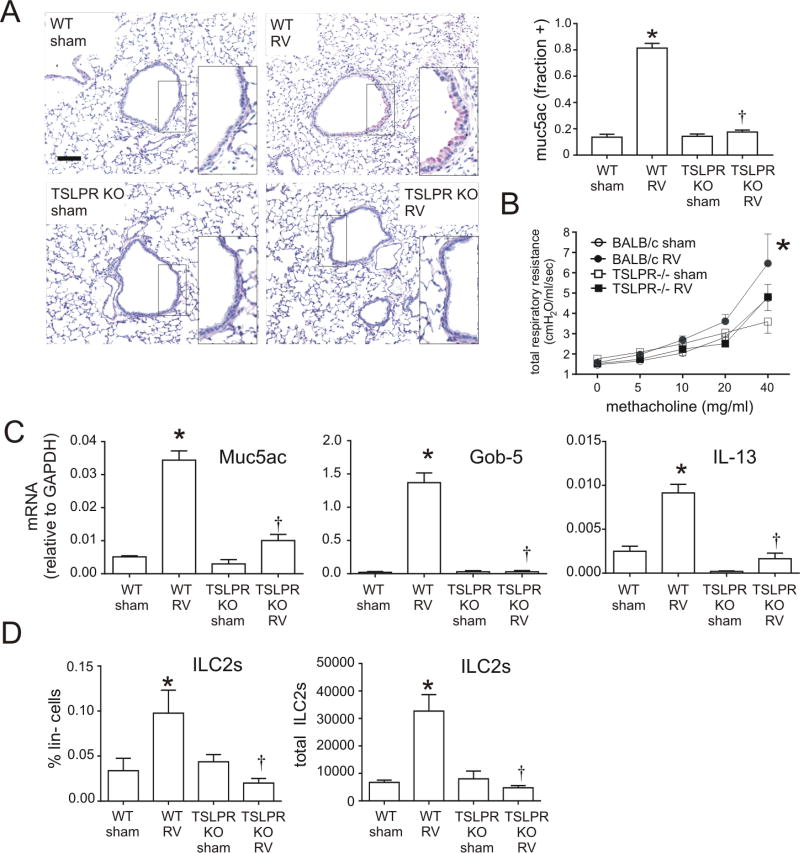
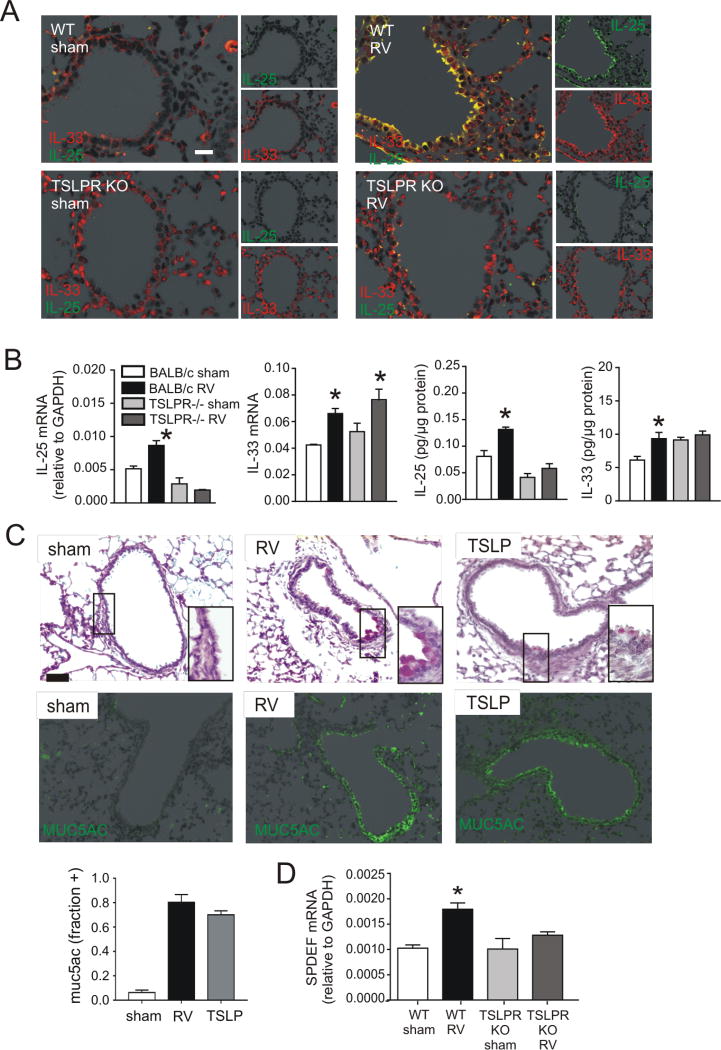
To examine the requirement of TSLP for RV-induced mucous metaplasia, we employed TSLPR KO mice. RV infection increased PAS staining and Muc5ac protein accumulation in wild-type mice, but no induction of mucus staining was found in TSLP receptor deficient mice (Figure 4A). Similarly, airways hyperresponsiveness to RV was blocked in the TLSPR KO mice (Figure 4B). Consistent with the reduction in PAS staining, induction of IL-13 and the mucus-related genes Muc5ac and Gob5 was significantly lower in TSLPR KO mice than in wild-type mice (Figure 4C). Utilizing TSLPR KO mice, we tested the requirement of TSLP for ILC2 expansion associated with early-life RV infection. Compared to wild-type mice in which ILC2 was increased, the ILC2 population was not expanded in TSLPR KO mice (Figure 4D). These results demonstrate that, like IL-33, TSLP is required for development of the RV-induced asthma phenotypes via regulation of ILC2 expansion.
FIG 4.
Inhibition of RV-induced mucous metaplasia and ILC2s expansion in TSLPR KO mice. A, Six-day-old BALB/c (WT) mice or TSLPR KO mice were inoculated with sham or RV. Lung sections were prepared 3 weeks after infection and stained with PAS solution or anti-Muc5ac. Representative lung sections of PAS-stained small airways are shown (scale bar, 50 µm). Muc5ac was quantified as the fraction of epithelium positively stained, as measured by NIH ImageJ software (N=3, mean±SEM, * different from sham + IgG, p<0.05; jdifferent from RV + IgG, p<0.05, one-way ANOVA). B, Airways hyperresponsiveness was measured in sham- or RV-treated wild type BALB/c or TSLPR KO mice 21 days after treatment. (N=4, mean±SEM, * different from sham, p<0.05, two-way ANOVA). C, Whole lung gene expression of the mucus-related genesMuc5ac, Gob5 and IL-13 was measured with quantitative PCR N=3–5, mean±SEM, *p<0.05 different from sham, †p<0.05 different from WT RV (one-way ANOVA). D Reduction of RV-induced ILC2 expansion in immature TSLPR KO mice. Six-day-old BALB/c mice and TSLPR KO mice were inoculated with sham or RV. Lung cells were collected 14 days after infection, stained and subjected to flow cytometry. The ILC2 percentage of lineage negative cells and total ILC2s per lung for each group are shown. N=4–5, mean±SEM, *different from sham, †different from WT RV, p<0.05, one-way ANOVA.
Requirement and sufficiency of IL-25, IL-33 and TSLP for innate cytokine production
To better assess the overlapping contributions of IL-33, TSLP (above) and IL-25 (21) to RV-induced mucous metaplasia, we examined the effects of anti-IL-33 and the TSLPR KO on RV-induced innate cytokine expression. As shown previously (21), immunofluorescence staining two days after early-life RV infection showed increased epithelial cell expression of IL-25 (Figure 5A). Administration of rIL-33 was sufficient for both IL-25 and TSLP staining. Anti-IL-33 blocked RV-induced IL-25 but not TSLP expression (Figure 5B). (TSLP levels, which peaked 14 days after infection, showed a trend towards inhibition at this time point.) Similarly, TSLPR KO mice showed no increase in RV-induced IL-25 protein expression (Figure 6A, 6B). BALB/c mice demonstrated IL-33 mRNA and protein induction following RV infection, whereas TSLPR KO mice showed constitutive IL-33 in both airway epithelial and subepithelial cells, with epithelial cells showing both perinuclear and cytoplasmic staining. Together, these results demonstrate that IL-33 and TSLP are required for RV-induced airway epithelial cell IL-25 expression. Finally, administration of recombinant TSLP was sufficient to induce mucous metaplasia, as evidenced by PAS staining and Muc5ac expression (Figure 6C).
FIG 5.
Innate cytokine expression in anti-IL-33- and rIL-33-treated immature mice after RV infection. Six-day-old BALB/c mice were inoculated with sham + IgG, RV + IgG or rIL-33. Anti-IL-33 was given 1 hour after infection to selected RV-treated mice. A. Two days after infection, lungs were stained for TSLP (red) and IL-25 (green; bar is 50 µm). B. Lung mRNA and protein expression were measured 2, 7, or 14 days later. (N=3–5, mean±SEM, *different from sham + IgG, † different from RV + IgG, one-way ANOVA.)
FIG 6.
Innate cytokine expression in immature TSLPR KO mice after RV infection. Six-day-old BALB/c or TSLPR KO mice were inoculated with sham or RV. A. Two days after infection, lungs were stained for IL-33 (red) and IL-25 (green; bar is 50 µm). B. Lung mRNA and protein expression were measured 2 days after infection (N=3–5, mean±SEM, *different from sham, one-way ANOVA). C. Six-day-old BALB/c mice were inoculated with sham, RV, or recombinant TSLP (rTSLP). Lung sections were prepared 3 weeks after infection and stained with PAS solution or anti-Muc5ac. Fractional airway epithelial staining for Muc5ac was measured (N=3, mean±SEM, * different from sham, p<0.05, one-way ANOVA). D. Lung SPDEF mRNA was measured 2 days after RV infection. (N=4, mean±SEM, *different from sham, one-way ANOVA).
Airway mucous cell differentiation is regulated by the transcription factor SAM-pointed domain-containing ETS-like factor (SPDEF). SPDEF, in turn, regulates the transcription of several mRNAs including IL13, Muc5ac, CCL20, CCL24, IL25, IL33 and TSLP (50). We examined the effects of RV infection on SPDEF mRNA expression in wild-type and TSLPR KO mice. In wild-type mice, SPDEF mRNA expression was significantly increased two days after RV infection (Figure 6D). Induction of SPDEF was absent in TSLPR KO mice, suggesting a mechanism by which TSLP may regulate airway epithelial cell IL-25 expression.
Requirements of innate cytokines for ILC2 proliferation, IL-13 production and innate cytokine receptor expression
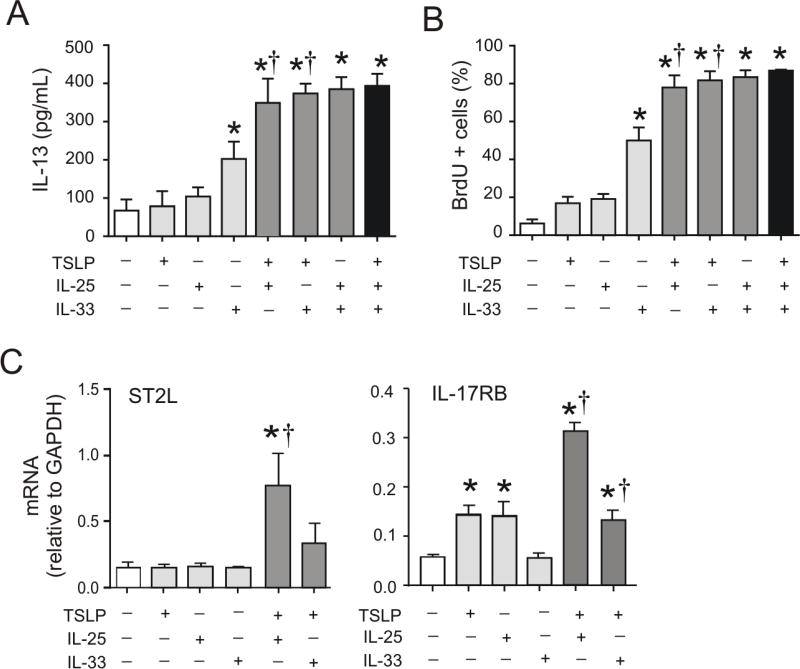
Interrelated requirements of IL-33, TSLP and IL-25 for RV-induced mucous metaplasia may also relate to effects on ILC2 expansion and function. To test this, lineage-negative, CD25 and CD127 double-positive ILC2s were sorted from the lungs of RV-infected baby mice and cultured them in the presence or absence of these cytokines, as well as IL-2 and IL-7, ex vivo. DNA synthesis was measured by BrdU uptake and IL-13 production was measured by ELISA. IL-33, but not TSLP or IL-25, significantly increased DNA synthesis and IL-13 production over that induced by IL-2 and IL-7 alone (Figures 7A and 7B). However, addition of TSLP significantly increased both IL-25- and IL-33-induced DNA synthesis and IL-13 production, with both combinations achieving a maximal response. IL-25 and IL-33, in combination with IL-2 and IL-7, also induced maximal ILC2 DNA synthesis and IL-13 production. Finally, we examined the effects of TSLP on ILC2 expression of IL17RB and IL1RL1, which encode the innate cytokine receptors IL-17RB and ST2L. TSLP significantly increased IL-25- and IL-33-induced IL17RB and IL1RL1 mRNA expression (Figure 7C).
FIG 7.
Cooperative effects of innate cytokines on ILC2 function. A–C. Lungs were collected from RV-infected immature mice, and cell suspensions were sorted for Lin− CD25+ CD127+ ILC2s by FACS. Sorted ILC2s were stimulated with a combination of IL-2, IL-7, IL-25, IL-33 or TSLP. DNA synthesis (A) and IL-13 protein production (B) of ILC2s were tested after 3 days of stimulation. The cell pellet was collected for mRNA expression by quantitative PCR (N=3–6/group). Effect of innate cytokines on ILC2 mRNA expression of IL17RB and IL1RL1 (*different from IL-2 + IL7, †different from rTSLP absence, one-way ANOVA).
Discussion
We demonstrated previously that early-life RV infection causes persistent mucus metaplasia and airway hyperresponsiveness which is dependent on IL-13 and IL-25 and associated with the expansion of IL-13-producing lung ILC2s (21). In the present study, we examined the contributions of two alternative innate cytokines, IL-33 and TSLP, to the observed asthma-like phenotype. RV infection increased lung IL-33 and TSLP levels. Specific enhancement of IL-33 expression was observed in both the airway epithelium and peribronchial cells, whereas TSLP expression was observed in the airway epithelium. Utilizing a neutralizing antibody to IL-33 and TSLPR KO mice, we showed that, compared to wild-type mice, RV-induced mucous metaplasia and airways hyperresponsiveness are significantly attenuated in anti-IL-33-treated wild-type and TSLPR KO mice. RV-induced ILC2 expansion was also significantly reduced. To understand the overlapping requirements of IL-25, IL-33 and TSLP for RV-induced mucous metaplasia, we examined the effects of anti-IL-33 and the TSLPR KO on IL-25 expression, as well as the requirements of these innate cytokines for ILC2 expansion and IL-13 production ex vivo. Both IL-33 and TSLP were required for airway epithelial cell IL-25 expression. IL-33 was sufficient for IL-25 and TSLP expression, mucous metaplasia and airways responsiveness. However, no single cytokine was sufficient for maximal ILC2 DNA synthesis or IL-13 expression. Together, these results demonstrate IL-25, IL-33 and TSLP play cooperative roles in the development of airway responses in mice with early-life RV infection.
Previous studies in mice have linked viral infection, IL-33, TSLP, ILC2s and the development of airways disease. In mice, IL-33-activated ILC2s mediate influenza-induced airways hyperresponsiveness (14). IL-33 is also required for ILC2-dependent restoration of airway epithelial integrity after influenza infection (51). Early-life pneumovirus infection induces an asthma-like phenotype in TLR7 KO mice which is accompanied by increased expression of IL-33 expression and expansion of lung ILC2s (52). Parainfluenza virus infection is followed by long-term induction of IL-33 expression and release from a subset of airway serous cells and alveolar type 2 cells linked to progenitor/stem cell function (53). IL-33 is necessary for respiratory syncytial virus (RSV)-induced bronchiolitis and ILC2s in neonatal mice (54). RSV infection activates IL-13-producing ILC2s through TSLP (55), and TSLP expression is required for IL-13 production, mucus production and airways hyperresponsiveness (56). TSLP and IL-33 expression in the mouse lung is induced by human metapneumovirus (hMPV) infection and TSLP is required for lung inflammation (57). We now report that IL-33 and TSLP are each required for RV-induced ILC2 expansion in a mouse model of developing asthma.
Few studies have addressed the overlapping functions of IL-33, TSLP and another epithelial-derived innate cytokine, IL-25, in models of airways disease. It has been suggested that the respective roles of IL-25, IL-33 and TSLP in allergic airways disease may vary depending on the type of antigen and route of antigen sensitization (i.e., intraperitoneal, intranasal or epicutaneous). However, more recent studies suggest this may not be the case (58). Instead, IL-25, IL-33 and TSLP appear to regulate expression of each other. In a house dust mite model of asthma, neutralization of IL-25 moderately reduces pulmonary eosinophilia and levels of type 2 cytokines while blocking IL-33 and TSLP expression (59). Vaccination against IL-33 also inhibits house dust mite-induced airway inflammation, including lung expression of IL-25 and TSLP expression (60). In the present study, we found that IL-33 and TSLP are each required for RV-induced IL-25 expression and deposition of IL-25 in the airway epithelium, localizing these cells as a site of cross-regulation. Anti-IL-33 had no effect on RV-induced lung TSLP levels, and the TSLPR KO had no effect on IL-33, suggesting that IL-33 and TSLP sit atop the epithelial cell-derived innate cytokine hierarchy that promotes Th2 cytokine responses. Consistent with this notion, administration of recombinant IL-33 and TSLP each induced mucous metaplasia. TSLP signaling was also required for RV-induced SPDEF mRNA expression, suggesting that epithelial-derived TSLP targets the epithelium in an autocrine manner, thereby regulating the induction of other innate cytokines via SPDEF. IL-25 has been shown to increase TSLP expression in MLE12 epithelial cells (61), additional evidence of crossregulation in the epithelium. Although RV has been previously noted to induce SPDEF expression in cultured airway epithelial cells (62), this has not been previously shown in vivo.
IL-25, IL-33 and TSLP may also have overlapping effects on immune target cells such as ILC2s. ILC2 cells produce large amounts of IL-5 and IL-13 when stimulated by IL-33 plus TSLP (16, 29, 63). To test this in the context of RV infection, we isolated ILC2s from the lungs of immature mice infected with RV. We found that addition of TSLP significantly increased both IL-25- and IL-33-induced DNA synthesis and IL-13 production, with both combinations achieving a maximal response. IL-25 and IL-33, in combination with IL-2 and IL-7, also induced maximal ILC2 DNA synthesis and IL-13 production. Such additive effects on IL-13 production may be based on the activation of common signaling pathways regulating ILC2 cytokine expression. For example, IL-33 and TSLP synergistically induce an interferon regulatory factor 4 (IRF4)-IL-9 program in ILC2s (64). In addition, we found that TSLP had additive effects on IL-25- and IL-33-induced mRNA expression of IL17RB and IL1RL1, which encode the IL-25 and IL-33 receptors IL-17RB and ST2L. Taken together, these data suggest that TSLP, IL-25 and IL-33 have additive effects on immature ILC2 function and augment cellular responsiveness to each other via upregulation of their cellular receptors.
Early-life wheezing-associated respiratory tract infections have long been considered risk factors for asthma. While initial attention focused on the potential role of RSV, evidence also exists for an association between early-life RV infection and asthma. In Finnish infants hospitalized for respiratory infection-associated wheezing, RV was associated with asthma development in contrast to RSV, which was negatively associated (17). Data from a birth cohort of high-risk infants from Wisconsin showed that wheezing-associated illness with RV is the most important risk factor for asthma development, higher than that of infants with allergen sensitization or RSV infection (18, 19). A population-based retrospective analysis of a Tennessee birth cohort showed an increased risk of early childhood asthma following bronchiolitis during RV-predominant non-winter months vs. RSV-predominant winter months (20). Finally, oral prednisolone treatment of infants hospitalized for their first episode of RV-induced wheezing decreased the risk of recurrent wheezing seven years later (65), Together, these data are consistent with the notion that early-life viral infections, including those with RV, perhaps in combination with other factors such as genetic background, allergen exposure and microbiome, modulate the immune response, increasing the likelihood of childhood asthma development.
We conclude that IL-33 and TSLP are required for epithelial cell IL-25 expression, mucus metaplasia and ILC2 expansion following early-life RV infection. Administration of intranasal IL-33, but not TSLP, was sufficient for mucous metaplasia. Finally, TSLP was required for maximal ILC2 gene expression in response to IL-25 and IL-33. The generation of mucous metaplasia in immature mice involves a complex interplay between the innate cytokines IL-25, IL-33 and TSLP.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 AI120526
The authors thank Dr. Steven Ziegler (Benaroya Research Institute, Seattle) for his gift of TSLPR KO mice. The authors also thank Drs. Nicholas Lukacs (University of Michigan Medical School) for his constructive criticism of this research.
Abbreviations
- RV
rhinovirus
- ILC2
type 2 innate lymphoid cells
- TSLP
thymic stromal lymphopoietin
- TSLPR KO
TSLP receptor knockout
- PAS
Periodic acid-Schiff
- SPDEF
SAM-pointed domain-containing ETS-like factor
- RSV
respiratory syncytial virus
- rIL-33
recombinant IL-33
- rTSLP
recombinant TSLP
References
- 1.Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK Cell Deficiency Predisposes to Viral-Induced Th2-Type Allergic Inflammation via Epithelial-Derived IL-25. J. Immunol. 2010;185:4681–4690. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 2.Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, Lloyd CM. Overexpression of Smad2 Drives House Dust Mite-mediated Airway Remodeling and Airway Hyperresponsiveness via Activin and IL-25. Am. J. Respir. Crit. Care Med. 2010;182:143–154. doi: 10.1164/rccm.200905-0725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, Waal-Malefyt Rd, Bazan F, Kastelein RA, Liu Y-J. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 5.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou B, Comeau MR, Smedt TD, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Wang Y-H, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX-F, Yao Z, Cao W, Liu Y-J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie ANJ. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 11.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie ANJ. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 2012;129:191–198. e194. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J-i, Ohtani M, Fujii H, Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 14.Chang Y-J, Kim HY, Albacker LA, Baumgarth N, McKenzie ANJ, Smith DE, DeKruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HY, Chang Y-J, Subramanian S, Lee H-H, Albacker LA, Matangkasombut P, Savage PB, McKenzie ANJ, Smith DE, Rottman JB, DeKruyff RH, Umetsu DT. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 2012;129:216–227. e216. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halim Timotheus YF, Krauß Ramona H, Sun Ann C, Takei F. Lung Natural Helper Cells Are a Critical Source of Th2 Cell-Type Cytokines in Protease Allergen-Induced Airway Inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Kotaniemi-Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J. Allergy Clin. Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemanske RF, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, Pappas TE, Dasilva DF, Tisler CJ, Gern JE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J. Allergy Clin. Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee W-M, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr Wheezing Rhinovirus Illnesses in Early Life Predict Asthma Development in High-Risk Children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, Hartert TV. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J. Allergy Clin. Immunol. 2009;123:964–966. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, Sajjan US, Hershenson MB. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J. Allergy Clin. Immunol. 2014;134:429–439. e428. doi: 10.1016/j.jaci.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, Bourne P, McClanahan TK, Pflanz S, de Waal Malefyt R. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int. Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 23.Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. Suppression of Interleukin-33 Bioactivity through Proteolysis by Apoptotic Caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Nabe T, Wakamori H, Yano C, Nishiguchi A, Yuasa R, Kido H, Tomiyama Y, Tomoda A, Kida H, Takiguchi A, Matsuda M, Ishihara K, Akiba S, Ohya S, Fukui H, Mizutani N, Yoshino S. Production of interleukin (IL)-33 in the lungs during multiple antigen challenge-induced airway inflammation in mice, and its modulation by a glucocorticoid. Eur. J. Pharmacol. 2015;757:34–41. doi: 10.1016/j.ejphar.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, Sudo K, Okumura K, Saito H, Nakae S. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci USA. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J. Allergy Clin. Immunol. 2014;134:583–592. e586. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WOCM. A Large-Scale, Consortium-Based Genomewide Association Study of Asthma. N Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belpinati F, Malerba G, Trabetti E, Galavotti R, Xumerle L, Pescollderungg L, Boner AL, Pignatti PF. Association of childhood allergic asthma with markers flanking the IL33 gene in Italian families. J. Allergy Clin. Immunol. 2011;128:667–668. doi: 10.1016/j.jaci.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Savenije OE, Mahachie John JM, Granell R, Kerkhof M, Dijk FN, de Jongste JC, Smit HA, Brunekreef B, Postma DS, Van Steen K, Henderson J, Koppelman GH. Association of IL33-IL-1 receptor-like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. J. Allergy Clin. Immunol. 2014;134:170–177. doi: 10.1016/j.jaci.2013.12.1080. [DOI] [PubMed] [Google Scholar]
- 31.Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemière C, Martin JG, Hamid Q. Increased Expression of IL-33 in Severe Asthma: Evidence of Expression by Airway Smooth Muscle Cells. J. Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 32.Saglani S, Lui S, Ullmann N, Campbell GA, Sherburn RT, Mathie SA, Denney L, Bossley CJ, Oates T, Walker SA, Bush A, Lloyd CM. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J. Allergy Clin. Immunol. 2013;132:676–685. e613. doi: 10.1016/j.jaci.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson DJ, Makrinioti H, Rana BMJ, Shamji BWH, Trujillo-Torralbo M-B, Footitt J, Jerico d-R, Telcian AG, Nikonova A, Zhu J, Aniscenko J, Gogsadze L, Bakhsoliani E, Traub S, Dhariwal J, Porter J, Hunt D, Hunt T, Hunt T, Stanciu LA, Khaitov M, Bartlett NW, Edwards MR, Kon OM, Mallia P, Papadopoulos NG, Akdis CA, Westwick J, Edwards MJ, Cousins DJ, Walton RP, Johnston SL. IL-33-Dependent Type 2 Inflammation during Rhinovirus-induced Asthma Exacerbations In Vivo. Am. J. Respir. Crit. Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, Himes BE, Levin AM, Mathias RA, Hancock DB, Baurley JW, Eng C, Stern DA, Celedon JC, Rafaels N, Capurso D, Conti DV, Roth LA, Soto-Quiros M, Togias A, Li X, Myers RA, Romieu I, Van Den Berg DJ, Hu D, Hansel NN, Hernandez RD, Israel E, Salam MT, Galanter J, Avila PC, Avila L, Rodriquez-Santana JR, Chapela R, Rodriguez-Cintron W, Diette GB, Adkinson NF, Abel RA, Ross KD, Shi M, Faruque MU, Dunston GM, Watson HR, Mantese VJ, Ezurum SC, Liang L, Ruczinski I, Ford JG, Huntsman S, Chung KF, Vora H, Li X, Calhoun WJ, Castro M, Sienra-Monge JJ, del Rio-Navarro B, Deichmann KA, Heinzmann A, Wenzel SE, Busse WW, Gern JE, Lemanske RF, Jr, Beaty TH, Bleecker ER, Raby BA, Meyers DA, London SJ, Gilliland FD, Burchard EG, Martinez FD, Weiss ST, Williams LK, Barnes KC, Ober C, Nicolae DL. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat. genetics. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Doi S, Fujita K, Miyatake A, Enomoto T, Miyagawa T, Adachi M, Tanaka H, Niimi A, Matsumoto H, Ito I, Masuko H, Sakamoto T, Hizawa N, Taniguchi M, Lima JJ, Irvin CG, Peters SP, Himes BE, Litonjua AA, Tantisira KG, Weiss ST, Kamatani N, Nakamura Y, Tamari M. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat. genetics. 2011;43:893–896. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, Miyagawa T, Doi S, Kameda M, Fujita K, Miyatake A, Enomoto T, Noguchi E, Masuko H, Sakamoto T, Hizawa N, Suzuki Y, Yoshihara S, Adachi M, Ebisawa M, Saito H, Matsumoto K, Nakajima T, Mathias RA, Rafaels N, Barnes KC, Himes BE, Duan QL, Tantisira KG, Weiss ST, Nakamura Y, Ziegler SF, Tamari M. Thymic Stromal Lymphopoietin Gene Promoter Polymorphisms Are Associated with Susceptibility to Bronchial Asthma. Am. J. Respir. Cell Mol. Biol. 2011;44:787–793. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M, Rogers L, Cheng Q, Shao Y, Fernandez-Beros ME, Hirschhorn JN, Lyon HN, Gajdos ZKZ, Vedantam S, Gregersen P, Seldin MF, Bleck B, Ramasamy A, Hartikainen A-L, Jarvelin M-R, Kuokkanen M, Laitinen T, Eriksson J, Lehtimäki T, Raitakari OT, Reibman J. Genetic Variants of TSLP and Asthma in an Admixed Urban Population. PLoS One. 2011;6:25099. doi: 10.1371/journal.pone.0025099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunninghake GM, Soto-Quirós ME, Avila L, Kim HP, Lasky-Su J, Rafaels N, Ruczinski I, Beaty TH, Mathias RA, Barnes KC, Wilk JB, O’Connor GT, James Gauderman W, Vora H, Baurley JW, Gilliland F, Liang C, Sylvia JS, Klanderman BJ, Sharma SS, Himes BE, Bossley CJ, Israel E, Raby BA, Bush A, Choi AM, Weiss ST, Celedón JC. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010;65:1566–1575. doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biagini Myers JM, Martin LJ, Kovacic MB, Mersha TB, He H, Pilipenko V, Lindsey MA, Ericksen MB, Bernstein DI, LeMasters GK, Lockey JE, Khurana Hershey GK. Epistasis between serine protease inhibitor Kazal-type 5 (SPINK5) and thymic stromal lymphopoietin (TSLP) genes contributes to childhood asthma. J. Allergy Clin. Immunol. 2014;134:891–899. e893. doi: 10.1016/j.jaci.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic Stromal Lymphopoietin Expression Is Increased in Asthmatic Airways and Correlates with Expression of Th2-Attracting Chemokines and Disease Severity. J. Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 42.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, Lee TH, Corrigan CJ. Expression and Cellular Provenance of Thymic Stromal Lymphopoietin and Chemokines in Patients with Severe Asthma and Chronic Obstructive Pulmonary Disease. J. Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 43.Gauvreau GM, O’Byrne PM, Boulet L-P, Wang Y, Cockcroft D, Bigler J, FitzGerald JM, Boedigheimer M, Davis BE, Dias C, Gorski KS, Smith L, Bautista E, Comeau MR, Leigh R, Parnes JR. Effects of an Anti-TSLP Antibody on Allergen-Induced Asthmatic Responses. N. Engl. J. Med. 2014;370:2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 44.Perez GF, Pancham K, Huseni S, Preciado D, Freishtat RJ, Colberg-Poley AM, Hoffman DP, Rose MC, Nino G. Rhinovirus infection in young children is associated with elevated airway TSLP levels. Eur. Respir. J. 2014;44:1075–1078. doi: 10.1183/09031936.00049214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newcomb DC, Sajjan U, Nanua S, Jia Y, Goldsmith AM, Bentley JK, Hershenson MB. Phosphatidylinositol 3-Kinase Is Required for Rhinovirus-induced Airway Epithelial Cell Interleukin-8 Expression. J. Biol. Chem. 2005;280:36952–36961. doi: 10.1074/jbc.M502449200. [DOI] [PubMed] [Google Scholar]
- 46.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, Lukacs NW, Johnston SL, Hershenson MB. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2008;177:1111–1121. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagarkar DR, Bowman ER, Schneider D, Wang Q, Shim J, Zhao Y, Linn MJ, McHenry CL, Gosangi B, Bentley JK, Tsai WC, Sajjan US, Lukacs NW, Hershenson MB. Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J. Immunol. 2010;185:2525–2535. doi: 10.4049/jimmunol.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han M, Hong JY, Jaipalli S, Rajput C, Lei J, Hinde JL, Chen Q, Hershenson NM, Bentley JK, Hershenson MB. IFN-γ blocks development of an asthma phenotype in rhinovirus-infected baby mice by inhibiting type 2 innate lymphoid cells. Am. J. Respir. Cell Mol. Biol. 2017;56:242–251. doi: 10.1165/rcmb.2016-0056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider D, Hong JY, Popova AP, Bowman ER, Linn MJ, McLean AM, Zhao Y, Sonstein J, Bentley JK, Weinberg JB, Lukacs NW, CurtisO JL, Sajjan US, Hershenson MB. Neonatal rhinovirus infection induces mucous metaplasia and airways hyperresponsiveness. J. Immunol. 2012;188:2894–2904. doi: 10.4049/jimmunol.1101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J. Clin. Invest. 2015;125:2021–2031. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaiko GE, Loh Z, Spann K, Lynch JP, Lalwani A, Zheng Z, Davidson S, Uematsu S, Akira S, Hayball J, Diener KR, Baines KJ, Simpson JL, Foster PS, Phipps S. Toll-like receptor 7 gene deficiency and early-life Pneumovirus infection interact to predispose toward the development of asthma-like pathology in mice. J. Allergy Clin. Immunol. 2013;131:1331–1339. e1310. doi: 10.1016/j.jaci.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 53.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard J-P, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, Holtzman MJ. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saravia J, You D, Shrestha B, Jaligama S, Siefker D, Lee GI, Harding JN, Jones TL, Rovnaghi C, Bagga B, DeVincenzo JP, Cormier SA. Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33. PLoS Pathog. 2015;11:1005217. doi: 10.1371/journal.ppat.1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, Quitalig M, Hotard AL, Moore ML, Hartert TV, Zhou B, McKenzie AN, Peebles RS., Jr Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2016;138:814–824. e811. doi: 10.1016/j.jaci.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee H-C, Headley MB, Loo Y-M, Berlin A, Gale M, Jr, Debley JS, Lukacs NW, Ziegler SF. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012;130:1187–1196. e1185. doi: 10.1016/j.jaci.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lay MK, Céspedes PF, Palavecino CE, León MA, Díaz RA, Salazar FJ, Méndez GP, Bueno SM, Kalergis AM. Human metapneumovirus infection activates the TSLP pathway that drives excessive pulmonary inflammation and viral replication in mice. Eur. J. Immunol. 2015;45:1680–1695. doi: 10.1002/eji.201445021. [DOI] [PubMed] [Google Scholar]
- 58.Morita H, Arae K, Unno H, Toyama S, Motomura K, Matsuda A, Suto H, Okumura K, Sudo K, Takahashi T, Saito H, Matsumoto K, Nakae S. IL-25 and IL-33 Contribute to Development of Eosinophilic Airway Inflammation in Epicutaneously Antigen-Sensitized Mice. PLoS One. 2015;10:0134226. doi: 10.1371/journal.pone.0134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregory LG, Jones CP, Walker SA, Sawant D, Gowers KHC, Campbell GA, McKenzie ANJ, Lloyd CM. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013;68:82–90. doi: 10.1136/thoraxjnl-2012-202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lei Y, Boinapally V, Zoltowska A, Adner M, Hellman L, Nilsson G. Vaccination against IL-33 Inhibits Airway Hyperresponsiveness and Inflammation in a House Dust Mite Model of Asthma. PLoS One. 2015;10:0133774. doi: 10.1371/journal.pone.0133774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angkasekwinai P, Park H, Wang Y-H, Wang Y-H, Chang SH, Corry DB, Liu Y-J, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korfhagen TR, Kitzmiller J, Chen G, Sridharan A, Haitchi H-M, Hegde RS, Divanovic S, Karp CL, Whitsett JA. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc. Natl. Acad. Sci USA. 2012;109:16630–16635. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang HE, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal. Immunol. 2016;9:275–286. doi: 10.1038/mi.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr. Allergy Immunol. 2013;24:237–243. doi: 10.1111/pai.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.