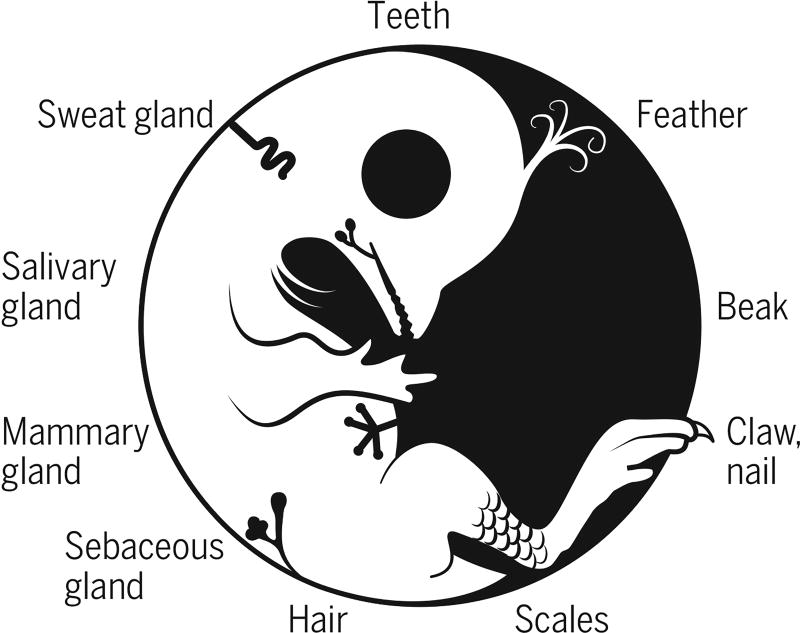
The integument forms the interface between an organism and its environment. It serves diverse functions such as communication, endothermy, defense, and flight. During vertebrate evolution, various integumentary organs, including hairs, feathers, glands, and teeth, have evolved to help animals adapt to evolving environmental changes (1) (see the first figure). These ectodermal organs form through epithelial-dermal interactions. Classic tissue recombination experiments have demonstrated that the dermis specifies the organ phenotypes within a developmental time window (2). Yet the underlying mechanisms remain unclear. On page 1551 of this issue, Lu et al. (3) reveal the specific molecular mechanism whereby an epithelial placode can be guided to form either a hair follicle for its architecture or a sweat gland for its secretory function.
Evo-devo of integuments.
A prototype animal with diverse forms of integumentary appendages.
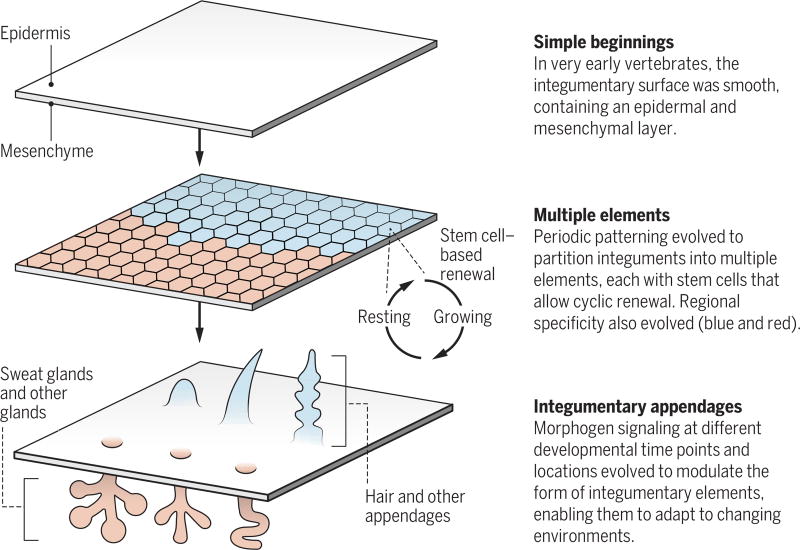
It is instructive to consider nature’s way of integumentary organ design—the “tao” of this process. Periodic patterning has evolved as an effective integument organization principle (4) (see the second figure). This is achieved by partitioning the integument into numerous elements (e.g., on average, >30,000 hair follicles in a mouse). Each element contains its own stem cells and undergoes cyclic regeneration, thus allowing it to regenerate after wear or injury. Further, each element can be independently controlled by modulating the stem cells to form different appendage types under different physiological or hormone conditions (e.g., rooster or hen feathers) and in different body regions (e.g., downy feathers become flight, tail, or contour feathers) (5). Collectively, this is an effective way to build complex yet adaptable integuments.
Organizing to adapt.
Periodic patterning enables the formation of complex yet adaptable integument organs.
Stem cells enable a hair or feather follicle to undergo cyclic renewal, and each cycle is composed of growing and resting phases. The length of the hair filament is a function of the duration of the growth phase, which is regulated by fibroblast growth factor 5 (FGF5) (6). This mechanism enables closely related species to quickly adapt to cold or warm environments. Yet this is a quantitative change. What about qualitative changes that alter organ functions?
Lu et al. demonstrate how hair follicle and sweat gland fates can be switched. In mice, sweat glands are confined to the paw regions, and the dorsal skin can only form hair follicles. Transciptome profiling suggested distinctly different amounts of several bone morphogenetic proteins (BMPs), WNT proteins, and FGF proteins in regions producing hair follicles versus those producing sweat glands. Previous experiments already showed that suppressing BMP signaling could convert sweat glands in mouse paws into hairs (7). Here, the authors used inducible and tissue-specific transgenic mouse and lentivirus technology to perturb morphogen signaling levels at different times and in different locations. Suppressing BMP signaling caused placodes in the ventral foot to switch fates to form hairs, whereas increasing BMP signaling guided placodes on the dorsal skin to switch fates toward sweat glands.
BMP works in a circuit with WNT, FGF, and sonic hedgehog (SHH), and the authors show that BMP signaling in the dermis differentially elevates WNT expression to higher levels in the ventral foot than in the dorsal skin. In WNT-responsive dermal cells, the expression of several FGFs are highly up-regulated in the ventral foot relative to the dorsal skin. Ectopic FGF18 increased expression of the glandular marker K18, decreased expression of the hair follicle marker K17, and caused hair loss in the dorsal skin. Furthermore, ectopic expression of FGF18, BMP4, and BMP5 in the dorsal epidermis induced Engrailed-1 expression in the ventral foot but not in the dorsal epithelium, and caused a more glandular fate. SHH is highly expressed in the hair relative to sweat gland placodes. Increasing SHH signaling in the ventral foot epidermis induced or repressed the expression of hair-specific markers and gland-specific markers, respectively. Thus, Lu et al. identify a molecular circuit in the ventral foot that works synergistically to form sweat glands.
One of the critical events in the evolution of early humans is the expansion of the sweat gland–producing region to cover the body (8). This alteration allowed people to have improved heat tolerance, which enabled them to run long distances in big game hunting while maintaining their body temperature. Does similar circuitry function in controlling hair versus sweat gland fate determination in humans? Lu et al. found high BMP activity spikes around 17 weeks of gestation, shifting placode fates from hairs to glands. Thus, similar sweat gland–forming circuitry is activated by spatial signals in mice but by temporal signals in humans. The next challenge will be to find out how BMPs are regulated spatiotemporally.
There are many more examples in which modification of the integument helps animals adapt to their changing environment. For example, the tooth changes shape in related rodents to enable optimal use of their diets (9). In filter feeding, baleen whales’ tooth formation is shut off and modified hair bundles form in the mouth cavity instead (see the photo). At the macroevolutionary level, changes in integument organs help define a whole new vertebrate class (10). For example, the conversion of scaly integument to feathers in feathered dinosaurs was the prelude to the birth of the bird as a whole new class (11, 12). And in mammals, the evolution of mammary glands helps define the mammalian class (13, 14). Thus, the ensemble of modules (hair or feather follicles) allows the generation of a complex integument that is highly adaptable. Modulation of integument phenotypes can occur at the genomic level with consequences at the evolutionary scale, or at the epigenetic level with metamorphic changes in the same individual, or in the same species but at different ages, sexes, and seasons (15).
For filter-feeding animals such as baleen whales, modification of the integument blocks tooth formation in the mouth cavity to permit hair bundles to form instead.
Understanding the “tao” of the integument can also lead to medical benefits. With the progress of stem cell biology, scientists now have access to ectodermal progenitors. These new insights will help our efforts to guide these progenitors into new integument organ phenotypes for regenerative medicine.
Acknowledgments
Supported by NIH grants AR42177 and AR60306 (C.-M.C.), grants from China Medical University Hospital, and a fellowship from National Taiwan University (Y.C.L.). We thank R. Widelitz and P. Wu for help with the manuscript.
References
- 1.Chuong C-M, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Landes Bioscience; Austin, TX: 1998. [Google Scholar]
- 2.Dhouailly D. Wilhelm Roux’s Arch. Dev. Biol. 1975;177:323. doi: 10.1007/BF00848183. [DOI] [PubMed] [Google Scholar]
- 3.Lu CP, Polak L, Keyes BE, Fuchs E. Science. 2016;354:aah6102. doi: 10.1126/science.aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuong C-M, Yeh CY, Ting-Xin J, Widelitz RB. Wiley Interdisc. Rev. Dev. Biol. 2013;2:97. doi: 10.1002/wdev.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuong C-M, et al. Physiology. 2012;27:61. doi: 10.1152/physiol.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebert JM, et al. Cell. 1994;78:1017. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 7.Plikus M, et al. Am. J. Pathol. 2004;164:1099. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamberov YG, et al. Proc. Natl. Acad. Sci. U.S.A. 2015;112:9932. doi: 10.1073/pnas.1511680112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jernvall J, Keränen SV, Thesleff I. Proc. Natl. Acad. Sci. U.S.A. 2000;97:14444. doi: 10.1073/pnas.97.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu P, et al. Int. J. Dev. Biol. 2004;48:249. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuong C-M, et al. J. Exp. Zool. B. 2003;298:42. doi: 10.1002/jez.b.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, et al. Science. 2014;346:1253293. doi: 10.1126/science.1253293. [DOI] [PubMed] [Google Scholar]
- 13.Oftedal OT. Animal. 2012;6:355. doi: 10.1017/S1751731111001935. [DOI] [PubMed] [Google Scholar]
- 14.Widelitz RB, et al. Semin. Cell Dev. Biol. 2007;18:255. doi: 10.1016/j.semcdb.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei M, Chuong C-M. Science. 2016;351:559. doi: 10.1126/science.aaf1635. [DOI] [PubMed] [Google Scholar]