Abstract
Caffeic acid phenethyl ester (CAPE), extracted from propolis, was proven to inhibit colon cancer. Caffeic acid p-nitro-phenethyl ester (CAPE-pNO2), a derivative of CAPE, was determined to be an anti-platelet agent and a protector of myocardial ischaemia with more potent effects. In the present study, CAPE-pNO2 showed stronger cytotoxic activity than CAPE. We revealed interactions between CAPE-pNO2 and experimental cells. CAPE-pNO2 induced apoptosis in HT-29 cells by up-regulating P53, cleaved-caspase-3, Bax, P38 and CytoC; CAPE-pNO2 also up-regulated P21Cip1 and P27Kip1 and down-regulated CDK2 and c-Myc to promote cell cycle arrest in G0/G1. In xenograft studies, CAPE-pNO2 remarkably suppressed tumour growth dose dependently and decreased the expression of VEGF (vascular endothelial growth factor) in tumour tissue. Moreover, HE staining showed that no observable toxicity was found in the heart, liver, kidney and spleen. In addition, metabolites of CAPE-pNO2 in HT-29 cells and organs were detected. In conclusion, para-nitro may enhance the anticancer effect of CAPE by inhibiting colon cancer cell viability, inducing apoptosis and cell cycle arrest via the P53 pathway and inhibiting tumour growth and reducing tumour invasion by decreasing the expression of VEGF; additionally, metabolites of CAPE-pNO2 showed differences in cells and organs.
Introduction
Colon cancer is a common malignant tumour of the digestive tract. In clinical settings, approximately 1.4 million colon cancer patients were diagnosed and more than 690 thousand patients died from colon cancer worldwide in 20121. Risk factors of colon cancer mainly include increasing age, male sex, a high-fat diet, an inadequate intake of fibre and a sedentary lifestyle. Nearly 50% of patients die from recrudescence and metastatic diseases two years after curative resections2, 3. Based on a series of statistical data, lower expression of Bcl-2 and overexpression of Bax contribute to the lower survival rate in colon cancer patients. Caspase-9 overexpression leads to cell apoptosis and G0/G1 arrest and reduces the secretion of carcinoembryonic antigen, and Caspase-3 can induce the damage of DNA4–7. The abnormal expression of cell cycle-associated proteins (c-Myc and CDK2) may promote the occurrence of colon cancer8–11. Wang, J. J. et al. reported that 1, 6-bis [4-(4-amino-3-hydroxyphenoxy) -phenyl] (DPD) inhibited colon cancer cell (HCT-116) activity and tumour growth through the P21 signalling pathway12. These studies implied that the occurrence of colon cancer is related to the P53 pathway.
Caffeic acid phenethyl ester (CAPE) has been identified as the main active component of propolis. It has been reported that CAPE possesses antioxidant, anti-inflammatory and anti-cancer effects13–15. It can inhibit prostate cancer by regulating the expression of Skp2, P27Kip1, P21Cip1 and P53, reduce VEGF secretion in MDA-231 cells in a dose-dependent manner16–18; up-regulate E2F-1, P53 and P21 expression in cervical cancer cells; and change the expression of Cyclin A, Cyclin B and Cyclin C in the cell cycle19. Additionally, CAPE exerts therapeutic effects on cholangiocarcinoma, lung cancer, liver cancer and oral cancer20–23. In our previous study, caffeic acid para-nitro-phenethyl ester (CAPE-pNO2) was developed and proven to be more effective than CAPE in acute myocardial ischaemic and reperfusion injury in rats and other blood-related diseases in previous studies24, 25. However, after the introduction of the nitro-group in CAPE, the structure-dependent changes in the anticancer activity of the CAPE derivatives against colon cancer are still unknown. Hence, we comparatively studied the anti-colon cancer effects of CAPE-pNO2 and CAPE, and detected the metabolites by LC-MS/MS in colon cancer cells, xenograft tumour and organs.
Results
CAPE and CAPE-pNO2 inhibit cell proliferation
The inhibitory effects of CAPE and CAPE-pNO2 on colon cancer cells were measured by the MTT assay. In HT-29 cells (Fig. 1B), CAPE and CAPE-pNO2 inhibited cell viability in a dose-dependent manner, the IC50 of CAPE and CAPE-pNO2 were 44.5 μM and 29.7 μM. For HCT-116 cells (Fig. 1C), the IC50 of CAPE and CAPE-pNO2 were 47.2 μM and 33.8 μM. The cell viability of HT-29 cells and HCT-116 cells was increased after PFT-α treating, and PFT-α decreased the effect of CAPE and CAPE-pNO2.
Figure 1.
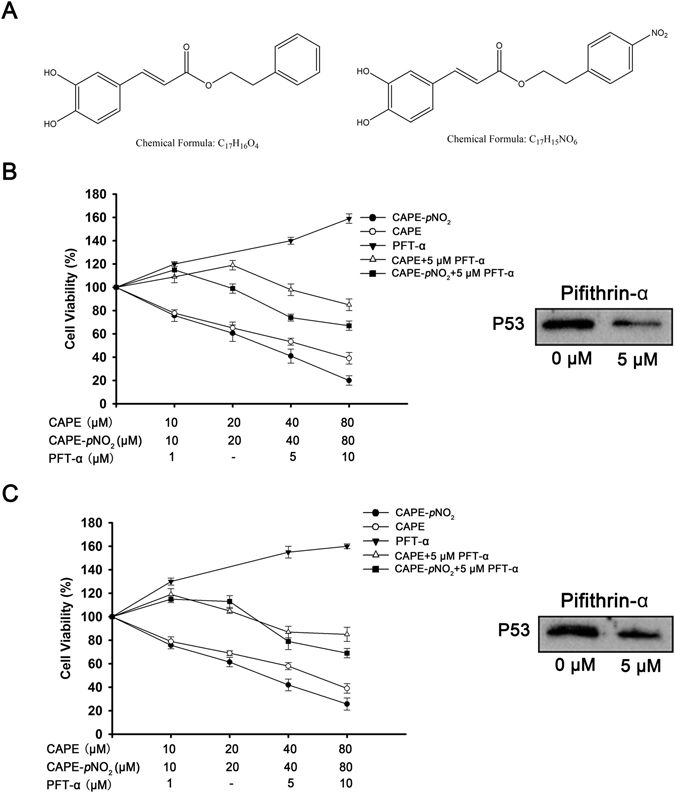
Chemical structure of the compounds used in the present study and results of the MTT assay. (A) Chemical structure of CAPE and CAPE-pNO2. HT-29 cells (B) and HCT-116 cells (C) were treated with CAPE and CAPE and CAPE-pNO2 for 48 h, and the expression of P53 after treatment by PFT-α. Values represented the means ± SD from three independent experiments, and error bars represented the STDEV (SD).
CAPE and CAPE-pNO2 induce colon cancer cell apoptosis
After 48 h of treatment, the apoptosis rates were identified by flow cytometry (Fig. 2C,D). The data showed that CAPE-pNO2 induced colon cancer cell apoptosis in a dose-dependent manner, and the effect of CAPE-pNO2 was distinctly higher than that of CAPE. At 40 μM, the apoptosis rates in the CAPE and CAPE-pNO2 treatment groups were 34.0% and 49.0%, respectively, in HT-29 cells and 39.0% and 47.0%, respectively, in HCT-116 cells (Fig. 2A,B). Hoechst 33342 staining was also used to measure cell apoptosis. From the microscopic vision fields, the number of HT-29 and HCT-116 cells was decreased, and the fluorescence intensity was increased with increasing concentrations of CAPE and CAPE-pNO2. The results suggested that the effect of CAPE-pNO2 is stronger than that of CAPE (p < 0.01) (Fig. 2E,F).
Figure 2.
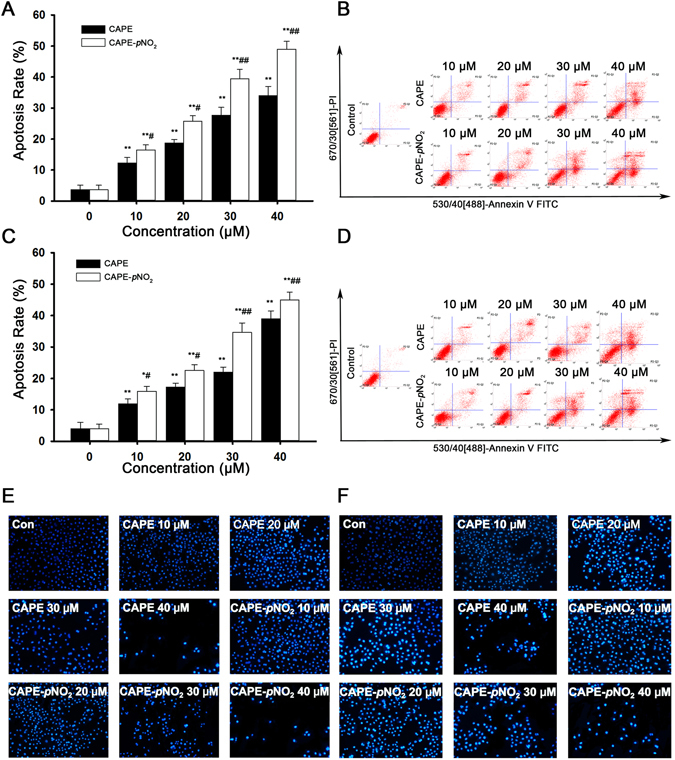
CAPE and CAPE-pNO2 induce apoptosis in colon cancer cells. HT-29 and HCT-116 cells were treated with 0, 10, 20, 30 and 40 μmol/L CAPE and CAPE-pNO2 for 48 h. The apoptosis rates of HT-29 cells (A) and HCT-116 cells (B) were calculated by SigmaPlot 12.5. Flow cytometry analysis in HT-29 (C) and HCT-116 (D) cells. HT-29 cells (E) and HCT-116 (F) cells (×200) were stained by Hoechst 33342 after treatment with different concentrations of CAPE and CAPE-pNO2 for 48 h. From the microscopic vision fields, the cell number was decreased, and fluorescence was increased by treatment for 48 h in a dose-dependent manner. Values represented the means ± SD from three independent experiments, and error bars represented the STDEV (SD). *p < 0.05, **p < 0.01: CAPE and CAPE-pNO2 compared with the control. # p < 0.05, ## p < 0.01: CAPE-pNO2 compared with CAPE at the same concentration.
CAPE and CAPE-pNO2 induce cell cycle arrest in G0/G1 in colon cancer cells
The cell cycle distribution was detected by flow cytometry. As shown in Fig. 3, the number of HT-29 cells (Fig. 3A) in G0/G1 phase increased in a dose-dependent manner from 31.9% to 77.3% and 86.5% after treatment with CAPE and CAPE-pNO2, respectively. Concerning HCT-116 cells (Fig. 3B), the G0/G1 phase increased from 31.0% to 80.1% and 84.5%, but the G2/M phase was barely changed in the present study. The results proved that the progression of cells from G1 to the S phase was interrupted more obviously by CAPE-pNO2 than by CAPE (p < 0.01) (Fig. 3C,D).
Figure 3.
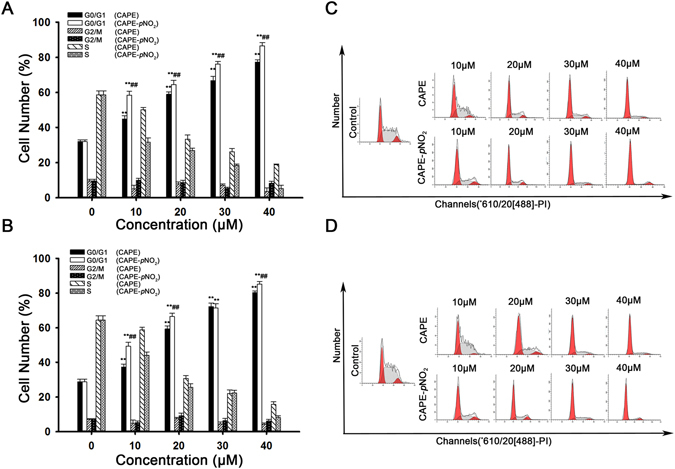
CAPE and CAPE-pNO2 induce cell cycle arrest at the G0/G1 phase in colon cancer cells. The percentage of cells in G0/G1, S and G2/M phase was calculated using Multicycle software. Changes in the cell cycle distribution in HT-29 (A) and HCT-116 (B) cells after treatment for 48 h with the concentration from 0 to 40 μmol/L. HT-29 (C) and HCT-116 (D) cells were stained with PI and were analysed by flow cytometry. The data represented the means ± SD from three independent experiments, and error bars represented with STDEV (SD). *p < 0.05, **p < 0.01: CAPE and CAPE-pNO2 compared with the control. # p < 0.05, ## p < 0.01: CAPE-pNO2 compared with CAPE at the same concentration.
CAPE and CAPE-pNO2 regulate the expression of P53 signalling pathway related proteins in HT-29 cell and tumours
The expression of related proteins in HT-29 cells was measured by western blot assay after treatment with CAPE and CAPE-pNO2 (Fig. 4). The results showed that these two drugs could down-regulate the expression of pro-caspase-3, which was reduced by 44.0% and 79.0% after treatment with CAPE and CAPE-pNO2, respectively, while cleaved-caspase-3 was increased 2.1- and 3.1-fold, respectively. CAPE and CAPE-pNO2 could up-regulate the expression of Bax, CytoC, P53 and P38, and the up-regulated specific data were 0.5- and 0.9-fold in Bax, 0.7- and 1.1-fold in CytoC, 0.7- and 1.0-fold in P53, and 0.43- and 0.78-fold in P38, respectively. The above effects occurred in a dose-dependent manner within 10 μM to 40 μM. These results showed that CAPE and CAPE-pNO2 induce HT-29 cell apoptosis likely through the P53 signalling pathway, and the effect of CAPE-pNO2 is stronger. CAPE and CAPE-pNO2 also regulated cell cycle-related proteins. After treatment with CAPE and CAPE-pNO2 at 40 μM for 48 h, C-Myc and CDK2 were reduced by 51.0%, 63.0% and 55.0%, 67.0%, while P21Cip1 and P27kip1 were increased 1.7- and 1.8-fold and 1.7- and 3.0-fold. In tumours, CAPE-pNO2 could regulate expression of P53 signaling pathway related proteins in a dose-dependent manner. CAPE and CAPE-pNO2 could up-regulated P53, P21Cip1, P27Kip1, CytoC and Cleaved Caspase-3, down-regulated c-Myc, CDK2 and Pro-Caspase-3. After CAPE and CAPE-pNO2 treating in the same dose of 10 mg/kg/day, the expressions of these proteins were up to 114% and 183% in P53, 180% and 260% in P27Kip1, 137% and 207% in P21Cip1, 113% and 183% in CytoC, 120% and 206% in Cleaved Caspase-3, while down to 63% and 49% in Pro-Caspase-3, 80% and 51% in CDK2, 82% and 53% in c-Myc, respectively (Fig. 7).
Figure 4.
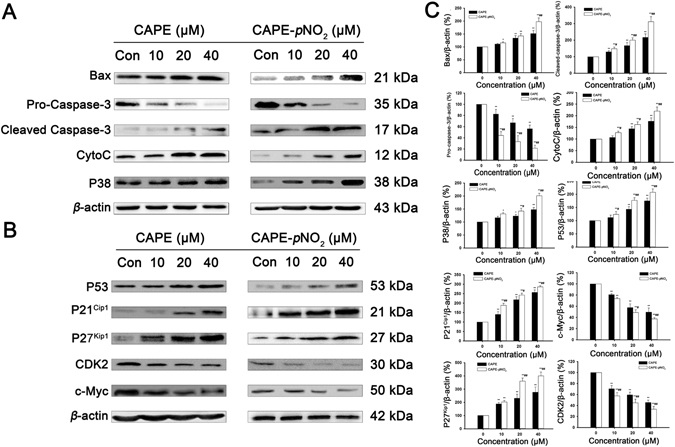
Regulation of apoptosis-related and cycle-related proteins by CAPE and CAPE-pNO2 treatment in HT-29 cells. HT-29 cells were treated with CAPE and CAPE-pNO2 at a dose of 0, 10, 20 or 40 μmol/L for 48 h. (A) Apoptosis-related proteins in the P53 signalling pathway such as Bax, Pro-Caspase-3, Cleaved-Caspase-3, CytoC and P38 were determined by western blotting. (B) The cell cycle-related protein expression levels of P21 Cip1, P27Kip1, P53, CDK2 and c-Myc were determined by western blotting. The blots were representative of three independent experiments. Data represented the means ± SD from three independent experiments, and error bars represented the STDEV (SD). *p < 0.05, **p < 0.01: CAPE and CAPE-pNO2 compared with the control. # p < 0.05, ## p < 0.01: CAPE-pNO2 compared with CAPE at the same concentration.
Figure 5.
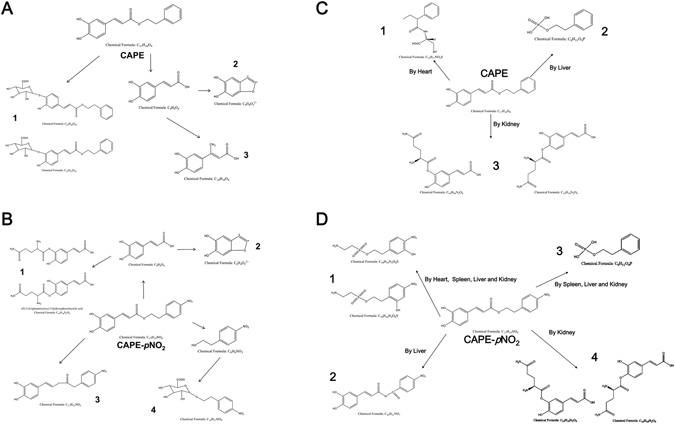
Metabolite analysis of CAPE and CAPE-pNO2 in HT-29 cells and in tumours, heart, liver, spleen and kidney. Metabolic processes of CAPE (A) and CAPE-pNO2 (B) in HT-29 cells. Metabolite analysis of CAPE (C) and CAPE-pNO2 (D) in tumours, heart, liver, spleen and kidney.
Metabolite analysis in HT-29 cells, xenograft tumour and several organs after treating with CAPE and CAPE-pNO2
The metabolites of CAPE and CAPE-pNO2 in HT-29 cells were analysed using the Triple TOFTM 4600 system and MetabolitePilot 1.5 software. Three metabolites of CAPE were detected: (2S,3S,4R,5R,6S)-3,4,5-trihydroxy-6-(2-hydroxy-5-((E)-3-oxo-3-phenethoxyprop-1-en-1-yl) phenoxy)tetrahydro-2H-pyran-2-carboxylic acid or (2S,3S,4R,5R,6S)-3,4,5-trihydroxy-6-(2- hydroxy-4-((E)-3-oxo-3-phenethoxyprop-1-en-1-yl)phenoxy)tetrahydro-2H-pyran-2-carboxylic acid (C 23 H 24 O 10 ), 5,6-dihydroxybenzofuran-2,3-diylium (C 8 H 4 0 3 2+ ) and (E)-3-(3,4- dihydroxyphenyl) but-2-enoic acid (C 10 H 10 O 4 ). Four metabolites of CAPE- p NO 2 were detected: (E)-3-(4-(glutaminyloxy)-3-hydroxyphenyl) acrylic acid or (E)-3-(3- (glutaminyloxy)-4-hydroxyphenyl) acrylic acid (C 10 H 10 O 4 ), 5,6-dihydroxybenzo-furan-2,3 -diylium (C 8 H 4 0 3 2+ ), (E)-5-(3,4-dihydroxyphenyl)-1-(4-nitrophenyl) pent-4-en-2-one (C 17 H 15 NO 5 ) and (2S,3S,4R,5R)-3,4,5-trihydroxy-6-(4-nitrophenethoxy)tetrahydro-2H -pyran -2-carboxylic acid (C 14 H 17 NO 9 ). Only C8H403 2+ was a common metabolite of CAPE and CAPE-pNO2 (Figs 5A,B and S1).
Figure 6.
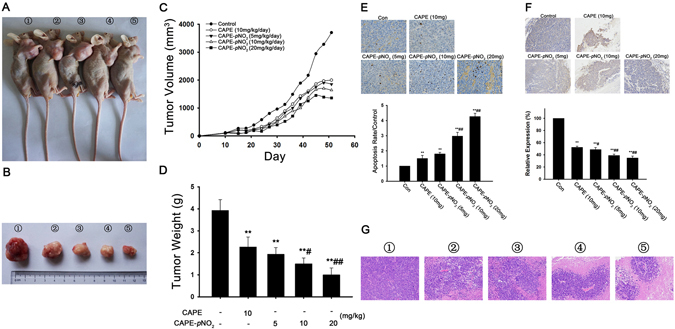
CAPE and CAPE-pNO2 inhibit tumour growth in vivo. After 41-day treatment, tumours were observed in vivo (A) and in vitro (B); ①, ②, ③, ④ and ⑤ represented the control, CAPE-10 mg/kg/day, CAPE-pNO2-5 mg/kg/day, CAPE-pNO2-10 mg/kg/day and CAPE-pNO2-20 mg/kg/day groups, respectively. (C) After nude mice were injected with HT-29 cells for 9 days, CAPE and CAPE-pNO2 were given intragastrically for 42 days. The tumour volume was measured every three days. (D) Inhibition rate of tumour growth after treatment with CAPE and CAPE-pNO2. Apoptosis and expression were detected by TUNEL and immunohistochemistry. (E) TUNEL staining in tumours cells (×400), and the relative apoptosis rate was calculated using Image-Pro Plus (IPP) software. (F) Results of immunohistochemistry (×200). The integrated option density (IOD) value was used to measure the expression level of VEGF. (G) The paraffin sections of tumours were stained with haematoxylin and eosin (HE), and the necrotic area and shrinking nucleus were increased after treatment in a dose-dependent manner; ①, ②, ③, ④ and ⑤ represented the control, CAPE-10 mg/kg/day, CAPE-pNO2-5 mg/kg/day, CAPE-pNO2-10 mg/kg/day and CAPE-pNO2-20 mg/kg/day groups. Values represented the means ± SD from three independent experiments, and error bars represented the STDEV (SD). *p < 0.05, **p < 0.01: CAPE and CAPE-pNO2 compared with the control. # p < 0.05, ## p < 0.01: CAPE-pNO2 (5, 10, 20 mg/kg/day) compared with CAPE (10 mg/kg/day).
CAPE was transformed into (2-phenylbutanoyl)-D-cysteine (C 13 H 17 NO 3 S) in the heart, phenethyl dihydrogen phosphate (C 8 H 11 O 4 P) in the liver, and (E)-3-(3-((L-glutaminyl) oxy)-4-hydroxyphenyl) acrylic acid or (E)-3-(4-((D-glutaminyl) oxy)-3-hydroxyphenyl) acrylic acid (C 14 H 16 N 2 O 6) in the kidney. However, CAPE-pNO2 was metabolized into 3-hydroxy-4-nitrophenethyl 2-aminoethane-1-sulfonate or 2-hydroxy-4-nitrophenethyl 2-aminoethane-1-sulfonate (C 10 H 14 N 2 O 6 S) in the heart, liver, spleen and kidney and phenethyl dihydrogen phosphate (C 8 H 11 O 4 P) in the liver, spleen and kidney. Additionally, (E)-(E)-3-(3,4-dihydroxyphenyl) acrylic 4-nitrobenzoic anhydride (C 16 H 11 NO 7) and (E)-3-(3-((L-glutaminyl) oxy)-4-hydroxyphenyl) acrylic acid or (E)-3-(4-((D-glutaminyl) oxy) -3-hydroxyphenyl) acrylic acid (C 14 H 16 N 2 O 6) were found in the liver and kidney, respectively (Figs 5C,D and S2,S3). Notably, all of the above metabolites were not found in xenograft tumours.
CAPE and CAPE-pNO2 inhibit tumour growth in nude mice
HT-29 cells were xenografted into the nude mice to evaluate the effects of CAPE and CAPE-pNO2 on tumour growth. As shown in Fig. 6, following the administration of CAPE and CAPE-pNO2 by gavage for 42 days, tumour growth in the CAPE and CAPE-pNO2 groups was inhibited remarkably. Compared with the control, the tumour growth inhibition ratios in the CAPE and CAPE-pNO2 groups (5 mg/kg/day, 10 mg/kg/day, 20 mg/kg/day) were 45.0%, 50.0%, 55.7% and 63.2%, respectively (Fig. 6D). As shown in Fig. 6B, the sizes of tumours after treatment with these two substances were significantly smaller than those of the control group. The results of the tumour and organs by HE staining is shown in Fig. 6G (tumour) and Fig. S4 (heart, liver, spleen and kidney), and they showed that the necrotic area of tumours was expanded after treatment with CAPE and CAPE-pNO2, but there were no morphological changes in other organs, indicating that CAPE and CAPE-pNO2 had no visible toxicity. The results of the TUNEL assay in tumours showed that the dose-dependent effects of cell apoptosis induced by CAPE-pNO2 are stronger than those induced by CAPE (Fig. 6E). Furthermore, immunohistochemistry assay indicated that the expression of VEGF was reduced by 44.0%, 49.0%, 58.0% and 62.0%, respectively, after treatment with CAPE (10 mg/kg) and CAPE-pNO2 (5 mg/kg, 10 mg/kg and 20 mg/kg) (Fig. 6F).
Figure 7.
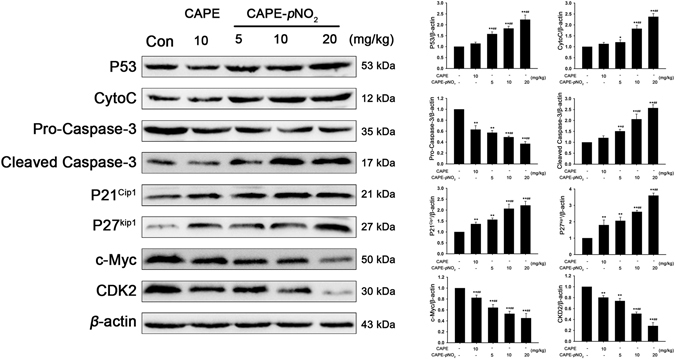
Regulation of proteins by CAPE and CAPE-pNO2 treatment in in tumours. The regulations of P53, Pro-Caspase-3, Cleaved Caspase-3, CytoC, P21Cip1, P21Kip1, c-Myc and CDK2 in tumours were detected by western blotting. The blots were representative of three independent experiments. Data represented the means ± SD from three independent experiments, and error bars represented the STDEV (SD). *p < 0.05, **p < 0.01: CAPE and CAPE-pNO2 compared with the control. # p < 0.05, ## p < 0.01: CAPE-pNO2 compared with CAPE at the same concentration.
Discussion
CAPE is a bioactive natural ingredient exacted from propolis and exhibits inhibition activity against various cancers, such as prostate cancer, breast cancer and colon cancer. In a previous study, the inhibitory effect of CAPE on colon cancer cells was determined. Wang D. et al.26 reported that CAPE induced HCT-116 cell cycle arrest in G0/G1 and apoptosis by decreasing the expression of β-catenin. Xiang D. et al.27 suggested that CAPE inhibited the proliferation of HCT-116 cells and SW480 cells via the β-catenin/T-cell signalling pathway by down-regulating cyclin D1 and c-myc, and En-Pei Isabel Chiang et al.28 discovered that caffeic acid phenylpropyl ester (CAPPE), a derivative of CAPE, could inhibit the growth of the HCT-116 and SW480 cells more significantly than CAPE through the PI3K/AKT and AMPK signalling pathways in vivo and in vitro. He, Y. J. et al.3 also proved that CAPE induced SW480 cell apoptosis by down-regulating PSMA1 and PSAT1 while up-regulating GNPDA1 and GPX-1. These studies proved that the signalling pathway that mediated the apoptosis of colon cancer cells and tumour after treatment with CAPE was not unique. P53 is one of the tumour suppressor proteins that has an intimate connection with the occurrence and progression of many tumours in humans, and it can induce apoptosis and cell cycle arrest of carcinoma29. The result in the above literature was not involved in the P53 signalling pathway after treatment with CAPE in colon cancer. By contrast, the P53 signalling pathway was selected to explore the anti-cancer mechanism of CAPE and CAPE-pNO2 in HT-29 cells.
CAPE-pNO2, a derivative of CAPE like CAPPE but with quite a different chemical structure, was synthesized by adding a nitro moiety to the para position in our laboratory. It was proven to be a platelet anticoagulant for collagen-induced platelet aggregation and a protector of acute myocardial ischaemia-reperfusion injury in previous studies24, 25, and these effects of CAPE-pNO2 were more potent than those of CAPE, but there is no report concerning the anti-cancer effect of CAPE-pNO2.
In this study, the MSI-type cell line HCT-116 and the MSS-type cell lines HT-29 and SW480 were selected to verify the anti-cancer effect of CAPE and explore the anti-cancer effect of CAPE-pNO2 in colon cancer. In the MTT assay, CAPE and CAPE-pNO2 inhibited cell proliferation in a dose-dependent manner, and the IC50 of CAPE-pNO2 was lower than that of CAPE. Moreover, the inhibitory effect of CAPE and CAPE-pNO2 was weaken when the expression of P53 was decreased by PFT-α, this result proved that P53 signalling pathway was one of the approaches which inhibited the proliferation of HT-29 and HCT-116 cells. Hoechst 33342 staining showed that the number of cancer cell was decreased, and the fluorescence intensity was increased with increasing concentrations of drugs. Compared with the CAPE treated group, after treated with CAPE-pNO2, the apoptosis rate of HT-29 cell and HCT-116 cells increased 15 and 8 percentage points (Fig. 2), the number of HT-29 cells and HCT-116 cells in G0/G1 phase were increased 10 and 5 percentage points (Fig. 3), these data showed that CAPE-pNO2 exhibited stronger inhibitory effect on HT-29 cells than that on HCT-116 cells. Based on the above results, we selected HT-29 cells of MSS type cell lines for the further study on anti-colon cancer mechanism of CAPE-pNO2 in vivo and in vitro.
The results of western blotting showed that both CAPE and CAPE-pNO2 could up-regulate P53, P38, Bax, CytoC and cleaved-caspase-3 and down-regulate pro-caspase-3. These proteins associated with the P53 signalling pathway were closely connected to the occurrence and growth of tumours30, and up-regulated CytoC proved that CAPE and CAPE-pNO2 could induce the mitochondrial apoptotic and active pro-caspase-3 to become cleaved-caspase-3, our results were consistent with those of Liu X. et al.31.
In a study of the cell cycle, CAPE and CAPE-pNO2 induced colon cancer cell cycle arrest in the G0/G1 phase; up-regulated P53, P21Cip1and P27Kip1; and down-regulated CDK2 and c-Myc. P53 mainly promoted tumour cell apoptosis and induced cell cycle arrest. When the cells of the body are damaged, P21Cip1 mRNA and protein expression levels are elevated following activation by P53 protein, and the cell cycle is blocked in G1, G2 or S phase32, 33. Down-regulated CDK2 by CAPE and CAPE-pNO2 treatment might induce pRb dephosphorylation to promote cancer cell ageing and prevent cell cycle progression from G1 to S phase34, 35. Up-regulated c-Myc induces cell cycle arrest in the G1 phase and inhibits the repair effects on telomeres, preventing the cells from being immortalized36, 37. Gao FH. et al. reported38 that oridonin can suppress colon cancer effectively by regulating the expression of c-Myc, P21Cip1 and P27Kip1. Compared with CAPE, CAPE-pNO2 more strongly induces up-regulation of Bax, cleaved-caspase-3, CytoC, P53, P38, P21Cip1 and P27Kip1 and down-regulation of pro-caspase-3, CDK2 and c-Myc. In one word, CAPE and CAPE-pNO2 inhibited proliferation of cells and suppressed tumours growth by regulating the P53 signalling pathway, and CAPE-pNO2 is more effective than CAPE in inhibiting cell growth, inducing apoptosis and cell cycle arrest in G0/G1 and suppressed tumours growth.
To explore the anticancer effect of CAPE-pNO2 in vivo, HT-29 cells were xenografted into nude mice. The turning point of the tumour growth curve appeared on the 37th, 35th and 33rd days after treating with CAPE-pNO2 at doses of 5, 10 and 20 mg/kg/day, respectively. However, the tumour growth curve in the CAPE (10 mg/kg/day) group showed a relatively steady trend on the 41st day. Based on the results in vitro, we used HT-29 cells to establish xenograft models. According to the report by En-Pei Isabel Chiang28, after injecting HCT-116 cells into nude mice, CAPE treatment lasted for six weeks. Although CAPE markedly inhibited the tumour growth, the growth trend of the treatment group showed a downward trend, a finding that was different from ours. The cause of this discrepancy might be the different cell lines used. Additionally, Wu J20 reported that the growth curves of xenograft tumours using MDA-231 cells and MCF-7 cells after treatment with CAPE were also different. At the end of experiment, all nude mice were euthanized, and the tumours were removed. The results of HE and TUNEL staining showed that CAPE and CAPE-pNO2 inhibited tumour growth through inducing tumour tissue necrosis and apoptosis. More importantly, CAPE-pNO2 exhibited more potent effects than CAPE on tumours. Interestingly, no morphological changes were found in the heart, liver, spleen and kidney after treatment with CAPE and CAPE-pNO2 for a long time. In clinical settings, many drugs used to cure colorectal cancer, such as 5-fluorouracil, have serious toxic and side effects and can even lead to patient death39, 40. Thus, CAPE-pNO2 might have great clinical application value. Meanwhile, the results of immunohistochemistry indicated that CAPE and CAPE-pNO2 decreased the expression of VEGF to disturb the pervasion and growth of tumours, while there was almost no expression of VEGF in normal colon tissues, and many reports have shown that the combination of VEGF with tyrosine kinases and neuropilins on the tumour cell surface promoted the progress of tumour invasion and cancer stem cell formation41–43, and VEGF could be related to the survival of patients with colorectal carcinoma and should be considered a predictor of the prognosis clinically44. Thus, CAPE-pNO2 may be regarded as a better inhibitor of VEGF in colon tumours (p < 0.01).
The nitro group at the para position was the only difference between CAPE and CAPE-pNO2. Consequently, our results imply that the anticancer effects of CAPE were enhanced by the para-nitro moiety. Similarly, it was confirmed that para-nitric oxide-donating acetylsalicylic acid was more purposeful in chronic lymphocytic leukaemia cells and more applicable to clinical treatment than NO-ASA45. For further study on the para nitro, LC-MS/MS was applied to investigate the difference in metabolites between CAPE and CAPE-pNO2 in HT-29 cells and in organs (tumour, heart, liver, spleen and kidney). In our results, the main difference is that CAPE can combine with the glucose acid, while para nitro-benzene alcohol combined with glucose acid after the hydrolysis of CAPE-pNO2. In CAPE, caffeic acid from CAPE hydrolysis was methylated; however, in CAPE-pNO2, caffeic acid from CAPE-pNO2 hydrolysis combined with L (+)-cysteine (maybe from the HT-29 cell line). C17H15NO5 only occurred in the metabolite of CAPE-pNO2.
On the other hand, the metabolites of CAPE and CAPE-pNO2 in HT-29 cells were different in the heart, liver, spleen and kidney. CAPE was transformed into C13H17NO3S, C8H11O4P and C14H16N2O6 in the heart, liver and kidney, respectively. Compared with CAPE, CAPE-pNO2 was transformed into 4 metabolites, C14H16N2O6, C8H11O4P, C10H14N2O6S and C16H11NO7. The differences in the metabolites in cells and organs may be due to the discrepancy of the species of metabolic enzyme and their types. However, there were no metabolites detected in tumours, implying that CAPE and CAPE-pNO2 were transformed to other compounds in tumours and need to be further studied in the future. Additionally, according to the pharmacokinetic analysis of CAPE and CAPE-pNO2 in rats, the half-lives (t1/2) of these compounds were 4.2 h and 20.9 h, respectively, and the area under concentration-time curve (AUCall) values were 1659 and 3239, respectively, indicating that the bioavailability of CAPE-pNO2 was higher than that of CAPE, and the metabolic processes of CAPE and CAPE-pNO2 would be different46. The most of above metabolites are inactive excrement of CAPE or CAPE-pNO2, and there are no literatures reported the anti-cancer effect of these metabolites detected in this study, the results of LC-MS/MS provided a basis for further study to explore the compounds to against colon cancer with stronger effect only.
Conclusion
In this study, para-nitro enhanced the anti-colon cancer activity of CAPE, and the present data showed that CAPE-pNO2 is more effective than CAPE in inducing colon cancer cell death, apoptosis and cell cycle arrest in the G0/G1 phase by regulating the relative proteins in the P53 pathway and inhibiting tumour growth. Moreover, CAPE-pNO2 significantly decreased the expression of VEGF. The metabolites of CAPE-pNO2 were different from those of CAPE in HT-29 cells and organs. This study contributes to further development of the pharmacological activity of CAPE-pNO2.
Materials and Methods
Materials
CAPE and p-nitro CAPE (CAPE-pNO2) were synthesized as previously described47. The chemical structures are shown in Fig. 1A and B. The human colon cancer cell line HT-29 and HCT-116 were purchased from the Cell Bank at the Chinese Academy of Sciences (Beijing, China). Dulbecco’s modified Eagle medium-high glucose (DMEM-H) and foetal bovine serum (FBS) were purchased from Gibco/Invitrogen (Carlsbad, CA, USA). Dimethyl sulfoxide (DMSO), trypsin, streptomycin, penicillin, 3-[4,5-dimethyl-2-thiazolyl]-2,5- diphenyl-2-tetrazolium bromide (MTT), propidium iodide (PI) and Hoechst 33342 were obtained from Sigma Aldrich (St. Louis, MO, USA). P53 inhibitor (Pifithrin-α) was purchased form Selleck (USA). An Annexin V-FITC Apoptosis Detection Kit was purchased from Keygen Biotech Co Ltd. (Nanjing, China). RNase A was acquired from Promega (Madison, WI). Bcl-2, Bax, cleaved-caspase-3, pro-caspase-3, P38, P21Cip1, P27Kip, P53, c-Myc, CDK2, VEGF and β-actin antibodies were purchased from Proteintech Group Inc. (Wuhan, China). For treatment, CAPE and CAPE-pNO2 were dissolved in DMSO and diluted to a final concentration with culture medium (DMSO concentration < 0.1%). All chemical reagents were used before the date of expiration.
Cell Culture
HCT-116 and HT-29 cells were maintained in DMEM-H supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C in a humidified incubator with 5% CO2 and 95% air.
Cell Viability Assay
HT-29 and HCT-116 cells were seeded at a density of 3 × 103 cells per well in 96-well plates and were incubated for 24 h. Next, different concentrations CAPE and CAPE-pNO2 were administered. For pifithrin-α (PFTα, P53 inhibitor) group, cells were treated with the above concentration of CAPE and CAPE-pNO2 after pre-treatment with 5 μM PFTα, and then the expression of P53 was detected by Western Bolt. After treatment with CAPE and CAPE-pNO2 for 48 h, MTT (5 μg/mL) was added to each well for 4 h at 37 °C. The amount of MTT crystals (formazan) dissolved by DMSO was measured using a Plate Reader (Bio Tek) at 570 nm. All experiments were repeated three times, and IC50 values were calculated.
Hoechst 33342 staining
Hoechst 33342 was dissolved in PBS, and the final concentration was 10 μg/mL. HT-29 and HCT-116 cells were treated with different concentrations of CAPE and CAPE-pNO2 after seeding in 6-well plates (8 × 104 per well). After 48 h, the cells were rinsed three times with PBS, and Hoechst 333342 was added. The cells were then incubated at 37 °C in the dark for 30 min. Thereafter, the dye liquor was removed, and the cells were washed three times with PBS. A fluorescence microscope (Olympus U-RFLT50, Tokyo, Japan) was used for the examination of each well at 6 different fields of view.
Cell apoptosis and cell cycle analysis by flow cytometry
HT-29 and HCT-116 cells were seeded in 6-well plates at a density of 8 × 104 per well. After 24 h, different concentrations CAPE and CAPE-pNO2 were added. Next, floating cells (digested by trypsin) were collected after treatment with CAPE and CAPE-pNO2 for 48 h. Cells were re-suspended in 500 μL of binding buffer after washing twice with PBS. Next, 5 μL of Annexin V-FITC and 5 μL of PI were added, and the cells were incubated for 1 h in the dark. Apoptosis was measured with a FACSCalibur flow cytometer (Keygen Biotech, Co Ltd, Nanjing, China). For cell cycle analysis, after treatment for 48 h, the cells were collected and then fixed with 70% ice-cold ethanol at 4 °C for 24 h. The stationary liquid was removed through centrifugation, and then 100 μL of RNase A and 400 μL of PI were added, followed by incubation at room temperature for 30 min without light. Next, a FACSCalibur flow cytometer (Becton-Dickinson) equipped with a 488-nm argon laser was used, and cell cycle analysis was performed using Cell Quest software and ModFit.
Western Blotting Analysis
The proteins of HT-29 cells were extracted after treatment with RIPA buffer and 1 mM PMSF (phenylmethylsulfonyl fluoride) on the ice for 30 min, the proteins of tumours were extracted after homogenate with RIPA buffer and 1 mM PMSF at 4 °C for 30 min. Proteins with different molecular weights were separated on 6.0%, 8.0% and 12% SDS-polyacrylamide gels. The proteins on the gel were transferred to a PVDF membrane, which was soaked for 10 seconds with methanol. Next, 5.0% skim milk prepared with PBST (0.1% Tween-20 and 99.9% PBS) was used to block the membrane for 1.5 h. Thereafter, the blots were incubated with primary antibody overnight at 4 °C. The blots were then incubated with the corresponding HRP-linked secondary antibody for 1.5 h after washing with PBST three times. The PVDF membranes were developed by ECL (enhanced chemiluminescence). The protein expression levels were determined by the grey values from the PVDF membrane that were calculated using software Quantity One.
Analysis of metabolites by LC-MS/MS
HT-29 cells were washed 3 times with PBS and then broken ultrasonically to collect metabolites after treatment for 48 h. Thereafter, centrifugation (1400 × g, 5 min) was applied to separate cell debris and metabolite liquid before injection into the LC-MS/MS instrument. Tissue samples were cut into small sections and then were mixed with liquid (methanol:acetonitrile:acetone:water = 3:3:3:1) to break cells; after homogenates were obtained, the mixed liquor was centrifuged (1600 × g, 10 min), and the supernatant was extracted for LC-MS/MS. LC-30AD (Shimadzu, Japan) conditions were as follows: a Kinetex XB-C18 column (2. 1 mm × 100 mm, 2.6 μm); a mobile phase of acetonitrile and ultrapure water with 0.1% formic acid; a flow rate of 300 μL/min; a column temperature of 30 °C; Triple TOF 4600 (Absciex USA): ionization mode included positive and negative; mass scanning range m/z: 50–300; sheath gas: 55 Pa; auxiliary gas: 55 Pa; curtain gas: 25 Pa; atomization temperature: 600 °C; scanning mode: TOF-MS-Product Ion-IDA; declustering potential: 80 V; CE collision energy 35 eV; CES collision energy: 35 ± 15 eV. Metabolites were analysed by the software Analyst TF 1.6, Peakview (Version 1.2, AB Sciex) and MetabolitePlot (Version 1.5, AB Sciex).
Xenografts in athymic mice
Four-week-old Male BALB/c nude mice were purchased from the Beijing HFK Bioscience Co Ltd. (Beijing China) and were placed in specific pathogen-free (SPF) conditions. Trypsinized HT-29 cells were injected into the right flanks of athymic mice at a density of 2 × 107/0.1 mL. Water and food for mice were sterilized. When the size of the tumour was approximately 100 mm3, the mice were divided into five groups of 10 mice each. These five groups were the control group, CAPE group (10 mg/kg/day), and CAPE-pNO2 groups (5 mg, 10 mg and 20 mg/kg/day). The tumour volumes were measured every 3 days using a Vernier calliper and were quantitated according to the formula (length × width2 × π)/648; the inhibition rate was calculate by the formula inhibition rate (%) = [1 − (the weight of tumours in the experimental/the weight of tumours in control)] × 100%. The tumours were removed and used for detecting other biochemical indexes after CAPE and CAPE-pNO2 were given for 42 days.
Haematoxylin and Eosin staining
After treatment, tumours and organs were removed from nude mice and were fixed with 4.0% paraformaldehyde. Paraffin sections were dewaxed to water for different experiments. For HE (haematoxylin and eosin) staining, sections were put into haematoxylin for 8 min and then washed twice before incubating with eosin for 3 min. Finally, the sections were sealed with neutral gum. Nuclei appeared bluish, while the cytoplasm appeared red through the microscope.
TUNEL staining
Paraffin sections were covered with broken membrane fluid for 20 min. The TUNEL kit reagents were added to the sections for 2 h at 37 °C while maintaining the humidity. Next, endogenous peroxidase was blocked by 3.0% hydrogen peroxide solution prepared in methanol for 15 min. DAB chromogen was used for staining; when positive cells became brown, the staining was stopped by washing with distilled water. Finally, the nuclei were stained with haematoxylin before sealing.
Immunohistochemistry
Antigen repair buffer (pH 9.0) was added before blocking endogenous peroxidase. The sections were sealed with 3.0% BSA for 30 min. The sections were incubated with the primary antibody (VEGF) overnight at 4 °C. After incubating with PBS three times, the sections were incubated with the corresponding secondary antibody for 50 min. Next, DAB and haematoxylin were used for staining as previously described. The IOD value measured the expression of VEGF.
Statistical analysis
All data were analysed by SPASS 16.0, the data normality was verified by K-S test, and if the P value was more than 0.05, the data obeyed a normal distribution. The data were presented as the means ± SD for at least three independent experiments. One-way ANOVA and Student’s t-test were performed for statistical analysis. A P value less than 0.05 was considered statistically significant.
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. All animal procedures were approved by the Ethical Committee for Animal Experiments of Southwest University (Permit Number: SYXK 2015-0002). All efforts were made to minimize suffering.
Electronic supplementary material
Acknowledgements
We would like to thank the Key Program Projects of the Beijing Hua Mu-weiye science and Technology Co. Ltd, China (grant No. SWU2010015) and Shanxi Zhaoyi Biological Co Ltd, China (Grant No. SWU2013130) for financial support.
Author Contributions
Z.L., H.T. and X.Y. designed the project; H.T. and X.Y. performed the experiments; Z.L., H.T. and X.Y. wrote the main manuscripts; H.T. and X.Y. prepared figures; all authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Hao Tang and Xiaofang Yao contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07953-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, et al. Global Cancer Statistics, 2012. CA-A Cancer Journal for Clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, et al. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 3.He YJ, et al. Identification of differential proteins in colorectal cancer cells treated with caffeic acid phenethyl ester. World J Gastroentero. 2014;20:11840–11849. doi: 10.3748/wjg.v20.i33.11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melincovici CS, et al. The prognostic significance of p53, Bax, Bcl-2 and cyclin E protein overexpression in colon cancer-an immunohistochemical study using the tissue microarray technique. Rom J Morphol Embryol. 2016;57:81–89. [PubMed] [Google Scholar]
- 5.Shirali S, et al. Adenosine induces cell cycle arrest and apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian cancer cell line OVCAR-3. Tumor Biol. 2013;34:1085–1095. doi: 10.1007/s13277-013-0650-1. [DOI] [PubMed] [Google Scholar]
- 6.Xu D, et al. Apoptotic block in colon cancer cells may be rectified by lentivirus mediated overexpression of caspase-9. Acta Gastro-ent Bel. 2013;76:372–380. [PubMed] [Google Scholar]
- 7.Brown MF, et al. Loss of Caspase-3 sensitizes colon cancer cells to genotoxic stress via RIP1-dependent necrosis. Cell Death & Disease. 2015;6:e1729. doi: 10.1038/cddis.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kugimiya N, et al. The c-MYC-ABCB5 axis plays a pivotal role in 5-fluorouracilresistance in human colon cancer cells. J. Cell. Mol. Med. 2015;19:1568–1581. doi: 10.1111/jcmm.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim TG, et al. Curcumin Suppresses Proliferation of Colon Cancer Cells by Targeting CDK2. Cancer Prevention Research. 2014;7:466–474. doi: 10.1158/1940-6207.CAPR-13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DE, et al. 6,7,4’-Trihydroxyisoflavone inhibits HCT-116 human colon cancer cell proliferation by targeting CDK1 and CDK2. Carcinogenesis. 2011;32:629–635. doi: 10.1093/carcin/bgr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, et al. c-Myc enhances colon cancer cell-mediated angiogenesis through the regulation of HIF-1 alpha. Biochem Bioph Res Co. 2013;430:505–511. doi: 10.1016/j.bbrc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang JJ, et al. Role of p21 as a determinant of 1,6-Bis[4-(4-amino-3-hydroxyphenoxy)phenyl] diamantane response in human HCT-116 colon carcinoma cells. Oncology Reports. 2012;27:529–534. doi: 10.3892/or.2011.1546. [DOI] [PubMed] [Google Scholar]
- 13.Sulaiman GM, Al-Amiery AA, Bagnati R, Sulaiman GM. Theoretical, antioxidant and cytotoxic activities of caffeic acid phenethyl ester and chrysin. Int J Food Sci Nutr. 2014;65:101–105. doi: 10.3109/09637486.2013.832174. [DOI] [PubMed] [Google Scholar]
- 14.Dos Santos JS, Monte-Alto-Costa A. Caffeic Acid Phenethyl Ester Improves Burn Healing in Rats Through Anti-Inflammatory and Antioxidant Effects. J Burn Care Res. 2013;34:682–688. doi: 10.1097/BCR.0b013e3182839b1c. [DOI] [PubMed] [Google Scholar]
- 15.Firat U, et al. The effects of caffeic acid phenethyl ester (CAPE) on bacterial translocation and inflammatory response in an experimental intestinal obstruction model in rats. Eur Rev Med Pharmacol Sci. 2015;19:1907–1914. [PubMed] [Google Scholar]
- 16.Lin HP, et al. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21(Cip1) and p27(Kip1) Oncotarget. 2015;6:6684–6707. doi: 10.18632/oncotarget.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan XJ, et al. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. Faseb J. 2008;22:3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, et al. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011;308:43–53. doi: 10.1016/j.canlet.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu TH, et al. Caffeic acid phenethyl ester induces E2F-1-mediated growth inhibition and cell-cycle arrest in human cervical cancer cells. Febs J. 2013;280:2581–2593. doi: 10.1111/febs.12242. [DOI] [PubMed] [Google Scholar]
- 20.Onori P, et al. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappa B and induction of apoptosis. Int J Cancer. 2009;125:565–576. doi: 10.1002/ijc.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulasli SS, et al. Anticancer Effects of Thymoquinone, Caffeic Acid Phenethyl Ester and Resveratrol on A549 Non-small Cell Lung Cancer Cells Exposed to Benzo(a)pyrene. Asian Pac J Cancer Prev. 2013;14:6159–6164. doi: 10.7314/APJCP.2013.14.10.6159. [DOI] [PubMed] [Google Scholar]
- 22.Beltran-Ramirez O, Perez RM, Sierra-Santoyo A, Villa-Trevino S. Cancer Prevention Mediated by Caffeic Acid Phenethyl Ester Involves Cyp2b1/2 Modulation in Hepatocarcinogenesis. Toxicol Pathol. 2012;40:466–472. doi: 10.1177/0192623311431947. [DOI] [PubMed] [Google Scholar]
- 23.Kuo YY, et al. Caffeic Acid phenethyl ester is a potential therapeutic agent for oral cancer. Int J Mol Sci. 2015;16:10748–10766. doi: 10.3390/ijms160510748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou K, et al. A CAPE analogue as novel antiplatelet agent efficiently inhibits collagen-induced platelet aggregation. Pharmazie. 2014;69:615–620. [PubMed] [Google Scholar]
- 25.Du Q, et al. Protective effects of p-nitro caffeic acid phenethyl ester on acute myocardial ischemia-reperfusion injury in rats. Exp Ther Med. 2016;11:1433–1440. doi: 10.3892/etm.2016.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, et al. Effect of caffeic acid phenethyl ester on proliferation and apoptosis of colorectal cancer cells in vitro. World J Gastroenterol. 2005;11:4008–12. doi: 10.3748/wjg.v11.i26.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang DB, et al. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the beta-catenin/T-cell factor signaling. Anticancer Drugs. 2006;17:753–762. doi: 10.1097/01.cad.0000224441.01082.bb. [DOI] [PubMed] [Google Scholar]
- 28.Chiang EPI, et al. Caffeic Acid Derivatives Inhibit the Growth of ColonCancer: Involvement of the PI3-K/Akt and AMPK Signaling Pathways. PLoS One. 2014;9:e99631. doi: 10.1371/journal.pone.0099631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ataie-Kachoie P, Pourgholami MH, Bahrami-B F, Badar S, Morris DL. Minocycline attenuates hypoxia-inducible factor-1α expression correlated with modulation of p53 and AKT/mTOR/p70S6K/4E-BP1 pathway in ovarian cancer: in vitro and in vivo studies. Am J Cancer Res. 2015;5:575–588. [PMC free article] [PubMed] [Google Scholar]
- 30.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 31.Liu XS, Kim CN, Yang J, Jemmerson R, Wang XD. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/S0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 32.Abbasi MM, Helli S, Monfaredan A, Jahanban-Esfahlan R. Hesa-A Improves Clinical Outcome of Oral Carcinoma by Affecting p53 Gene Expression in vivo. Asian Pac J Cancer Prev. 2015;16:4169–72. doi: 10.7314/APJCP.2015.16.10.4169. [DOI] [PubMed] [Google Scholar]
- 33.Ataie-Kachoie P, Pourgholami MH, Bahrami-B F, Badar S, Morris DL. Minocycline attenuates hypoxia-inducible factor-1 alpha expression correlated with modulation of p53 and AKT/mTOR/p70S6K/4E-BP1 pathway in ovarian cancer: in vitro and in vivo studies. Am J Cancer Res. 2015;5:575–588. [PMC free article] [PubMed] [Google Scholar]
- 34.Burns DM, D’Ambrogio A, Nottrott S, Richter JD. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature. 2011;473:105–U125. doi: 10.1038/nature09908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esteras N, et al. Downregulation of extracellular signal-regulated kinase 1/2 activity by calmodulin KII modulates p21(Cip1) levels and survival of immortalized lymphocytes from Alzheimer’s disease patients. Neurobiol Aging. 2013;34:1090–1100. doi: 10.1016/j.neurobiolaging.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Xu DW, et al. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc Natl Acad Sci U S A. 2001;98:3826–3831. doi: 10.1073/pnas.071043198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson LG, Henriksson MA. The Yin and Yang functions of the Myc oncoprotein in cancer development and as targets for therapy. Exp Cell Res. 2010;316:1429–1437. doi: 10.1016/j.yexcr.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 38.Gao FH, et al. Oridonin induces apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c-myc. BMC Cancer. 2010;10:610. doi: 10.1186/1471-2407-10-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: Current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol. 2004;22:2214–2232. doi: 10.1200/JCO.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Mazzuca F, et al. Pre-treatment evaluation of 5-fluorouracil degradation rate: association of poor and ultra-rapid metabolism with severe toxicity in a colorectal cancer patients cohort. Oncotarget. 2016;7:20612–20620. doi: 10.18632/oncotarget.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bendardaf R, et al. VEGF-1 Expression in Colorectal Cancer is Associated with Disease Localization, Stage, and Long-term Disease-specific Survival. Anticancer Res. 2008;28:3865–3870. [PubMed] [Google Scholar]
- 42.Ahluwalia A, Jones MK, Szabo S, Tarnawski AS. Aberrant, ectopic expression of VEGF and VEGF receptors 1 and 2 in malignant colonic epithelial cells. Implications for these cells growth via an autocrine mechanism. Biochem Biophys Res Commun. 2013;437:515–520. doi: 10.1016/j.bbrc.2013.06.096. [DOI] [PubMed] [Google Scholar]
- 43.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Razavi, R. Nitric Oxide-Donating Acetylsalicylic Acid Induces Apoptosis in Chronic Lymphocytic Leukemia Cells and Shows Strong Antitumor Efficacy In vivo. Clin Cancer Res. 17, 286–293, doi:10.1158/1078-0432.CCR-10-1030 (2011). [DOI] [PubMed]
- 45.Bendardaf R, El-Serafi A, Syrjanen K, Collan Y, Pyrhonen S. The effect of vascular endothelial growth factor-1 expression on survival of advanced colorectal cancer patients. Libyan J Med. 2017;12:1290741. doi: 10.1080/19932820.2017.1290741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gou J, et al. Absorption properties and effects of caffeic acid phenethyl ester and its p-nitro-derivative on P-glycoprotein in Caco-2 cells and rats. Pharm Biol. 2016;54:2960–2967. doi: 10.1080/13880209.2016.1197284. [DOI] [PubMed] [Google Scholar]
- 47.Liu TL, et al. Novel Caffeic Acid Phenethyl Ester (CAPE) Analogues as Immunoregulatory Agents: Synthesis and SAR Study. Lat Am J Pharm. 2013;32:329–334. [Google Scholar]
- 48.Chiu YW, et al. Baicalein inhibits the migration and invasive properties of human hepatoma cells. Toxicol Appl Pharmacol. 2011;255:316–326. doi: 10.1016/j.taap.2011.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


